In 2002, Osteoporosis Canada published clinical practice guidelines for the diagnosis and management of osteoporosis in Canada.1 At the time, there was considerable evidence for the use of anti-resorptive agents in the management of osteoporosis, including estrogen, the selective estrogen receptive modulator, raloxifene, several approved bisphosphonates (alendronate, etidronate and risedronate) and calcitonin. For these agents, much information exists to document their primary efficacy in preventing fractures, the most important outcome of osteoporosis.
In contrast to anti-resorptive drugs, anabolic agents result in the formation of new bone in both trabecular and cortical envelopes and thus partially repair the deterioration in micro architecture that leads to the increased fragility of adult osteoporotic bone. Sodium fluoride was the first of such agents to be evaluated in randomized controlled trials, but the results showed that the bone of patients receiving the drug was of poor quality and that the incidence of fractures was not reduced even though large measurable increments in bone mineral density (BMD) were seen. In the 2002 guidelines, parathyroid hormone (PTH) was mentioned only briefly because the pivotal phase III trial of teriparatide (PTH[ 1–34]) had only just been published,2 and regulatory approval for its clinical use had not yet been obtained in Canada.
Osteoporosis Canada updates its clinical guidelines at intervals, when a sufficient body of evidence becomes available to allow a considered interpretation of the place new therapies should occupy in the management of osteoporosis. It has now been possible for the Clinical Guidelines Committee of Osteoporosis Canada to perform a systemic review of trials evaluating PTH for the treatment of osteoporosis. Its findings appear in this issue of CMAJ (see page 52).3 Both the systematic review and the recommended guidelines were reviewed by the Scientific Advisory Council of Osteoporosis Canada (members of which include primary care physicians), together with a panel of patients with osteoporosis. Teriparatide has been most widely used. There is only limited information on the full sequence hormone PTH(1–84), given that the results of the phase III antifracture trial have yet to be published in full. Another analogue, cyclic PTH(1–31) amide, is currently undergoing phase II evaluation.
The systematic review was undertaken to review studies of the efficacy and safety of PTH for the treatment of osteoporosis in men and postmenopausal women and of osteoporosis caused by glucocorticoids. The primary search focused on outcomes that included either BMD or fractures. Secondary outcomes were back pain and quality of life. There was level 1 evidence that teriparatide treatment for 21 months resulted in significant reductions in both vertebral and nonvertebral fractures in postmenopausal women with previous vertebral fractures. Consistent with its anabolic action, teriparatide and intact PTH significantly increased BMD at all skeletal sites except the radius (level 1 evidence). There were initial concerns that the neutral effects of PTH on radial BMD might translate into increased fragility of tubular long bones, but these have not been realized. PTH leads to periosteal new bone formation along the outer cortical surfaces of long bones. According to biomechanical theory, small increments in the external diameter of a cylindrical structure translate into marked increments in mechanical strength. This is consistent with the findings of the reduced incidence of nonvertebral fractures seen in the phase III RCT of teriparatide.2
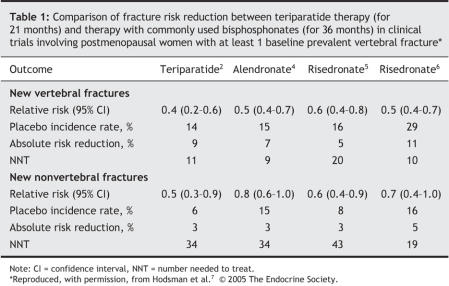
There remain a number of important issues surrounding the use of PTH and its interactions with bisphosphonates. First, PTH has not yet proven to be more effective than bisphosphonates in reducing incident osteoporotic fractures during adequate head-to-head comparator trials. Thus far, the only head-to-head trials have been small comparisons between teriparatide and alendronate, and they have shown a greater efficacy on BMD and a trend toward improved fracture incidence in favour of teriparatide. Table 1 summarizes the historical antifracture efficacy of teriparatide and the 2 most commonly used bisphosphonates in Canada, alendronate and risedronate.2,4–7 Second, prior or concurrent use of alendronate appears to diminish the increments in BMD and biochemical markers of bone turnover induced by teriparatide. Until this interaction is clarified, the optimal use of teriparatide should be in bisphosphonate-naive patients. This is a dilemma for insurers, who typically require failure of less expensive treatments before considering coverage of more expensive options.
Table 1
Since teriparatide's approval in Canada in June 2004 for the treatment of osteoporosis, its uptake has been slow. In 2005, the number of prescriptions for teriparatide accounted for less than 1% of all prescriptions for drugs used in the treatment of osteoporosis. In large part, this may be due to lack of formulary access and the high cost of the therapy. In Canada, treatment with teriparatide is limited to a maximum of 18 months, in part because of carcinogenicity studies demonstrating an increased incidence of osteosarcoma in rats treated from “adolescence” through to senescence. The current direct medication cost for an 18-month course of teriparatide is $15 875, although the manufacturer has recently introduced a discounted pricing system available to patients with limited ability to pay for their own drug supply. In December 2004, the Canadian Expert Drug Advisory Committee recommended that provinces not include teriparatide in their formularies because of the high cost of treatment and the lack of a convincing cost-utility analysis to justify its inclusion.8 At the time of writing, there was no access to teriparatide under any of the provincial formularies except in Quebec. The objectives of the systematic review of PTH conducted by Osteoporosis Canada did not include a review of cost-utility analyses, but it is appropriate to take these realities into account in formulating our guidelines.
One way to compare the cost-effectiveness of osteoporosis therapies is to determine the number needed to treat (NNT) to prevent a fracture. Table 1 shows the historical antifracture efficacy of teriparatide and the 2 most widely used bisphosphonates, alendronate and risedronate, as reported in the primary phase III clinical trials of each drug. These data are not derived from head-to-head comparisons. They were are taken from RCTs involving postmenopausal women who had at least 1 prevalent vertebral fracture. In the 4 trials cited, the mean age of the study cohorts ranged from 69 to 71 years and treatment duration from 21 months (teriparatide) to 3 years (alendronate and risedronate). Although there may have been other factors influencing future fracture risk, the 4 study populations are comparable. In the absence of head-to-head trials, this is a pragmatic way to compare efficacy, because age and the prevalence of fractures before initiating therapy were primary determinants of fracture rates and therefore greatly influence the NNT. As can be seen in Table 1, the apparent relative efficacy of teriparatide and bisphosphonates is similar in this population. However, we believe that simple comparisons of NNT calculations should not be the only criterion by which to compare the benefits of teriparatide with bisphosphonates, especially in this case in which the underlying fracture risk in the RCT of teriparatide may in fact have been much higher, and the duration of treatment much shorter, than that in the RCTs evaluating alendronate and risedronate; both factors may have obscured the superiority of teriparatide over these bisphosphonates.9
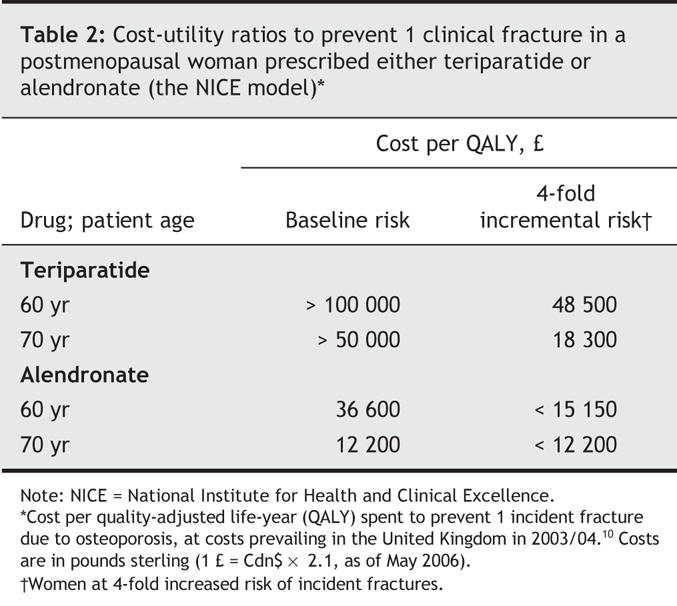
The National Institute for Health and Clinical Excellence (NICE) in the United Kingdom has compared the cost-utility ratios between the bisphosphonates, raloxifene and teriparatide using a modified individual Markov approach. The baseline model examined the cost-utility ratio of these agents in postmenopausal women with at least 1 prevalent vertebral fracture and a T-score of less than –2.5, stratified by ages 50 through 80 years. The modelled risk was adjusted for the severity of underlying osteoporosis, such that patients with either (a) 2 or more fractures, a T-score of less than –3.0 and an additional major but nonmodifiable risk factor or (b) an “extremely” low T-score of less than –4.0 would have a 4-fold higher risk of fracture than that expected of the baseline model. For simplicity, Table 2 outlines the cost-utility ratios (calculated in pounds sterling per quality-adjusted life-year [QALY]) to prevent 1 clinical fracture in a postmenopausal woman treated with either teriparatide or alendronate.10 As can be seen, the cost-utility ratio becomes more favourable when treatment is given to older patients, and the ratio for teriparatide approaches that for alendronate only among women at 4-fold increased risk of incident fractures. The NICE analysis has the advantage that generally agreed on quantifiable risks and benefits were applied within a single health care system (the United Kingdom); the much higher cost-utility ratio for teriparatide as compared with the ratio for the bisphosphonate is driven by the high cost of teriparatide rather than its efficacy. However, in the absence of head-to-head fracture trials, this analysis may well underestimate the potential superiority of teriparatide over bisphosphonates.
Table 2
Recommendations
On the basis of these considerations, the Clinical Guidelines Committee of Osteoporosis Canada suggests the following recommendations for the use of teriparatide, recognizing that these may or may not be applicable to the use of other PTH analogues as more data become available. The grading of recommendations involved the use of consensus techniques with a panel of expert consultants and followed the same grading technique used in the 2002 clinical practice guidelines.1 Recommendations were assigned a grade of D if they were based only on a committee consensus in the absence of clear supporting evidence or when the evidence was weak. All recommendations were reviewed by the guidelines committee, and if appropriate, the assigned level of evidence or grade of recommendation was modified.
• On the basis of fracture efficacy data, teriparatide should be recommended as a first-line therapy for women 65 years or older who have prevalent vertebral fractures and low BMD (T-score ≤ –2.5) (grade A). Given the evolving evidence that bisphosphonates might blunt the effectiveness of teriparatide, patients selected for treatment should ideally be bisphosphonate-naive (grade B).
• On the basis of the results of a cost-effectiveness study, teriparatide should be reserved for the most severely affected patients (those with more than 1 fragility fracture and very low BMD) (grade B). Until there is direct comparative evidence that teriparatide has superior antifracture efficacy to bisphosphonates, the latter are a more appropriate initial choice for patients with less severe osteoporosis (grade D).
• Other potential candidates for teriparatide include postmenopausal women with very low BMD (T-score ≤ –3.5) and those who continue to have fragility fractures despite an adequate trial of bisphosphonates (2-year period) (grade D).
• Teriparatide should be considered as a second-line therapy for men 65 years or older who have severe osteoporosis and prevalent fragility fractures (grade B) or patients who are taking long-term corticosteroid therapy and have corticosteroid-induced osteoporosis and prevalent fractures (grade D).
• On the basis of BMD outcomes, it is recommended that alendronate (grade B) and probably other bisphosphonates (grade D) should be discontinued before starting therapy with teriparatide. There is no evidence on whether there should be a washout period.
• Therapy with an anti-resorptive agent after completing teriparatide therapy is recommended to maintain or increase BMD (grade B).
• Baseline serum levels of calcium, PTH, uric acid, creatinine and 25-hydroxyvitamin D [25(OH)D] should be measured — and confirmed to be within acceptable normal limits — before therapy with teriparatide is started. Serum calcium levels should be measured again — before injection — after the first month of therapy, since mild hypercalcemia will develop in a minority (about 10%) of patients during treatment. Hypercalcemia can usually be managed by reducing the intake of oral calcium supplements and, if necessary, by reducing the teriparatide injection frequency to alternate days (grade D). In patients with a history of renal stones, calcium levels in 24-hour urine collections should be measured (grade D).
• The duration of teriparatide therapy should be limited to a maximum of 18 months because of the lack of long-term safety data (grade D).
• Supplemental calcium intake should be limited to 500 mg/d to minimize the risk of hypercalcemia. Vitamin D supplements of 800 IU/d are particularly important in preventing subclinical vitamin D deficiency during teriparatide treatment (grade D).
• Use of teriparatide is contraindicated in children and adolescents and in people with a history of skeletal irradiation or Paget's disease, or both (grade D).
• Teriparatide should be avoided in patients who have primary hyperparathyroidism or who have significant renal impairment or vitamin D deficiency. It should be used with caution in people with a history of gout (grade D).
@ See related article page 52
Acknowledgments
The Clinical Guidelines Committee of Osteoporosis Canada received administrative support from Osteoporosis Canada to hold the in-person meetings needed to create the guidelines and to cover the costs for the literature review and analysis. Contributing sponsors to Osteoporosis Canada included Procter & Gamble Canada (now known as Sanofi-Aventis Canada), Eli-Lilly Canada and Merck Frosst Canada. Funds were used as unrestricted grants to further development of clinical practice guideline by Osteoporosis Canada following the publication of the first set of clinical practice guidelines in 2002; they were not donated for the specific purpose of creating the guidelines for parathyroid hormone therapy.
Footnotes
Contributors: All of the authors contributed equally to the writing and revision of the manuscript and approved the final version submitted for publication.
Competing interests: Ann Cranney has been a member of the medical advisory boards for Merck Frosst Canada and Zelos Therapeutics and has received speaker fees from Aventis, Procter & Gamble Pharmaceuticals, Eli Lilly and Merck Frosst Canada. Anthony Hodsman is a member of the medical advisory boards of Eli Lilly Canada, Sanofi-Aventis, Zelos Therapeutics, NPS Allelix, Novartis Canada, Servier Canada and Merck Frosst Canada. He has participated in clinical trials funded by Eli Lilly, Novartis, NPS Allelix, Sanofi-Aventis and Zelos Therapeutics. Alexandra Papaioannou has received speaker fees from Eli Lilly Canada, Novartis, Merck Frosst Canada, Amgen, Sanofi-Aventis and Procter & Gamble Pharmaceuticals; she has received unrestricted educational grants from Eli Lilly Canada, Merck Frosst Canada, Sanofi-Aventis and Procter & Gamble Pharmaceuticals.
Correspondence to: Dr. Anthony Hodsman, Division of Nephrology, Rm. F-215, St. Joseph's Health Centre, 268 Grosvenor St., London ON N6A 4V2; anthony.hodsman@sjhc.london.on.ca
REFERENCES
- 1.Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ 2002;167(10 Suppl):S1-S34. [PMC free article] [PubMed]
- 2.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434-41. [DOI] [PubMed]
- 3.Cranney A, Papaioannou A, Zytaruk N, et al; Clinical Guidelines Committee of Osteoporosis Canada. Parathyroid hormone for the treatment of osteoporosis: a systematic review. CMAJ 2006;175(1):52-9. [DOI] [PMC free article] [PubMed]
- 4.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348:1535-41. [DOI] [PubMed]
- 5.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postemopausal osteoporosis. JAMA 1999;282:1344-52. [DOI] [PubMed]
- 6.Reginster JY, Minne HW, Sorenson OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 2000;11:83-91. [DOI] [PubMed]
- 7.Hodsman AB, Bauer DC, Dempster D, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 2005;26:688-703. [DOI] [PubMed]
- 8.Canadian Expert Drug Advisory Committee (CEDAC). CEDAC final recommendation on reconsideration and reasons for recommendation: teriparatide (ForteoTM– Eli Lilly Canada Inc.). Ottawa: Canadian Agency for Drugs and Technologies in Health; 2004. Available: www.cadth.ca/media/cdr/complete/cdr_complete_Forteo_2004Dec22.pdf (accessed 2006 June 8).
- 9.Schechtman E. Odds ratio, relative risk, absolute risk reduction, and the number needed to treat — Which of these should we use? Value Health 2002;5:431-6. [DOI] [PubMed]
- 10.National Institute for Health and Clinical Excellence. Osteoporosis — secondary prevention: the clinical effectiveness and cost effectiveness of technologies for the secondary prevention of osteoporotic fractures in postmenopausal women [Technology Appraisal no TA087]. London (UK): National Institute for Health and Clinical Excellence; 2005. Available: www.nice.org.uk/page.aspx?o=TA087 (accessed 2006 Jun 5).