Abstract
A novel regulatory gene, trh, which is involved in hrp gene expression, is identified in the plant pathogen Xanthomonas oryzae pv. oryzae. In the trh mutant, expression of HrpG, which is a key regulator for hrp gene expression, is reduced both under the in vitro hrp-inducing condition and in planta.
Xanthomonas oryzae pv. oryzae is the causal agent of bacterial leaf blight of rice (23). Like other gram-negative phytopathogenic bacteria in the genera Erwinia, Pseudomonas, and Ralstonia, Xanthomonas spp. possess clustered hypersensitive response and pathogenicity (hrp) genes that play important roles for pathogenicity on host plants and for triggering a hypersensitive response on nonhost plants (1). The most conserved genes in the hrp cluster, called hrc (hrp conserved) genes (2), encode core components of a type III secretion system (TTSS) that delivers virulence factors from bacteria to host cells (6, 7, 20).
Expression of hrp genes is highly regulated and is generally suppressed in complex media but induced in planta and in certain nutrient-poor synthetic media (4, 21, 26, 33, 37). Although many hrp-regulatory genes have been isolated and a complicated regulatory system has been revealed in Pseudomonas syringae, Erwinia spp., and Ralstonia solanacearum (1, 3, 4, 5, 6, 12, 31, 32, 37, 38), only two hrp-regulatory genes, hrpG and hrpX, have been identified in xanthomonads so far (18, 34, 35). HrpG is predicted to be a response regulator, belonging to the OmpR family of a two-component regulatory system, although the corresponding kinase gene has not been identified (36). Phosphorylated HrpG is, therefore, predicted to regulate the expression of another hrp-regulatory gene, hrpX, the product of which belongs to the AraC regulator family, followed by transcriptional activation of other hrp genes (including TTSS structural genes) and genes encoding effector proteins secreted via a TTSS (34, 35).
To isolate and identify novel hrp-regulatory genes in X. oryzae pv. oryzae, we first conducted transposon mutagenesis using an EZ::TN transposome-mediated insertion system (Epicentre, Madison, WI) (27) on 74HrcQ::GUS, in which a promoterless β-glucuronidase (GUS) gene was inserted at +42 (+1 represents A of the initiation codon) in hrcQ (the first gene of the hrpD operon) in the genomic DNA of X. oryzae pv. oryzae strain T7174R (8, 29). Approximately 1,000 kanamycin-resistant clones were incubated in a hrp-inducing medium, XOM2 (26), at 28°C for 15 h, and GUS activity in each clone was measured (14, 26). In one clone, NRH867, GUS activity was significantly reduced compared to that of 74HrcQ::GUS, although the activity was not completely lost (Fig. 1). Growth of NRH867 in nutrient-rich NBY medium (28) and nutrient-poor XOM2 was similar to that of the parental strain 74HrcQ::GUS (data not shown).
FIG. 1.
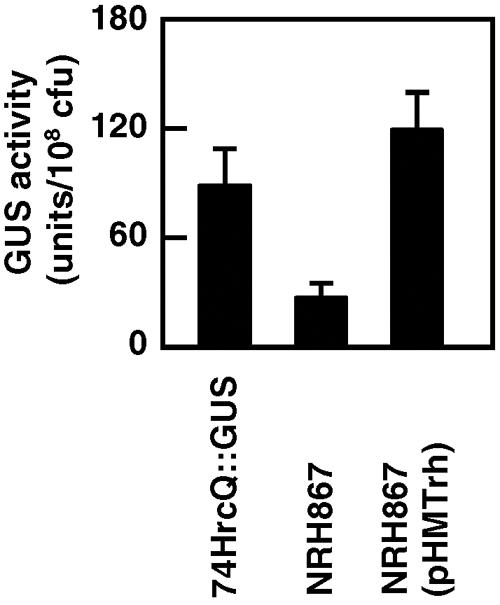
Expression of hrcQ::gus in trh mutant NRH867. X. oryzae pv. oryzae strains were incubated in the hrp-inducing medium XOM2 for 15 h, and GUS activity per 1 × 108 CFU of bacteria was measured. Strain 74HrcQ::GUS contains a hrcQ::gus fusion gene in the genome, and NRH867, a derivative of 74HrcQ::GUS, has a transposon inserted in trh. Plasmid pHMTrh harbors a trh gene. Values are averages ± standard deviations (n = 3).
Sequence analysis revealed that, in NRH867, the transposon was inserted at +310 in a putative transcriptional regulator gene (trh [transcriptional regulator for hrp]) (XOO0783 in the genomic database of X. oryzae pv. oryzae T7174 [17]). The coding sequence of trh is predicted to be 729 bp long (242 amino acids), and in motif analysis using ExPASy (http://www.expasy.org/prosite/), the product was predicted to be a member of the GntR regulator family with a helix-turn-helix motif in the N-terminal region of the protein (+31 to +50) (11). Transcriptional regulators of the family include activators, repressors, and molecules that both activate and repress a wide range of bacterial operons (19). The reduced GUS activity in NBH867 was complemented by introduction of plasmid pHMTrh, which harbors a ∼900-bp PvuII-SphI fragment containing a trh gene inserted into the broad-host-range vector pHM1 (13) (Fig. 1). Higher GUS activity was observed in the transformant NRH867 (pHMTrh) than in 74HrcQ::GUS, which is probably due to overexpression of trh from multiple copies of the gene by introduction of the plasmid.
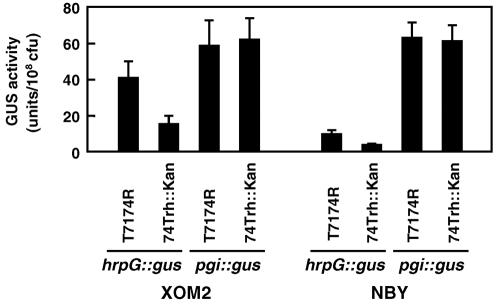
We cloned a ∼7.9-kb SacI-NotI fragment containing a trh gene inserted with a kanamycin resistance gene from the genomic DNA of NRH867 in pBluescript II SK(+) (Stratagene, La Jolla, CA) and generated the trh mutant 74Trh::Kan from the wild-type strain T7174R by marker exchange mutagenesis using the plasmid. Then expression of hrcU, hrpXo, and hrpG in 74Trh::Kan was investigated using plasmids harboring each hrp gene fused with a promoterless gus gene. GUS activity in all of the transformants, even in that transformed with the plasmid harboring the hrp-regulatory gene hrpG fused with gus, was reduced, but not completely lost, compared to that in those derived from T7174R after a 15-h incubation in XOM2 (Fig. 2) (data not shown). The expression level of a phosphoglucose isomerase gene (pgi) in 74Trh::Kan, which is independent of a hrp-regulatory system (27) and is used as a control, was similar to that in T7174R.
FIG. 2.
Effects of mutation in trh on expression of hrpG. A plasmid harboring hrpG::gus fusion genes inserted into the broad-host-range vector pHM1 (13) was introduced into T7174R and 74Trh::Kan. Each transformant was incubated in XOM2 and NBY for 15 h, and GUS activity per 108 CFU was measured. As a control, a plasmid harboring a gus-fused phosphoglucose isomerase gene (pgi), expression of which is independent of hrp (27), was used. Values are averages ± standard deviations (n = 3).
To investigate the involvement of Trh in transcription of hrpG, real-time PCR was conducted with total RNA extracted from bacterial cells cultured in XOM2 for 15 h using a QuanTitect SYBR green real-time PCR kit (QIAGEN, Valencia, CA). The time course of the amplification of the PCR products was measured by the iCycler iQ real-time PCR System (Bio-Rad, Richmond, VA). As shown in Table 1, transcription of hrpG in 74Trh::Kan was reduced compared to that in T7174R. Accumulation of the hpa1 transcript, which is expressed in a hrp-dependent manner, also decreased in 74Trh::Kan. On the other hand, accumulation of the DNA gyrase subunit B (gyrB) transcript, which is used for a reference, was not reduced in the mutant in comparison to that in the wild-type strain. These results indicate that Trh directly or indirectly activates hrpG transcription, followed by increased expression of other hrp genes.
TABLE 1.
Accumulation of hpa1 and hrpG transcripts in the trh mutant of X. oryzae pv. oryzaea
| Strain | Relative expression valueb
|
||
|---|---|---|---|
| gyrB | hpa1 | hrpG | |
| T7174R | 104.1 ± 23.1 | 24,863.7 ± 5,509.1 | 193.6 ± 62.1 |
| 74Trh::Kan | 144.4 ± 33.1 | 3,895.7 ± 1,709.6 | 29.1 ± 16.1 |
Total RNA (10 ng) extracted from each bacterial strain after incubation in XOM2 was used as a template. The primers were designed with Beacon Designer software, version 2.0 (Bio-Rad), as follows: for gyrB, the sense primer was 5′-GGCGAGCACAATGGCATT-3′ and the antisense primer was 5′-CCATCCTTCTGCGGGATGT-3′; for hpa1, the sense primer was 5′-AAGCCAGGACACAACGTTCG-3′ and the antisense primer was 5′-GAAGCAGGGCCGAGATGAG-3′; and for hrpG, the sense primer was 5′-AGGCACTGACCCACTTTC-3′ and the antisense primer was 5′-ATCGGAAGCACCACTCTC-3′.
The threshold cycle (CT) was determined using iCycler iQ real-time detection system software, version 3.0 (Bio-Rad), and the relative expression value was calculated as 1/2CT × 108. Values are averages ± standard deviations for three independent experiments.
Interestingly, expression of hrpG increased after incubation in XOM2 (hrp inducing) even without trh compared with incubation in NBY (not hrp inducing), although expression of hrpG was higher in the presence of trh (Fig. 2). And after incubation in NBY, hrpG expression was lower but up-regulated by Trh. These results suggest that at least two factors are involved in transcriptional activation of hrpG. One is mediated by Trh, which functions both under the nutrient-rich non-hrp-inducing condition (NBY) and under the nutrient-poor hrp-inducing condition (XOM2). The other is mediated by an unknown factor(s), which functions only under hrp-inducing conditions. In R. solanacearum, four genes, prhA, prhI, prhR, and prhJ, have been identified as regulatory genes involved in hrpG expression (4, 5, 16). The regulatory cascade in which these genes are involved is reported to function specifically during plant-bacterium interactions. Although there have been no reports of genes corresponding to prh genes in xanthomonads, a regulator(s) other than Trh controlling hrpG expression may be present, which specifically functions under hrp-inducing conditions, including during plant-bacterium interactions.
Expression of trh under different growth conditions was examined by a real-time PCR method using total RNA extracted from T7174R incubated in XOM2 or NBY. Although accumulation of the hpa1 transcript seemed to be much lower after incubation in NBY than in XOM2, the amount of the specifically amplified fragment for trh, as well as that for gyrB, did not significantly differ between the two growth conditions (Table 2), suggesting constitutive expression of the trh gene.
TABLE 2.
Accumulation of trh and hpa1 transcripts under hrp-inducing (XOM2) and non-hrp-inducing (NBY) conditionsa
| Medium | Relative expression valueb
|
||
|---|---|---|---|
| gyrB | trh | hpa1 | |
| XOM2 | 104.1 ± 23.1 | 4.7 ± 0.6 | 24,863.7 ± 5,509.1 |
| NBY | 113.7 ± 67.1 | 5.4 ± 0.6 | 73.3 ± 28.2 |
Total RNA (10 ng) extracted from each bacterial strain after incubation in XOM2 was used as a template. The primers used for trh were 5′-AACAACTTGAAGCCGAAGG-3′ (sense primer) and 5′-CCACGTACTGCATGAAACC-3′ (antisense primer). Primers for detection of hpa1 and gyrB transcripts are given in Table 1, footnote a.
The threshold cycle (CT) was determined, and the relative expression value was calculated as 1/2CT × 108. Values are averages ± standard deviations for three independent experiments.
We also investigated the expression and secretion in 74Trh::Kan of HrpE1 and Hpa1, which have been reported to be major components of the hrp pilus and a harpin-like protein, respectively, and to be secreted to the culture supernatant under the hrp-inducing condition (9, 10, 30). We incubated 74Trh::Kan under the hrp-inducing condition, and bacterial proteins in 1 ml culture were separated into intracellular and extracellular fractions by centrifugation at 10,000 × g for 5 min. One hundred microliters of supernatant, with bacteria completely removed by filtration, was used for an enzyme-linked immunosorbent assay (ELISA). Proteins in bacterial cells were extracted with 300 μl of B-PER bacterial protein extraction reagent (Pierce, Rockford, IL), and the concentrations were measured with a protein assay kit (Bio-Rad) using bovine serum albumin as a reference. The samples (5 μg) were added to 150 μl Laemmli buffer (15) and used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by an immunoblot assay to detect Hpa1 and HrpE1, respectively. Accumulation of these proteins in the extracellular fraction from the trh mutant was greatly reduced compared with that from the wild type, likely because of less expression of their own genes and of the genes encoding components of the type III secretion apparatus (Fig. 3). The lower level of secretion of the protein was restored by introduction of pHMTrh. We also confirmed lower levels of accumulation of Hpa1 and HrpE1 in the intracellular fraction of 74Trh::Kan and complementation by introduction of plasmid pHMTrh (Fig. 3).
FIG. 3.
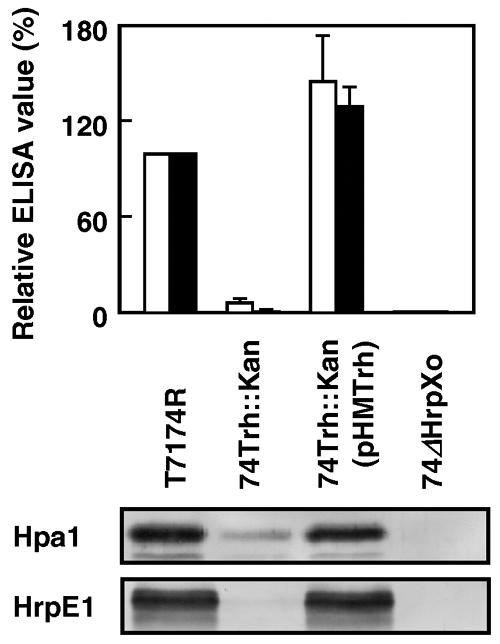
Accumulation of Hpa1 and HrpE1 in the culture supernatant and in bacterial cells. (Top) For quantification of Hpa1 and HrpE1 secreted in the culture supernatant, an ELISA was conducted. The values shown are ELISA values (ΔA405/h) relative to those from T7174R and are averages ± standard deviations for five independent experiments. White and black bars, Hpa1 and HrpE1, respectively. (Bottom) For detection of Hpa1 and HrpE1 in the bacterial cells, Western blot analysis was conducted using 5 μg bacterial proteins per lane. For each assay, polyclonal antibodies against Hpa1 and HrpE1 (unpublished data) were used as the primary antibody and an alkaline phosphatase-conjugated goat anti-rabbit antibody (Bio-Rad) was used as the secondary antibody. Reactions were visualized using p-nitrophenyl phosphate (1 mg/ml) for ELISA and nitroblue tetrazolium (337.5 μg/ml) and 5-bromo-4-chloro-3-indolyl phosphate (175 μg/ml) for the immunoblot assay. Strain 74ΔHrpXo is a hrpXo-deficient mutant described previously (25).
Finally, we investigated the involvement of trh in the expression of hrp genes in rice leaves by using strain 74Hpa1::Lux and the trh mutant 74Hpa1::Lux/Trh::Kan, in which a fragment containing a promoterless lux operon derived from pUCD623 (22) was inserted at the EcoRI site located downstream of the hpa1 promoter. Expression of the gene is regulated by HrpG and is specifically expressed under the hrp-inducing condition (9). The virulence of hpa1 mutants is lower, but they are still pathogenic on rice (9, 39). The strains were diluted in water (optical density at 600 nm [OD600], 0.3; approximately 1 × 108 CFU/ml) and inoculated by the clipping method (25) onto flag leaves and the next leaves of rice (Oryza sativa L. cv. IR24) grown in a greenhouse at 28 ± 5°C. Expression of hpa1 was examined by measuring bioluminescence using a video-intensified microscope camera and analyzed by ARGUS-100 (Hamamatsu Photonics, Hamamatsu, Japan) (24). During infection, accumulation of bioluminescence from rice leaves inoculated with 74Hpa1::Lux/Trh::Kan was lower than that from leaves inoculated with 74Hpa1::Lux. At 3 days after inoculation, the level of bioluminescence from the trh mutant strain was ca. five times lower than that from the trh+ strain (Table 3). Simultaneously, the bacterial numbers in 1-cm-long leaf sections including the inoculation site were measured by plating on the medium. Unexpectedly, there was no significant difference in the increase in the number of bacteria between the two strains. Moreover, leaves infected with each strain showed disease symptoms from 6 days after inoculation, and there was no significant difference in lesion lengths between the two strains by 2 weeks after inoculation.
TABLE 3.
Decrease of hpa1 expression in the trh-defective mutant during growth in rice leaves and pathogenicity of the mutants
| Straina | Bioluminescence (cpm)b,c | No. of bacteria (107 CFU)c,d | Lesion length (mm)c,e |
|---|---|---|---|
| 74Hpa1::Lux | 4,802.8 ± 1,521.3 | 4.7 ± 3.2 | 40.0 ± 7.8 |
| 74Hpa1::Lux/Trh::Kan | 910.0 ± 434.0 | 4.0 ± 2.2 | 37.8 ± 9.2 |
Each bacterium was used to inoculate the susceptible rice cultivar IR24.
Bioluminescence from 1-cm-long leaf sections, including the inoculation site, was measured 3 days after inoculation, and photons per minute are shown.
Values are averages ± standard deviations for five inoculated leaves.
Bacterial numbers from 1-cm-long leaf sections, including the inoculation site, were measured by plating on the medium 3 days after inoculation.
Measured 2 weeks after inoculation.
For further comparison of virulence between two strains with or without trh, dilution series of a bacterial suspension (1/5, 1/52, 1/53, 1/54, and 1/55; original OD600 of suspension, 0.3) of T7174R and 74Trh::Kan were prepared and inoculated onto rice leaves, and lesion lengths were measured 2 weeks after inoculation. With decreasing bacterial concentrations, the appearance of disease symptoms was delayed and lesion lengths became shorter in leaves inoculated with each strain. There were no significant differences in lesion formation between the two strains (Table 4). These results suggest that expression of hrp genes (at least hpa1) in the trh mutant is decreased (but not completely lost) not only under the in vitro hrp-inducing condition but also during bacterial growth in rice leaves and that even reduced expression of hrp genes and reduced secretion of TTSS effectors due to a mutation in trh may be sufficient for bacterial growth in host plants and the development of disease symptoms, although the possibility that expression of hrp genes other than hpa1 does not decrease, unlikely in the case of XOM2, cannot be excluded. In R. solanacearum, mutations in prh genes do not necessarily result in loss of pathogenicity, suggesting that there are multiple cascades to induce expression and activation of HrpG (4, 5, 16). In X. oryzae pv. oryzae, expression of hrp genes may be induced by an unknown cascade(s) to a level sufficient for pathogenicity on rice even without Trh.
TABLE 4.
Lesion lengths on rice leaves inoculated with dilution series of 74Trh::Km
| Strain | Lesion length (mm)a on rice leaves inoculated with the following dilution of a bacterial suspensionb:
|
|||||
|---|---|---|---|---|---|---|
| ×1 | ×5 | ×52 | ×53 | ×54 | ×55 | |
| T7174R | 76.83 ± 16.49 | 56.33 ± 20.26 | 54.50 ± 5.54 | 47.83 ± 9.28 | 31.00 ± 12.88 | 23.76 ± 16.06 |
| 74Trh::Km | 62.67 ± 15.08 | 54.83 ± 23.40 | 47.17 ± 16.62 | 40.67 ± 16.01 | 31.50 ± 19.60 | 22.50 ± 8.41 |
Measured 2 weeks after inoculation. Values are averages ± standard deviations for six inoculated leaves.
A bacterial suspension concentrated to an OD600 of 0.3 was diluted 5, 52, 53, 54, and 55 times and used to inoculate the rice cultivar IR24.
In this work we showed that a putative transcriptional regulatory gene, trh, is involved in expression of a hrp-regulatory gene, hrpG, in X. oryzae pv. oryzae, although whether the regulation is direct or indirect remains unknown. The trh gene is located far from both the hrp gene cluster and hrp-regulatory genes hrpG and hrpXo, although prh genes are located adjacent to the hrp gene cluster in R. solanacearum. Moreover, trh is located near the gene cluster for the type II secretion system (XOO0771 to XOO0781), which implies the possibility of involvement of trh in expression of genes other than hrp, especially genes for construction of the type II secretion system. However, we found no difference between the wild type and the trh mutant in the extracellular activity of cellulase, which is secreted via the type II secretion system, under either hrp-inducing or non-hrp-inducing conditions (data not shown). To clarify direct or indirect transcriptional activation of hrpG by Trh and involvement of Trh in expression of genes other than hrpG, further investigation is required.
Acknowledgments
We thank A. J. Bogdanove for critical reading of the manuscript.
This work was supported by a Grant-in-Aid for Scientific Research [(B) 13460024] from the Ministry of Education, Science, Sports, and Culture of Japan and by the Mishima Kaiun Memorial Foundation.
REFERENCES
- 1.Alfano, J. R., and A. Collmer. 1997. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J. Bacteriol. 179:5655-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdanove, A. J., S. V. Beer, U. Bonas, C. A. Boucher, A. Collmer, and D. L. Coplin. 1996. Unified nomenclature for broadly conserved hrp genes of phytopathogenic bacteria. Mol. Microbiol. 20:681-683. [DOI] [PubMed] [Google Scholar]
- 3.Bretz, J., L. Losada, K. Lisboa, and S. W. Hutcheson. 2002. Lon protease functions as a negative regulator of type III protein secretion in Pseudomonas syringae. Mol. Microbiol. 45:397-409. [DOI] [PubMed] [Google Scholar]
- 4.Brito, B., M. Marenda, P. Baberis, C. Boucher, and S. Genin. 1999. prhJ and hrpG: two new components of the plant signal-dependent regulatory cascade controlled by PrhA in Ralstonia solanacearum. Mol. Microbiol. 31:237-251. [DOI] [PubMed] [Google Scholar]
- 5.Brito, B., D. Aldon, P. Baberis, C. Boucher, and S. Genin. 2002. A signal transfer system through three compartments transduces the plant cell contact-dependent signal controlling Ralstonia solanacearum hrp genes. Mol. Plant-Microbe Interact. 15:109-119. [DOI] [PubMed] [Google Scholar]
- 6.Büttner, D., and U. Bonas. 2002. Getting across—bacterial type III effector proteins on their way to the plant cell. EMBO J. 21:5313-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis, G. R., and F. V. Gijsegem. 2000. Assembly and function of type III secretion systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 8.Ezuka, A., and O. Horino. 1974. Classification of rice varieties and Xanthomonas oryzae strains on the basis of their differential interactions. Bull. Tokai Kinki Natl. Agric. Exp. Stn. 27:1-19. [Google Scholar]
- 9.Furutani, A., S. Tsuge, T. Oku, K. Tsuno, Y. Inoue, H. Ochiai, H. Kaku, and Y. Kubo. 2003. Hpa1 secretion via type III secretion system in Xanthomonas oryzae pv. oryzae. J. Gen. Plant Pathol. 69:271-275. [Google Scholar]
- 10.Furutani, A., S. Tsuge, K. Ohnishi, Y. Hikichi, T. Oku, K. Tsuno, Y. Inoue, H. Ochiai, H. Kaku, and Y. Kubo. 2004. Evidence for HrpXo-dependent expression of type II secretory proteins in Xanthomonas oryzae pv. oryzae. J. Bacteriol. 186:1374-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haydon, D. J., and J. R. Guest. 1991. A new family of bacterial regulatory proteins. FEMS Microbiol. Lett. 63:291-295. [DOI] [PubMed] [Google Scholar]
- 12.Hutcheson, S. W., J. Bretz, T. Sussan, S. Jin, and K. Pak. 2001. Enhancer-binding proteins HrpR and HrpS interact to regulate hrp-encoded type III protein secretion in Pseudomonas syringae strains. J. Bacteriol. 183:5589-5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Innes, R. W., M. A. Hirose, and P. L. Kuempel. 1988. Induction of nitrogen-fixing nodules on clover requires only 32 kilobase pairs of DNA from the Rhizobium trifolii symbiosis plasmid. J. Bacteriol. 170:3793-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jefferson, R. A., T. A. Kavanagh, and M. W. Bevan. 1987. GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6:3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Marenda, M., B. Brito, D. Callard, S. Genin, P. Barberis, C. A. Boucher, and M. Arlat. 1998. PrhA controls a novel regulatory pathway required for the specific induction of Ralstonia solanacearum hrp genes in the presence of plant cells. Mol. Microbiol. 27:437-453. [DOI] [PubMed] [Google Scholar]
- 17.Ochiai, H., Y. Inoue, M. Takeya, A. Sasaki, and H. Kaku. 2005. Genome sequence of Xanthomonas oryzae pv. oryzae suggests contribution of large numbers of effector genes and insertion sequences to its race diversity. Jpn. Agric. Res. Q. 39:275-287. [Google Scholar]
- 18.Oku, T., A. M. Alvarez, and C. I. Kado. 1995. Conservation of the hypersensitivity-pathogenicity regulatory gene hrpX of Xanthomonas campestris and X. oryzae. DNA Seq. 5:245-249. [DOI] [PubMed] [Google Scholar]
- 19.Peekhaus, N., and T. Conway. 1998. Positive and negative transcriptional regulation of the Escherichia coli gluconate regulon gene gntT by GntR and the cyclic AMP (cAMP)-cAMP receptor protein complex. J. Bacteriol. 180:1777-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plano, G. V., J. B. Day, and F. Ferracci. 2001. Type III export: new uses for an old pathway. Mol. Microbiol. 40:284-293. [DOI] [PubMed] [Google Scholar]
- 21.Schulte, R., and U. Bonas. 1992. A Xanthomonas pathogenicity locus is induced by sucrose and sulfur-containing amino acid. Plant Cell 4:79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw, J. J., L. G. Settles, and C. I. Kado. 1987. Transposon Tn4431 mutagenesis of Xanthomonas campestris pv. campestris: characterization of a nonpathogenic mutant and cloning of a locus for pathogenicity. Mol. Plant-Microbe Interact. 1:39-45. [Google Scholar]
- 23.Swings, J., M. Van den Mooter, L. Vauterin, B. Hoste, M. Gillis, T. W. Mew, and K. Kersters. 1990. Reclassification of causal agents of bacterial blight (Xanthomonas campestris pv. oryzae) and bacterial leaf streak (Xanthomonas campestris pv. oryzicola) of rice as pathovars of Xanthomonas oryzae (ex Ishiyama 1922) sp. nov., nom. rev. Int. J. Syst. Bacteriol. 40:309-311. [Google Scholar]
- 24.Tsuge, S., Y. Ikawa, Y. Hikichi, Y. Nakazawa-Nasu, K. Suzuki, Y. Kobo, and H. Horino. 1999. Behavior of bioluminescent transconjugants of Xanthomonas oryzae pv. oryzae in compatible and incompatible rice leaves. Ann. Phytopathol. Soc. Jpn. 65:93-99. [Google Scholar]
- 25.Tsuge, S., A. Furutani, R. Fukunaka, Y. Kubo, and O. Horino. 2001. Growth complementation of hrpXo mutants of Xanthomonas oryzae pv. oryzae by virulent strains in rice cultivars resistant and susceptible to the parental strain. J. Gen. Plant Pathol. 67:51-57. [Google Scholar]
- 26.Tsuge, S., A. Furutani, R. Fukunaka, T. Oku, K. Tsuno, H. Ochiai, Y. Inoue, H. Kaku, and Y. Kubo. 2002. Expression of Xanthomonas oryzae pv. oryzae hrp genes in a novel synthetic medium, XOM2. J. Gen. Plant Pathol. 68:363-371. [Google Scholar]
- 27.Tsuge, S., H. Ochiai, Y. Inoue, T. Oku, K. Tsuno, H. Kaku, and Y. Kubo. 2004. Involvement of phosphoglucose isomerase in pathogenicity of Xanthomonas oryzae pv. oryzae. Phytopathology 94:478-483. [DOI] [PubMed] [Google Scholar]
- 28.Vidaver, A. K. 1967. Synthetic and complex media for the rapid detection of phytopathogenic pseudomonads: effect of the carbon source. Appl. Microbiol. 15:1523-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watabe, M., M. Yamaguchi, I. Furusawa, and O. Horino. 1993. Virulence and bacterial multiplication and movement in rice leaves of Xanthomonas campestris pv. oryzae mutants impaired in productivity of extracellular polysaccharide. Ann. Phytopathol. Soc. Jpn. 59:544-550. [Google Scholar]
- 30.Weber, E., T. Ojanen-Reuhs, E. Huguet, G. Hause, M. Romantschuk, T. K. Korhonen, U. Bonas, and R. Koebnik. 2005. The type III-dependent Hrp pilus is required for productive interaction of Xanthomonas campestris pv. vesicatoria with pepper host plants. J. Bacteriol. 187:2458-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei, Z. M., and S. V. Beer. 1995. hrpL activates Erwinia amylovora hrp gene transcription and is a member of the ECF subfamily of δ factors. J. Bacteriol. 177:6201-6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei, Z. M., J. F. Kim, and S. V. Beer. 2000. Regulation of hrp genes and type III protein secretion in Erwinia amylovora by HrpX/HrpY, a novel two-component system, and HrpS. Mol. Plant-Microbe Interact. 13:1251-1262. [DOI] [PubMed] [Google Scholar]
- 33.Wengelnik, K., C. Marie, M. Russel, and U. Bonas. 1996. Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J. Bacteriol. 178:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wengelnik, K., and U. Bonas. 1996. HrpXv, an AraC-type regulator, activates expression of five of six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J. Bacteriol. 178:3462-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wengelnik, K., G. Van den Ackerveken, and U. Bonas. 1996. HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria, is homologous to two-component response regulators. Mol. Plant-Microbe. Interact. 9:704-712. [DOI] [PubMed] [Google Scholar]
- 36.Wengelnik, K., O. Rossier, and U. Bonas. 1999. Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J. Bacteriol. 181:6828-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao, Y., Y. Lu, S. Heu, and S. W. Hutcheson. 1992. Organization and environmental regulation of the Pseudomonas syringae pv. syringae 61 hrp cluster. J. Bacteriol. 174:1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao, Y., S. Heu, Y. Jinseong, Y. Lu, and S. W. Hutcheson. 1994. Identification of a putative alternative sigma factor and characterization of a multicomponent regulatory cascade controlling the expression of Pseudomonas syringae pv. syringae Pss61 hrp and hrmA genes. J. Bacteriol. 176:1025-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu, W., M. M. Magbanua, and F. F. White. 2000. Identification of two novel hrp-associated genes in the hrp gene cluster of Xanthomonas oryzae pv. oryzae. J. Bacteriol. 182:1844-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]