Abstract
Fragility fractures at the trochanter (TR) and the femoral neck (FN) have distinct etiologies, but the underlying age-related structural changes at these proximal femoral sub-regions are poorly understood. 28 young (41 ± 3 years) and 124 elderly (74 ± 3 years) healthy Caucasian women underwent volumetric quantitative computed tomography at the hip. Integral (i), cortical (c) and trabecular (t) bone mineral density and content (BMD, BMC) were measured. Geometric parameters included cross sectional area (CSA), and volumes of the integral, cortical and trabecular regions (VOL). Structural measures included indices of compressive (Compstr) and bending (BSI) strength. After adjusting for height and weight, an F-test was used to compare the TR and the FN mean values between young and elderly and to test for interaction to compare logarithmic difference of young and elderly (log(Young)-log(Elderly), Y/Ed) between the FN and the TR in an ANOCOVA model. All BMC, iBMD and tBMD values were significantly lower in elderly than in young women, with the largest Y/Edin the FN tBMC and tBMD (P < 0.0011 and P < 0.0001). cBMD in young and elderly groups was not significantly different at the TR while at the FN it was greater (P = 0.0075) in elderly than young women, showing significant Y/Ed(P = 0.0003) dependence on skeletal site. Elderly women had significantly larger iVOL and CSA values (0.0001 < P < 0.0051), except for the FN iVOL. cVOL values were smaller in elderly than young women (P < 0.0001). Y/Edin bone geometry differed by sub-region only for cVOL measures (P = 0.0267). Despite larger CSA and iVOL measures in elderly, the younger women had greater Compstr (P < 0.0001) and BSI (P = 0.0051). Thus, although both the TR and the FN appear to increase in size with age, this enlargement is insufficient to protect against loss of bone strength.
Keywords: Computed tomography, Bone mineral density, Bone volume, Bone strength, Osteoporosis-pathophysiology
Introduction
Throughout life, the skeleton adapts itself to the changes that occur in its loading environment [15,25,34]). In load bearing bones, regional muscle contractions generate loads at the attachment site that are greater than body weight [6,27]. Therefore the loading environment of each skeletal site will be determined not only by body weight, but also by the loads generated by the contractions of muscles attached to that particular skeletal site. This idea is evidenced by the sub-regional variations in the structure of the proximal femur. The trochanter (TR) and the femoral neck (FN) constitute two sub-regions of the proximal femur that are structurally different and that are subjected to different loading conditions. While both the TR and the FN are subject to weight bearing and muscle forces, only the TR is a muscle attachment site. Because the bone geometry and sub-regional material properties are adapted to sustain these local applied loads, the TR and the FN sites may show different age-related changes in geometry and volumetric bone mineral density.
Epidemiologic data showing clearly distinct etiologies for the TR and the FN fractures underscore the relevance of the structural differences of these two sub-regions of the hip. In white women, but not in African-American women or men, the ratio of TR to FN hip fractures increases with age, and in both genders and all ethnicities, the TR fractures are more common in frailer individuals [11,14,16-18,20,22,28].
Despite these clear differences, and the fact that trochanteric fractures account for over half the hip fracture incidence in Caucasian women over age 70 [8], structural investigations of aging in the hip have mainly focused on the femoral neck. Various reports have supported the role of periosteal apposition as a mechanism to preserve bone strength in the context of age-related trabecular and cortical bone loss[1,3,7,9,12,19,30,31,33,36,37]. However, none of these reports have focused on examining age-related material and geometric changes in the trochanter and comparing these changes to those observed in the femoral neck.
To investigate age-related changes in volumetric bone mineral density (vBMD) and geometry in the TR and the FN, we performed a cross-sectional comparison of Caucasian young and elderly women. We used volumetric quantitative computed tomography (vQCT) to estimate bone mass, vBMD, bone size and indices of structural strength. The use of vQCT allowed us to separately analyze changes in cortical and trabecular bone in those two sub-regions. We hypothesized that in healthy women, the TR and the FN would show different age-related changes in bone mass, density and geometry in the cortical and trabecular compartments. We also hypothesized that these changes would tend to protect the structural strength of the hip against age-related bone loss.
Materials and methods
Subjects
In this retrospective cross-sectional study, we included two groups of healthy Caucasian women living in the San Francisco Area recruited independently for two different studies. For each study we considered only the control group. A young group was comprised of women between 35 and 45 years of age and an elderly group was comprised of women aged 70-80 years. Exclusion criteria were as follows: pregnancy; history of malignant or any bone disease, vertebral fractures, juvenile (type I or insulin dependent) diabetes, any history of trauma at the measurement sites, confinement to a wheel chair or use of canes, previous or current treatment with corticosteroids, chemotherapy, bisphosphonates, calcitonin and/or tamoxifen.
There was only one visit and informed consent was obtained prior to any study-related procedures. The University of California San Francisco Committee for Human Research approved the study protocol. Demographic information, such as age, medical history, menopausal status, prior fractures, overall health assessment, and medications were collected. After measurement of height and weight, the patients underwent vQCT of the proximal femur.
Scanning
All subjects were scanned using a GE9800Q CT system (GE Medical Systems, Milwaukee, WI) using a protocol in which the proximal femoral region from the superior aspect of the acetabulum to 3-5 mm inferior to the lesser trochanter was encompassed with contiguous 3-mm thick images. Calibration of the scanner Hounsfield units to equivalent concentration of calcium hydroxyapatite was obtained using a calibration phantom placed under the hips of the subjects (Image Analysis, Columbia KY). Because the studies were done at two different times and the study protocol was optimized during that time, the young and elderly cohorts were imaged with slightly different protocols. The younger subjects were imaged with 80 kVp/280 mAs and the elderly cohort with 120 kVp/150 mAs. Image size was 0.937 mm × 0.937 mm × 3 mm, in-plane resolution were matrices of 512 × 512 pixels. The maximal spatial resolution of the system was in the order of 0.7/0.8 mm. To cross-calibrate the two scanning protocols, a phantom (CIRS, Norfolk, VA) containing inserts with variable BMD was imaged with both settings. For comparative purpose only we have included values of total femur BMD obtained with DXA (Lunar, GE Systems).
vQCT BMD analysis
CT images were transferred to a computer workstation and processed to extract measures of bone mineral content (BMC), volumetric bone mineral density (vBMD) and bone volume (VOL) using analysis techniques described previously [23,24]. The processing task included calibration of the CT images from the native scanner Hounsfield Units to equivalent concentration (g/cm3) of calcium hydroxyapatite (HA) and determination of integral (whole bone), cortical and trabecular regions of interest from vQCT scans of the hip as described in detail in a previous publication (Fig. 1) [23]. The regions of interest included volumes of integral, cortical and trabecular bone approximately matched to the trochanteric (TR) and the femoral neck (FN) regions imaged on DXA systems in the proximal femur [24]. The TR was computed by subtracting the FN from the overall proximal femoral region [24]. For each region, we computed vBMD (g/cm3), BMC (g) and VOL (cm3). The trabecular and cortical vBMD values represent the equivalent HA concentration averaged over the voxels contained in the trabecular and cortical regions of interest respectively. Because the spatial resolution of the CT system is larger than the thickness of a trabeculum or a very thin cortex (such as the medial aspect of the anterior-superior cortex of the femoral neck cortices), the cortical and trabecular regions contain non-bone components. Therefore, when cortical and trabecular vBMD are estimated the final average value also includes voxels containing medullary tissue. In our laboratory, the in vivo precision for vBMD determinations based on repeated measurements of 10 subjects range from 0.72% to 1.56%, except for cortical vBMD (2.89%) and trabecular vBMD (5.85%) at the FN [26].
Fig. 1.
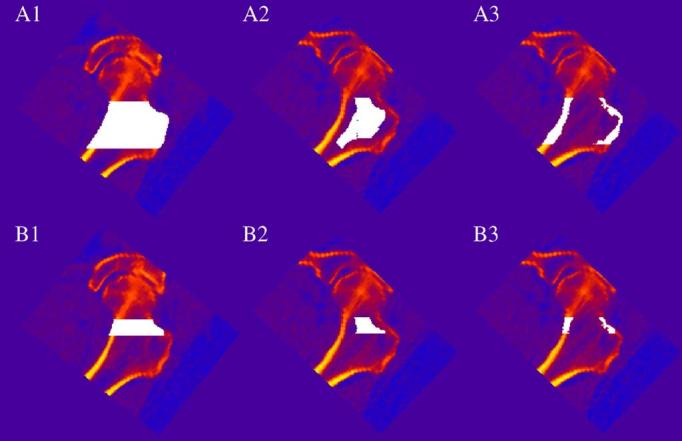
vQCT regions of interest in the proximal femur. Regions of interest are white pixels superimposed on image data. vQCT images showing (1) iBMD (2) tBMD and (3) cBMD in the (A) overall proximal femur and in (B) the FN. The TR is the region outside the FN but within the overall proximal femoral region.
vQCT geometric and strength analysis
We measured the cross-sectional areas (CSA, cm2) of the femoral neck and mid-trochanter (Fig. 2). The volumes of the TR and the FN integral regions of interest, which included all voxels contained within the outer bone margin, were employed as measures of integral bone volume (iVOL). The volumes of the TR and the FN cortical regions of interest were utilized as measures of cortical bone volume (cVOL). As estimates of bone area, we located the positions along the femoral neck axis of minimum and maximum CSA, which respectively corresponded to the smallest CSA of the femoral neck and the largest CSA through the trochanteric region. The in vivo precision for our CSA measurements based on repeated measurements of 10 subjects are 1.4% at the FN and 2.18% at the TR [26].
Fig. 2.
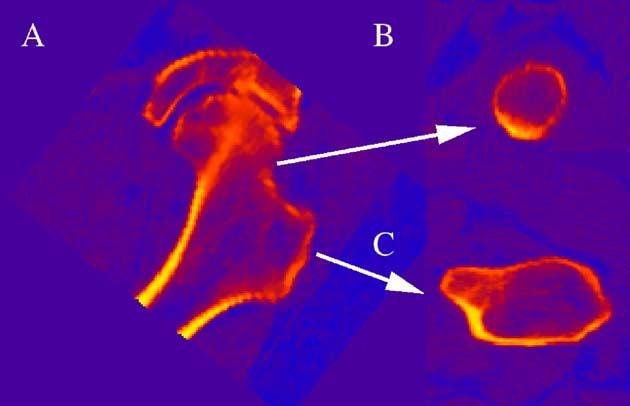
Definitions of proximal femoral planes for geometric measurements and strength estimates. vQCT images corresponding to (A) Coronal projection through hip with arrows pointing to (B) image of FN CSA and (C) image of TR CSA.
To estimate the mechanical competence of the proximal femur we computed structural indices of compressive strength (Compstr, g2/cm4) at the TR region and compressive (Compstr, g2/cm4) and bending strength (BSI, cm3) at the FN. These indices were calculated as previously described [23]. Briefly, the compressive strength indices (Compstr) were computed according to methods described by Sievanen [35]. To calculate BSI, a 2-mm thick section centered at the location of minimum CSA was reconstructed. BSI was computed as an effective polar moment of inertia divided by a measure of the femoral neck width. To account for heterogeneity, the contribution of each voxel to the polar moment of inertia was weighted by the elastic modulus computed from relationships reported by Keyak et. al. [21].
Statistical analysis
Statistical analysis was done using JMP (v5.0.1, SAS Institute, Cary, NC, USA). Mean and standard deviation (SD) were calculated for each one of the variables included in this study. First, we compared mean values for young and elderly women between the TR and the FN compartments by F-test after adjusting for height and weight in an analysis of covariance (ANOCOVA) model. We further performed logarithmic transformation of bone parameters not only to improve normal distribution of inter-regional data, but also to better account for differences between two different skeletal regions with distinctive anatomical characteristics. To compare logarithmic differences of young and elderly (log(Young)-log(Elderly), Y/Ed) between FN and TR we used the F-test for interaction in an ANOCOVA model after adjusting for height and weight. A significant interaction between age groups and compartments suggested an age-related change that differed between compartments. P values greater than 0.05 were considered not significant.
Results
Subjects
Study subjects’ characteristics are summarized in Table 1. Height, but not body weight, of the elderly and the young groups were significantly different. Values for total femur BMD, as measured by DXA, were significantly different between young women (1.02 ± 0.093 g/cm2) and elderly women (0.88 ± 0.13 g/cm2, P < 0.001) after adjusting for height and weight.
Table 1.
Study population characteristics
| n | Age (years) | Height (cm) | Weight (kg) | |
|---|---|---|---|---|
| Young | 28 | 41.14 ± 3.09a | 163.74 ± 6.24a | 68.18 ± 12.29b |
| Elderly | 124 | 74.42 ± 3.44 | 158.59 ± 6.45 | 69.38 ± 12.24 |
Age, height and weight mean S.D. Statistical significance levels for comparison of young with elderly women are given as follows:
P < 0.001;
P > 0.01;
cP < 0.05;
ns: P > 0.05.
Bone mineral content
All BMC values were significantly lower in the elderly group than in the young group, with the largest difference between young and elderly observed for the FN tBMC (Table 2). The Y/Edin tBMC was significantly larger at the FN than at the TR (P = 0.0053).
Table 2.
Bone mineral content
| iBMC (g) | cBMC (g) | tBMC (g) | |
|---|---|---|---|
| Trochanteric region | |||
| Young | 19.38 ± 3.56a | 14.58 ± 2.94b | 2.72 ± 0.69b |
| Elderly | 16.47 ± 4.03 | 11.90 ± 2.99 | 2.08 ± 0.84 |
| Femoral neck region | |||
| Young | 5.05 ± 0.87b | 4.24 ± 0.74b | 0.35 ± 0.15b |
| Elderly | 4.03 ± 0.97 | 3.27 ± 0.71 | 0.17 ± 0.20 |
| P value young/elderly differences | |||
| Trochanteric vs. femoral neck | 0.3206 | 0.4013 | 0.0053 |
Integral (iBMC), cortical (cBMC) and trabecular (tBMC) bone mineral content mean SD. Statistical significance levels for comparing young with elderly women after adjusting for height and weight are given as follows:
P < 0.001;
P < 0.01;
cP < 0.05;
ns: P > 0.05. Statistically significant Y/Ed between TR and FN after adjusting for height and weight are in bold in the last row.
Volumetric bone mineral density
All iBMD and tBMD values were significantly lower in the elderly group than in the young group (Table 3). However, this was not the case for cBMD values. The FN cBMD was significantly greater (P < 0.05) in elderly women, but the TR cBMD values for the two groups did not differ significantly. Significance levels in Y/Edbetween the TR and the FN varied as a function of bone compartment considered. Y/Edin tBMD (P = 0.0034) and cBMD (P = 0.0003) differed significantly as a function of proximal femoral sub-region, but Y/Edin iBMD did not.
Table 3.
Volumetric bone mineral density
| iBMD (g/cm3) | cBMD (g/cm3) | tBMD (g/cm3) | |
|---|---|---|---|
| Trochanteric region | |||
| Young | 0.313 ± 0.04a | 0.541 ± 0.03b | 0.130 ± 0.03a |
| Elderly | 0.247 ± 0.04 | 0.525 ± 0.03 | 0.086 ± 0.03 |
| Femoral neck region | |||
| Young | 0.351 ± 0.04a | 0.515 ± 0.04c | 0.130 ± 0.04a |
| Elderly | 0.272 ± 0.04 | 0.539 ± 0.04 | 0.049 ± 0.048 |
| P value young/elderly differences | |||
| Trochanteric vs. femoral neck | 0.6625 | 0.0003 | 0.0034 |
Integral (iBMD), cortical (cBMD) and trabecular (tBMD) volumetric bone mineral density mean SD. Statistical significance levels for comparing young with elderly women after adjusting for height and weight are given as follows:
P < 0.001;
P < 0.01;
P < 0.05;
ns: P > 0.05. Statistically significant Y/Ed between TR and FN after adjusting for height and weight are in bold in the last row.
Bone geometry
All iVOL and tVOL measurements were significantly larger in the elderly women at the TR but not at the FN, which showed a non-significant trend toward greater values in the elderly subjects (Table 4). In contrast, cVOL values were significantly lower in elderly women in both of the studied regions. Among all volume measures, only the Y/Edfor cVOL values differed by proximal femoral skeletal site, with the FN showing a larger Y/Edthan the TR (P = 0.0267).
Table 4.
Bone volume
| iVOL (cm3) | cVOL (cm3) | tVOL (cm3) | |
|---|---|---|---|
| Trochanteric region | |||
| Young | 62.24 ± 10.71a | 26.94 ± 5.02b | 21.53 ± 5.33b |
| Elderly | 66.63 ± 12.04 | 22.52 ± 4.72 | 24.78 ± 5.54 |
| Femoral neck region | |||
| Young | 14.55 ± 2.75c | 8.24 ± 1.42b | 2.65 ± 1.08c |
| Elderly | 14.96 ± 3.49 | 6.06 ± 1.22 | 3.06 ± 1.35 |
| P value young/elderly differences | |||
| Trochanteric vs. femoral neck | 0.4367 | 0.0267 | 0.9560 |
Integral (iVOL), cortical (cVOL) and trabecular (tVOL) bone volume mean SD. Statistical significance levels for comparing young with elderly women after adjusting for height and weight are given as follows:
P < 0.001;
P < 0.01;
P < 0.05;
ns: P > 0.05. Statistically significant Y/Ed between TR and FN after adjusting for height and weight are in bold in the last row.
CSA at the FN and at the TR showed significantly higher values in the elderly group (Table 5), but the Y/Eddid not significantly depend on proximal femoral skeletal site.
Table 5.
Cross-sectional area and structural strength estimates
| CSA (cm2) | Compstr (g2/cm4) | BSI (cm3) | ||||
|---|---|---|---|---|---|---|
| Trochanteric region | ||||||
| Young | 23.88 ± 2.72a | 2.36 ± 0.58a | Elderly | 27.91 ± 3.39 | 1.74 ± 0.58 | |
| Femoral neck region | ||||||
| Young | 8.73 ± 1.16a | 1.07 ± 0.22a | 0.51 ± 0.09b | |||
| Elderly | 9.80 ± 1.65 | 0.73 ± 0.23 | 0.45 ± 0.11 | |||
| P value young/elderly differences | ||||||
| Trochanteric vs. femoral neck | 0.2421 | 0.0335 | ||||
Cross-sectional area (CSA), compressive strength index (Compstr) and bending strength index (BSI) mean SD. Statistical significance levels for comparing young with elderly women after adjusting for height and weight are given as follows:
P < 0.001;
P < 0.01;
cP < 0.05;
ns: P > 0.05. Statistically significant Y/Ed between TR and FN after adjusting for height and weight are in bold in the last row.
Structural properties
All bone structural indices examined in this study were significantly lower in the elderly women compared to the young women (Table 5). Y/Edin compressive strength depended on proximal femoral skeletal site (P = 0.0335) showing a larger Y/Eddecrease in Compstr at the FN.
Discussion
In this study, we compared in vivo differences in proximal femoral compartmental vBMD, BMC, and indices of bone geometry and structural strength between healthy young women near the peak of bone mass and healthy elderly women. While a recently published study based on QCT has reported cross-sectional changes in femoral neck BMD and geometry in men and women aged 20-90 [33], our study was novel in that it examined in vivo how young-elderly differences in these parameters depend on two distinct functional sub-regions of the proximal femur, the TR and the FN. We found that the TR showed the largest inter-group difference in bone size and the FN showed the largest difference in bone mass. The greater values for the geometric parameters may be insufficient to completely compensate for age-related BMD and BMC loss, resulting in a substantial age-related decrease in indices of bone structural strength that differs between the two functional regions of interest.
Bone mass was estimated by BMC. The almost two-fold lower values of tBMC at the FN in elderly women compared with young women, and the fact that Y/Edbetween the TR and the FN were significant, suggest that the loss of trabecular bone mass that occurs with age at the FN is greater than the loss of trabecular bone mass that occurs in the TR. Since bone adapts itself throughout life to the changes that occur in the loading environment [15,25,34], the trabecular bone in the FN may be less important for load bearing than trabecular bone at the TR and therefore its decrease in values for mass is greater.
Cortical vBMD reflects the average concentration of bone mineral in the cortical region (Fig. 1) and the porosity of the cortex. Because of partial volume averaging, its value depends on cortical thickness especially at those locations of the proximal femoral cortex where the cortical thickness is less than 2-3 mm. The higher value of cBMD at the FN in the elderly women, compared to the TR (which decreased with age), may be due to the presence of highly mineralized areas in the FN cortex of elderly women. These results are consistent with findings reporting increased mineralized tissue, areas of higher mineral content than the adjacent cortex, at the FN in elderly populations [4,5,37]. The origin of these highly mineralized areas may be due to subperiosteal calcification derived from the periosteum [11], necrotic tissue where minerals continue to accumulate [5] or calcified fibrocartilagenous tissue at points of attachments of tendons or capsules[5,37]. The presence of fully mineralized tissue areas may have implications for fracture strength and it has been reported that these highly mineralized regions make the formation and expansion of cracks easier [10]. However, our cortical BMD results should be interpreted with caution, as the cortical regions are very heterogeneous in thickness and porosity, and much of the cortical volume encompasses the thin antero-superior cortex, which is subject to partial volume averaging. At the same time, local increases in cortical thickness in the load bearing inferior cortex [29] might reflect higher values for cBMD at the FN in the elderly, considering the effect of partial volume averaging. Further studies are necessary to clarify this finding.
TBMD at the FN was strikingly lower in elderly women compared to young women, and the difference between young and elderly values of tBMD was two-fold higher at the FN than at the TR. These results are similar to the pattern observed for tBMC and probably reflect the sharp decrease in bone mass that occurs in this region with age. Y/Edstrongly depended on site (FN vs. TR). These observations indicate that estimations of total proximal femur bone mass might not accurately reflect and in fact underestimate the magnitude of trabecular bone mass loss in the femoral neck.
The TR, but not the FN, anatomically constitutes an important muscle insertion site. It is well known that muscle forces place high physiological loads on bones throughout life and that the skeleton adapts to changes in this mechanical loading [6,13]. Therefore, intermittent loads imposed on bone by the muscles attached at the TR could induce an increase in bone size, due to periosteal bone formation. However, we cannot rule out that part of this effect may not have structural relevance and might be related to hypertrophic degenerative effects around the trochanter that occur with age. Hypermineralized areas reported in the elderly population have been related to calcified fibrocartilagenous tissue at the points of attachments of tendons and capsules and also to periosteal calcification derived from the periosteum [5,37,38]. Both of these effects can be reflected in an apparent increase in the cVOL through vQCT imaging. The similar Y/Edin tVOL between the FN and the TR suggests that endocortical resorption is similar in the TR and the FN. However, enhanced periosteal apposition at the TR may lead to a smaller reduction in cVOL values at this site.
As reported by Riggs et al. [33], both the CSA at the TR and at the FN were larger in the elderly women. This increase in CSA values was consistent with that of iVOL at the TR, but not at the FN where iVOL was not significantly larger in the elderly women. Since CSA is measured in a single slice through the FN this may be due to heterogeneous changes along the FN.
Because differences in whole bone strength derive from differences in both density and geometry, we calculated indices of compressive and bending strength from the vQCT measurements. The estimated compressive strength values were consistently smaller in the elderly women at both the TR and the FN. The inter site difference in Y/Edis the consequence of a non significant larger decrease in values for iBMD and a smaller increase in CSA values at the FN than at the TR. However, the elderly subjects had only slightly smaller FN BSI values despite having greatly reduced cVOL values. This may be due to the protective effect of the larger values of CSA at the FN in the elderly, although alternatively, it may be partly explained by a lesser degree of endocortical resorption at the FN CSA position.
The results described above suggest that the TR and the FN present different patterns of age-related change in the cortical and trabecular compartments, and each can potentially be evaluated and monitored in the clinical setting. Site dependence was not observed for the integral vQCT measurements. Thus, integral bone measurements, which combine the cortical and trabecular compartments, may be limited in their ability to identify and describe age-related changes in the proximal femur. Further study is required to better understand the importance of site dependence on the age-related patterns of change in the cortical and trabecular compartments for overall bone strength, and its ultimate clinical relevance.
This study has several limitations. First, although our young and elderly groups were ethnically and geographically matched, and reasonably comparable in body size, it is important to take into account that our cross sectional study design entails some cohort effects for which we cannot correct. Also, though this study was performed in a normal population with no reported vertebral fractures, elderly women have reduced inter-vertebral disc space, which we cannot adjust for, introducing a source of error in height adjustment. In addition, the fact that the elderly group presents lower height values, but similar weight values, may indicate a normal increase in fat mass that occurs with age. This type of limitation might result in an underestimation of periosteal apposition as well as in volume measurements in elderly. Second, degenerative changes, such as calcification of ligaments attaching at the cortical surface, may partially explain measurements of increased bone size in our elderly group. Further, areas of focal high mineralization in the femoral neck, which have been observed in elderly subjects, may contribute to our observation of higher apparent cortical BMD in the femoral neck in our elderly group. As with all studies of the proximal femur using vQCT, measurements of the cortex are affected by partial volume averaging, which results in systematic overestimation of cortical volume and underestimation of cortical BMD in the thin antero-superior portion of the femoral neck cortex. Changes in cortical thickness N1.2 mm have been reported using a helical CT scanner [32]. Our cortical region of interest is primarily composed of the inferomedial cortex of the proximal femur, for which thickness values exceeding 3-4 mm have been reported in humans, but also includes the thin superomedial cortex (thickness 0.3 mm) [2]. Thus, it is likely that the cortical volume changes observed in this study primarily reflect changes occurring in the thick inferomedial cortex. Another limitation of our study is that the indices of bone strength have not been validated as predictors of fracture risk and should not be taken as such. Rather, they are interpretive tools, which assist in estimating the effect of bone density and geometry changes on changes in bone strength.
In conclusion, we hypothesized that in healthy women, the TR and the FN would show different age-related changes in bone mass, density and geometry at the cortical and trabecular compartments. Indeed, we observed anatomic heterogeneity in Y/Edin proximal femoral BMC, vBMD and bone size. With age there was greater loss of trabecular and cortical bone in the FN than in the TR, and a greater increase in bone size at the TR compared to the FN. For compressive strength indices the larger bone size in the elderly women could not compensate for lower BMD. However, changes in the FN geometry in the elderly women appear to protect estimated bending strength but not compressive strength despite BMD and cortical volume loss. These findings showing sub-regional dependence in age related structural changes at the proximal femur contribute to building the knowledge for understanding the distinctive etiologies in fragility fractures between the TR and the FN.
Acknowledgments
This study was supported by NASA, grant number NAS-9-99055 and the National Institute of Health, grant number RO1-AR46197. The authors are thankful for the excellent technical assistance of Vesta March CRT, Faye Wong CRT, Cynthia Hayashi CRT ARRT and Mary Sherman CRT. The authors thank Bernadette de Guzman and Bernard Smith for assistance in scheduling CT scans. Funding source: NASA (Contract NAS-9-99055 from NASA Johnson Spaceflight Center) and NIH R01-AR46197.
References
- 1.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–34. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 2.Bagi CM, Wilkie D, Georgelos K, Williams D, Bertolini D. Morphological and structural characteristics of the proximal femur in human and rat. Bone. 1997;21:261–7. doi: 10.1016/s8756-3282(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 3.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res. 2000;15:2297–304. doi: 10.1359/jbmr.2000.15.12.2297. [DOI] [PubMed] [Google Scholar]
- 4.Bousson V, Peyrin F, Bergot C, Hausard M, Sautet A, Laredo JD. Cortical bone in the human femoral neck: three-dimensional appearance and porosity using synchrotron radiation. J Bone Miner Res. 2004;19:794–801. doi: 10.1359/JBMR.040124. [DOI] [PubMed] [Google Scholar]
- 5.Boyce TM, Bloebaum RD. Cortical aging differences and fracture implications for the human femoral neck. Bone. 1993;14:769–78. doi: 10.1016/8756-3282(93)90209-s. [DOI] [PubMed] [Google Scholar]
- 6.Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12:1547–51. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- 7.Carter vdMM D, Beauptre G. skeletal development: mechanical consequences of growth, aging and disease. In: Marcus FDR, Kelsey J, editors. Academic Press; Osteoporosis. San Diego, CA, USA: 1996. pp. 333–50. [Google Scholar]
- 8.Chrischilles E, Shireman T, Wallace R. Costs and health effects of osteoporotic fractures. Bone. 1994;15:377–86. doi: 10.1016/8756-3282(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree N, Lunt M, Holt G, Kroger H, Burger H, Grazio S, et al. Hip geometry, bone mineral distribution, and bone strength in European men and women: the EPOS study. Bone. 2000;27:151–9. doi: 10.1016/s8756-3282(00)00300-8. [DOI] [PubMed] [Google Scholar]
- 10.Currey J. The mechanical consequences of variation in the mineral content of bone. J Biomech. 1969;2:1–11. doi: 10.1016/0021-9290(69)90036-0. [DOI] [PubMed] [Google Scholar]
- 11.Dretakis EK, Christodoulou NA. Significance of endogenic factors in the location of fractures of the proximal femur. Acta Orthop Scand. 1983;54:198–203. doi: 10.3109/17453678308996556. [DOI] [PubMed] [Google Scholar]
- 12.Duan Y, Beck TJ, Wang XF, Seeman E. Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res. 2003;18:1766–74. doi: 10.1359/jbmr.2003.18.10.1766. [DOI] [PubMed] [Google Scholar]
- 13.Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporosis and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2:73–85. [PubMed] [Google Scholar]
- 14.Gallagher JC, Melton LJ, Riggs BL, Bergstrath E. Epidemiology of fractures of the proximal femur in Rochester, Minnesota. Clin Orthop. 1980:163–71. [PubMed] [Google Scholar]
- 15.Goodship AE, Lanyon LE, McFie H. Functional adaptation of bone to increased stress. An experimental study. J Bone Jt Surg, Am. 1979;61:539–46. [PubMed] [Google Scholar]
- 16.Hinton RY, Lennox DW, Ebert FR, Jacobsen SJ, Smith GS. Relative rates of fracture of the hip in the United States. Geographic, sex, and age variations. J Bone Jt Surg, Am. 1995;77:695–702. doi: 10.2106/00004623-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Hinton RY, Smith GS. The association of age, race, and sex with the location of proximal femoral fractures in the elderly. J Bone Jt Surg, Am. 1993;75:752–9. doi: 10.2106/00004623-199305000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18:57S–63S. doi: 10.1016/8756-3282(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 19.Kaptoge S, Dalzell N, Loveridge N, Beck TJ, Khaw KT, Reeve J. Effects of gender, anthropometric variables, and aging on the evolution of hip strength in men and women aged over 65. Bone. 2003;32:561–70. doi: 10.1016/s8756-3282(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 20.Karagas MR, Lu-Yao GL, Barrett JA, Beach ML, Baron JA. Heterogeneity of hip fracture: age, race, sex, and geographic patterns of femoral neck and trochanteric fractures among the US elderly. Am J Epidemiol. 1996;143:677–82. doi: 10.1093/oxfordjournals.aje.a008800. [DOI] [PubMed] [Google Scholar]
- 21.Keyak JH, Lee IY, Skinner HB. Correlations between orthogonal mechanical properties and density of trabecular bone: use of different densitometric measures. J Biomed Mater Res. 1994;28:1329–36. doi: 10.1002/jbm.820281111. [DOI] [PubMed] [Google Scholar]
- 22.Koval KJ, Aharonoff GB, Rokito AS, Lyon T, Zuckerman JD. Patients with femoral neck and intertrochanteric fractures. Are they the same. Clin Orthop. 1996:166–72. doi: 10.1097/00003086-199609000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 24.Lang TF, Keyak JH, Heitz MW, Augat P, Lu Y, Mathur A, et al. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone. 1997;21:101–8. doi: 10.1016/s8756-3282(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 25.Lanyon LE. Functional strain as a determinant for bone remodeling. Calcif Tissue Int. 1984;36(Suppl 1):S56–61. doi: 10.1007/BF02406134. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Sode M, Saeed I, Lang T. Automated registration of hip and spine for longitudinal QCT studies: integration with 3 D densitometric and structural analysis. Bone. 2006;38:273–9. doi: 10.1016/j.bone.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu TW, Taylor SJ, O’Connor JJ, Walker PS. Influence of muscle activity on the forces in the femur: an in vivo study. J Biomech. 1997;30:1101–6. doi: 10.1016/s0021-9290(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 28.Mautalen CA, Vega EM, Einhorn TA. Are the etiologies of cervical and trochanteric hip fractures different. Bone. 1996;18:133S–7S. doi: 10.1016/8756-3282(95)00490-4. [DOI] [PubMed] [Google Scholar]
- 29.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, et al. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–35. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 30.McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Jt Surg, Am. 1993;75:1193–205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Power J, Loveridge N, Rushton N, Parker M, Reeve J. Evidence for bone formation on the external “periosteal” surface of the femoral neck: a comparison of intracapsular hip fracture cases and controls. Osteoporos Int. 2003;14:141–5. doi: 10.1007/s00198-002-1333-8. [DOI] [PubMed] [Google Scholar]
- 32.Prevrhal S, Engelke K, Kalender WA. Accuracy limits for the determination of cortical width and density: the influence of object size and CT imaging parameters. Phys Med Biol. 1999;44:751–64. doi: 10.1088/0031-9155/44/3/017. [DOI] [PubMed] [Google Scholar]
- 33.Riggs BL, Melton Iii LJ, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–54. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 34.Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol. 1984;107:321–7. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- 35.Sievanen H. A physical model for dual-energy X-ray absorptiometry-derived bone mineral density. Invest Radiol. 2000;35:325–30. doi: 10.1097/00004424-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Smith RW, Jr, Walker RR. Femoral expansion in aging women: implications for osteoporosis and fractures. Science. 1964;145:156–7. doi: 10.1126/science.145.3628.156. [DOI] [PubMed] [Google Scholar]
- 37.Vajda EG, Bloebaum RD. Age-related hypermineralization in the female proximal human femur. Anat Rec. 1999;255:202–11. doi: 10.1002/(SICI)1097-0185(19990601)255:2<202::AID-AR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Zagba-Mongalima G, Goret-Nicaise M, Dhem A. Age changes in human bone: a microradiographic and histological study of subperiosteal and periosteal calcifications. Gerontology. 1988;34:264–76. doi: 10.1159/000212965. [DOI] [PubMed] [Google Scholar]


