Abstract
Cell-to-cell communication in bacteria is mediated by quorum-sensing systems (QSS) that produce chemical signal molecules called autoinducers (AI). In particular, LuxS/AI-2-dependent QSS has been proposed to act as a universal lexicon that mediates intra- and interspecific bacterial behavior. Here we report that the model organism Bacillus subtilis operates a luxS-dependent QSS that regulates its morphogenesis and social behavior. We demonstrated that B. subtilis luxS is a growth-phase-regulated gene that produces active AI-2 able to mediate the interspecific activation of light production in Vibrio harveyi. We demonstrated that in B. subtilis, luxS expression was under the control of a novel AI-2-dependent negative regulatory feedback loop that indicated an important role for AI-2 as a signaling molecule. Even though luxS did not affect spore development, AI-2 production was negatively regulated by the master regulatory proteins of pluricellular behavior, SinR and Spo0A. Interestingly, wild B. subtilis cells, from the undomesticated and probiotic B. subtilis natto strain, required the LuxS-dependent QSS to form robust and differentiated biofilms and also to swarm on solid surfaces. Furthermore, LuxS activity was required for the formation of sophisticated aerial colonies that behaved as giant fruiting bodies where AI-2 production and spore morphogenesis were spatially regulated at different sites of the developing colony. We proposed that LuxS/AI-2 constitutes a novel form of quorum-sensing regulation where AI-2 behaves as a morphogen-like molecule that coordinates the social and pluricellular behavior of B. subtilis.
Bacteria not only behave as self-sufficient individuals but also act as communities capable of cell-cell communication (7, 15, 20, 42, 53). This social interaction leads to the coordination of communitarian activities that resemble, in their complexity, the behaviors observed in multicellular organisms (20, 42, 49, 53, 57). This microbial phenomenon is known as quorum sensing, a process by which bacteria monitor their cell population density by measuring the concentration of small secreted signal molecules called autoinducers (57). Even though a vast number of diverse quorum-sensing systems exist (41, 46, 57), they can be divided into two established paradigms that regulate the intraspecific behavior in many bacteria: (i) LuxI/LuxR-type quorum-sensing systems in gram-negative bacteria responsible for the production of N-acyl-l-homoserine lactone autoinducers or type I autoinducers and (ii) oligopeptide/two component-type quorum-sensing circuits in gram-positive bacteria responsible for the production of autoinducer peptides (4, 17, 26, 32, 44).
However, in recent years, a considerable amount of information has been gained on the existence of another type of quorum-sensing system that not only seems to participate in intraspecific bacterial behavior but also seems to regulate the interspecific interactions among bacteria of different genera. This system involves the production of autoinducer-2 (AI-2) signal molecules, mediated by the activity of the LuxS enzyme, in response to cell density (15, 46, 59, 62). It has been demonstrated in numerous studies that the AI-2 molecules present in the supernatant of luxS-positive bacteria have the capacity to induce the AI-2 reporter species Vibrio harveyi, the model organism originally selected for the study of AI-2-dependent quorum sensing (57). LuxS/AI-2 have been shown to control a variety of cellular processes, such as production of pathogenicity factors, toxin production, biofilm formation, and swarming motility (14, 16, 28, 31, 34, 37, 38, 50, 58). More recently, it has been shown under laboratory conditions that interspecific luxS-dependent quorum sensing from E. coli modulated luxS-dependent quorum sensing in V. harveyi and pathogenic V. cholerae (63). Therefore, LuxS and its product AI-2 have been proposed to serve as a universal language (bacterial Esperanto or universal bacterial lexicon) used by bacteria to mediate their intra- and interspecific behaviors (59, 62).
Alternatively, it has been proposed that LuxS, the synthase for AI-2, has another role in the cell, in which it functions as an integral component of the activated methyl cycle (AMC) to avoid (jointly with the activity of the Pfs enzyme; see below) the intracellular accumulation of the toxic metabolite S-adenosyl-l-homocysteine (SAH). In fact, AI-2 is a by-product of the AMC, which recycles S-adenosyl-l-methionine (SAM), the main methyl donor in cells (35, 51, 55, 60, 61). As part of the AMC, SAM is converted to S-adenosyl-l-homocysteine (SAH), which is subsequently detoxified by the Pfs enzyme to generate adenosine and the sole intracellular source of substrate for LuxS, S-ribosyl-homocysteine (SRH). LuxS then produces the precursor of AI-2, 4,5-dihydroxy-2,3-pentanedione (DPD) during the conversion of SRH to homocysteine. DPD is able to undergo spontaneous cyclization to either of the two forms (R or S) of 2,4-dihydroxy-2-methylhydro-3-furanone (DHMF). Hydration of S-DHMF yields S-TMHF that subsequently forms a diester with boric acid to generate the first characterized active form of AI-2 with a key role in the regulation of quorum sensing of marine V. harveyi (11, 47). On the other hand, R-DHMF hydrates to form R-THMF, the active form of enteric AI-2 that has recently been cocrystallized with its putative cellular transporter/receptor LsrB (39, 54). Furthermore, R-THMF, once internalized, is phosphorylated to sequester it into the cytoplasm, where R-THMF-phosphate might function as an active intracellular autoinducer (39, 54). The findings that more than one type of furanone-derived molecule, depending on the bacterial species and the surrounding environment, can behave as the AI-2 in quorum sensing made it necessary to use the expression “AI-2” as a generic term to indicate a family of interconverting molecules with autoinducer activity (R-THM-furanone, S-THM-furanone-borate, and R-THM-furanone-phosphate) (see the legend to Fig. 2A for details and references 55 and 57).
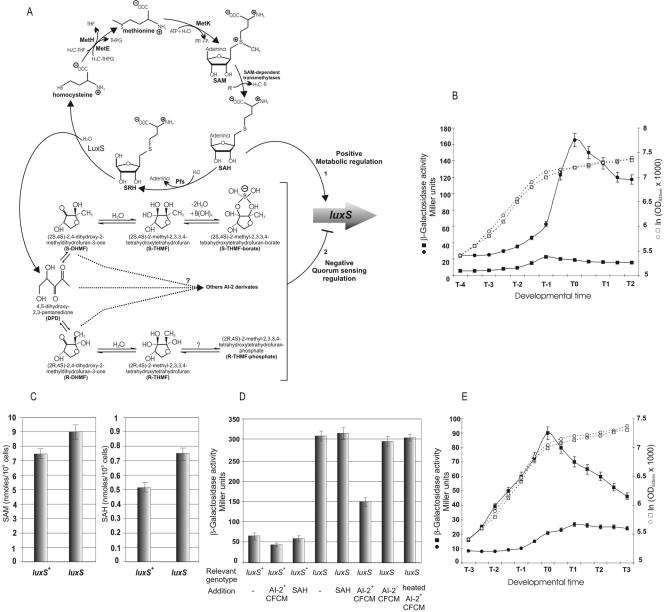
FIG. 2.
LuxS activity regulates luxS expression in B. subtilis. (A) A diagram of the activated methyl cycle (AMC) of the cell pointing out the nature of the products with AI-2 activity derived from the activity of the LuxS enzyme. Also shown are two possibilities, discussed in the text, for the regulation of luxS expression. One is by metabolic regulation mediated by metabolites such as SAH (possibility 1), and the other is by quorum-sensing regulation by AI-2 (possibility 2). (B) Up-regulation of luxS expression in AI-2-deficient B. subtilis cells. Growth and β-galactosidase activity were measured as indicated. Growth (□) and luxS activity (▪) of the RG1338 culture (wild-type strain, AI-2-positive) and growth (○) and luxS activity (•) of the RG1341 culture (luxS-negative mutant strain, AI-2 deficient) are shown. Essentially the same results were obtained using the B. subtilis natto strains RG4366 and RG4369 instead of RG1338 and RG1341, respectively (data not shown). (C) Intracellular levels of SAM and SAH accumulated by AI-2-proficient (strain RG1337) and AI-2-deficient (strain RG1339) cells. A representative set of experiments made by triplicate is shown. Essentially the same levels of SAH and SAM were detected in cultures of the B. subtilis natto strains RG4365 and RG4367 (data not shown). (D) Regulation of luxS expression by CFCM containing (or not containing) AI-2 activity. The figure shows the β-galactosidase activity accumulated until 1 h after T0 by cultures of the strains RG1338 (luxS+, AI-2-proficient) and RG1341 (luxS negative, AI-2 deficient) with each of them harboring a reporter luxS-lacZ fusion at the nonessential amyE locus (Table 1). Each culture of the strains RG1338 and RG1341 was grown in LB in the presence of the additions indicated in the figure. SAH was added at a final concentration of 1 mM; CFCM were added at a final concentration ranging from 1 to 5% (vol/vol) in each set of experiments repeated in triplicate (a representative set of data is shown). AI-2-positive CFCM and AI-2-deficient CFCM were derived from RG1337 and RG1339 cultures, respectively, as indicated in Materials and Methods. Essentially the same results were obtained using CFCM from cultures of the B. subtilis natto strains RG4365 (instead of CFCM from RG1337 cells) and RG4367 (instead of CFCM from RG1339 cells), and the results were corroborated by using the reporter natto strains RG4366 and RG4369 (data not shown). (E) In vivo down-regulation of luxS expression by up-regulation of AI-2 production. Growth and β-galactosidase activities expressed by the strain RG1341 harboring the multicopy plasmid pER3 (○ and •, respectively) overexpressing AI-2 and the control plasmid pHT315 (□ and ▪, respectively).
So far, LuxS/AI-2-dependent quorum sensing has been studied in diverse gram-positive and gram-negative bacteria (55, 57), but it is far from clear how widely this AI-2 family of autoinducers is used in bacterial signaling during the expression of wild behaviors in nature (33, 35, 51, 55, 57, 61). One microorganism that serves as an excellent model to study the regulation of cell development and social behavior is the gram-positive spore-forming soil-bacterium Bacillus subtilis (5, 10, 12, 21, 56). At present, there are two well-studied cell density-dependent signaling systems that have been described in this bacterium. These two QSS depend on the production of specific autoinducer peptides that are encoded by com and phr genes that control sporulation and competence development (21, 26, 32, 44). However, it has been recently reported that the detection of a LuxS-dependent signaling in Bacillus anthracis and Bacillus cereus with implicitness in density-dependent gene expression and pathogenesis for both bacteria (3, 27). Both luxS orthologs, from B. cereus and B. anthracis, show a high degree of identity with a putative luxS ortholog located on the chromosome of the sequenced reference strain 168 of B. subtilis. Therefore, we were intrigued to know whether or not a LuxS/AI-2-dependent signaling system operates in B. subtilis and, if this is the case, what consequences it would have on the social behavior and developmental potential of this model organism.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The two wild-type B. subtilis strains used in this study were the domesticated, laboratory-reference strain RG1337 (JH642 laboratory stock; James A. Hoch, The Scripps Research Institute, La Jolla, Calif.) and the undomesticated and wild B. subtilis natto strain RG4365 (laboratory stock; Akira Nakamura, University of Tsukuba, Japan). These two strains, as well as their isogenic derivates (Table 1), were grown in Luria-Bertani broth (LB), Schaeffer's sporulation medium (SM), or Spizizen minimal salts medium supplemented with glucose (0.5%) plus the supplements as indicated. The antibiotics, when required, were used at the following final concentrations: 5 μg/ml chloramphenicol, 100 μg/ml spectinomycin, 1 μg/ml erythromycin, and 2.5 μg/ml phleomycin. For sporulation efficiency, B. subtilis strains were grown in SM for 20 h and then treated with 10% CHCl3 for 15 min before being plated as previously described (2). Transformation of B. subtilis, to obtain isogenic derivates of the parental strains, was carried out as previously described (18). β-Galactosidase assays from B. subtilis strains harboring lacZ fusions were assayed as described previously and the specific activity was expressed in Miller units (2). The β-galactosidase experiments described in the figure legends were independently repeated three to five times, and a representative set of results is shown in each figure. All cultures were grown at 37°C. V. harveyi BB170 was kindly provided by B. Bassler (Princeton University) and grown in AB medium overnight at 30°C as described previously (6, 19). AB medium consists of 10 mM K2HPO4 (pH 7.0), 0.3 M NaCl, 0.05 M MgSO4, 0.2% vitamin-free Casamino Acids (Difco), 2% glycerol, and 1 mM l-arginine.
TABLE 1.
Bacillus subtilis strains
| Strain | Relevant genotype | Comments and/or source (reference)a |
|---|---|---|
| RG1337 (JH642) | Wild-type domesticated | Laboratory stock (2) |
| RG1338 | amyE::luxS-lacZ cat | This study |
| RG1339 | luxS::pJM103 cat | This study |
| RG1340 | luxS::pJM103 cat::spc | pCm::Spc (BGSC)b→RG1339 |
| RG1341 | amyE::luxS-lacZ cat luxS::pJM103 cat::spc | RG1338→RG1339 |
| RG4365 | Wild-type undomesticated natto strain | Laboratory stock (A. Nakamura) |
| RG4366 | amyE::luxS-lacZ cat | This study |
| RG4367 | luxS::pJM103 cat | This study |
| RG4368 | amyE::sspB-lacZ cat | RG348 (36)→RG4365 |
| RG4369 | amyE::luxS-lacZ cat luxS::pJM103 cat::spc | This study |
| RG12604 | amyE::abrB-lacZ cat | Laboratory stock (2) |
| RG1342 | amyE::abrB-lacZ cat luxS::pJM103 cat::spc | RG12604→RG1340 |
| RG438 | amyE::sinR-lacZ cat | Laboratory stock (18) |
| RG1343 | amyE::sinR-lacZ cat luxS::pJM103 cat::spc | RG438→RG1340 |
| RG19005 | amyE::spo0A-lacZ cat | Laboratory stock (36) |
| RG1344 | amyE::spo0A-lacZ cat luxS::pJM103 cat::spc | RG19005→RG1340 |
| RG1345 | amyE::luxS-lacZ cat ΔabrB::spc | RG1338→RG12607 (2) |
| RG1346 | amyE::luxS-lacZ cat ΔsinR::pheo | RG1338→RG432 (18) |
| RG1347 | amyE::luxS-lacZ cat Δspo0A::ery | RG955 (36)→RG1338 |
| RG4380 | amyE::luxS-lacZ cat Δspo0A::ery | RG955 (36)→RG4366 |
| RG4381 | amyE::luxS-lacZ cat ΔsinR::pheo | RG432 (18)→RG4366 |
Strain construction is indicated by an arrow. Chromosomal DNA or plasmid DNA listed at the tail of the arrow was used to transform the strains listed at the head of the arrow.
BGSC, Bacillus Genetic Stock Center, Ohio State University, Columbus, Ohio.
For the swarming analysis, we used B medium (1), which contains 15 mM (NH4)2SO4, 8 mM MgSO4 · 7H2O, 27 mM KCl, 7 mM sodium citrate · 2H2O, and 50 mM Tris-HCl (pH 7.5) supplemented with 0.6 mM KH2PO4, 2 mM CaCl2 · 2H2O, 1 mM FeSO4 · 7H2O, 10 mM MnSO4 · 4H2O, 4.5 mM glutamic acid, 780 μM tryptophan, 860 μM lysine, and 0.2% (wt/vol) glucose. All plates were prepared by supplementing the medium with 0.7% of agar and used during the day.
Detection of SAM and SAH.
For the detection of SAM and SAH, B. subtilis strains were grown in Spizizen minimal salts medium until 1 h after the end of the exponential phase. Standard reagents, SAM iodide salt, SAH, and 1-heptanosulfonic acid sodium salt were all obtained from Sigma Chemical Company. SAM and SAH levels were determined by reverse-phase ion-pair high-performance liquid chromatography (ΔKTA basic high-performance liquid chromatograph [Amersham]). To prepare the high-performance liquid chromatography extracts, frozen pellets from cultures were resuspended in chilled 0.01 N hydrochloric acid (HCl) and sonicated on ice. After centrifugation, supernatants were filtered through a 0.2-μm filter (Orange Scientific) at 4°C. The mobile phase consisted of 87% 5 mM heptanesulfonic acid (Sigma Co.), with the pH adjusted to 3.2 with HCl and 13% acetonitrile (EM Science). The flow rate was 1 ml/min through an Altech C18 column (4.6 by 250 mm), and the absorbance was monitored at 254 nm. The amounts of SAM and SAH in the samples were determined by comparison with a standard solution of 10 μM each of SAM and SAH in 0.01 N HCl. All runs, including standards, were done three times. SAM and SAH levels were expressed as picomoles per 1 × 109 CFU of the corresponding B. subtilis culture.
Plasmids and strains constructions.
Strains RG1338 and RG4366 are isogenic derivates from RG1337 and RG4365, respectively, that carry a transcriptional reporter fusion to the luxS promoter region (Table 1). To obtain the promoter region of luxS, we proceeded to a PCR amplification of chromosomal DNA from strain RG1337 using the oligonucleotides 5′ TTCTTCAGGTACCTTTCTGATGCAAG 3′ and 5′ TTATTTAGGATCCCGTCTGTTCCCAC 3′ (the introduced restriction sites for KpnI and BamHI are underlined; boldface indicates substitutions used to create the restriction site). The PCR product (230 bp) containing the promoter region of luxS was cloned into the mcs of the vector pJM116 to make a transcriptional fusion to the lacZ gene of E. coli (2, 56). The resulting plasmid (pER1) (Fig. 1A) was linearized and integrated into the chromosome of wild-type competent cells via double crossover at the amyE locus by transformation and selection for resistance to chloramphenicol (2, 56). For the construction of B. subtilis luxS-negative mutant strains (RG1339 and RG4367), we amplified a 420-bp internal fragment of luxS by using chromosomal DNA from strain RG1337 as a template and the oligonucleotides 5′ ggTTgTggATCCATATgTAAgACATTgCg 3′ and 5′ TTCTTCTggATCCTgTgAAAgCCAgAAACgC 3′. The amplified PCR fragment was cloned into the integrative vector pJM103 (2, 57) at the unique BamHI site, generating pER2 (Fig. 1A). This plasmid was used to transform competent cells of RG1337 and RG4365 and to select, by a single-crossover event, chloramphenicol-resistant colonies that were screened by Southern blotting and PCR to corroborate the integration of pER2 into the luxS locus. To change the antibiotic resistance marker (chloramphenicol resistance) of the strains to one of a spectinomycin resistance, we used the plasmid pCm::Spc isolated from the E. coli ECE74 strain (Bacillus Genetic Stock Center). For the overexpression of luxS in the multicopy vector pHT315 (25 to 50 copies/cells), we amplified from strain RG1337 a truncated version of luxS lacking its promoter region but containing its own ribosomal binding and ATG sites (plasmid pER3) (Fig. 1A). To this end, we used oligonucleotides 5′ gAAATTACATTTCTgCagAAggggAgAg 3′ and 5′ CCTAAATggTACCAAACgCTTACAgC 3′ to clone the amplified PCR fragment between the PstI and KpnI restriction sites of pHT315, giving rise to the multicopy plasmid pER3 (Fig. 1A). This plasmid and the vector pHT315 were introduced into competent cells of the luxS-negative mutant reporter strain RG1341 (Table 1) and selected for resistance to erythromycin (15 μg/ml).
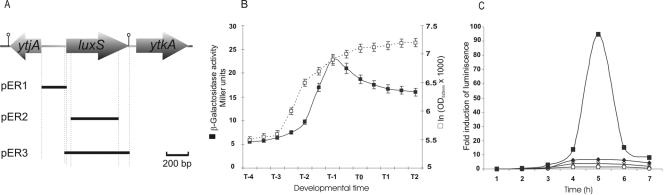
FIG. 1.
Organization and biological activity of luxS of B. subtilis. (A) Representation of the DNA region containing luxS and its adjacent genes. In addition, we showed the location and size of the DNA segments amplified and used for plasmid and strain constructions (see Materials and Methods for details). Rho-independent transcriptional terminators and the relevant restriction sites used for cloning are shown on the diagram. (B) The wild-type reporter strain RG1338 (pluxS-lacZ::amyE) was grown in SM and growth was measured as the increase in optical density at 525 nm; samples were collected at the indicated times and assayed for β-galactosidase activity expressed in Miller units (2, 36). T0 represents the transition from vegetative to stationary phase. Essentially, the same pattern of luxS expression (with a maximum of 30 to 45 Miller units at T−1) was observed with the B. subtilis natto strain RG4366 (data not shown). (C) Induction of bioluminescence in V. harveyi strain BB170 by CFCM prepared from wild-type RG1337 cells and its isogenic luxS-negative derivate RG1338 (Table 1). At time zero, CFCM from the different strains was added to the V. harveyi culture at a final concentration of 10% (vol/vol) and light production was recorded. Symbols for the addition of CFCM at each experimental condition are as follows: ▪, V. harveyi plus CFCM from RG1337 cells; ○, V. harveyi plus CFCM from RG1338 cells; ⧫, V. harveyi plus heated (80°C, 20 min) CFCM from RG1337 cells; ×, V. harveyi without CFCM addition. Data for a representative set of experiments are shown. Essentially, the same results as the ones obtained with CFCM from RG1337 and RG1338 cells were obtained using CFCM from the B. subtilis natto strains RG4365 and RG4367, respectively, and the V. harveyi BB170 strain (data not shown).
AI-2 bioassay.
B. subtilis strains were cultured in LB for 12 h (to stationary phase). The stationary-phase cultures were then inoculated into LB at a 1:20 dilution and grown until mid-exponential phase. The culture supernatants were collected by centrifugation at 8,000 × g and passed through a 0.45-μm filter (Orange Scientific) to remove cells. This cell-free conditioned medium (CFCM) was stored at −20°C until studied. The bioassay was performed essentially as described previously (63). Briefly, the V. harveyi BB170 luxN::Tn5, AI-1 sensor-negative AI-2 sensor-positive (6, 63) reporter strain was grown for 16 h with aeration at 30°C in AB medium, and then diluted 1:10,000 in fresh AB medium. Cell-free culture supernatants of B. subtilis strains to be tested for AI-2 activity were then added to the diluted V. harveyi culture at a 10% (vol/vol) final concentration. The cultures were shaken al 30°C and light production was measured every hour.
Biofilm (pellicle) formation.
Overnight cultures of B. subtilis were grown in LB to stationary phase. Then, 50 μl of these cultures were diluted in 2 ml of fresh LB supplemented with 10% yeast extract and incubated at 37°C for 48 h.
Swarming motility.
B medium (1) fortified with 0.7% agar was inoculated with 1 μl of 2 × 108 cells/ml grown to mid-log phase at 37°C in LB. The inoculated petri dishes were then incubated at 37°C for 24 h.
Fruiting body formation.
For colony architecture analysis, the luxS+ and luxS-negative natto strains were grown for 12 h in LB, reaching a cell density of 5 × 107 viable cells/ml, and then 5 μl of each culture were placed onto SM-agar plates supplemented with 10% yeast extract plus (when indicated) 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal, 80 μg/ml) and incubated at 37°C.
Microscope observations.
The developed swarm, biofilm and fruiting-body plates were visualized with a Stemi 2000 (Carl Zeiss, Germany) stereomicroscope using a KL1500LCD (Carl Zeiss, Germany) illumination system. A Power-Shot A80 system (Canon) was used to capture the photographs for swarm, fruiting bodies, and biofilm images.
RESULTS
B. subtilis luxS gene is actively transcribed, and its product, AI-2, mediates interspecific signaling with V. harveyi.
AI-2-dependent quorum sensing signaling is currently found in more than 55 gram-positive and gram-negative bacterial species, leading to the suggestion that AI-2 constitutes a universal language for interspecies communication (15, 46, 50, 53, 57, 59). To uncover the situation in B. subtilis, we characterized the pattern of expression of the B. subtilis luxS ortholog and the supposed biological activity of its product (AI-2) using the V. harveyi bioassay. An examination of the annotation of the complete genome sequence of the B. subtilis European strain 168 (http://genolist.pasteur.fr/SubtiList) showed only one ortholog (formerly named ytjB) of the V. harveyi luxS gene (data not shown). luxS of B. subtilis seems to be organized as a monocistronic unit of 471 bp flanked by rho-independent transcriptional terminators and not linked to any specific gene class (Fig. 1A and data not shown). To examine the in vivo activity of luxS in B. subtilis, we cloned the 5′ end of this gene as a lacZ transcriptional fusion (strain RG1338) (Table 1). As shown in Fig. 1B, the use of this construct permitted demonstration that luxS was actively transcribed. luxS expression showed a continuous augmentation of its activity during the exponential growth of B. subtilis in LB, reaching a maximum value of 20 to 25 Miller units of β-galactosidase activity one hour before the onset of the stationary phase (T0), and decreased upon entry into stationary phase (Fig. 1B).
The temporally regulated activity of the luxS promoter opened the possibility that B. subtilis operates a previously undetected LuxS-dependent quorum-sensing signaling system apart from the well-described autoinducer peptide-dependent quorum-sensing systems that regulate sporulation and competence development (21). Therefore, to confirm that B. subtilis produced active AI-2, we recur to the use of the V. harveyi AI-2 reporter assay (6, 63). This AI-2 assay takes advantage of a deficiency in AI-1 sensor signaling in the V. harveyi strain BB170. Without the luxN AI-1-encoded sensor, strain BB170 can exhibit only bioluminescence in response to AI-2 (6, 63). When an overnight culture of the V. harveyi strain BB170 is diluted 1:10,000 (to yield low cell density), the level of endogenous AI-2 is reduced below the threshold required for luminescence. Under these experimental conditions, the addition of exogenous cell-free supernatants from bacteria possessing LuxS activity (and, hence, producing AI-2) can restore the bioluminescence phenotype of the BB170 cells (6, 57, 63). Therefore, we prepared CFCM from the wild-type strain RG1337 grown in LB until 1 h before T0. This CFCM was added to the BB170 cells prepared as indicated above, and the luminescence induced by B. subtilis culture supernatants was measured (Fig. 1C). As observed, the cell-free supernatant from the wild-type strain RG1337 significantly induced the luminescence of BB170 (Fig. 1C). In contrast, the luminescence of BB170 was induced neither by heat-treated CFCM (to destroy AI-2-dependent activity) of wild-type RG1337 cells nor by supernatant of the RG1339 isogenic luxS-negative mutant strain (absence of AI-2 activity). These results indicated that B. subtilis harbors an active luxS gene (Fig. 1B) that codes for an AI-2-mediated activity able to be recognized as the AI-2-specific signaling pathway of V. harveyi (Fig. 1C).
Transcription of luxS is regulated by a negative autoregulatory feedback loop.
The LuxS enzyme is responsible for the last enzymatic step of AI-2 synthesis (43, 45, 47, 55, 57), but at the same time, it has an important function within central metabolism as part of the activated methyl cycle (AMC) of the cell for recycling (jointly with Pfs) of the toxic intermediate SAH (51, 55, 57, 61, 62). In contrast to what has been reported in Salmonella enterica serovar Typhimurium and other bacteria for which luxS expression is constitutive (8, 55, 57), we demonstrated that in B. subtilis, luxS activity was temporally regulated (Fig. 1B). Therefore, it was interesting to analyze whether the synchronized expression of luxS was growth-phase regulated as a reflection of cell density (quorum sensing) or, in contrast, if luxS was regulated by central metabolism in order to maintain appropriate nontoxic levels of the intermediates of the AMC (Fig. 2A). To this end, we recurred to the use of an RG1337-isogenic luxS-negative mutant strain that harbored a luxS-lacZ reporter fusion at the nonessential amyE locus (strain RG1341) (Table 1). This construct permitted analysis of the expression of luxS in the absence of AI-2-dependent quorum sensing. First, we noticed that the absence of LuxS activity did not produce any detrimental effect on growth or final cellular yield of the luxS-negative mutant strain, in comparison to the wild-type strain (Fig. 2B and data not shown). More interesting, as is shown in Fig. 2B, the luxS-dependent β-galactosidase levels of the AI-2-deficient RG1341 culture far exceeded the levels of β-galactosidase activity accumulated by a culture of AI-2-proficient cells throughout the growth period (strain RG1338) (Table 1). Since the growth of the luxS-negative mutant strain was indistinguishable from the growth of the wild-type strain (Fig. 2B) and the two cultures reached similar cellular yields (data not shown), it can be assumed that the accumulation of intermediates of the AMC did not reach growth-toxic levels. To support this conclusion, we measured the intracellular levels of SAM and SAH accumulated by LuxS-proficient and LuxS-deficient cells. As shown in Fig. 2C, even though the intracellular levels of both intermediates of the AMC were a little higher in LuxS-deficient cells, their concentrations (less than nM) were far lower than the concentrations reported to be toxic in bacteria (33, 40, 43, 51, 55). Therefore, the dramatic difference in activity of the luxS gene in favor of the AI-2-deficient mutant strain (Fig. 2B) was not due to intracellular toxicity.
In contrast, we hypothesized that the regulation of luxS transcription was (i) metabolically regulated by metabolites of the AMC and/or (ii) cell density-regulated by AI-2 (Fig. 2A). In order to distinguish between these possibilities, we measured the activity of the luxS-lacZ reporter fusion in LuxS-proficient and LuxS-deficient cells grown in the presence of intermediates of the AMC or in the presence of CFCM prepared from wild-type (presence of AI-2) or luxS-negative (absence of AI-2) cultures. As shown in Fig. 2D, the activity of the luxS promoter in the wild-type strain was not affected by the addition of SAH (or other intermediates of the AMC, such as SAM and Met [data not shown]) or by the addition of CFCM prepared from a luxS-negative culture. In contrast, the addition of wild-type-derived CFCM (presence of AI-2) to a wild-type culture produced a slight, but reproducible, inhibition of luxS activity (Fig. 2D). Moreover, the addition of CFCM containing AI-2, but not CFCM prepared from a luxS-negative culture (absence of AI-2), to a LuxS-deficient culture produced a significant reduction of the activity of the luxS promoter. These results strongly indicated that it was not the accumulation of intermediates of the AMC (possibility 1 in Fig. 2A) that resulted in the higher activity of the luxS promoter and suggested the existence of a negative AI-2-dependent autoregulatory loop operating on luxS expression. Supporting this view, the heat treatment (to destroy the activity of AI-2 molecules) of CFCM derived from wild-type cells resulted in the complete loss of the original inhibitory effect on the activity of the luxS promoter (Fig. 2D).
If, as we suggested, AI-2 has an inhibitory effect on luxS expression (possibility 2 in Fig. 2A), it can be postulated that the overproduction or accumulation of AI-2 should decrease luxS transcription. To test this hypothesis, we overexpressed luxS without its native promoter region (to avoid intrinsic regulation and/or titer effects) in the multicopy vector pHT315 (see Fig. 1A and Materials and Methods). This multicopy plasmid has been shown to overexpress cloned genes from constitutive internal promoters (2, 26, 56). As is shown in Fig. 2E, the growth of the culture harboring the multicopy plasmid overexpressing luxS (pER3) (Fig. 1A) was not affected in comparison with the growth of the culture harboring pHT315 without insert. Confirming the original hypothesis of the existence of an AI-2-dependent negative feedback loop on luxS, the expression of luxS driven from its chromosomal copy with its native promoter showed a dramatic reduction throughout growth in the presence of the plasmid overproducing AI-2 (Fig. 2E).
AI-2 production is under the control of the master regulatory proteins of social behavior, Spo0A and SinR.
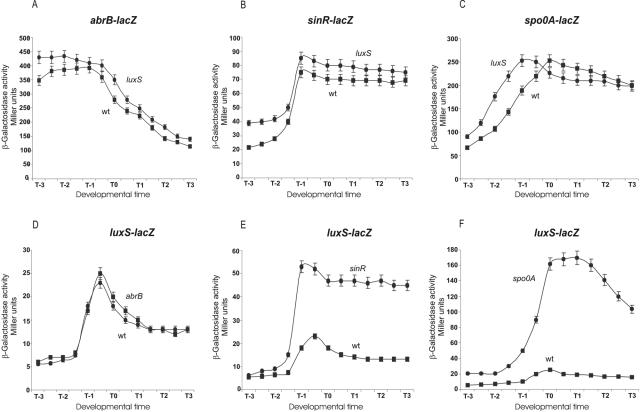
The observation that luxS expression reached a maximum level at the end of the exponential phase of growth and declined after the commencement of the stationary phase (Fig. 1B) led us to further examine if key B. subtilis transition state regulators are involved in the regulation of luxS expression and vice versa (9, 10, 21). In particular, we investigated the B. subtilis transcription factors (Spo0A, AbrB, and SinR) that participate in quorum-sensing regulation of biofilm, swarming, and spore development (9, 10, 22, 29, 30). As shown in Fig. 3, the levels of expression of the developmental genes abrB, sinR, and spo0A were essentially the same in LuxS-proficient and LuxS-deficient cells, discarding the possibility that AI-2 would be affecting the expression of those master regulatory proteins (Fig. 3A to C). On the other hand, while luxS activity was not affected in AbrB-deficient cells (Fig. 3D), luxS expression was dramatically induced in Spo0A- and SinR-deficient cultures (Fig. 3E and F), showing the existence of a Spo0A/SinR-dependent negative-regulatory circuit decreasing luxS expression. Moreover, these findings suggested the possibility of a physiologic connection between AI-2-dependent quorum sensing and pluricellular social behaviors regulated by Spo0A and SinR (see below).
FIG. 3.
Spo0A and SinR are negative regulators of AI-2 quorum sensing. (A to C) Activity of the regulatory genes abrB, sinR, and spo0A in the presence and absence of LuxS activity. (A) RG12604 and RG1342; (B) RG438 and RG1343; (C) RG19005 and RG1344. Symbols: ▪, LuxS-proficient cells; •, LuxS-deficient cells. (D to F) Effects of AbrB, SinR, and Spo0A on luxS expression. (D) RG1338 (▪) and RG1345 (•); (E) RG1338 (▪) and RG1346 (•); (F) RG1338 (▪) and RG1347 (•). Cells were grown in LB and samples were collected at the indicated times and assayed for β-galactosidase activity expressed in Miller units (18).
LuxS activity is required for biofilm formation and swarming motility in B. subtilis.
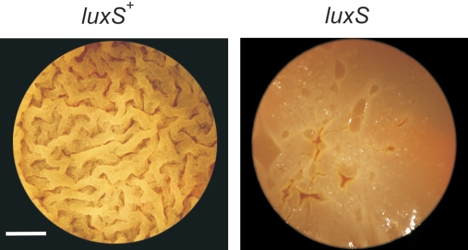
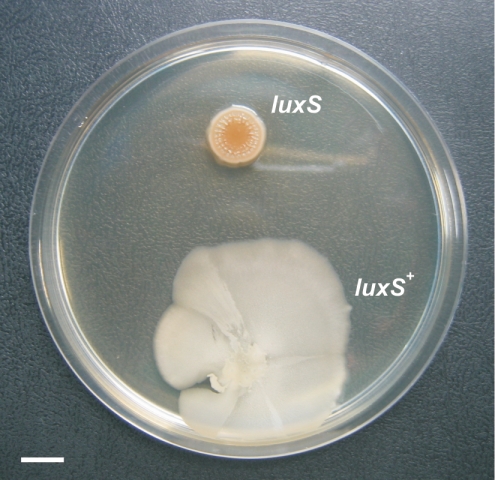
LuxS and/or AI-2 have been shown to affect normal biofilm formation and swarming motility in different pathogenic bacteria (13, 57). The domesticated B. subtilis strains routinely used in research laboratories (i.e., strains 168 and JH642) do not develop a high degree of spatial organization and do not form highly structured or surface-associated communities. However, it has been recently reported that wild, undomesticated strains of B. subtilis, which were not subjected to random mutagenesis or to repeated rounds of selections for fast growth in rich culture media, were capable of social and multicellular behaviors (9, 10, 12, 22, 29, 30). At the moment, only the undomesticated B. subtilis NCIB 3610 isolate (or Marburg strain, from which the strains 168 and JH642 are derived) has been selected for the studies of multicellular behavior in bacilli (9, 10, 29). However, wild behaviors should be common and well-distributed attributes of most bacteria in nature (20, 42, 49). One type of undomesticated B. subtilis isolate whose social behavior is unreported and is a model of study in our laboratory because of its probiotic properties, is the Japanese B. subtilis natto strain RG4365 (Table 1 and references 5, 24, and 48). We confirmed that the pattern of expression of luxS in the B. subtilis natto strain RG4366 (Table 1) and its up-regulation in an AI-2-deficient background (strain RG4369) were indistinguishable from the results obtained with the domesticated strains RG1338 and RG1341, respectively (data not shown). In light of these findings, we were motivated to know to what extent LuxS would be required for the full manifestation of two previously described social behaviors of B. subtilis NCIB 3610 that were under Spo0A and SinR regulation: biofilm formation and swarming migration (10, 12, 29, 30). First, we noted that the wild-type B. subtilis natto strain (RG4365) formed, in standing liquid cultures incubated at 37°C for 48 h, robust and highly structured biofilms that were very similar to the previously reported thick and vein-like biofilms formed by strain NCIB 3610 (9, 10). In contrast, biofilm formation of the luxS-negative RG4367 natto strain was delayed and morphologically altered showing a clear final defect on pellicle formation and biofilm maturation (Fig. 4).
FIG. 4.
LuxS activity is required for biofilm formation in B. subtilis. Top-down images of pellicle (biofilms) made by LuxS-proficient (left panel) cells (strain RG4365) and LuxS-deficient (right panel) cells (strain RG4367) in LB fortified with 10% yeast extract after 2 days at 37°C without shaking. Note the differences in rugosity and architecture between both biofilms. Scale bar, 1 cm.
When we analyzed the ability of the LuxS-proficient and LuxS-deficient B. subtilis natto cells (strains RG4365 and RG4367, respectively) to swarm on solid surfaces, we found that LuxS activity was also essential for this cell density-dependent social motility behavior (Fig. 5). These results strongly argue for a novel level of regulation and complexity (LuxS-dependent quorum sensing) apart from the previously reported effects of Spo0A and SinR (12, 22), acting on the manifestation of the social and pluricellular behaviors of wild B. subtilis isolates.
FIG. 5.
LuxS activity is required for swarming motility in B. subtilis. Swarming ability of AI-2-proficient (strain RG4365) and AI-2-deficient (RG4367) cells after 24 h of incubation in B medium at 37°C. See Materials and Methods for details. Scale bar, 1 cm.
LuxS activity produces a morphogen-like developmental signal crucial for pluricellular aerial architecture in B. subtilis.
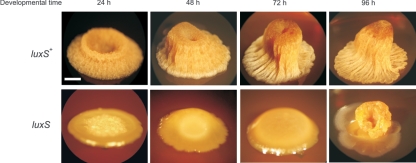
While colonies of the domesticated B. subtilis strains (i.e., 168 and JH642) are flat, smooth, and poorly differentiated (9, 10), colonies of the wild-type B. subtilis natto strain form thick and very sophisticated aerial structures. In Fig. 6, we show the time course of formation and maturation of the aerial colony architecture produced by the wild-type B. subtilis natto strain RG4365. Early in morphogenesis, the borders of the developing colony acquired a vein-like architecture with avenues of cells centrally converging to give rise, at later times (48 h to 72 h of development), to a palisade of cells that formed a central cylinder emerging from the base of the colony (Fig. 6). During the development of this elegant arrangement of differentiating cells, the cavity of the elevating cylinder (or cellular tower) remained open, full of a viscous fluid containing few trapped cells (data not shown). Later in development (96 h of development), the crest of the aerial tower was covered and closed with several layers of cells (Fig. 6) that finally differentiated into mature spores (see below and Fig. 7A).
FIG. 6.
LuxS activity is required for aerial colony architecture in B. subtilis. Time course development of colony morphogenesis of AI-2-proficient (strain RG4365) and AI-2-deficient (strain RG4367) cells on solid SM fortified with 10% yeast extract. Scale bar, 1 mm.
FIG. 7.
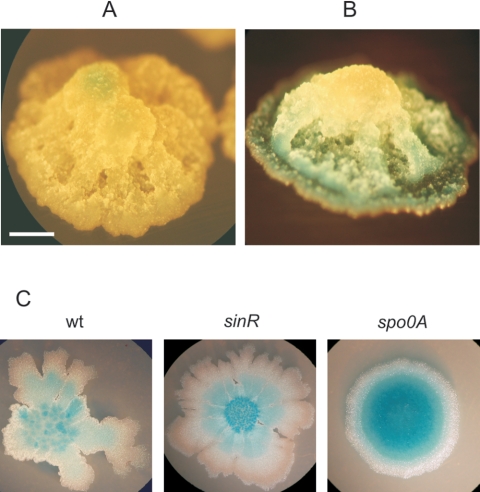
Spore development and AI-2-dependent cell-to-cell communication during fruiting body morphogenesis of wild-type B. subtilis cells. (A) Expression of the late sporulation gene sspB (strain RG4368) indicating that spore development takes place at the top of the developing colony in an AI-2-independent process (see the legend to Fig. 3C and the text for details). Spore morphogenesis is indicated by development of the blue color. (B) Expression of luxS (strain RG4366) from the bottom of the developing aerial colony, suggesting the formation of an ascending gradient of AI-2 molecules. Scale bar, 1 mm. (C) Expression of luxS in the absence of SinR (strain RG4381) and Spo0A (strain RG4380) after 16 h of incubation in LB at 37°C.
Microbial aerial structures are typically sites for sporulation in fungi and filamentous bacteria. This is also the case for the microscopic fruiting bodies that were shown to project from the colonies of the wild strain NCIB 3610 (10). To prove that wild-type B. subtilis natto cells would also differentiate into spores in special regions of the developing aerial colony (Fig. 6), we used a construct for which the promoter region of the sporulation-specific gene sspB, which is expressed late in sporulation, was fused to the lacZ gene and integrated in a single copy into the chromosome of the wild-type B. subtilis natto strain RG4365 (strain RG4368) (Table 1). This reporter strain was grown on solid medium that contained the X-Gal indicator of LacZ activity to point out the sites in the aerial colony where the spores were formed. As observed in Fig. 7A, spore morphogenesis was restricted to the pinnacle of the colony, a finding that indicated that spore development took place in a temporal and spatially regulated process that was highly coordinated with the formation of a sophisticated aerial colony. In fact, the extremely differentiated natto colony resembled a giant fruiting body (Fig. 7A). In the undomesticated NCIB 3610 (and also in myxobacteria), fruiting bodies are microscopic aerial projections of the colony (10), but in the case of B. subtilis natto, the whole aerial colony behaved as a unique and enormous fruiting body visible at first glance (Fig. 6 and 7A). Interestingly, when the behavior of the luxS-negative B. subtilis natto mutant strain was analyzed on solid medium, a tremendous defect in colony morphogenesis was immediately observed (Fig. 6). It took longer for the LuxS-deficient cells to make a structured colony (72 h or more) that finally failed to fully differentiate. In fact, LuxS-deficient cells were unsuccessful in their commitment to aerial-colony development because, at longer times of incubation (96 h or more), the immature AI-2-deficient aerial structure collapsed (Fig. 6).
In eukaryotes, during the course of development, cells of many tissues differentiate in response to the presence of gradients of substances that act as morphogens (52, 64). These developmental messenger molecules emanate from a restricted part of the tissue and spread away from their source to form a concentration gradient (52, 64). Since the pattern and the appearance of aerial fruiting body formation of the wild-type B. subtilis natto strain resembled the behavior and cell-coordination of a differentiating tissue, we were intrigued to know whether the pattern of luxS expression, and hence AI-2 production, was temporal and spatially regulated during development (25). Therefore, we analyzed the expression of luxS in B. subtilis natto during fruiting body morphogenesis. As observed in Fig. 7B, luxS was clearly expressed at the base of the fruiting body, supporting the view that AI-2 production was spatially restricted (probably by action of Spo0A and SinR; see Discussion and Fig. 7A and C) to a specific region of the developing colony from where it would be able to emanate. This result suggested the formation of an AI-2 gradient (bacterial morphogen) (25) that would diffuse or migrate through the incipient aerial colony (bacterial tissue) to coordinate appropriate gene expression during fruiting body morphogenesis.
DISCUSSION
luxS of B. subtilis shares a high degree of homology with luxS orthologs found in different bacteria, including luxS genes from Escherichia coli, S. enterica serovar Typhimurium, and V. harveyi, in which LuxS-dependent quorum sensing has been extensively studied (3, 23, 27, 45). Furthermore, the overexpression of the B. subtilis luxS gene in E. coli and the use of the produced B. subtilis LuxS enzyme made it possible to prove unambiguously that LuxS is a ribosylhomocysteinase that catalyzes the conversion of SRH to homocysteine and DPD, the precursor of all known active forms of AI-2 (23, 43, 45, 47, 60). However, the presence of an AI-2-dependent activity in B. subtilis and the impact of LuxS on the regulation of quorum sensing in this model bacterium have not yet been reported. Precisely, the main contributions of this work are (i) that it showed for the first time that luxS of B. subtilis was an active gene responsible for the production of active AI-2 able to be recognized by the AI-2-dependent signaling system of V. harveyi (Fig. 1) and (ii) the numerous evidence that demonstrated the key role of luxS in the cell density-dependent multicellular behavior of B. subtilis.
Even though AI-2-dependent quorum sensing has been analyzed in many bacteria, very little is known about the regulation of luxS expression (5, 55). Here, we demonstrated that luxS transcription was under AI-2-dependent quorum-sensing regulation (Fig. 2B). The dramatic overexpression of luxS in an AI-2-deficient background was not due to the accumulation of the toxic metabolite SAH of the AMC (Fig. 2A), because, as it was shown, the growth of the luxS-negative mutant strain (completely deficient in AI-2-dependent activity; see Fig. 1C) was the same as the growth of the wild-type strain (Fig. 2B). While SAH and SAM accumulated to a higher level in luxS-deficient cells than the levels of the AMC metabolites in wild-type cells (Fig. 2C), they were far lower than the levels of SAH reported to be toxic in bacteria (55, 60). These results (Fig. 2B and C) indicated that the levels of accumulated metabolites from the AMC in the luxS-deficient strain were not toxic and suggested that the concentration of the intermediates of the AMC would be cell regulated (i.e., at transcriptional level of the genes involve in the AMC and/or stability of the AMC metabolites) in order to keep these compounds at low, nontoxic concentrations.
The lack of a detrimental effect on growth resulting from the absence of LuxS activity suggested a role for this enzyme in cell-cell signaling and not in AMC-related metabolism to decrease (jointly with the activity of Pfs) the concentration of SAH. We obtained further evidence for a quorum-sensing or growth-phase regulation of luxS from the experiments in which we measured the effects on luxS expression after the addition of cell-free supernatants (CFCM) derived from AI-2-positive and AI-2-negative cultures (Fig. 2D). The observed results strongly argued for an AI-2-dependent negative inhibitory feedback loop controlling luxS transcription (Fig. 2A, possibility 2). In order to test this hypothesis, we predicted that an overaccumulation or overproduction of AI-2 should decrease luxS expression. To prove this, we overproduced AI-2 by expressing the B. subtilis luxS gene in the multicopy plasmid pHT315. As shown in Fig. 2E, and as was predicted from the original hypothesis, luxS expression was severely decreased in the strain that harbored the luxS-multicopy plasmid (without any detrimental effect on growth) confirming that AI-2 regulates luxS expression in B. subtilis (Fig. 2A, possibility 2).
So far, the best-characterized and most complex quorum-sensing-dependent phenomenon operating in B. subtilis is the process of spore development (2, 21). Therefore, we were intrigued to know whether an interrelationship would exist between sporulation and AI-2-dependent quorum sensing. As shown in Fig. 3A, LuxS activity did not affect the expression of the master regulatory protein Spo0A, and as expected from this result, spore efficiency in the luxS-negative mutant strain was also not affected (data not shown). In addition, LuxS did not affect the expression (Fig. 3B and C) of abrB and sinR, two other key developmental genes previously shown to be required for complete biofilm development and pluricellular behaviors in B. subtilis (12, 22).
In contrast, we found that Spo0A and SinR (Fig. 3E and F), but not AbrB (Fig. 3D), have a notorious negative effect on luxS expression. These results indicated that the regulation of luxS expression in B. subtilis was complex and intricate, being under the control of an autoregulatory AI-2-dependent feedback loop (Fig. 2) and under the control of the master regulatory proteins Spo0A and SinR (Fig. 3). Therefore, we wondered to what extent LuxS would affect the pluricellular behavior of wild isolates of B. subtilis. We decided to study the role of LuxS on pluricellularity by using B. subtilis natto, a probiotic and health-promoting strain for human consumption in Asian countries, as a model (24). We observed that AI-2-dependent activity was required for the formation of mature, robust, and highly differentiated biofilms (Fig. 4). Moreover, a significant defect of the LuxS-dependent ability of B. subtilis natto cells to swarm on solid medium (Fig. 5) was also noted. These results on biofilm formation and swarming motility, plus the previously shown inhibitory feedback loop on luxS (Fig. 2B), strongly argue in favor of a role of AI-2 as a signaling molecule in B. subtilis.
An equally impressive result was obtained when we analyzed the impact of LuxS-dependent activity on the ability of B. subtilis to make highly structured and complex communities on solid surfaces (bacterial colonies or colony biofilms). Effectively, wild-type cells of B. subtilis natto were able to develop into very complex and sophisticated giant aerial colonies (Fig. 6) in which spore development was temporal and spatially confined to the top of the giant colony (Fig. 7A). Importantly, we demonstrated that AI-2 activity was essential for this developmental process. Cells deficient in AI-2 production needed more time for organization to try to make the aerial colony (Fig. 6). However, this fruiting body was weaker and more undifferentiated than the fruiting bodies made by wild-type cells at all times during development. Finally, after longer incubation, the fruiting body made by the AI-2-deficient cells fell down and collapsed similar to a Babel Tower (Fig. 6). Since AI-2 expression was restricted to a specific region of the developing colony (Fig. 7B), we propose that the LuxS-produced signal (AI-2) behaves as a bacterial morphogen (25, 52, 64) that would be able to migrate (Fig. 7B) along the incipient, but well-organized, bacterial community in order to regulate an appropriate morphogenesis and synchronization of cell activities during development (Fig. 6 and 7) (52, 64). Importantly, the master regulatory proteins Spo0A and SinR restricted the production of AI-2 to the bottom of the fruiting body (Fig. 7B). In fact, we showed that luxS expression was repressed by SinR (Fig. 3E) and Spo0A (Fig. 3F), and we also showed that Spo0A (sporulation) was expressed only at the top of the developing colony (Fig. 7A). Therefore, it was plausible to envision that Spo0A (and SinR) would restrict the production of the morphogen to the bottom of the developmentally committed colony from where AI-2 migrates to orchestrate cell-to-cell communication. Effectively, this prediction was confirmed by the observation of a less spatial restriction of LuxS activity in colonies deficient in spo0A and sinR expression (Fig. 7C).
Up to today, three distinct DPD-derived signals have been identified: S-THMF-borate (in V. harveyi) and R-THMF/R-THMF-P (in S. enterica serovar Typhimurium) (55, 57). S-THMF-borate and R-THMF chemical species may exist in equilibrium and rapidly interconvert (55, 57). Other DPD derivates might also exist and be biologically active (see Fig. 2A and reference 57). Since Vibrio, Salmonella, and Bacillus organisms are inhabitants of completely different niches (sea, gut, and soil, respectively) exciting tasks for the future include elucidation of the chemical structure of the AI-2 molecule made by the soil bacterium B. subtilis and understanding of the AI-2-dependent signaling pathway of cell-to-cell communication that operates in this model bacterium.
Acknowledgments
We are thankful for the insights of Roberto Kolter (whom we were delighted to meet in Iguazú Falls, Argentina, in 2004) and to Klaus Winzer for helpful and encouraging advice and Bonnie Bassler for the V. harveyi BB170 strain.
This work was supported by national grants to R.G. from FONCyT, CONICET, and Fundación Antorchas. E.L., A.R., and A.A. are doctoral fellows from CONICET, and R.G. is a career researcher from CONICET and a former Pew Latin American Scholar (Pew, Philadelphia, Pa.) and Fulbright Scholar (Washington, D.C.).
REFERENCES
- 1.Antelmann, H., S. Engelmann, R. Schmid, A. Sorokin, A. Lapidus, and M. Hecker. 1997. Expression of stress- and starvation-induced dps/pexB homologous gene is controlled by the alternative sigma factor σB in Bacillus subtilis. J. Bacteriol. 179:7251-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arabolaza, A., A. Nakamura, M. E. Pedrido, L. Martelotto, L. Orsaria, and R. Grau. 2003. Characterization of a novel inhibitory feedback of the anti-anti-sigma factor SpoIIAA on activation of Spo0A transcription factor during development in Bacillus subtilis. Mol. Microbiol. 47:1251-1263. [DOI] [PubMed] [Google Scholar]
- 3.Auger, S., E. Krin, S. Aymerich, and M. Gohar. 2006. Autoinducer 2 affects biofilm formation by Bacillus cereus. Appl. Environ. Microbiol. 72:937-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon Schneider, K., T. M. Palmer, and A. D. Grossman. 2002. Characterization of comQ and comX, two genes required for production of ComX pheromone in Bacillus subtilis. J. Bacteriol. 184:410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barák, I., E. Ricca, and S. Cutting. 2005. From fundamental studies of sporulation to applied spore research. Mol. Microbiol. 55:330-338. [DOI] [PubMed] [Google Scholar]
- 6.Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:773-786. [DOI] [PubMed] [Google Scholar]
- 7.Bassler, B. L. 2002. Small talk: cell-cell communication in bacteria. Cell 109:421-424. [DOI] [PubMed] [Google Scholar]
- 8.Beeston, A. L., and M. G. Surette. 2002. pfs-dependent regulation of autoinducer 2 production in Salmonella enterica serovar typhimurium. J. Bacteriol. 184:3450-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branda, S., J. E. Gonzalez-Pastor, E. Dervyn, D. Ehrlich, R. Losick, and R. Kolter. 2004. Genes involved in formation of structurated multicellular communities by Bacillus subtilis. J. Bacteriol. 186:3970-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branda, S., J. E. Gonzalez-Pastor, S. Ben-Yehuda, R. Losick, and R. Kolter. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98:11621-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, X., S. Schauder, N. Potier, A. van Dorsselaer, I. Pelczer, et al. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 12.Chu, F., D. Kearns, S. Branda, R. Kolter, and R. Losick. 2006. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 59:1216-1228. [DOI] [PubMed] [Google Scholar]
- 13.Daniels, R., J. Vanderleyden, and J. Michiels. 2004. Quorum sensing and swarming migration in bacteria. FEMS Microbiol. Rev. 28:261-289. [DOI] [PubMed] [Google Scholar]
- 14.Day, W. A., Jr., and A. T. Maurelli. 2001. Shigella flexneri LuxS quorum-sensing system modulates virB expression but is not essential for virulence. Infect. Immun. 69:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Federle, M. J., and B. L. Bassler. 2003. Interspecies communication in bacteria. J. Clin. Investig. 112:1291-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsyth, M., and T. Cover. 2000. Intercellular communication in Helicobacter pylori: luxS is essential for the production of an extracellular signaling molecule. Infect. Immun. 68:3193-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuqua, C., and P. E. Greenberg. 2002. Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 3:685-695. [DOI] [PubMed] [Google Scholar]
- 18.Gottig, N., M. E. Pedrido, M. Méndez, E. Lombardía, A. Rovetto, V. Phillipe, L. Orsaria, and R. Grau. 2005. The Bacillus subtilis SinR and RapA developmental regulators are responsible for the inhibition of spore development by alcohol. J. Bacteriol. 187:2662-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg, E. P., J. W. Hastings, and S. Ulitzur. 1979. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch. Microbiol. 120:87-91. [Google Scholar]
- 20.Greenberg, E. P. 2003. Bacterial communication and group behaviour. J. Clin. Investig. 112:1288-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman, A. D. 1995. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu. Rev. Genet. 29:477-508. [DOI] [PubMed] [Google Scholar]
- 22.Hamon, M. A., and B. A. Lazazzera. 2001. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 42:1199-1209. [DOI] [PubMed] [Google Scholar]
- 23.Hilgers, M. T., and M. L. Ludwig. 2001. Crystal structure of the quorum-sensing protein LuxS reveals a catalytic metal site. Proc. Natl. Acad. Sci. USA 98:11169-11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosoi, T., and K. Kiuchi. 2004. Production and probiotic effects of natto, chapter 12. In E. Ricca, A. O. Henriques, and S. Cutting (ed.), Bacterial spore formers: probiotics and emerging applications. Horizon Bioscience, Norwich, United Kingdom.
- 25.Jelback, L., and L. Søgaard-Andersen. 2002. Pattern formation by a cell surface-associated morphogen in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 99:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jian, M., R. Grau, and M. Perego. 2000. Differential processing of propeptide inhibitors of Rap phosphatases in Bacillus subtilis. J. Bacteriol. 182:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, M. B., and M. J. Blaser. 2003. Detection of luxS-signaling molecule in Bacillus anthracis. Infect. Immun. 71:3914-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joyce, E. A., et al. 2004. LuxS is required for persistent pneumococcal carriage and expression of virulence and biosynthesis genes. Infect. Immun. 72:2964-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kearns, D., F. Chu, R. Rudner, and R. Losick. 2004. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol. Microbiol. 52:357-369. [DOI] [PubMed] [Google Scholar]
- 30.Kearns, D., and R. Losick. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49:581-590. [DOI] [PubMed] [Google Scholar]
- 31.Kim, S., et al. 2003. Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol. Microbiol. 48:1647.1664. [DOI] [PubMed] [Google Scholar]
- 32.Lazazzera, B. A., J. A. Solomon, and A. D. Grossman. 1997. An exported peptide functions intracellularly to contribute to cell density signaling in Bacillus subtilis. Cell 89:917-925. [DOI] [PubMed] [Google Scholar]
- 33.Manefield, M., and S. L. Turner. 2002. Quorum sensing in context: out of molecular biology and into microbial ecology. Microbiology 148:3762-3764. [DOI] [PubMed] [Google Scholar]
- 34.Marouni, M. J., and S. Sela. 2003. The luxS gene of Streptococcus pyogenes regulates expression of genes that affect internalization by epithelial cells. Infect. Immun. 71:5633-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNab, R., and R. J. Lamont. 2003. Microbial dinner-party conversations: the role of LuxS in interspecies communication. J. Med. Microbiol. 52:541-545. [DOI] [PubMed] [Google Scholar]
- 36.Méndez, M., L. Orsaria, V. Phillipe, M. E. Pedrido, and R. Grau. 2004. Novel roles of the master transcription factors Spo0A and σB for survival and sporulation of Bacillus subtilis at low growth temperature. J. Bacteriol. 186:989-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merritt, J., F. Qi, S. Goodman, M. Anderson, and W. Shi. 2003. Mutation in luxS affects biofilm formation in Streptococcus mutants. Infect. Immun. 71:1972-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merritt, J., J. Kreth, W. Shi, and F. Qi. 2005. LuxS control bacteriocin production in Streptococcus mutants through a novel regulatory component. Mol. Microbiol. 57:960-969. [DOI] [PubMed] [Google Scholar]
- 39.Miller, S. T., K. B. Xavier, S. R. Campagna, M. E. Taga, M. F. Semmelhack, et al. 2004. A novel form of the bacterial quorum sensing signal AI-2 recognized by Salmonella typhimurium receptor LsrB. Mol. Cell 15:677-687. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto, S., A. Lezhava, T. Hosaka, Y. Okamoto-Hosoya, and K. Ochi. 2003. Enhanced expression of S-adenosylmethionine synthetase causes overproduction of actinorhodin in Streptomyces coelicolor A3(2). J. Bacteriol. 185:601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pappas, K., C. Weingart, and S. Winans. 2004. Chemical communication in proteobacteria: biochemical and structural studies of signal synthases and receptors required for intercellular signaling. Mol. Microbiol. 53:756-769. [DOI] [PubMed] [Google Scholar]
- 42.Parsek, M. R., and E. P. Greenberg. 2005. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 13:27-33. [DOI] [PubMed] [Google Scholar]
- 43.Pei, D., and J. Zhu. 2004. Mechanism of action of S-ribosylhomocysteinase (LuxS). Curr. Opin. Chem. Biol. 8:492-497. [DOI] [PubMed] [Google Scholar]
- 44.Perego, M. 1997. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc. Natl. Acad. Sci. USA 94:8612-8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruzheinikov, S. N., S. K. Das, S. E. Sedelnikova, et al. 2001. The 1.2 Å structure of a novel quorum-sensing protein, Bacillus subtilis LuxS. J. Mol. Biol. 313:111-122. [DOI] [PubMed] [Google Scholar]
- 46.Schauder, S., and B. L. Bassler. 2001. The language of bacteria. Genes Dev. 15:1468-1480. [DOI] [PubMed] [Google Scholar]
- 47.Schauder, S., K. Shokat, M. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 48.Senesi, S. 2004. Bacillus spores as probiotic products for human use, chapter 11. In E. Ricca, A. O. Henriques, and S. Cutting (ed.), Bacterial spore formers: probiotics and emerging applications. Horizon Bioscience, Norwich, United Kingdom.
- 49.Shapiro, J. A. 1998. Thinking about bacterial populations as multicellular organisms. Annu. Rev. Microbiol. 52:81-104. [DOI] [PubMed] [Google Scholar]
- 50.Sperandio, V., A. Torres, B. Jarvis, J. Nataro, and J. Kapers. 2003. Bacterial-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun, J., R. Daniel, I. Wagner-Dobler, and A.-P. Zeng. 2004. Is autoinducer-2 a universal signal for interspecies communication: a comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol. Biol. 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabata, T., and Y. Takei. 2004. Morphogens, their identification and regulation. Development 131:703-712. [DOI] [PubMed] [Google Scholar]
- 53.Taga, M. E., and B. L. Bassler. 2003. Chemical communication in bacteria. Proc. Natl. Acad. Sci. USA 100:14549-14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taga, M. E., S. T. Miller, and B. L. Bassler. 2003. Lsr-mediated transport and processing of AI-2 in Salmonella typhimurium. Mol. Microbiol. 50:1411-1427. [DOI] [PubMed] [Google Scholar]
- 55.Vendeville, A., K. Winzer, K. Heurlier, C. M. Tang, and K. R. Hardie. 2005. Making “sense” of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat. Rev. Microbiol. 3:383-396. [DOI] [PubMed] [Google Scholar]
- 56.Wang, L., R. Grau, M. Perego, and J. A. Hoch. 1997. A novel histidine kinase inhibitor regulating development in Bacillus subtilis. Genes Dev. 11:2569-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waters, C. M., and B. L. Bassler. 2005. Quorum sensing: cell to cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21:319-346. [DOI] [PubMed] [Google Scholar]
- 58.Wen, Z. T., and R. A. Burne. 2004. LuxS-mediated signaling in Streptococcus mutants is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 186:2682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winans, S. C. 2002. Bacterial Esperanto. Nat. Struct. Biol. 9:83-84. [DOI] [PubMed] [Google Scholar]
- 60.Winzer, K., K. R. Hardie, N. Burgess, N. Doherty, D. Kirke, M. T. Holden, R. Linforth, et al. 2002. LuxS: its role in central metabolism and the in vivo synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 148:909-922. [DOI] [PubMed] [Google Scholar]
- 61.Winzer, K., K. R. Hardy, and P. Williams. 2002. Bacterial cell-to-cell communication: sorry, can't talk now—gone to lunch! Curr. Opin. Microbiol. 5:216-222. [DOI] [PubMed] [Google Scholar]
- 62.Xavier, K. B., and B. L. Bassler. 2003. LuxS quorum sensing: more than just a numbers game. Curr. Opin. Microbiol. 6:191-217. [DOI] [PubMed] [Google Scholar]
- 63.Xavier, K. B., and B. L. Bassler. 2005. Interference with AI-2-mediated bacterial cell-cell communication. Nature 437:750-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu, A. J., and M. P. Scott. 2004. Incredible journey: how do developmental signals travel through tissue? Genes Dev. 18:2985-2997. [DOI] [PubMed] [Google Scholar]