Abstract
The protective major surface protein 1 (MSP1) complex of Anaplasma marginale is a heteromer of MSP1a and MSP1b, encoded by a multigene family. The msp1β sequences were highly conserved throughout infection. However, liquid chromatography-tandem mass spectrometry analysis identified only a single MSP1b protein, MSP1b1, within the MSP1 complex.
The outer membrane proteins (OMPs) of bacterial pathogens mediate interaction with the host, including the immune system. Importantly, the function of OMPs is dependent upon both the conformation of the individual proteins, including oligomerization, and supramolecular interaction with other OMPs through covalent and noncovalent interactions. Among certain intracellular pathogens, most notably those of the genus Chlamydia, there is a high degree of intermolecular cross-linking in the outer membrane (7, 19, 24, 37). A similar pattern occurs in the rickettsial pathogen Anaplasma marginale, in which the outer membrane proteins associate through extensive disulfide cross-links, forming homomeric and heteromeric complexes (35). The immunologic importance of these complexes is illustrated by the solid protection against A. marginale challenge induced by immunization using isolated outer membranes (13, 34), protection which is not fully achieved by immunization with individual proteins alone or in a mixture (1, 27, 29).
In contrast to the failure of individual A. marginale OMPs to consistently induce protection, immunization with the major surface protein 1 (MSP1) complex provides significant protection against high-level rickettsemia and disease (26, 27). This MSP1 complex is composed of proteins encoded by genes of two distinct chromosomal loci, msp1α and msp1β (9). The precise composition of the immunoprotective MSP1 complex has remained undefined, largely due to the presence of multiple msp1β genes and partial genes (6, 8, 9, 14, 36), an arrangement that could lend itself to segmental gene conversion, as has been reported for MSP2 and MSP3 (10-12, 23), other immunodominant proteins of A. marginale. The recent report of the complete genome sequence of the St. Maries strain of A. marginale has provided the unambiguous full complement of msp1α and msp1β genes and facilitates the mapping of the individual components of the MSP1 complex to the genome (9). The goal of the present study was to characterize the complexity of the MSP1b component in the immunoprotective MSP1 complex.
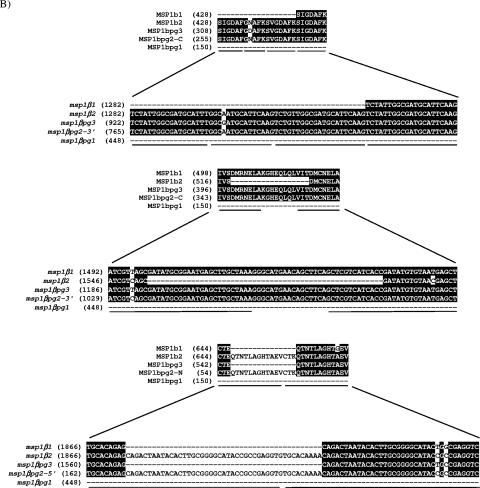
The genomic organization of the msp1α and msp1β genes is schematically represented in Fig. 1. Although MSP1a and MSP1b are covalently linked through disulfide bonds (35), the encoding loci are separated by 320 kb in the St. Maries strain genome (9). MSP1a is encoded by a single-copy gene, msp1α, and is conserved among strains, with the exception of a set of tandem repeats of 84 or 87 bp at the 5′ end, which vary in number and sequence (4, 8, 21, 25). In contrast, MSP1b is encoded by the polymorphic msp1β family, consisting of five paralogous genes (6, 8, 9, 14, 36). Vector NTI (Invitrogen, Carlsbad, CA) was used to align the predicted amino acid sequences of the msp1β genes from the St. Maries strain to determine the identities among the genes. The deduced amino acid sequences of the two full-length genes, msp1β1 and msp1β2, are 92% identical, while the predicted amino acid sequence of the longest partial gene (pg), msp1βpg3, shares 97% and 75% identity with MSP1b1 and MSP1b2, respectively. For optimal alignment (Fig. 2A), the predicted amino acid sequence of msp1βpg2 is divided into two regions (MSP1bpg2-N and MSP1bpg2-C). The N-terminal region of MSP1bpg2 is 66% and 81% identical to the C-terminal region of MSP1b1 and MSP1b2, respectively, while the C-terminal region of MSP1bpg2 is 85% and 82% identical to the N-terminal region of MSP1b1 and MSP1b2, respectively. When the predicted amino acid sequence of the shortest partial gene, msp1βpg1, is compared to those of MSP1b1 and MSP1b2, the N-terminal region of MSP1bpg1 is 94% identical to the N-terminal regions of both MSP1b1 and MSP1b2, and the C-terminal region of MSP1bpg1 is 53% identical to those of both MSP1b1 and MSP1b2. Three types of repeats have been identified in MSP1b (5), as in the amino acid alignment of the five MSP1b sequences (Fig. 2A). The repeat regions (Fig. 2B) create an opportunity for recombination events to occur and may lead to duplication or deletion of repeats during replication.
FIG. 1.
Genomic organization of the A. marginale msp1α and msp1β loci in the St. Maries strain genome (9). The white arrow indicates msp1α; black arrows indicate full-length msp1β genes; gray arrows indicate msp1β partial genes pg1, pg2, and pg3.
FIG.2.
Predicted amino acid sequences of the MSP1b proteins from A. marginale strain St. Maries. For the aligned sequences of MSP1b1, MSP1b2, MSP1bpg3, MSP1bpg2, and MSP1bpg1, identical amino acids are indicated by white letters on a black background, and repeat regions are underlined (A). Nucleotide sequences and corresponding predicted amino acid sequences of the repeat regions (underlined) were identified for the msp1β genes (B). Numbers in parentheses indicate the relative positions in the protein or gene sequence.
In A. marginale, recombination between full-length functional genes and partial genes occurs frequently, as has been described for msp2 and msp3 (10-12, 23), generating structural and antigenic variants (1-3, 16-18). To determine if deletion or duplication events occur at the repeat regions in the msp1β genes or if recombination occurs among the msp1β genes and is selected for during the transmission cycle, we sequenced all five msp1β genes at different time points during acute and persistent infection and following tick transmission. Calf C942bl was infected intravenously with an A. marginale strain St. Maries stabilate, and ticks (Dermacentor andersoni) were allowed to feed on C942b1 and subsequently transmit the infection to calf C956bl. Both calves had peak infections of ≥109 A. marginale organisms per ml of blood. Calf C956bl was splenectomized 11 months after tick transmission and experienced a second peak of rickettsemia of ≥109 A. marginale organisms per ml of blood. DNA was isolated from the calves during all three peaks of rickettsemia (≥109 organisms/ml of blood) and at one time point during persistent infection, in which the rickettsemia was <107 organisms/ml of blood, and then sequenced. Primers specific for each msp1β gene were used (Table 1). Table 2 shows the number of clones sequenced from each calf at each time point. The sequences from these clones were aligned with the sequences derived from the St. Maries strain genome (9) using vector NTI. No clones were sequenced for msp1βpg1 and msp1βpg2 during persistent infection due to insufficient amounts of DNA. Although the repeat regions in the msp1β genes provide preferential sites for recombination, the clones had no duplications or deletions in the repeat regions. Polymorphisms were limited to nonclustered single-nucleotide substitutions, which occurred at an average of 3 × 10−4 errors per base pair, a rate that is within the range expected for Taq polymerase-based amplification. In contrast to the frequent recombinations observed in the msp2 and msp3 expression site copies (10-12, 17, 18, 23), our data indicate that either recombination within and among the msp1β genes is rare or that there is no strong selective pressure that allows organisms expressing recombined sequences to predominate in the population.
TABLE 1.
Primers used for PCR amplification of msp1β genes
| Primera | Primer sequence (5′-3′) | Product amplified | Product size (bp) |
|---|---|---|---|
| Msp1b1 TF | CTTGACCAGAGCATTGACGCAC | msp1β1 transcript | 1,875 |
| Msp1b1/pg3 TR | AGAAGACTGCTGTTGTTGCTGC | Probe | |
| Msp1b2 TF | GTAACGAGCTTGCACAAATATGTGGG | msp1β2 transcript | 695 |
| Msp1b2 TR | GGAAGAAGACTGCTGTTGTGCAG | ||
| Msp1bpg3 TF | GTCTATTGGCGATGCATTTGGCG | msp1βpg3 transcript | 979 |
| Msp1b1/pg3 TR | AGAAGACTGCTGTTGTTGCTGC | ||
| Msp1bpg2 TF | CCCACCTTGTGTGCATGGC | msp1βpg2 transcript | 1,182 |
| Msp1bpg2 TR | CGCCAAAGTACACCAAAGGCC | ||
| Msp1bpg1 TF | CAGAATTAGAGTATATCGCCCTGTATGCATG | msp1βpg1 transcript | 536 |
| Msp1bpg1 TR | CCCAGTACGGGGGTTTCCC | ||
| Msp1b1/b2 OF | CGGTTATCAAGACATTGTTAAGTAGGTAGGTG | msp1β1 ORF | 2,530 |
| Msp1b1 OR | GCCGAATATGCGCAGATGGC | ||
| Msp1b1/b2 OF | CGGTTATCAAGACATTGTTAAGTAGGTAGGTG | msp1β2 ORF | 2,338 |
| Msp1b2 OR | CTACCACGTTCTTGTATCCACACAAGG | ||
| Msp1bpg3 OF | GCATAGGGAAGATTGAAGTACCAGC | msp1βpg3 ORF | 2,096 |
| Msp1bpg3 OR | CTACACCGTGTCAGTTAAAGGTAGGG | ||
| Msp1bpg2 OF | CACAGCAGCATATTCAGGAATGTTGAAG | msp1βpg2 ORF | 1,466 |
| Msp1bpg2 OR | GCGATTTTCGCCTCACAGAGC | ||
| Msp1bpg1 OF | CATCATCTCGCAGATGAGCACTCG | msp1βpg1 ORF | 827 |
| Msp1bpg1 OR | GCGATACAACAGCCACAAAGCG | ||
| Msp1b1/b2 EF | ATGACAGAAGACGACAAGCAACAAC | Probe |
TF and TR indicate forward and reverse primers designed to amplify specific msp1β transcripts. OF and OR indicate forward and reverse primers designed for the amplification of each msp1β open reading frame (ORF). EF indicates a forward primer designed for expression of MSP161 and MSP162.
TABLE 2.
Numbers of clones sequenced for each msp1β gene during infection
| Clone | No. of clones for indicated calf and time of samplinga
|
|||
|---|---|---|---|---|
| C942bl, acute | C956bl
|
|||
| Acute | Persistent | Postsplenectomy (acute rickettsemia) | ||
| msp1β1 | 13 | 32 | 13 | 25 |
| msp1β2 | 29 | 22 | 12 | 28 |
| msp1βpg1 | 26 | 10 | 0 | 10 |
| msp1βpg2 | 13 | 31 | 0 | 29 |
| msp1βpg3 | 13 | 19 | 6 | 5 |
Blood was obtained from calf C942bl during acute infection (acute) and from C956bl during acute and persistent (persistent) stages of infection and following splenectomy (postsplenectomy), when the rickettsemia again peaked.
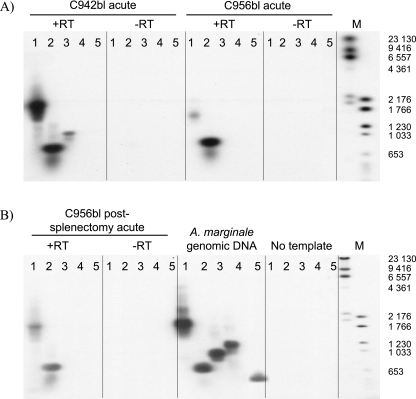
To determine which of the five msp1β genes were transcribed, RNA from all three peaks of rickettsemia was isolated and reverse transcribed using random hexamers (Invitrogen). The lack of recombination at the gene level allowed the use of genome data for designing specific primers for individual msp1β transcripts to avoid cross-reactive priming. The optimal annealing temperature for each primer set was determined by performing gradient PCR with the cloned genes as templates (results not shown). PCRs for msp1β1, msp1β2, and msp1βpg2 were performed at an annealing temperature of 60°C, while reactions for msp1βpg1 and msp1βpg3 were performed at 70°C and 71.8°C, respectively. The PCR products were visualized on ethidium bromide-stained gels, transferred to nylon membranes, and hybridized with an msp1β digoxigenin (DIG)-labeled probe. Primers used to amplify the 2.2-kb probe were designed to anneal to the conserved ends of the full-length genes to ensure that the probe would bind to all five msp1β genes. Detection was performed with a DIG luminescent detection kit and CDP-Star (Roche Applied Science, Indianapolis, IN) and visualized by autoradiography. Transcripts for both msp1β1 and msp1β2 were detected during peak infections in calf C942bl and calf C956bl (Fig. 3). A faint band for msp1βpg3 was observed in the Southern blot for calf C942bl (Fig. 3A), and in two additional experiments, a similar band for msp1βpg3 was detected for calf C956bl during the postsplenectomy peak of rickettsemia (data not shown). To rule out cross-reactivity and to confirm the specificity of the transcripts, the bands for msp1β1, msp1β2, and msp1βpg3 were cloned and sequenced. The sequences were identical to the genome sequences and confirmed the specificity of amplification.
FIG. 3.
Analysis of msp1β transcripts by Southern blotting with (+RT) and without (−RT) reverse transcription. A. marginale strain St. Maries RT-PCR products were hybridized with a DIG-labeled msp1β probe. cDNA samples collected from calf C942b1 during acute rickettsemia (A) and from calf C956bl presplenectomy (A) and postsplenectomy (B) were amplified with primers specific for each msp1β transcript (Table 1). A. marginale genomic DNA was used as a positive control (B). Lanes 1 to 5 represent the primer sets used: 1, msp1β1; 2, msp1β2; 3, msp1βpg3; 4, msp1βpg2; 5, msp1βpg1. For the A. marginale genomic DNA (B), the image in lane 5 is the result of a longer exposure of the same gel compared to the images of lanes 1 to 4. The sizes of digoxigenin-labeled markers (lane M) are indicated in base pairs on the right of the same gel as shown.
To determine if MSP1b1, MSP1b2, and/or MSP1bpg3 associate with MSP1a in the MSP1 complex, our strategy was to immunoprecipitate the MSP1 complex with a monoclonal antibody (MAb), Ana22B1, specific for MSP1a (21), followed by analysis using liquid chromatography-tandem mass spectrometry (LC-MS-MS). To confirm first that LC-MS-MS could distinguish between MSP1b1 and MSP1b2, full-length msp1β1 and msp1β2 were amplified by PCR and cloned into pTrcHis (Invitrogen). The proteins were expressed in Escherichia coli and purified with Probond resin (Invitrogen). A FLAG-tag (DYKDDDDK) (22) incorporated at the C-terminal end allowed for additional purification by affinity chromatography using anti-FLAG M2-agarose (Sigma, St. Louis, MO). Ten micrograms of each purified protein was separated by sodium dodecyl sulfide-polyacrylamide gel electrophoresis, and the Coomassie blue-stained bands (Fig. 4) were excised for in-gel trypsin digestion (32). The trypsin-digested recombinant proteins were used for LC-MS-MS analysis, which was performed using an LC Packings 180-μm C18 100-Å PepMap column (Dionex, Sunnyvale, CA), an Esquire HCT electrospray ion trap (Bruker Daltonics, Billerica, MA), and an LC Packings Ultimate Nano high-performance liquid chromatography system. A flow rate of 800 nl/min with a 0% to 60% gradient of 0.1% trifluoroacetic acid in 95% acetonitrile was used for 95 min followed by an additional 15 min with a 100% solution of 0.1% trifluoroacetic acid in 95% acetonitrile. The Mascot search engine (Matrix Science) was used to search the peptide fingerprints against the A. marginale strain St. Maries genome (9, 20). The Mascot search was performed with carbamido-methyl as the fixed modification of cysteine and variable oxidation of methionine. One missed trypsin cleavage was allowed during the search. Twenty-seven peptides identified for MSP1b1 covered approximately 49% of the MSP1b1 amino acid sequence, with a Mascot score of 2,324. Thirty-one peptides identified for MSP1b2 covered approximately 56% of the MSP1b2 amino acid sequence, with a Mascot score of 4,433. In Mascot, the score for an MS-MS match is based on the absolute probability (P) that the observed match between the experimental data and the database sequence is a random event. The individual ions score is the absolute probability that the observed match is a random event and is reported as −10log10(P), where P is the absolute probability. A probability of 10−20 thus becomes a score of 200. Individual ions scores of >19 indicate significant identity or extensive homology (P < 0.05). The Mascot scores reported are the sums of nonredundant individual ions scores of identified peptides. Mascot scores are derived from ions scores as a nonprobabilistic basis for ranking proteins. Six peptides unique for MSP1b1 and seven peptides unique for MSP1b2 were identified (Table 3). Therefore we could confirm that, although MSP1b1 and MSP1b2 are 92% identical, unique peptides for each full-length MSP1b could be identified by LC-MS-MS from in-gel trypsin-digested proteins.
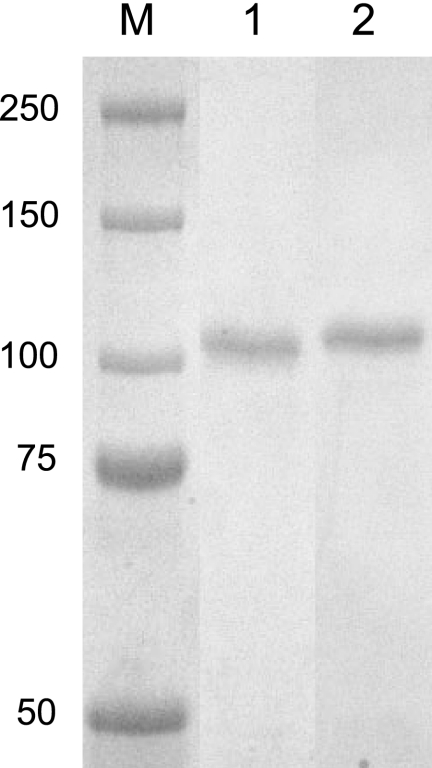
FIG. 4.
Expression of recombinant MSP1b1 and MSP1b2. Proteins were purified with an anti-FLAG affinity column. Lane M, dual-color molecular size markers (in kDa); lane 1, recombinant MSP1b1; lane 2, recombinant MSP1b2.
TABLE 3.
Unique peptides identified by LC-MS-MS analysis of recombinant MSP1b1 and MSP1b2
| Peptide (aa positions) | Sequence of peptide | Ions score of peptide detected at protein amt ofb:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 32 pmol | 6 pmol | 1.2 pmol | ||||||
| MSP1b1 | ||||||||
| 461-478 | EADRVQAEQQAEEQAMTKa | 16 | ||||||
| 465-478 | VQAEQQAEEQAMTK | 77 | ||||||
| 497-508 | TIVSDMRNELAK | 4 | ||||||
| 669-683 | LDDAQGLQEATPEAK | 85 | 27 | |||||
| 684-712 | GVEGINPEELEQAAEGLATAVNEASADGK | 57 | 19 | |||||
| 713-739 | IQSLNQQESQIAQGEQQQQQQSSGWSR | 114 | 45 | 58 | ||||
| Mascot scorec
|
2,324 | 250 | 60 | |||||
| MSP1b2
|
||||||||
| 428-438 | SIGDAFGNAFK | 35 | ||||||
| 483-496 | AQAEQQAEEQAMTK | 112 | 74 | |||||
| 515-535 | TIVSDMCNELAQICGLSQAER | 82 | 5 | |||||
| 662-676 | QTNTLAGHTAEVQAK | 53 | 6 | |||||
| 684-698 | FDDAQGLQEATPEAK | 93 | 16 | |||||
| 699-727 | GVEGINQEELEQAAEGLATAVNEASAEGK | 102 | 57 | 6 | ||||
| 728-756 | IQSLNQQESQIAQGGQHAAQQQSSSGWSR | 116 | 67 | |||||
| Mascot score | 4,433 | 837 | 68 | |||||
This peptide sequence is the same as the peptide sequence at positions 465 to 478 but has four additional N-terminal amino acids because of a missed trypsin cleavage site.
Ions score is −10log(P), where P is the probability that the observed match is a random event. Individual ions scores of >25 (32 pmol) and >18 (6 and 1.2 pmol) indicate identity or extensive homology (P < 0.05).
Mascot scores reported are the sums of nonredundant individual ions scores of identified peptides.
To immunoprecipitate the MSP1 complex, A. marginale organisms were isolated (28) during the postsplenectomy peak of infection from calf C956bl and incubated with MSP1a-specific MAb Ana22B1-coupled Sepharose beads (21, 27, 35). Affinity chromatography using Sepharose-coupled MAb Ana22B1 was previously used to isolate the native MSP1 complex for vaccine trials (26, 27); thus, this immunoprecipitated material mimics the protective immunogen. The immunoprecipitated complex was separated on a 7.5% Criterion gel (Bio-Rad, Hercules, CA) and stained with SYPRO ruby (Bio-Rad) for LC-MS-MS. Stained bands were excised from the gel and pooled from three identical lanes for in-gel trypsin digestion (20, 32). The trypsin-digested samples were analyzed by LC-MS-MS using a flow rate of 1.2 μl/min and a gradient of 10% to 100% of 0.1% trifluoroacetic acid in 95% acetonitrile for 50 min. Mascot was used to search the peptide mass fingerprints against the St. Maries strain genome, as done previously. A band at 75 kDa was confirmed to be MSP1a by a Mascot score of 299, with seven peptides matched and 20% amino acid coverage. The area between 90 and 150 kDa was excised as a block and found to be positive for MSP1b1 with a Mascot score of 58, with two peptides matched and 6% amino acid coverage. Two unique MSP1b1 peptides, LDDAQGLQEATPEAK (ions score, 43) and GVEGINPEELEQAAEGLATAVNEASADGK (ions score, 29), were identified, and no unique MSP1b2 peptides were identified. Immunoprecipitation with MAb AmR38A6, which recognizes MSP1b (21, 35) and both recombinant MSP1b1 and MSP1b2 proteins (data not shown), was also performed, and the area between 100 and 150 kDa again scored positive for MSP1b1, with a Mascot score of 35, with two peptides matched and 3% amino acid coverage. Only one unique MSP1b1 peptide, LDDAQGLQEATPEAK (ions score, 35), was identified.
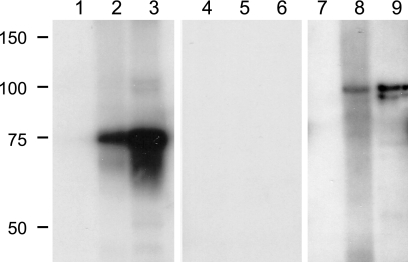
To enrich for MSP1 in the sample used for immunoprecipitation (20), outer membranes were isolated from A. marginale organisms (28) obtained from calf C956bl during the postsplenectomy peak of infection and used for immunoprecipitation with MSP1a-specific MAb Ana22B1-coupled Sepharose beads (21, 27, 35). The immunoprecipitated complex was then separated on a 7.5% Criterion gel and either transferred to a nylon membrane for immunoblotting or stained with SYPRO ruby for LC-MS-MS. MSP1a-specific MAb Ana22B1 recognized the predicted 75-kDa protein on the immunoblot (Fig. 5, lane 3), while MSP1b-specific MAb AMR38A6 recognized a predominant band at approximately 100 kDa (Fig. 5, lane 9), indicating that the MSP1 complex could be immunoprecipitated as shown by the presence of bands of the expected size and antigenic specificity. No reaction was observed with MAb Tryp1E1 (specific for Trypanosoma brucei), which was used as a negative control (Fig. 5, lanes 4 through 6). A. marginale strain St. Maries organisms were used as a positive control (Fig. 5, lanes 2, 5, and 8), and uninfected erythrocytes were used as a negative control on the immunoblot (Fig. 5, lanes 1, 4, and 7). All but one of the MSP1b1- and MSP1b2-specific peptides identified by LC-MS-MS are present in MSP1bpg3, so it would be difficult to distinguish MSP1bpg3 from MSP1b1 and MSP1b2 by peptide fingerprinting. However, MSP1bpg3, if translated, is predicted to be 68 kDa, which would allow differentiation between MSP1bpg3, MSP1b1, and MSP1b2 by size. A band of this size was not observed in either the immunoblots or SYPRO ruby-stained gels, suggesting that MSP1bpg3 is either not translated or not part of the MSP1 complex.
FIG. 5.
Detection of MSP1a and MSP1b in A. marginale outer membranes immunoprecipitated with MSP1a-specific antibody. Lanes 1, 4, and 7, 1 μg of uninfected red blood cells; lanes 2, 5, and 8, 5 μg of A. marginale strain St. Maries organisms; lanes 3, 6, and 9, 30 μg of A. marginale strain St. Maries outer membranes immunoprecipitated with MAb Ana22B1 specific for MSP1a. Molecular size markers are indicated in kDa. The Western blot was developed with MAb Ana22B1 specific for MSP1a (lanes 1 to 3), MAb Tryp1E1 specific for T. brucei (lanes 4 to 6), and MAb AmR38A6 specific for MSP1b (lanes 7 to 9).
Two sequential gel slices representing the region between 95 and 100 kDa were excised from the SYPRO ruby-stained gel, digested with trypsin, and analyzed using LC-MS-MS. Analysis of the upper band revealed peptides from MSP1b1 (Mascot score, 184; five peptides matched and 13% amino acid coverage), with LDDAQGLQEATPEAK (ions score, 21) and GVEGINPEELEQAAEGLATAVNEASADGK (ions score, 56) as the discriminatory MSP1b1-specific peptides. Peptides from an unrelated protein, MSP3 (Mascot score, 292; eight peptides matched and 15% amino acid coverage), were also identified in the upper band. The lower band was positive for MSP3 (Mascot score, 502; 12 peptides matched and 25% amino acid coverage) and also had a lower-scoring hit to MSP1b1 (Mascot score, 36). This could explain the presence of the second lower-intensity band in the Western blot (Fig. 5, lane 9). In a repeated immunoprecipitation with MAb Ana22B1, the LDDAQGLQEATPEAK peptide was again identified for MSP1b1, but MSP1b2-specific peptides were not detected in any of the immunoprecipitations. In a further attempt to identify MSP1b2 in outer membranes not subjected to immunoprecipitation, outer membranes were separated on a 7.5% Criterion gel, and proteins were electroeluted from a block corresponding to 75 to 100 kDa. The electroeluted sample was further separated on a 7.5% Criterion gel, and three bands (approximately 80, 90, and 100 kDa) were excised (data not shown). All three bands were positive for MSP3. The 90-kDa band was also positive for MSP1b1 (Mascot score, 28), with LDDAQGLQEATPEAK (ions score, 20) as the distinguishing peptide. No MSP1b2-specific peptides were identified.
In general, the limit of protein identification by mass spectrometry is in the low-femtomolar range when proteolytic fragments are introduced into the instrument. The practical sensitivity for identification of proteins in gels is much less because a smaller portion of the protease digestion is analyzed. Radiolabeled recombinant proteins used to systematically evaluate peptide recoveries from in-gel trypsin digestion indicated that at least 80% of the labeled tryptic peptides could be extracted from gel bands containing 1 to 10 pmol of protein (33). To determine the sensitivity of detection in our system, serial dilutions of purified recombinant MSP1b1 and MSP1b2 proteins were separated on a 7.5% Criterion gel. Samples of 10, 2, 0.4, 0.08, and 0.016 μg of protein were loaded per well. The bands were excised and processed for LC-MS-MS. Based on 100% sample recovery and the volume injected for LC-MS-MS, the maximum protein amounts analyzed for MSP1b1 and MSP1b2 were 32 pmol and 31.2 pmol, respectively. At the highest protein amount injected for MSP1b1, six specific peptides were detected (Table 3); at 6.4 pmol, only three peptides specific for MSP1b1 were detected; and at 1.2 pmol, only one specific peptide was detected (Table 3). For MSP1b2, seven specific peptides were detected at 31.2 pmol, the highest amount injected (Table 3); five specific peptides were detected at 6.2 pmol; and two specific peptides were detected at 1.25 pmol (Table 3). At the two lowest amounts of input protein (0.25 and 0.05 pmol), no specific peptides could be detected for either MSP1b1 or MSP1b2. The ions scores of the two MSP1b2-specific peptides detected using 1.25 pmol of input protein are not greater than the statistically significant limit of 18 (P < 0.05). However, a significant protein match, with a Mascot score of 68 (P < 0.05), could still be established for MSP1b2. Thus, if MSP1b1 and MSP1b2 are present in the MSP1 complex, they should be detected at an amount of 0.96 to 1.25 pmol, based on 80% and 100% recovery, respectively. To determine if any of the unique peptides identified for MSP1b2 were present at low intensities in the MSP1 complex, the m/z values of the seven distinguishing peptides of MSP1b2 (listed in Table 3) were extracted from the raw LC-MS-MS data for our samples. However, no peaks for the MSP1b2 peptides were identified. Since unique peptides with similar ions scores were detectable for both MSP1b1 and MSP1b2 using the recombinant proteins, both MSP1b1 and MSP1b2 should be detectable in the MSP1 complex if they were present at the same level. We conclude that if MSP1b2 is linked to MSP1a in the complex, it is below the level of detection.
Additional bands that were observed at 55, 75, and 90 kDa on the SYPRO ruby-stained gel of the immunoprecipitated A. marginale outer membranes (data not shown) were also analyzed using LC-MS-MS. The 75-kDa band was confirmed to be MSP1a (Mascot score, 327; 12 peptides matched and 22% amino acid coverage), and the 90-kDa band was positive for MSP3 (Mascot score, 380; 14 peptides matched and 17% amino acid coverage). MSP3 is a highly abundant membrane protein, and its molecular size varies, depending on the length of the central hypervariable domain of a specific variant (23). Thus, detection of MSP3 at molecular sizes of 90, 95, and 100 kDa is consistent with the sizes of the variant domains previously reported and reflects the presence of multiple variants within the A. marginale population (3, 23). The detection of MSP3 in the MSP1 complex likely reflects both its abundance in the membrane and its nearest-neighbor relationship with MSP1 (35). Proteins in the MSP1 complex contain glutamine-rich segments, which are thought to form a structural framework for the formation of multimeric complexes (15, 31). The 29- or 30-amino-acid repeat regions of MSP1a contain three consecutive glutamine residues plus an additional one or two glutamine residues (4, 30). MSP1b1 contains four glutamine residues in the first 10 amino acids and an additional four glutamine residues in the next 30 amino acids; at the C-terminal end, 9 of the last 30 amino acids are glutamine (Fig. 2A). These glutamine-rich regions may be responsible for the previously reported noncovalent interaction between the MSP1 complex and MSP3 (35).
The band at 55 kDa was excised to determine if it could be a product of msp1βpg3 but was identified by LC-MS-MS as VirB10 (Mascot score, 107; six peptides matched and 20% amino acid coverage). VirB10 is a protein that forms part of the type IV secretion system and was recently identified by LC-MS-MS analysis of antigenic A. marginale outer membranes separated by two-dimensional electrophoresis (20). The detection of VirB10 in the complex is novel, as prior studies (35) were limited by the lack of knowledge regarding the full complement of membrane proteins and were restricted to identifying interactions among six known outer membrane proteins using MAbs. How VirB10 is linked to the MSP1 complex and what its immunological significance is are unknown.
It has been proposed that several cysteine-rich proteins in Chlamydia spp., including the major outer membrane proteins which contain 7 to 10 cysteine residues (37), form disulfide bonds to provide rigidity to the chlamydial cell wall (7, 19, 37). The A. marginale MSP1 complex proteins also contain a notable number of cysteine residues; MSP1a contains nine cysteine residues (4, 30), while MSP1b1 has seven (Fig. 1A). The MSP1 complex has been shown to be disulfide linked, and MSP2 and MSP5 can each occur as monomers or disulfide-linked multimers, consistent with a highly cross-linked outer membrane. MSP1, MSP2, MSP3, and MSP4 are nearest neighbors, and MSP1, MSP2, and MSP5 are also associated through noncovalent interactions (35). Our current findings support the presence of additional outer membrane proteins within these complexes that can now be identified using the complete genome sequence. Analyzing the complexity of and the interactions among membrane proteins is necessary for future immunological studies designed to fully understand the protective nature of A. marginale outer membranes.
Acknowledgments
We thank Kimberly Kegerreis, Ralph Horn, Shelley Whidbee, and Bev Hunter for excellent technical assistance and Junzo Norimine for helpful discussions and assistance.
This research was supported by National Institutes of Health grant AI053692.
REFERENCES
- 1.Abbott, J. R., G. H. Palmer, K. A. Kegerreis, P. F. Hetrick, C. J. Howard, J. C. Hope, and W. C. Brown. 2005. Rapid and long-term disappearance of CD4+ T lymphocyte responses specific for Anaplasma marginale major surface protein-2 (MSP2) in MSP2 vaccinates following challenge with live A. marginale. J. Immunol. 174:6702-6715. [DOI] [PubMed] [Google Scholar]
- 2.Alleman, A. R., and A. F. Barbet. 1996. Evaluation of Anaplasma marginale major surface protein 3 (MSP3) as a diagnostic test antigen. J. Clin. Microbiol. 34:270-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alleman, A. R., G. H. Palmer, T. C. McGuire, T. F. McElwain, L. E. Perryman, and A. F. Barbet. 1997. Anaplasma marginale major surface protein 3 is encoded by a polymorphic, multigene family. Infect. Immun. 65:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allred, D. R., T. C. McGuire, G. H. Palmer, S. R. Leib, T. M. Harkins, T. F. McElwain, and A. F. Barbet. 1990. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc. Natl. Acad. Sci. USA 87:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbet, A. F., and D. R. Allred. 1991. The msp1β multigene family of Anaplasma marginale: nucleotide sequence analysis of an expressed copy. Infect. Immun. 59:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbet, A. F., G. H. Palmer, P. J. Myler, and T. C. McGuire. 1987. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect. Immun. 55:2428-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bavoil, P., A. Ohlin, and J. Schachter. 1984. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect. Immun. 44:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowie, M. V., J. de la Fuente, K. M. Kocan, E. F. Blouin, and A. F. Barbet. 2002. Conservation of major surface protein 1 genes of Anaplasma marginale during cyclic transmission between ticks and cattle. Gene 282:95-102. [DOI] [PubMed] [Google Scholar]
- 9.Brayton, K. A., L. S. Kappmeyer, D. R. Herndon, M. J. Dark, D. L. Tibbals, G. H. Palmer, T. C. McGuire, and D. P. Knowles, Jr. 2005. Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc. Natl. Acad. Sci. USA 102:844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brayton, K. A., D. P. Knowles, T. C. McGuire, and G. H. Palmer. 2001. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proc. Natl. Acad. Sci. USA 98:4130-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brayton, K. A., P. F. M. Meeus, A. F. Barbet, and G. H. Palmer. 2003. Simultaneous variation of the immunodominant outer membrane proteins, MSP2 and MSP3, during Anaplasma marginale persistence in vivo. Infect. Immun. 71:6627-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brayton, K. A., G. H. Palmer, A. Lundgren, J. Yi, and A. F. Barbet. 2002. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol. Microbiol. 43:1151-1159. [DOI] [PubMed] [Google Scholar]
- 13.Brown, W. C., V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer. 1998. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 66:5406-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camacho-Nuez, M., M. de Lourdes Muñoz, C. E. Suarez, T. C. McGuire, W. C. Brown, and G. H. Palmer. 2000. Expression of polymorphic msp1β genes during acute Anaplasma marginale rickettsemia. Infect. Immun. 68:1946-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courey, A. J., and R. Tjian. 1988. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55:887-898. [DOI] [PubMed] [Google Scholar]
- 16.Eid, G., D. M. French, A. M. Lundgren, A. F. Barbet, T. F. McElwain, and G. H. Palmer. 1996. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect. Immun. 64:836-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French, D. M., W. C. Brown, and G. H. Palmer. 1999. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 67:5834-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French, D. M., T. F. McElwain, T. C. McGuire, and G. H. Palmer. 1998. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect. Immun. 66:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatch, T. P. 1996. Disulfide cross-linked envelope proteins: the functional equivalent of peptidoglycan in chlamydiae? J. Bacteriol. 178:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez, J. E., W. F. Siems, G. H. Palmer, K. A. Brayton, T. C. McGuire, J. Norimine, and W. C. Brown. 2005. Identification of novel antigenic proteins in a complex Anaplasma marginale outer membrane immunogen by mass spectrometry and genomic mapping. Infect. Immun. 73:8109-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuire, T. C., G. H. Palmer, W. L. Goff, M. I. Johnson, and W. C. Davis. 1984. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect. Immun. 45:697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIlhinney, R. A. 2004. Generation and use of epitope-tagged receptors. Methods Mol. Biol. 259:81-98. [DOI] [PubMed] [Google Scholar]
- 23.Meeus, P. F., K. A. Brayton, G. H. Palmer, and A. F. Barbet. 2003. Conservation of a gene conversion mechanism in two distantly related paralogues of Anaplasma marginale. Mol. Microbiol. 47:633-643. [DOI] [PubMed] [Google Scholar]
- 24.Newhall, W. J., IV 1987. Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect. Immun. 55:162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberle, S. M., G. H. Palmer, A. F. Barbet, and T. C. McGuire. 1988. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect. Immun. 56:1567-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer, G. H., A. F. Barbet, G. H. Cantor, and T. C. McGuire. 1989. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect. Immun. 57:3666-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer, G. H., A. F. Barbet, W. C. Davis, and T. C. McGuire. 1986. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science 231:1299-1302. [DOI] [PubMed] [Google Scholar]
- 28.Palmer, G. H., and T. C. McGuire. 1984. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J. Immunol. 133:1010-1015. [PubMed] [Google Scholar]
- 29.Palmer, G. H., S. M. Oberle, A. F. Barbet, W. L. Goff, W. C. Davis, and T. C. McGuire. 1988. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect. Immun. 56:1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer, G. H., F. R. Rurangirwa, and T. F. McElwain. 2001. Strain composition of the ehrlichia Anaplasma marginale within persistently infected cattle, a mammalian reservoir for tick transmission. J. Clin. Microbiol. 39:631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson, M. G., N. Tanese, B. F. Pugh, and R. Tjian. 1990. Functional domains and upstream activation properties of cloned human TATA binding protein. Science 248:1625-1630. [DOI] [PubMed] [Google Scholar]
- 32.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 33.Speicher, K. D., O. Kolbas, S. Harper, and D. W. Speicher. 2000. Systematic analysis of peptide recoveries from in-gel digestions for protein identifications in proteome studies. J. Biomol. Tech. 11:74-86. [PMC free article] [PubMed] [Google Scholar]
- 34.Tebele, N., T. C. McGuire, and G. H. Palmer. 1991. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect. Immun. 59:3199-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidotto, M. C., T. C. McGuire, T. F. McElwain, G. H. Palmer, and D. P. Knowles, Jr. 1994. Intermolecular relationships of major surface proteins of Anaplasma marginale. Infect. Immun. 62:2940-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viseshakul, N., S. Kamper, M. V. Bowie, and A. F. Barbet. 2000. Sequence and expression analysis of a surface antigen gene family of the rickettsia Anaplasma marginale. Gene 253:45-53. [DOI] [PubMed] [Google Scholar]
- 37.Yen, T. Y., S. Pal, and L. M. de la Maza. 2005. Characterization of the disulfide bonds and free cysteine residues of the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Biochemistry 44:6250-6256. [DOI] [PubMed] [Google Scholar]