Abstract
Many Helicobacter pylori isolates contain a 40-kb region of chromosomal DNA known as the cag pathogenicity island (PAI). The risk for development of gastric cancer or peptic ulcer disease is higher among humans infected with cag PAI-positive H. pylori strains than among those infected with cag PAI-negative strains. The cag PAI encodes a type IV secretion system that translocates CagA into gastric epithelial cells. To identify Cag proteins that are expressed by H. pylori during growth in vitro, we compared the proteomes of a wild-type H. pylori strain and an isogenic cag PAI deletion mutant using two-dimensional difference gel electrophoresis (2D-DIGE) in multiple pH ranges. Seven Cag proteins were identified by this approach. We then used a yeast two-hybrid system to detect potential protein-protein interactions among 14 Cag proteins. One heterotypic interaction (CagY/7 with CagX/8) and two homotypic interactions (involving H. pylori VirB11/ATPase and Cag5) were similar to interactions previously reported to occur among homologous components of the Agrobacterium tumefaciens type IV secretion system. Other interactions involved Cag proteins that do not have known homologues in other bacterial species. Biochemical analysis confirmed selected interactions involving five of the proteins that were identified by 2D-DIGE. Protein-protein interactions among Cag proteins are likely to have an important role in the assembly of the H. pylori type IV secretion apparatus.
Helicobacter pylori is a gram-negative, microaerophilic bacterium that colonizes the human stomach. Despite the development of a gastric mucosal inflammatory response and an H. pylori-specific humoral immune response, H. pylori can persistently colonize the stomach for decades or for life. Most individuals harboring H. pylori remain asymptomatic, but the presence of this organism is a risk factor for the development of peptic ulceration, gastric mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma (64).
H. pylori strains isolated from different humans exhibit a high level of genetic diversity (14). One of the most striking variations among H. pylori strains is the presence or absence of a 40-kb region of chromosomal DNA known as the cag pathogenicity island (PAI). This region of DNA is found in about 50 to 60% of H. pylori strains isolated in Western countries (21) and in over 90% of H. pylori isolates from Japan (40). Epidemiologic studies indicate that gastric cancer and peptic ulcer disease occur more frequently in persons infected with cag PAI-positive H. pylori strains than in persons infected with cag PAI-negative strains (14, 15, 68). Moreover, in a Mongolian gerbil model of H. pylori infection, wild-type (WT) strains harboring an intact cag PAI cause more severe gastric inflammation than do isogenic mutant strains in which cag genes are disrupted or deleted (33, 47, 51).
One protein encoded by the H. pylori cag PAI is CagA, an effector protein that is translocated into gastric epithelial cells (7, 12, 30, 46, 57, 63). CagA is the only known effector protein encoded by the cag PAI. Upon translocation into gastric epithelial cells, CagA causes a wide array of cellular alterations. These include dephosphorylation of cellular proteins, altered morphology of gastric epithelial cells (known as the “hummingbird phenotype”), activation of the Ras/MEK/extracellular signal-regulated kinase pathway, cell scattering, cell proliferation, a motogenic response, activation of β-catenin, and alterations of tight junctions (4, 10, 18, 22, 28, 30, 31, 42, 46, 48, 57-60, 62).
About 18 genes within the H. pylori cag PAI are required for translocation of CagA into gastric epithelial cells (7, 12, 27, 46, 57, 63). Several cag genes that are essential for CagA translocation are homologous to genes that encode components of type IV secretion systems (T4SSs) in other gram-negative bacterial species (2, 21, 27, 66). Therefore, it is presumed that the H. pylori cag PAI encodes a T4SS that mediates the translocation of CagA into host cells (17). Electron microscopic studies indicate that upon attachment of H. pylori to gastric epithelial cells, pilus-like structures are formed between the bacteria and host cells (52, 65). It seems likely that these structures may be involved in the translocation of CagA into gastric epithelial cells. In addition to translocating CagA into host cells, there is evidence that the H. pylori cag PAI-encoded T4SS may translocate peptidoglycan into host cells (69).
The T4SS of Agrobacterium tumefaciens, which translocates DNA and proteins into plant cells, is considered the prototype of the type IV secretion systems (19, 72). The A. tumefaciens T4SS comprises 11 VirB proteins (VirB1 to VirB11) encoded by the virB operon as well as the VirD4 protein. The H. pylori cag PAI contains about seven genes that are homologous to genes encoding the A. tumefaciens T4SS, and mutagenesis experiments indicate that each of these seven H. pylori genes is essential for CagA translocation into host cells (27).
Very little is known about the H. pylori cag PAI-encoded T4SS, the expression of Cag proteins in H. pylori, and the functions of individual proteins encoded by the cag PAI. Previous studies that used a conventional two-dimensional (2D) gel electrophoresis methodology detected the expression of only four Cag proteins (Cag3, CagX/8, CagM/16, and CagA/26) (9, 36). Three Cag proteins (CagY/7, CagX/8, and CagT/12) were reported to be associated with pilus structures that form between H. pylori and host cells (52, 65). High-resolution structural data are available for only two Cag proteins (VirB11/ATPase and CagZ/6). H. pylori VirB11/ATPase self-associates to form hexameric rings (39, 55, 71) and is predicted to have ATPase activity. CagZ/6 is unrelated to any other known proteins, and its function is unknown (20).
In this study, we used 2D difference gel electrophoresis (2D-DIGE) methodology to identify Cag proteins that are expressed by H. pylori during growth in vitro. We then used a yeast two-hybrid system and biochemical analyses to identify potential protein-protein interactions among proteins encoded by the H. pylori cag PAI. We propose that these protein-protein interactions are important for the assembly and function of the H. pylori cag PAI-encoded T4SS.
MATERIALS AND METHODS
Bacterial strains.
H. pylori strain 26695 was grown on trypticase soy agar plates containing 5% sheep blood at 37°C in the presence of 5% CO2. A single colony (26695 Sc#7), which was catalase positive, competent for transformation, and able to translocate CagA into AGS cells, was selected for further analysis. To generate a mutant derivative of 26695 Sc#7 that lacked the entire cag PAI, we used the methodology previously described by Ilver et al. (32). In brief, this methodology involved the transformation of H. pylori 26695 Sc#7 with a nonreplicating plasmid containing a chloramphenicol resistance cassette flanked by 0.5-kb sequences that flank the cag PAI in H. pylori 26695. Chloramphenicol-resistant transformants were isolated, and a single colony, designated 26695ΔcagPAI, was selected for further analysis. In a PCR using primers OP9451 (5′-ACATTTTGGCTAAATAAACACTG-3′) and OP9452 (5′-CATGCGAGCGGCGATGTG-3′), derived from genes that flank the cag PAI in wild-type strain 26695, a 2-kb PCR product was amplified from 26695ΔcagPAI, whereas no product was amplified from the wild-type strain, thereby confirming that the cag PAI had been deleted in the mutant strain. Immunoblot analysis with anti-CagA serum (Austral Biologicals) indicated that CagA was produced by the wild-type strain but not by 26695ΔcagPAI.
Escherichia coli DH5α was used for plasmid propagation and expression of recombinant proteins. Transformants containing yeast two-hybrid plasmid pBD were grown at 37°C in Luria-Bertani (LB) broth or on LB agar supplemented with 24 μg of chloramphenicol/ml. Transformants containing yeast two-hybrid plasmid pAD or expression plasmid pGEX or pMAL were grown at 37°C in LB broth or on LB agar supplemented with 50 μg of ampicillin/ml (LB-AMP). For protein expression, LB-AMP was supplemented with 0.2% glucose (LB-AMP-GLU).
CagA translocation assay.
AGS human gastric cells were cultured in six-well plates (5 × 105 cells per well), and H. pylori was added at a multiplicity of infection of 100:1 for 6 h at 37°C in the presence of 5% CO2. Cells were lysed in NP-40 lysis buffer (150 mM NaCl, 1% NP-40, 50 mM Tris-HCl, pH 8) containing Complete Mini EDTA-free protease inhibitor cocktail (Roche) and 2 mM sodium orthovanadate for 1 h at 4°C. Lysates were collected and centrifuged to separate the soluble and insoluble fractions. Proteins in the soluble fraction of the lysates were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a nitrocellulose membrane for immunoblotting. The membrane was blocked with Tris-buffered saline containing 0.1% Tween, 3% bovine serum albumin, and 2 mM sodium orthovanadate (TBS-Tween-BSA-O) for 30 min. The membrane was incubated with mouse anti-phosphotyrosine antibody PY99 (Santa Cruz Biotechnology) diluted 1:3,000 in TBS-Tween-BSA-O for 1 h and washed with TBS-Tween containing 2 mM sodium orthovanadate. The membrane was incubated for 1 h with horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin G (IgG) antibody (Amersham Biosciences), diluted 1:6,000 in TBS-Tween-BSA-O, and washed three times in TBS-Tween containing 2 mM sodium orthovanadate. Signals were detected using enhanced chemiluminescence methodology (Amersham Biosciences) and X-ray film. After incubation of the membrane with a stripping buffer, the membrane was immunoblotted with an anti-CagA antibody (Austral Biologicals), followed by horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad), each used at a dilution of 1:6,000 in TBS-Tween containing 2% nonfat powdered milk.
2D-DIGE.
2D gel instrumentation and reagents were from Amersham Biosciences/GE Healthcare unless otherwise noted. H. pylori strains 26695 Sc#7 and 26695ΔcagPAI were lysed with lysis buffer {7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} for 30 min at room temperature. The Micro BCA protein assay reagent kit (Pierce) was used to determine the concentration of protein precipitated from aliquots of each lysate. Prior to coresolution on the same 2D gel, 250 to 500 μg of bacterial lysates from each strain was differentially labeled with cyanine fluorescent dyes (Cy3 and Cy5) as described previously (29). First-dimensional isoelectric focusing (IEF) was carried out using 24-cm immobilized pH gradient strips after either passive rehydration for IEF at pH 4 to 7 or anodic cup loading at pH 7 to 11 using DeStreak reagent with a manifold-equipped IPGphor IEF unit for both procedures according to the manufacturer's instructions. Second-dimensional SDS-PAGE was performed on hand-cast 12% SDS-PAGE gels that were preferentially affixed to a presilanized glass plate to ensure the accuracy of subsequent robotic protein excision, and electrophoresis was carried out at 0.5 W/gel for 2 h followed by 20 W/gel until completion using a DALT-12 unit. The differentially labeled coresolved proteome maps were imaged separately by dye-specific excitation and emission wavelengths using a Typhoon 9400 variable-mode imager.
Image analysis and protein identification.
DeCyder software (version 5.0; Amersham Biosciences/GE Healthcare) and Melanie-Viewer (version 3.06; Swiss Institute of Bioinformatics, Geneva, Switzerland) were used to directly compare Cy3-labeled wild-type H. pylori strain 26695 and Cy5-labeled isogenic cag PAI deletion mutant strain (ΔcagPAI) proteome maps without interference from gel-to-gel variation. The 2D gels were poststained with Sypro Ruby (Molecular Probes/Invitrogen) to ensure accurate automated protein spot excision. Protein spot features exhibiting all-or-none differences between the wild-type and the ΔcagPAI strains were picked for in-gel protease digestion with trypsin, peptide extraction, and sample target spotting using standard methods with an integrated Spot-Handling-Workstation (Amersham Biosciences/GE Healthcare). Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) was used to acquire peptide mass maps of the intact molecular peptide ions (M + H) to within 20 ppm, and data-dependent TOF/TOF tandem MS/MS was used to fragment selected ions to generate amino acid sequence information using a Voyager 4700 mass spectrometer (Applied Biosystems) for both procedures. Both types of mass spectral data were then collectively used for the interrogation of protein databases using GPS Explorer software (Applied Biosystems) and the MASCOT database search algorithm (Matrix Science). Data presented in support of protein identifications include (i) combined molecular weight search scores, (ii) the number of matching peptides in the peptide mass map, (iii) the number of matching peptides with supporting MS/MS fragmentation spectra, and (iv) the percent coverage of the identified protein.
Plasmid construction.
Fourteen cag genes were selected for analysis in a yeast two-hybrid system. Each of these genes was PCR amplified from genomic DNA of H. pylori strain 26695. Primer pairs used for PCR amplification contained either EcoRI and SalI restriction sites or SalI and PstI restriction sites for forward and reverse primers, respectively (Table 1). Amplicons were digested with the appropriate restriction endonucleases. For the yeast two-hybrid study, digested amplicons were cloned into plasmids encoding the transcription activation domain (pAD) or the DNA binding domain (pBD) of the GAL4 Two-Hybrid Phagemid system (Stratagene). In most cases, full-length cag genes were cloned. In the cases of genes that are predicted to encode a signal sequence (HP0522, HP0528, HP0532, HP0537, HP0540, and HP0542, encoding Cag3, CagX/8, CagT/12, CagM/16, CagI/19, and CagG/21, respectively), only the portion of the gene encoding the mature protein (excluding the signal sequence) was cloned in order to avoid the possibility that the hydrophobic signal sequence might interfere with nuclear translocation of proteins expressed in the yeast two-hybrid system (49). Because of the large size of HP0527 (6 kb), which encodes CagY/7, this gene was cloned into both vectors in multiple overlapping segments (HP0527A, HP0527B, and HP0527C). HP0527A is a 1.5-kb region of the gene encoding the N terminus of CagY/7 [CagY/7(A)], HP0527B is a 3-kb segment encoding the middle portion of CagY/7 [CagY/7(B)], and HP0527C is a 1.5-kb region encoding the C terminus of CagY/7 [CagY/7(C)]. DNA sequence analysis was used to confirm that the desired cag sequence was successfully cloned into each plasmid as expected.
TABLE 1.
Oligonucleotides used to PCR amplify genes selected for study in the yeast two-hybrid system
| Protein | Gene | Primera | Primer sequence (5′-3′) |
|---|---|---|---|
| Cag3(A)b | HP0522A | BAR1971 | CCCCGAATTCATGTTTAGAAAACTAGCAACC |
| BAR1972 | CCCCGTCGACTTACTTTGAATCTTTCAGTAA | ||
| Cag3(B) | HP0522B | AND7073 | CCCCGAATTCAAAGAAATAAGTGAAGCCGATA |
| BAR1972 | CCCCGTCGACTTACTTTGAATCTTTCAGTAA | ||
| Cag5 | HP0524 | AND7078 | CCCCGTCGACTCATGGAAGACTTTTTGTATAACAC |
| AND7079 | CCCCCTGCAGTCACAGTTCACTTGAACCCA | ||
| VirB11/ATPase | HP0525 | BAR1969 | CCCCGAATTCATGACTGAAGACAGATTGAGT |
| BAR1970 | CCCCGTCGACCTACCTGTGTTTGATATAAAATT | ||
| CagZ/6 | HP0526 | OPE370 | CCCCGAATTCATGGAACTCGGTTTCAATGAAG |
| OPE371 | CCCCGTCGACTTATTCCAAATTTAATTTTAATTGGG | ||
| CagY/7(A)b | HP0527A | AND6956 | CCCCGAATTCATGAATGAAGAAAACGATAAACT |
| AND7075 | CCCCGTCGACAGGTTTTCATCTTTGATTAAATC | ||
| CagY/7(B) | HP0527B | AND7074 | CCCCGAATTCCAGATGAAAAAGACTCTAGAG |
| AND7077 | CCCCGTCGACTGTGTCCTTTTATTTTGGATTTC | ||
| CagY/7(C) | HP0527C | AND7076 | CCCCGAATTCGAAAAGGCTGTTGCGGATTG |
| AND6957 | CCCCGTCGACTTAATTGCCACCTTTGGGG | ||
| CagX/8 | HP0528 | AND7082 | CCCCGAATTCGCAGCACTTGACATTAAGAAT |
| AND7083 | CCCCGTCGACTTATTTATCTCTGACAAGAGG | ||
| CagV/10 | HP0530 | AND7084 | CCCCGAATTCATGTTAGGGAAAAAAAACGAAG |
| OP9258 | CCCCGTCGACTTATTTATTTAATGCCTTATTTTTTG | ||
| CagT/12 | HP0532 | AND7080 | CCCCGAATTCAAAAAAGTGGTGAAACAAAAGAA |
| AND7081 | CCCCGTCGACTCACTTACCACTGAGCAAAC | ||
| CagS/13 | HP0534 | BAR3711 | CCCCGAATTCATGAGTAATAACATGCGAAAAC |
| BAR3712 | CCCCGTCGACTTACAGTCCTTTTTCTTTCATG | ||
| CagM/16 | HP0537 | BAR3509 | CCCCGAATTCATGCTTGCAAAAATCGTTTTTAG |
| BAR3510 | CCCCGTCGACCTATTCAAAGGGATTATTCTTG | ||
| CagI/19 | HP0540 | OPE372 | CCCCGAATTCGGCACAGAAGTAGTAATAACG |
| OPE373 | CCCCGTCGACTCATTTGACAATAACTTTAGAGC | ||
| CagG/21 | HP0542 | OPE374 | CCCCGAATTCGACCAAAACACGGATATAAAAG |
| OPE375 | CCCCGTCGACTTAATACCCTAAGATCGGTGG | ||
| CagF/22 | HP0543 | OPE376 | CCCCGAATTCATGAAACAAAGTTTGCGCGAAC |
| OPE377 | CCCCGTCGACTCAATCGTTACTGTTGTTTTGATT | ||
| CagE/23/PicB | HP0544 | OP9261 | CCCCGTCGACTCGTGTTTGTGGCAAGCAAAC |
| OP9262 | CCCCCTGCAGTTAATACTCCTTTATTTGTTGATA |
For each gene, the forward primer is listed first, followed by the reverse primer. Forward primers contain an EcoRI or SalI site, and reverse primers contain a SalI or PstI site.
Cag3(A) indicates the full-length protein, and Cag3(B) indicates a fragment lacking the predicted signal sequence. CagY/7(A), CagY/7(B), and CagY/7(C) are three different fragments of CagY/7.
Selected cag genes were also cloned into E. coli expression vectors (either pMAL-c2X [New England Biolabs], which encodes maltose binding protein [MBP], or pGEX-5-X [Amersham Biosciences], which encodes glutathione S-transferase [GST]). Primers for PCR amplification of the cag genes were the same as those described above, except that reverse primers encoding a tag sequence of six histidine residues were used for amplification of genes that were cloned into pGEX-5-X. Expression plasmids were transformed into E. coli DH5α.
Yeast two-hybrid assay.
To screen for protein-protein interactions among H. pylori cag-encoded proteins, we used the GAL4 Two-Hybrid Phagemid system (Stratagene). In this system, the transcription activation domain (AD) and the DNA binding domain (BD) of the yeast GAL4 transcription activator are encoded by two different plasmids, termed pAD and pBD, respectively. Each plasmid encodes a selectable marker (LEU2 by pAD and TRP1 by pBD). The yeast strain used in this system, Saccharomyces cerevisiae strain YRG-2, encodes two reporter genes, HIS3 (imidazoleglycerol phosphate dehydratase, which catalyzes the sixth step in histidine biosynthesis) and lacZ (β-galactosidase). The HIS3 reporter gene construct consists of the GAL1 upstream activating sequence (UASGAL1) and the TATA portion of the GAL1 promoter, which are fused to the HIS3 reporter gene and regulate its expression. The lacZ reporter construct consists of three copies of the GAL4 17-mer consensus sequence and the TATA portion of the iso-1-cytochrome c promoter, which are fused to the lacZ reporter and regulate its expression. The GAL4 BD hybrid protein binds to both the UASGAL1 and the GAL4 17-mer sequences present upstream of the reporter genes. In this system, if two fusion proteins interact, the GAL4 transcription activation domain and the GAL4 DNA binding domain are brought into close proximity, and this initiates transcription of the reporter genes. Protein-protein interactions are therefore detected by the ability of cotransformed yeast cells to grow in selective medium (synthetic defined [SD] minimal medium) lacking tryptophan, leucine, and histidine and by production of β-galactosidase activity. Yeast cells were grown in rich medium (yeast extract-peptone-dextrose) or in SD minimal medium (supplemented with the required amino acids and glucose) at 30°C as described in the GAL4 Two-Hybrid Phagemid manual (Stratagene). Yeast strain YRG-2 was cotransformed with 400 ng of individual plasmids using the lithium acetate method (1). Cotransformed yeasts were selected by growth on SD medium containing glucose and lacking tryptophan and leucine at 30°C. For a positive protein-protein interaction control, we cotransformed the yeast with pAD-WT and pBD-WT plasmids (Stratagene), which encode fusion proteins consisting of amino acids 132 to 236 of wild-type lambda cI, fragment C, and either the GAL4 AD or GAL4 BD, respectively. The lambda cI gene product naturally forms homodimers. We also cotransformed yeast with pAD-MUT and pBD-MUT plasmids (Stratagene), which encode fusion proteins consisting of amino acids 132 to 236 of E233K mutant lambda cI, fragment C, and either the GAL4 AD or GAL4 BD, respectively. The cI-E223K mutation encodes a substitution in the gene product that interferes with the interaction between the homodimers, resulting in a relatively low-affinity protein-protein interaction. The wild-type fusion proteins are designated GAL4AD-WT and GAL4BD-WT, and the mutant fusion proteins are designated GAL4AD-MUT and GALBD-MUT. For a negative control, we cotransformed yeast with the pAD-WT plasmid and a pBD-pLamin C plasmid (Stratagene), which expresses a fusion protein consisting of the BD of GAL4 and amino acids 60 to 230 of human lamin C. The proteins encoded by these two plasmids do not interact in the yeast two-hybrid system.
One colony of each cotransformant was grown in 2 ml of SD medium containing 2% glucose and lacking tryptophan and leucine at 30°C with aeration for ∼18 h. Cultures were then normalized to an optical density at 600 nm (OD600) of about 0.26 in a final volume of 100 μl of SD medium lacking tryptophan, leucine, and histidine and 10-fold serially diluted into SD medium lacking tryptophan, leucine, and histidine in a microtiter plate as described previously (67). These serial dilutions of cultures were seeded onto selection plates lacking Trp and Leu as well as plates lacking Trp, Leu, and His and were incubated at 30°C for 3 to 7 days. This approach permitted a comparison of yeast growth on the two types of selective media.
β-Galactosidase assay.
All yeast cotransformants that grew on medium lacking Trp, Leu, and His were analyzed further by testing for β-galactosidase activity. Individual cotransformants were grown in 2 ml of SD medium containing 2% glucose and lacking tryptophan and leucine at 30°C with aeration for 16 to 24 h until the cultures reached mid-log phase. Aliquots (150 μl) of the cultures were then mixed with 75 μl of 4 mg/ml of o-nitrophenyl-β-d-galactopyranoside in Z buffer (60 mM Na2HPO4 · 7H2O, 40 mM NaH2PO4 · H2O, 10 mM KCl, 1 mM MgSO4 · 7H2O, and 50 mM β-mercaptoethanol) and 75 μl of Y-PER reagent (Pierce) and incubated at 37°C until solutions turned yellow (∼1 to 4 h). At this point, the reactions were stopped by the addition of 50 μl of β-galactosidase assay stop solution (1 M sodium carbonate), and the incubation time was recorded. The optical densities (OD410) of the samples were measured in a 96-well plate, and the β-galactosidase activity was calculated by using the Miller equation. One Miller unit, or 1 unit of β-galactosidase activity, is defined as the activity that hydrolyzes 1 μmol of o-nitrophenyl-β-d-galactopyranoside to o-nitrophenol and d-galactose per minute per cell (41). The positive control (yeast expressing GAL4AD-WT and GAL4BD-WT) consistently expressed higher levels of β-galactosidase activity than did the mutant positive control (yeast expressing GAL4AD-MUT and GAL4BD-MUT), and both positive controls consistently expressed higher levels of β-galactosidase activity than did the negative control (yeast expressing GAL4AD-WT and GAL4BD-pLaminC).
Expression and detection of recombinant Cag fusion proteins.
E. coli DH5α strains containing plasmids encoding Cag fusion proteins or plasmids encoding MBP or GST alone were inoculated into LB-AMP-GLU and grown at 37°C overnight with shaking. These cultures were diluted 1:100 into LB-AMP-GLU and incubated at 37°C until they reached an OD600 of 0.5. Cultures were then induced with a final isopropyl-β-d-thiogalactopyranoside (IPTG) concentration of 0.5 mM and incubated at 37°C for 2 h. Fifty milliliters of the IPTG-induced cultures was pelleted, resuspended in 2.5 ml MBP column buffer (20 mM Tris-HCl, pH 7.4, 200 mM NaCl, 1 mM EDTA), and stored overnight at −20°C. Suspensions were thawed on ice and sonicated on ice using 10 10-s pulses at 5 W. Insoluble debris was pelleted, and the soluble E. coli extracts containing the Cag fusion proteins or MBP or GST alone were collected and stored at 4°C until use. The presence of MBP or GST fusion proteins in E. coli soluble extracts was detected by immunoblotting using a monoclonal anti-MBP antibody (Sigma) or a polyclonal anti-GST antibody (53) diluted 1:6,000 and 1:30,000, respectively, in TBS-Tween containing 2% nonfat powdered milk. The secondary antibodies were sheep anti-mouse IgG antibody (Amersham Biosciences) and goat anti-rabbit IgG (Bio-Rad), respectively, diluted 1:6,000 in TBS-Tween containing 2% nonfat powdered milk. Signals were detected as described above.
Affinity purification of protein complexes.
Equal volumes of E. coli extracts containing Cag fusion proteins (GST-Cag or MBP-Cag) or GST or MBP alone were mixed and incubated at 4°C for 30 min. Glutathione-Sepharose 4B beads (Amersham Biosciences) previously equilibrated in GST extraction buffer (50 mM Tris-HCl, pH 8.5, 100 mM NaCl, 1 mM EDTA) were added (1 ml of 50% slurry per liter of bacterial culture) and incubated at 4°C for 1 h. Samples were placed in Handee Spin Cup columns (Pierce) for wash steps. Columns were washed with 10 bed volumes of GST extraction buffer containing 0.5% Triton X-100 and 10 bed volumes of GST extraction buffer containing 0.1% Triton X-100 followed by five subsequent washes with GST extraction buffer alone. Affinity-purified proteins were analyzed by SDS-PAGE and immunoblotting with anti-MBP and anti-GST antibodies, as described above.
RESULTS
Construction and analysis of an H. pylori mutant strain lacking the cag pathogenicity island.
In order to study proteins encoded by the H. pylori cag PAI, we selected H. pylori strain 26695 for analysis (66). This strain has a functional cag PAI-encoded T4SS that can translocate CagA into AGS gastric epithelial cells (11, 27). We isolated a single colony of strain 26695 (Sc#7) that was catalase positive, competent for transformation, and able to translocate CagA into AGS cells. We then generated an isogenic mutant derivative of 26695 Sc#7 (26695ΔcagPAI) in which the cag PAI was replaced with a chloramphenicol acetyltransferase cassette, as described in Materials and Methods. PCR analysis confirmed that the entire cag PAI had been deleted in the mutant strain, and immunoblot analysis indicated that CagA was produced by the wild-type strain but not the mutant strain (data not shown). Western blot analysis of AGS cells incubated with the wild-type H. pylori strain revealed a ∼140-kDa tyrosine-phosphorylated band corresponding to CagA, whereas this band was not observed when cells were incubated with the mutant strain (Fig. 1). Moreover, when the wild-type H. pylori strain was incubated with AGS cells, a change in cell shape known as the “hummingbird phenotype” was observed, whereas AGS cells incubated with strain 26695ΔcagPAI did not exhibit this alteration (data not shown).
FIG. 1.
Translocation of CagA into AGS cells. AGS gastric cells were cocultured with a wild-type H. pylori strain (H. pylori strain 26695 Sc#7) (WT), with an isogenic mutant strain that lacked the cag PAI (ΔcagPAI), or without bacteria. After 6 h, the cells were lysed, and the proteins in the lysates were analyzed by immunoblotting with an anti-phosphotyrosine (anti-PY99) antibody (top panel). The membrane was then stripped and immunoblotted with an anti-CagA antibody (bottom panel). The wild-type strain expressed a CagA protein that underwent tyrosine phosphorylation (CagAp-Tyr), and the isogenic cag PAI deletion mutant strain did not express CagA.
Expression of proteins encoded by the cag pathogenicity island.
The cag PAI in H. pylori strain 26695 is predicted to encode about 27 proteins (2, 21, 27, 66), but the expression of most of these proteins has not been confirmed experimentally. In order to detect proteins encoded by the cag PAI that are expressed under in vitro culture conditions, we compared the proteomes of the wild-type strain (26695 Sc#7) and the isogenic cag PAI mutant strain (26695ΔcagPAI) using 2D-DIGE. In brief, this methodology involves differentially labeled proteins from the two strains with two different cyanine fluorescent dyes (Cy3 and Cy5; Amersham Biosciences) prior to 2D gel electrophoresis. These samples were then separated together on the same gel and imaged individually using dye-specific excitation/emission spectra. This approach eliminates gel-to-gel artifacts, because the two differentially labeled samples are coresolved on a single gel and imaged individually using dye-specific excitation/emission spectra. Since the goal of these experiments was to identify Cag proteins expressed by the wild-type strain and absent from the proteome of the cag PAI mutant strain, proteins exhibiting all-or-none differences in expression (likely to represent proteins encoded by the cag PAI) were selected for analysis. Since proteins exhibiting less-pronounced differences in levels of expression were not investigated, pooled-sample internal standards (3, 29) were not included in replicate gels.
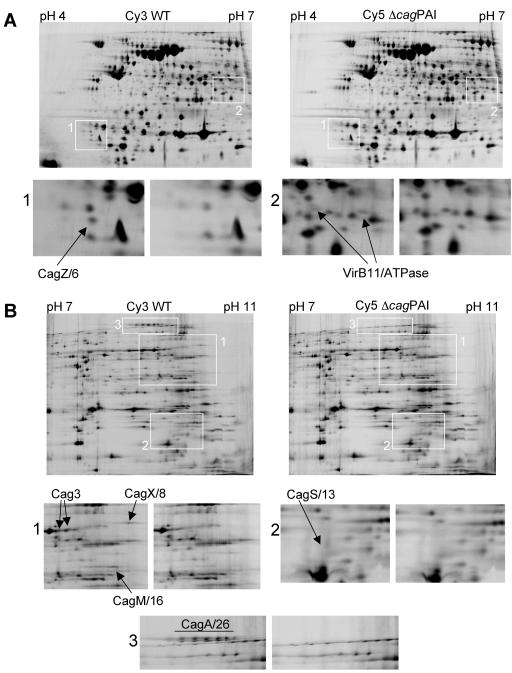
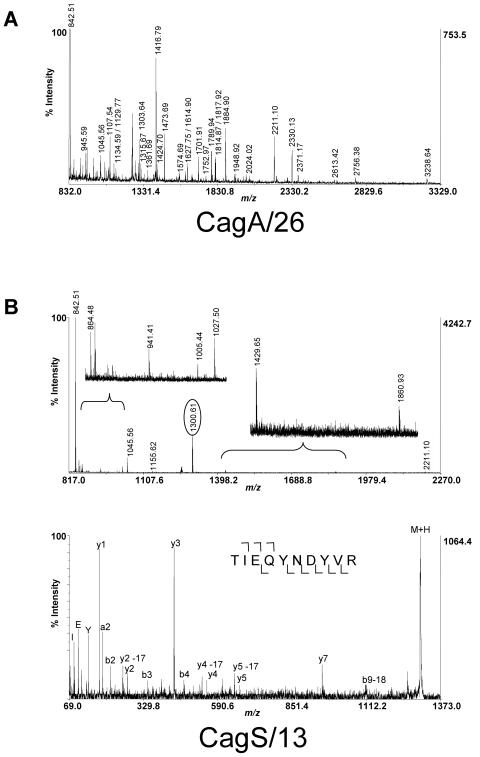
Using gels with pH ranges of pH 4 to 7 and pH 7 to 11, we were able to identify seven proteins expressed by the wild-type strain but not the cag PAI mutant strain (Fig. 2) and one protein expressed by the cag PAI mutant strain but not the wild-type strain. These differences in protein expression were observed in multiple independent lysates throughout the course of these experiments (data not shown). These proteins were excised and analyzed by mass spectrometry as described in Materials and Methods. The single protein expressed by the mutant strain but not the wild-type strain was identified as chloramphenicol acetyltransferase, a selectable antibiotic resistance marker used in the process of generating the mutant strain (data not shown). As expected, all seven proteins expressed by the wild-type strain but absent in the mutant were products of the cag PAI (Table 2 and Fig. 2 and 3). Each of these seven Cag proteins was unambiguously identified with statistical confidence using peptide mass maps and fragmentation spectra of individual peptides using tandem mass spectrometry (Table 2 and Fig. 2 and 3). This was true even for CagS/13 (encoded by HP0534), which was the weakest feature that was excised for identification by mass spectrometry and bioinformatics. The 2D gel migration of all identified proteins was consistent with the predicted molecular masses and isoelectric points (Table 2 and Fig. 2). Several different isoforms of CagA were repeatedly visible in the 2D gels (Fig. 2B, panel 3), which is consistent with the idea that CagA may undergo some form of posttranslational modification in H. pylori (11, 43). In addition, VirB11/ATPase and Cag3 could each be resolved into two isoforms (Fig. 2A, panel 2, and B, panel 1). Two of the seven proteins identified by 2D-DIGE analysis (VirB11/ATPase and CagX/8) are homologous to components of the Agrobacterium T4SS (VirB11 and VirB9, respectively) (Table 2).
FIG. 2.
Expression of proteins encoded by the cag pathogenicity island. H. pylori strain 26695 Sc#7 (WT) and an isogenic cag PAI deletion mutant strain (ΔcagPAI) were lysed, and the proteomes of these strains were analyzed by 2D-DIGE/MS, as described in Materials and Methods. Seven H. pylori Cag proteins (Cag3, VirB11/ATPase, CagZ/6, CagX/8, CagS/13, CagM/16, and CagA/26) (Table 2) were expressed by the wild-type strain but not by the mutant strain. (A) Representative data from a 2D-DIGE experiment using a gel with pH range of 4 to 7. CagZ/6 and VirB11/ATPase are indicated. VirB11/ATPase was detected in two isoforms (panel 2). (B) Representative data from a 2D-DIGE experiment using a gel with a pH range of 7 to 11. Cag3, CagX/8, CagM/16, CagS/13, and CagA/26 are indicated. Cag3 was detected in two isoforms (panel 1), and CagA/26 was detected in multiple isoforms (panel 3).
TABLE 2.
H. pylori proteins detected by 2D-DIGE/MS analysis
| H. pylori 26695 gene | Protein | Agrobacterium homologue | Protein identification dataa | Predicted molecular mass (kDa) | Predicted pI | Predicted signal sequence |
|---|---|---|---|---|---|---|
| HP0522 | Cag3 | 45, 7, 0, 23b | 55 | 8.87 | Yes | |
| HP0525 | VirB11/ATPase | VirB11 | 253, 14, 5, 46 | 37 | 6.43 | No |
| HP0526 | CagZ/6 | 233, 11, 4, 44 | 23 | 5.00 | No | |
| HP0528 | CagX/8 | VirB9 | 428, 28, 7, 41 | 60 | 9.42 | Yes |
| HP0534 | CagS/13 | 190, 9, 4, 37 | 23 | 8.97 | No | |
| HP0537 | CagM/16 | 269, 15, 5, 35 | 44 | 9.25 | Yes | |
| HP0547 | CagA/26 | 515, 37, 9, 16 | 132 | 8.82 | No |
The four values reported are the combined MS and MS/MS molecular weight search scores (MASCOT algorithm, scores above 57 are within the 95th percentile confidence interval), the number of peptides matched, the number of peptides with MS/MS data, and percentage of the amino acids accounted for by the matching peptides (coverage), respectively. Searches were performed without constraining taxonomy or molecular mass/pI and accounted for up to one missed trypsin cleavage, partial oxidation of methionine, and carbamidomethylation of cysteine sulfhydryls.
Scores are generally lower for Cag3 due to the presence of peptide ions from an H. pylori-encoded protein that comigrates with Cag3.
FIG. 3.
Identification of Cag proteins by mass spectrometry. Examples of MALDI-TOF and tandem TOF/TOF mass spectra. (A) Peptide mass map spectrum for CagA/26 displaying intact peptide ion masses (M + H) to within a 20-ppm mass accuracy. (B) Similar peptide mass map spectrum for CagS/13, with accompanying MS/MS fragmentation spectrum for an ion at m/z 1,300.61 (circled). Fragment ions containing the carboxyl terminus (y ions) are denoted below the peptide sequence (numbering follows the peptide right to left), and fragment ions containing the amino terminus (b ions) are denoted above the peptide sequence (numbering follows the peptide left to right). CagS/13 was the faintest of the seven Cag proteins identified by 2D-DIGE analysis.
Among the seven Cag proteins detected by 2D-DIGE, three (Cag3, CagX/8, and CagM/16) are predicted to undergo cleavage of amino-terminal signal sequences based on an analysis using the SignalP program (44, 45). The remaining proteins (VirB11/ATPase, CagZ/6, CagS/13, and CagA/26) are not predicted to have amino-terminal signal sequences. In the mass spectral data for the seven Cag proteins, peptides corresponding to the amino-terminal 25 amino acids were identified for CagZ/6 and CagS/13, which is consistent with the prediction that these two proteins do not undergo cleavage of signal sequences. No peptides corresponding to the amino-terminal 25 amino acids were identified in mass spectrometry analysis of the remaining five Cag proteins (data not shown).
Interactions among proteins encoded by the cag pathogenicity island.
Based on what is known about type IV secretion systems in other bacterial species (19, 72), it may be presumed that numerous protein-protein interactions occur among H. pylori Cag proteins in order to form a type IV secretion apparatus. In an effort to understand more about the functions of H. pylori Cag proteins and their role in the type IV secretion process, we sought to determine whether any of the proteins identified by 2D-DIGE physically interacted with other H. pylori Cag proteins. In addition to six proteins detected by 2D-DIGE (Cag3, VirB11/ATPase, CagZ/6, CagX/8, CagS/13, and CagM/16), we analyzed products of eight cag genes that were previously reported to be essential for the translocation of CagA into host cells (23, 72) (Table 3). Five of these (Cag5, CagY/7, CagV/10, CagT/12, and CagE/23/PicB) are homologues of components in the Agrobacterium T4SS.
TABLE 3.
H. pylori genes selected for analysis using a yeast two-hybrid system
| H. pylori 26695 gene | Protein | Agrobacterium homologue | Identified by 2D-DIGEa | Necessary for CagAp-Tyra,b | Necessary for IL-8 secretiona,b |
|---|---|---|---|---|---|
| HP0522 | Cag3 | + | + | + | |
| HP0524 | Cag5 | VirD4 | + | − | |
| HP0525 | VirB11/ATPase | VirB11 | + | + | + |
| HP0526 | CagZ/6 | + | − | − | |
| HP0527 | CagY/7 | VirB10 | + | + | |
| HP0528 | CagX/8 | VirB9 | + | + | + |
| HP0530 | CagV/10 | VirB8 | + | + | |
| HP0532 | CagT/12 | VirB7 | + | + | |
| HP0534 | CagS/13 | + | − | − | |
| HP0537 | CagM/16 | + | + | + | |
| HP0540 | CagI/19 | + | − | ||
| HP0542 | CagG/21 | + | − | ||
| HP0543 | CagF/22 | + | − | ||
| HP0544 | CagE/23/PicB | VirB4 | + | + |
+, yes; −, no.
Based on analysis of interactions between H. pylori strain 26695 and AGS cells (27).
To screen for protein-protein interactions among H. pylori cag PAI-encoded proteins, we elected to use the GAL4 Two-Hybrid Phagemid system (Stratagene). This system expresses bait and prey fusion proteins at relatively low levels, which is thought to reduce the number of false-positive interactions (34). Each cag gene selected for study (Table 3) was cloned into both a pAD and a pBD plasmid, as described in Materials and Methods. To identify protein-protein interactions among the selected cag PAI-encoded proteins, we tested all possible combinations of plasmids in the yeast two-hybrid system. Each possible interaction among cag PAI-encoded proteins was analyzed by testing two reciprocal pairs of plasmids (one pair in which the two cag genes were cloned into pAD and pBD plasmids and one pair in which the same two genes were cloned vice versa). Cotransformed yeast cells were selected as described in Materials and Methods. Single colonies were picked, and serial dilutions of the cultures were then plated onto SD medium lacking Trp and Leu as well as SD medium lacking Trp, Leu, and His in order to allow a comparison of yeast growth on the two types of selective media. As expected, all of the cotransformed yeast cells grew well on the former medium. The growth of cotransformed yeast on the latter medium provided evidence of a possible interaction between the proteins encoded by the cotransformed plasmids (see Fig. 4 and 5 for representative results).
FIG. 4.
Protein-protein interactions among H. pylori Cag proteins. Yeast cells were cotransformed with plasmids encoding the indicated fusion proteins. Cotransformed yeast cells (normalized based on optical density) were 10-fold serially diluted. Identical inocula were plated onto two types of media (SD medium lacking tryptophan and leucine and SD medium lacking tryptophan, leucine, and histidine). All of the cotransformed yeast cells grew on SD plates containing 2% glucose and lacking tryptophan and leucine. Growth of the cotransformed yeast cells on SD medium lacking tryptophan, leucine, and histidine provided evidence for the occurrence of protein-protein interactions. Panel A depicts three of the four homotypic interactions identified in the current study. Cag3(A) is a full-length protein, and Cag3(B) is a gene fragment lacking the predicted signal sequence. Panel B shows representative heterotypic interactions. GAL4AD-WT plus GAL4BD-WT are a positive control, and GAL4AD-WT plus GAL4BD-pLC are a negative control.
FIG. 5.
Interaction of H. pylori CagY/7 (VirB10 homologue) with H. pylori CagX/8 (VirB9 homologue). (A) Alignment of the full-length H. pylori CagY protein with A. tumefaciens VirB10. Three CagY/7 fragments [CagY/7(A), CagY/7(B), and CagY/7(C)] were expressed in the yeast two-hybrid system, as shown. The C-terminal region of CagY/7 [CagY/7(C)] is homologous to VirB10 from A. tumefaciens. (B) Yeast cells were cotransformed with the plasmids encoding the indicated fusion proteins and plated as described in the Fig. 4 legend. Yeast cotransformed with plasmids encoding GAL4BD-CagY/7(C) (the C terminus of CagY/7, which is homologous to VirB10) and GAL4AD-CagX/8 (a VirB9 homologue) grew on SD medium lacking tryptophan, leucine, and histidine, indicating an interaction between the two proteins encoded by these genes.
Four homotypic interactions (involving Cag3, Cag5, VirB11/ATPase, and CagM/16) were detected (Table 4 and Fig. 4A). The remaining 36 interactions were heterotypic (Table 4 and Fig. 4B). Four interactions [CagX/8 with CagY/7(C), CagX/8 with CagM/16, CagT/12 with CagM/16, and CagZ/6 with CagF/22] were identified using two different pairs of plasmids (i.e., reciprocal plasmid pairs), and the remaining 32 interactions were identified using only one plasmid pair.
TABLE 4.
Interactions among cag PAI-encoded proteins detected in a yeast two-hybrid systema
| Interacting protein partnersb
|
β-Galactosidase activityc | |
|---|---|---|
| GAL4AD- | GAL4BD- | |
| Cag3(B) | Cag3(B) | ++ |
| Cag3(B) | CagT/12 | ++ |
| Cag3(B) | CagM/16 | + |
| Cag3(B) | CagG/21 | ++ |
| Cag5 | Cag5 | ++ |
| Cag5 | CagZ/6 | + |
| Cag5 | CagM/16 | + |
| Cag5 | CagI/19 | + |
| Cag5 | CagG/21 | + |
| VirB11/ATPase | VirB11/ATPase | +++ |
| VirB11/ATPase | CagY/7(B) | ++ |
| CagZ/6 | CagS/13 | + |
| CagZ/6 | CagM/16 | + |
| CagZ/6 | CagI/19 | ++ |
| CagZ/6 | CagG/21 | ++ |
| CagZ/6* | CagF/22* | ++ |
| CagY/7(A) | CagF/22 | +++ |
| CagY/7(C) | CagZ/6 | + |
| CagY/7(C)* | CagX/8* | +++ |
| CagY/7(C) | CagM/16 | + |
| CagX/8 | CagZ/6 | + |
| CagX18* | CagY/7(C) | +++ |
| CagX/8* | CagM/16* | + |
| CagV/10 | Cag3(A) | ++ |
| CagV/10 | CagZ/6 | + |
| CagV/10 | CagT/12 | ++ |
| CagV/10 | CagM/16 | + |
| CagT/12 | CagZ/6 | + |
| CagT/12* | CagM/16* | + |
| CagM/16* | CagX/8* | ++ |
| CagM/16* | CagT/12* | + |
| CagM/16 | CagS/13 | + |
| CagM/16 | CagM/16 | ++ |
| CagI/19 | CagT/12 | + |
| CagI/19 | CagG/21 | ++ |
| CagG/21 | CagT/12 | + |
| CagG/21 | CagM/16 | ++ |
| CagG/21 | CagF/22 | ++ |
| CagF/22* | CagZ/6* | + |
| CagF/22 | CagT/12 | + |
| CagF/22 | CagM/16 | + |
| CagE/23/PicB | CagZ/6 | + |
| CagE/23/PicB | CagY/7(C) | + |
| CagE/23/PicB | CagM/16 | + |
Cotransformation with the indicated plasmids resulted in the growth of yeast on plates lacking Trp, Leu, and His. β-Galactosidase activity is indicated in the right column.
Asterisks indicate interactions that were detected with two reciprocal pairs of plasmids.
+++ indicates highest activity, similar to the positive control; ++ indicates medium activity, similar to the mutant positive control; + indicates low activity, lower than the mutant positive control but higher than the negative control.
Several of the interactions between H. pylori Cag proteins correspond to interactions that have been detected between components of the Agrobacterium VirB-D4 T4SS (Table 4). For example, homotypic interactions were detected for both H. pylori Cag5 and VirB11/ATPase, similar to what has been observed for the Agrobacterium homologues (VirD4 and VirB11, respectively) (72). Also, the C-terminal portion of H. pylori CagY/7 [CagY/7(C)] interacted with CagX/8, similar to the Agrobacterium homologues (VirB10 and VirB9, respectively) (Fig. 5) (26). H. pylori CagY/7 is predicted to have a molecular mass of 219 kDa, whereas A. tumefaciens VirB10 is predicted to have a molecular mass of 45 kDa. The C-terminal portion of H. pylori CagY/7 is homologous to Agrobacterium VirB10, but other portions of CagY/7 are not homologous to VirB10 proteins from Agrobacterium or other bacterial species that have type IV secretion systems (6). Thus, it is notable that the C-terminal portion of H. pylori CagY/7 (the portion homologous to Agrobacterium VirB10) interacted with CagX/8 (VirB9 homologue) (Fig. 5).
Each of the cotransformed yeasts that grew on selection medium lacking Trp, Leu, and His was tested for the expression of the second reporter gene, lacZ, as described in Materials and Methods. For this assay, a positive control (yeast expressing GAL4AD-WT plus GAL4BD-WT), a negative control (yeast expressing GAL4AD-WT plus GAL4BD-pLaminC), and a mutant positive control (yeast expressing GAL4AD-MUT plus GAL4BD-MUT) were used. The proteins encoded by the positive-control mutant plasmids exhibited a relatively low-affinity interaction compared to that of the wild-type positive-control proteins. All of the cotransformed yeasts that grew on selection plates (SD medium lacking tryptophan, leucine, and histidine) produced levels of β-galactosidase activity that were higher than those of the negative control, a result that is consistent with the occurrence of protein-protein interactions. Notably, there was considerable variation in the levels of β-galactosidase activity produced by different cotransformed yeasts (Table 4; see representative results in Fig. 6). The variation in levels of β-galactosidase activity may reflect various strengths of protein-protein interactions. All of the above-mentioned interactions among H. pylori Cag proteins that resemble interactions previously reported for A. tumefaciens homologues yielded medium or high levels of β-galactosidase activity (scored ++ or +++, respectively, in Table 4). Low levels of β-galactosidase activity (scored + in Table 4) may be indicative of weak protein-protein interactions. The biological significance of these weak interactions is uncertain.
FIG. 6.
Analysis of β-galactosidase activity. Yeast cells cotransformed with the indicated plasmid pairs (AD indicates the GAL4 activation domain, and BD indicates the GAL4 binding domain) were tested in triplicate for β-galactosidase activity as described in Materials and Methods. β-Galactosidase activity provides evidence for protein-protein interactions. The activity produced by each putative interacting pair was classified into one of three categories as shown in Table 4. High activity (+++) is comparable to that of the positive control, medium activity (++) is comparable to that of the mutant positive control, and low activity (+) is lower than that of the mutant positive control but higher than that of the negative control. Representative data from each of the three categories are shown.
Biochemical evidence of protein-protein interactions.
Yeast two-hybrid screening is a useful tool for detecting protein-protein interactions, but it is known to generate false-positive and false-negative results. Therefore, we undertook biochemical studies designed to confirm a subset of interactions identified by yeast two-hybrid screening. For these studies, we prioritized an investigation of interactions involving five proteins that were detected by 2D-DIGE (Cag3, CagZ/6, CagX/8, CagS/13, and CagM/16). Two Cag proteins (Cag3 and CagZ/6) were expressed in E. coli as GST fusion proteins, and five Cag proteins (CagX/8, CagV/10, CagS/13, CagM/16, and CagI/19) were expressed as MBP fusion proteins. As negative controls, GST alone and MBP alone were expressed in E. coli. Immunoblot analysis using antibodies to GST or MBP confirmed that each of the proteins was successfully expressed (Fig. 7A). E. coli lysates containing GST-Cag fusion proteins or the GST control and MBP-Cag fusion proteins or the MBP control were mixed as described in Materials and Methods, and GST fusion proteins and GST alone were then isolated from the E. coli lysates by affinity chromatography. A GST-Cag fusion protein or the GST control protein was successfully isolated from each sample, as expected, based on immunoblot analysis of the affinity-purified protein samples with an anti-GST antibody (Fig. 7B, lower panels). To detect potential interactions of GST-Cag fusion proteins with MBP-Cag fusion proteins, the affinity-purified protein samples were analyzed by immunoblotting with an anti-MBP antibody (Fig. 7B, upper panels). This analysis showed that two proteins (MBP-CagV/10 and MBP-CagM/16) interacted with GST-Cag3 and that five proteins (MBP-CagV/10, MBP-CagM/16, MBP-CagX/8, MBP-CagS/13, and MBP-CagI/19) interacted with GST-CagZ/6 (Fig. 7B). As expected, the GST control protein did not interact with the MBP control protein or with any of the MBP-Cag fusion proteins, and the MBP control protein did not interact with either of the GST-Cag fusion proteins (Fig. 7B). Thus, the results of these biochemical experiments suggested that the corresponding interactions detected in the yeast two-hybrid system were not false-positive results.
FIG. 7.
Detection of interactions among Cag fusion proteins. (A) The indicated proteins were expressed in E. coli, and E. coli lysates were then immunoblotted with anti-GST or anti-MBP antibodies. (B) E. coli extracts containing Cag fusion proteins, a GST control protein without a fused Cag protein, or an MBP control protein without a fused Cag protein were mixed as indicated. In each case, a GST-Cag fusion protein or GST control was mixed with an MBP-Cag fusion protein or MBP control. GST-Cag fusion proteins or GST without a fused Cag protein and any interacting MBP fusion proteins were affinity purified using glutathione-Sepharose beads. Affinity-purified proteins were analyzed by SDS-PAGE and immunoblotting. Each membrane was first analyzed by anti-MBP immunoblotting and then stripped and analyzed by anti-GST immunoblotting, as shown. MBP-CagV/10 and MBP-CagM/16 copurified with GST-Cag3 but not with GST alone. MBP-CagV/10, MBP-CagM/16, MBP-CagX/8, MBP-CagS/13, and MBP-CagI/19 copurified with GST-CagZ/6 but not with GST alone.
DISCUSSION
The H. pylori cag PAI is predicted to encode about 27 proteins (2, 21, 27, 66). Although only 7 of the 27 putative Cag proteins were identified in the current study by using 2D-DIGE methodology (Fig. 2 and Table 2), this finding compares favorably with the small number of Cag proteins that have been previously identified using conventional 2D gel electrophoresis methodologies (Cag3, CagX/8, CagM/16, and CagA/26) (9, 36). We present here the first experimental evidence indicating that CagZ/6 and CagS/13 are expressed during H. pylori growth in vitro. The Cag proteins that we identified by 2D-DIGE may represent a subset of Cag proteins that are produced in higher relative abundance than nonidentified Cag proteins. Some nonidentified Cag proteins, such as those with a very high molecular mass (including CagY/7) and those with molecular masses below 10 kDa, may not have been captured on the 2D gels, and Cag proteins that comigrate with a highly expressed H. pylori protein may not have been identified (despite separating the bacterial lysates over two medium-range pH gradients). It is possible that some Cag proteins are not expressed under the in vitro conditions used in these studies. Transcription of several genes in the H. pylori cag PAI has been reported to be regulated in response to environmental conditions (16, 35, 37), and transcription of T4SS genes in several other bacterial species is regulated in response to specific environmental signals (8, 54). Potentially, some H. pylori Cag proteins are expressed only in response to particular environmental stimuli that H. pylori encounters in vivo, including cell-cell contact or other host signals.
Six of the seven Cag proteins identified by 2D-DIGE and eight additional cag PAI-encoded proteins were analyzed further using the yeast two-hybrid methodology (Table 3). Each of these proteins displayed at least one detectable interaction with another cag PAI-encoded protein included in the study (Table 4). In total, we detected four homotypic interactions and 36 heterotypic interactions (Table 4). Four of the heterotypic interactions were detected using two different pairs of plasmids (i.e., reciprocal plasmid pairs), and the remaining 32 interactions were detected with only one plasmid pair (Table 4). It is not unusual for an interaction between two proteins to be detected with one yeast two-hybrid plasmid pair but not with the reciprocal pair (49, 70). Presumably, in these cases, the expressed protein interferes with the action of the activation domain or binding domain in one of the plasmids.
Two of the interactions detected in the current study have been detected previously using independent methods. The homotypic interaction of H. pylori VirB11/ATPase (encoded by pAD-HP0525 and pBD-HP0525) is consistent with previous data indicating that this protein self-associates to form a homohexameric structure (39, 55, 71). The homotypic interaction of H. pylori Cag5 (encoded by pAD-HP0524 and pBD-HP0524) is consistent with results of gel filtration experiments, which indicate that Cag5 forms multimers of four or five subunits (56).
Most of the other interactions among H. pylori Cag proteins described here have not been reported previously. In one large study that attempted to generate a comprehensive protein-protein interaction map for H. pylori, 10 cag PAI-encoded proteins (Cag3, VirB11/ATPase, CagY/7, CagX/8, CagT/12, CagM/16, CagL/18, CagI/19, CagE/23, and CagA/26) were reported to interact with H. pylori proteins not encoded by the cag PAI (49). An interaction of H. pylori VirB11/ATPase with itself was detected in that study, but no additional interactions of cag PAI-encoded proteins with other cag PAI-encoded proteins were reported (49). It is possible that genes of the cag PAI may have been underrepresented in the bait constructs used in the previous study. Whereas the “prey” library was comprised of 2 × 106 fragments from H. pylori genomic DNA, the 285 bait constructs were derived from only 261 H. pylori open reading frames (49).
Although yeast two-hybrid screening represents a valuable tool for identifying protein-protein interactions, this methodology is known to generate both false-positive and false-negative results. Therefore, it is important that interactions identified in yeast two-hybrid screens be verified using independent biochemical methods. In the current study, yeast two-hybrid studies indicated that 22 of the 36 heterotypic interactions detected by yeast two-hybrid screening were interactions involving either CagZ/6 or CagM/16, and many of these interactions yielded low levels of β-galactosidase activity. Because of a concern that these results might represent false-positive results, we investigated several of the interactions involving CagZ/6 or CagM/16 by analyzing interactions among recombinant proteins expressed in E. coli. As shown in Fig. 7, we were able to validate multiple interactions of CagZ/6 or CagM/16 with other Cag proteins. This suggests that the corresponding interactions identified in the yeast two-hybrid system were not false-positive results. Nevertheless, it is possible that some of these interactions do not occur among the corresponding proteins expressed in H. pylori. In future studies, it will be important to investigate the localization of Cag proteins in H. pylori and to validate the occurrence of interactions among native Cag proteins expressed in H. pylori.
The T4SS of A. tumefaciens may be viewed as the prototype for bacterial type IV secretion systems (19, 72). A model for the organization of the Agrobacterium T4SS proposes that the proteins comprising this secretion apparatus can be categorized into three groups: cytoplasmic or inner membrane proteins, a core complex located in the periplasm or membrane, and a pilus or surface structure that projects beyond the outer membrane (72). Two ATPases (VirB4 and VirB11) and VirD4 (a “coupling protein”) are classified as cytoplasmic or inner membrane proteins. VirB11 and VirD4 proteins assemble into hexameric structures, whereas VirB4 is a homodimer (24, 50). VirB7, VirB8, VirB9, and VirB10 are important components of the core complex. The pilus of the Agrobacterium T4SS is composed of VirB2 and VirB5, which are considered major and minor subunits, respectively. Protein-protein interactions among components of the Agrobacterium core complex (VirB7, VirB8, VirB9, and VirB10) have been demonstrated previously by several methods, including yeast two-hybrid methodology, affinity chromatography, immunoprecipitation, and chemical cross-linking (5, 13, 25, 26, 38, 61).
Based on experimental evidence accumulated from the study of type IV secretion systems in Agrobacterium and other bacterial species, H. pylori Cag5, VirB11/ATPase, and CagE/23/PicB (homologous to Agrobacterium VirD4, VirB11, and VirB4 proteins, respectively) are likely to be cytoplasmic or inner membrane-associated proteins. The formation of homooligomeric structures by H. pylori Cag5 and VirB11/ATPase is consistent with the formation of homooligomeric structures by the corresponding Agrobacterium homologues (72). In contrast to what might be predicted based on the Agrobacterium system, we did not detect a homotypic interaction with CagE/23/PicB (a VirB4 homologue).
Based on experimental evidence accumulated from the study of type IV secretion systems in Agrobacterium and other bacterial species, it is likely that H. pylori CagT/12, CagV/10, CagX/8, and CagY/7 (homologous to Agrobacterium VirB7, VirB8, VirB9, and VirB10, respectively) comprise part of the core complex of the H. pylori cag PAI-encoded T4SS, analogous to the VirB7-8-9-10 core complex in Agrobacterium. As described in Results, we found that several of these H. pylori Cag proteins can be involved in protein-protein interactions that are similar to the interactions of their Agrobacterium homologues (Table 4).
The four proteins that we predict to be localized to the core complex of the H. pylori cag PAI-encoded T4SS (CagT/12, CagV/10, CagX/8, and CagY/7, homologous to Agrobacterium VirB7, VirB8, VirB9, and VirB10, respectively) each displayed interactions in the yeast two-hybrid system with several H. pylori proteins that do not have homologues in other bacterial species (Table 4). We propose that several of these H. pylori proteins (including Cag3, CagM/16, CagI/19, CagG/21, and CagF/22) may represent novel species-specific components of the core complex of the H. pylori cag PAI-encoded T4SS. The genes that encode these proteins are reported to be required for CagA translocation (27), which is consistent with an important role for these proteins in the H. pylori cag PAI-encoded T4SS.
Two groups have previously reported that H. pylori can produce a pilus-like structure that represents an extracellular portion of the cag PAI-encoded T4SS apparatus (52, 65). A major component of the H. pylori pilus, analogous to Agrobacterium VirB2, has not yet been identified. However, three Cag proteins (CagT/12, CagX/8, and CagY/7) have been reported to be associated with H. pylori pilus structures (52, 65). These three H. pylori proteins are homologues of Agrobacterium VirB7, VirB9, and VirB10, which are considered components of the Agrobacterium T4SS core complex (72). The composition of the extracellular portion of the H. pylori cag T4SS may be considerably different from that of the A. tumefaciens T4SS pilus (17, 19). Potentially, a novel feature of the H. pylori cag PAI-encoded T4SS is a structural organization that permits certain proteins to be localized in a core complex as well as with cell surface structures.
In summary, at present, very little is known about the assembly and structural organization of the T4SS encoded by the cag PAI. The demonstration of protein-protein interactions among cag PAI-encoded proteins, as described in this study, represents a useful starting point for investigations of this topic. It will be important in future studies to analyze the localization of Cag proteins and protein-protein interactions among Cag proteins expressed in H. pylori.
Acknowledgments
We thank J. Forrest Busler and Corbin A. Whitwell for technical assistance with yeast two-hybrid analysis and 2D-DIGE/MS, respectively. We are grateful to John P. Donahue for the gift of anti-GST antibody.
This work was supported by NIH R01 DK53623, the Vanderbilt-Ingram Cancer Center, 5P30 DK58404 (Digestive Diseases Research Center), 5 T32 AI07611-05 (Cellular and Molecular Microbiology training grant), the Vanderbilt Academic Venture Capital Fund, and the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Agatep, R., R. D. Kirkpatrick, D. L. Parchaliuk, R. A. Woods, and R. D. Gietz. 1998. Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylene glycol (LiAc/ss-DNA/PEG) protocol. Technical Tips Online. [Online.] http://tto.trends.com.
- 2.Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37-53. [DOI] [PubMed] [Google Scholar]
- 3.Alban, A., S. O. David, L. Bjorkesten, C. Andersson, E. Sloge, S. Lewis, and I. Currie. 2003. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics 3:36-44. [DOI] [PubMed] [Google Scholar]
- 4.Amieva, M. R., R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson, L. B., A. V. Hertzel, and A. Das. 1996. Agrobacterium tumefaciens VirB7 and VirB9 form a disulfide-linked protein complex. Proc. Natl. Acad. Sci. USA 93:8889-88894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aras, R. A., W. Fischer, G. I. Perez-Perez, M. Crosatti, T. Ando, R. Haas, and M. J. Blaser. 2003. Plasticity of repetitive DNA sequences within a bacterial (type IV) secretion system component. J. Exp. Med. 198:1349-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahi, M., T. Azuma, S. Ito, Y. Ito, H. Suto, Y. Nagai, M. Tsubokawa, Y. Tohyama, S. Maeda, M. Omata, T. Suzuki, and C. Sasakawa. 2000. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med. 191:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachman, M. A., and M. S. Swanson. 2001. RpoS co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase. Mol. Microbiol. 40:1201-1214. [DOI] [PubMed] [Google Scholar]
- 9.Backert, S., T. Kwok, M. Schmid, M. Selbach, S. Moese, R. M. Peek, Jr., W. Konig, T. F. Meyer, and P. R. Jungblut. 2005. Subproteomes of soluble and structure-bound Helicobacter pylori proteins analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 5:1331-1345. [DOI] [PubMed] [Google Scholar]
- 10.Backert, S., S. Moese, M. Selbach, V. Brinkmann, and T. F. Meyer. 2001. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 42:631-644. [DOI] [PubMed] [Google Scholar]
- 11.Backert, S., E. C. Muller, P. R. Jungblut, and T. F. Meyer. 2001. Tyrosine phosphorylation patterns and size modification of the Helicobacter pylori CagA protein after translocation into gastric epithelial cells. Proteomics 1:608-617. [DOI] [PubMed] [Google Scholar]
- 12.Backert, S., E. Ziska, V. Brinkmann, U. Zimny-Arndt, A. Fauconnier, P. R. Jungblut, M. Naumann, and T. F. Meyer. 2000. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2:155-164. [DOI] [PubMed] [Google Scholar]
- 13.Baron, C., Y. R. Thorstenson, and P. C. Zambryski. 1997. The lipoprotein VirB7 interacts with VirB9 in the membranes of Agrobacterium tumefaciens. J. Bacteriol. 179:1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaser, M. J., and D. E. Berg. 2001. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Investig. 107:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaser, M. J., G. I. Perez-Perez, H. Kleanthous, T. L. Cover, R. M. Peek, P. H. Chyou, G. N. Stemmermann, and A. Nomura. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55:2111-2115. [PubMed] [Google Scholar]
- 16.Boonjakuakul, J. K., D. R. Canfield, and J. V. Solnick. 2005. Comparison of Helicobacter pylori virulence gene expression in vitro and in the Rhesus macaque. Infect. Immun. 73:4895-4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourzac, K. M., and K. Guillemin. 2005. Helicobacter pylori-host cell interactions mediated by type IV secretion. Cell. Microbiol. 7:911-919. [DOI] [PubMed] [Google Scholar]
- 18.Brandt, S., T. Kwok, R. Hartig, W. Konig, and S. Backert. 2005. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 102:9300-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cascales, E., and P. J. Christie. 2003. The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 1:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cendron, L., A. Seydel, A. Angelini, R. Battistutta, and G. Zanotti. 2004. Crystal structure of CagZ, a protein from the Helicobacter pylori pathogenicity island that encodes for a type IV secretion system. J. Mol. Biol. 340:881-889. [DOI] [PubMed] [Google Scholar]
- 21.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Churin, Y., L. Al-Ghoul, O. Kepp, T. F. Meyer, W. Birchmeier, and M. Naumann. 2003. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol. 161:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 24.Dang, T. A., X. R. Zhou, B. Graf, and P. J. Christie. 1999. Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter. Mol. Microbiol. 32:1239-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das, A., L. B. Anderson, and Y. H. Xie. 1997. Delineation of the interaction domains of Agrobacterium tumefaciens VirB7 and VirB9 by use of the yeast two-hybrid assay. J. Bacteriol. 179:3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das, A., and Y. H. Xie. 2000. The Agrobacterium T-DNA transport pore proteins VirB8, VirB9, and VirB10 interact with one another. J. Bacteriol. 182:758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer, W., J. Puls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42:1337-1348. [DOI] [PubMed] [Google Scholar]
- 28.Franco, A. T., D. A. Israel, M. K. Washington, U. Krishna, J. G. Fox, A. B. Rogers, A. S. Neish, L. Collier-Hyams, G. I. Perez-Perez, M. Hatakeyama, R. Whitehead, K. Gaus, D. P. O'Brien, J. Romero-Gallo, and R. M. Peek, Jr. 2005. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. USA 102:10646-10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman, D. B., S. Hill, J. W. Keller, N. B. Merchant, S. E. Levy, R. J. Coffey, and R. M. Caprioli. 2004. Proteome analysis of human colon cancer by two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics 4:793-811. [DOI] [PubMed] [Google Scholar]
- 30.Hatakeyama, M. 2004. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4:688-694. [DOI] [PubMed] [Google Scholar]
- 31.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683-686. [DOI] [PubMed] [Google Scholar]
- 32.Ilver, D., A. Arnqvist, J. Ogren, I. M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Boren. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 33.Israel, D. A., N. Salama, C. N. Arnold, S. F. Moss, T. Ando, H. P. Wirth, K. T. Tham, M. Camorlinga, M. J. Blaser, S. Falkow, and R. M. Peek, Jr. 2001. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J. Clin. Investig. 107:611-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James, P. 2001. Yeast two-hybrid vectors and strains. Methods Mol. Biol. 177:41-84. [DOI] [PubMed] [Google Scholar]
- 35.Joyce, E. A., J. V. Gilbert, K. A. Eaton, A. Plaut, and A. Wright. 2001. Differential gene expression from two transcriptional units in the cag pathogenicity island of Helicobacter pylori. Infect. Immun. 69:4202-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jungblut, P. R., D. Bumann, G. Haas, U. Zimny-Arndt, P. Holland, S. Lamer, F. Siejak, A. Aebischer, and T. F. Meyer. 2000. Comparative proteome analysis of Helicobacter pylori. Mol. Microbiol. 36:710-725. [DOI] [PubMed] [Google Scholar]
- 37.Kim, N., E. A. Marcus, Y. Wen, D. L. Weeks, D. R. Scott, H. C. Jung, I. S. Song, and G. Sachs. 2004. Genes of Helicobacter pylori regulated by attachment to AGS cells. Infect. Immun. 72:2358-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krall, L., U. Wiedemann, G. Unsin, S. Weiss, N. Domke, and C. Baron. 2002. Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 99:11405-11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krause, S., M. Barcena, W. Pansegrau, R. Lurz, J. M. Carazo, and E. Lanka. 2000. Sequence-related protein export NTPases encoded by the conjugative transfer region of RP4 and by the cag pathogenicity island of Helicobacter pylori share similar hexameric ring structures. Proc. Natl. Acad. Sci. USA 97:3067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maeda, S., H. Yoshida, T. Ikenoue, K. Ogura, F. Kanai, N. Kato, Y. Shiratori, and M. Omata. 1999. Structure of cag pathogenicity island in Japanese Helicobacter pylori isolates. Gut 44:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 42.Mimuro, H., T. Suzuki, J. Tanaka, M. Asahi, R. Haas, and C. Sasakawa. 2002. Grb2 is a key mediator of Helicobacter pylori CagA protein activities. Mol. Cell 10:745-755. [DOI] [PubMed] [Google Scholar]
- 43.Moese, S., M. Selbach, U. Zimny-Arndt, P. R. Jungblut, T. F. Meyer, and S. Backert. 2001. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: processing or breakage? Proteomics 1:618-629. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581-599. [DOI] [PubMed] [Google Scholar]
- 46.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 47.Ogura, K., S. Maeda, M. Nakao, T. Watanabe, M. Tada, T. Kyutoku, H. Yoshida, Y. Shiratori, and M. Omata. 2000. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 192:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puls, J., W. Fischer, and R. Haas. 2002. Activation of Helicobacter pylori CagA by tyrosine phosphorylation is essential for dephosphorylation of host cell proteins in gastric epithelial cells. Mol. Microbiol. 43:961-969. [DOI] [PubMed] [Google Scholar]
- 49.Rain, J. C., L. Selig, H. De Reuse, V. Battaglia, C. Reverdy, S. Simon, G. Lenzen, F. Petel, J. Wojcik, V. Schachter, Y. Chemama, A. Labigne, and P. Legrain. 2001. The protein-protein interaction map of Helicobacter pylori. Nature 409:211-215. [DOI] [PubMed] [Google Scholar]
- 50.Rashkova, S., X. R. Zhou, J. Chen, and P. J. Christie. 2000. Self-assembly of the Agrobacterium tumefaciens VirB11 traffic ATPase. J. Bacteriol. 182:4137-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rieder, G., J. L. Merchant, and R. Haas. 2005. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 128:1229-1242. [DOI] [PubMed] [Google Scholar]
- 52.Rohde, M., J. Puls, R. Buhrdorf, W. Fischer, and R. Haas. 2003. A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol. Microbiol. 49:219-234. [DOI] [PubMed] [Google Scholar]
- 53.Rojas, M., J. P. Donahue, Z. Tan, and Y. Z. Lin. 1998. Genetic engineering of proteins with cell membrane permeability. Nat. Biotechnol. 16:370-375. [DOI] [PubMed] [Google Scholar]
- 54.Rouot, B., M. T. Alvarez-Martinez, C. Marius, P. Menanteau, L. Guilloteau, R. A. Boigegrain, R. Zumbihl, D. O'Callaghan, N. Domke, and C. Baron. 2003. Production of the type IV secretion system differs among Brucella species as revealed with VirB5- and VirB8-specific antisera. Infect. Immun. 71:1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savvides, S. N., H. J. Yeo, M. R. Beck, F. Blaesing, R. Lurz, E. Lanka, R. Buhrdorf, W. Fischer, R. Haas, and G. Waksman. 2003. VirB11 ATPases are dynamic hexameric assemblies: new insights into bacterial type IV secretion. EMBO J. 22:1969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schroder, G., S. Krause, E. L. Zechner, B. Traxler, H. J. Yeo, R. Lurz, G. Waksman, and E. Lanka. 2002. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J. Bacteriol. 184:2767-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Segal, E. D., S. Falkow, and L. S. Tompkins. 1996. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc. Natl. Acad. Sci. USA 93:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segal, E. D., C. Lange, A. Covacci, L. S. Tompkins, and S. Falkow. 1997. Induction of host signal transduction pathways by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 94:7595-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selbach, M., S. Moese, R. Hurwitz, C. R. Hauck, T. F. Meyer, and S. Backert. 2003. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 22:515-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spudich, G. M., D. Fernandez, X. R. Zhou, and P. J. Christie. 1996. Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport apparatus. Proc. Natl. Acad. Sci. USA 93:7512-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stein, M., F. Bagnoli, R. Halenbeck, R. Rappuoli, W. J. Fantl, and A. Covacci. 2002. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43:971-980. [DOI] [PubMed] [Google Scholar]
- 63.Stein, M., R. Rappuoli, and A. Covacci. 2000. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA 97:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka, J., T. Suzuki, H. Mimuro, and C. Sasakawa. 2003. Structural definition on the surface of Helicobacter pylori type IV secretion apparatus. Cell. Microbiol. 5:395-404. [DOI] [PubMed] [Google Scholar]
- 66.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 67.Torres, V. J., M. S. McClain, and T. L. Cover. 2004. Interactions between p-33 and p-55 domains of the Helicobacter pylori vacuolating cytotoxin (VacA). J. Biol. Chem. 279:2324-2331. [DOI] [PubMed] [Google Scholar]
- 68.van Doorn, L. J., C. Figueiredo, R. Sanna, A. Plaisier, P. Schneeberger, W. de Boer, and W. Quint. 1998. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology 115:58-66. [DOI] [PubMed] [Google Scholar]
- 69.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 70.Vidal, M., and P. Legrain. 1999. Yeast forward and reverse ‘n’-hybrid systems. Nucleic Acids Res. 27:919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeo, H. J., S. N. Savvides, A. B. Herr, E. Lanka, and G. Waksman. 2000. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol. Cell 6:1461-1472. [DOI] [PubMed] [Google Scholar]
- 72.Yeo, H. J., and G. Waksman. 2004. Unveiling molecular scaffolds of the type IV secretion system. J. Bacteriol. 186:1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]