Abstract
In budding yeast, a signaling network known as the mitotic exit network (MEN) triggers exit from mitosis. We find that hypertonic stress allows MEN mutants to exit from mitosis in a manner dependent on the high osmolarity glycerol (HOG) mitogen-activated protein (MAP) kinase cascade. The HOG pathway drives exit from mitosis in MEN mutants by promoting the activation of the MEN effector, the protein phosphatase Cdc14. Activation of Cdc14 depends on the Cdc14 early anaphase release network, a group of proteins that functions in parallel to the MEN to promote Cdc14 function. Notably, exit from mitosis is promoted by the signaling branch defined by the Sho1 osmosensing system, but not by the Sln1 osmosensor of the HOG pathway. Our results suggest that the stress MAP kinase pathway mobilizes programs to promote completion of the cell cycle and entry into G1 under unfavorable conditions.
INTRODUCTION
In response to a sudden increase in extracellular osmolarity, cells induce an elaborate program that includes changes in transcription and translation as well as cell cycle progression to allow cells to adapt. Central to this response is a mitogen-activated protein (MAP) kinase signaling pathway known as the high osmolarity glycerol (HOG) pathway in budding yeast and the p38 pathways in more complex eukaryotes (reviewed in Hohmann, 2002).
The HOG pathway is composed of the MAP kinase Hog1, its MAP kinase kinase Pbs2 as well as two upstream regulatory branches that are defined by two putative osmosensors, Sln1 and Sho1. The Sln1 branch of the pathway is thought to sense changes in turgor pressure (Reiser et al., 2003) through the Ypd1-Ssk1 multicomponent phospho-relay system and the redundant Ssk2/Ssk22 protein kinases (Posas et al., 1996; Posas and Saito, 1998). The signal sensed by the Sho1 branch is not known but activation of Pbs2 through Sho1 requires proteins that are also involved in the cells’ response to mating pheromone and include Cdc42, Ste50, Ste20, and Ste11 (Maeda et al., 1995; Posas and Saito, 1997; Raitt et al., 2000; Reiser et al., 2000).
Activation of either the Sln1 or Sho1 branch of the pathway is sufficient to elicit adaptation to high osmolarity and lead to phosphorylation of Hog1 on strictly conserved T and Y residues in the catalytic domain of the kinase, thereby activating it. Activated Hog1 then transiently accumulates in the nucleus (Reiser et al., 1999) where it modulates the expression of genes involved in adaptation to high osmolarity by modulating activity of several transcription factors as well as through chromatin remodeling (Rep et al., 1999; Alepuz et al., 2001; Proft and Struhl, 2002; de Nadal et al., 2003, de Nadal et al., 2004). One of the key transcriptional outputs is the stimulation of intracellular glycerol production, which helps to rebuild the osmotic equilibrium between the extracellular and intracellular environment (reviewed in Hohmann 2002).
Activation of the HOG pathway also leads to a transient cell cycle arrest in G1 or G2. The transient G1 arrest is thought to be brought about by two mechanisms. First, the HOG pathway down-regulates the transcription of key regulators of the G1–S phase transition. Cyclin-dependent kinases (Cdc28 in budding yeast; CDKs) promote entry into the cell cycle when complexed with Cln1, -2, and -3 cyclin subunits (Cln-CDKs). Activation of the HOG pathway causes inhibition of CLN1 and CLN2 transcription by an unknown mechanism (Escote et al., 2004). Second, Hog1 directly phosphorylates an inhibitor of DNA replication, Sic1, thereby preventing the protein’s degradation and entry into DNA replication (Escote et al., 2004). Activation of the HOG pathway also delays cell cycle progression in G2 (Alexander et al., 2001) by inhibiting the mitosis-inducing Clb-CDKs transcriptionally and posttranscriptionally. How HOG pathway activation leads to inhibition of CLB cyclin transcription and activation of Swe1, which inhibits Clb-CDKs is unknown.
Hypertonic stress also seems to regulate exit from mitosis, the cell cycle transition from mitosis into G1. The failure of certain mutants to execute this transition can be suppressed by a high osmotic environment (Grandin et al., 1998). Exit from mitosis is triggered by the protein phosphatase Cdc14, which brings about this cell cycle transition by antagonizing Clb-CDKs (reviewed in Stegmeier and Amon, 2004). The protein phosphatase itself is tightly regulated by a competitive inhibitor Cfi1/Net1. The inhibitor holds Cdc14 inactive in the nucleolus during G1 phase, S phase, and early mitosis. During anaphase, Cdc14 is released from its inhibitor and spreads throughout the nucleus and cytoplasm to induce exit from mitosis. The release of Cdc14 from its inhibitor is brought about by two regulatory networks, the Cdc14 early anaphase release (FEAR) network and the mitotic exit network (MEN). The FEAR network promotes Cdc14 activation during early anaphase but is not essential for Cdc14 to promote exit from mitosis. The MEN functions during later stages of anaphase to activate Cdc14. This pathway’s Cdc14-promoting functions are essential for exit from mitosis to occur. Cells carrying temperature-sensitive mutations in components of the MEN arrest in late anaphase, with Cdc14 sequestered in the nucleolus. The cell cycle arrest of two temperature-sensitive MEN mutants, cdc15-2 and dbf2-2 has been shown to be partially suppressed by a high osmotic environment (Grandin et al., 1998). The mechanism underlying this suppression was unknown.
Here, we show that hypertonic stress allows MEN mutants to release Cdc14 from the nucleolus and to exit from mitosis in a manner dependent on the HOG MAP kinase cascade. Release of Cdc14 from its inhibitor depends on the FEAR network, suggesting that the HOG pathway regulates the FEAR network or functions in parallel to it to promote exit from mitosis in MEN mutants. Interestingly, exit from mitosis is promoted by the signaling branch defined by the Sho1 osmosensing system but not by the Sln1 branch of the HOG pathway. Our results suggest that the stress MAP kinase pathway promotes completion of the cell cycle and entry into G1 in a hypertonic environment and defines the first extracellular signal regulating Cdc14 and exit from mitosis.
MATERIALS AND METHODS
Strains
All strains are derivative of W303 (K699). Deletions of genes were performed by the PCR-based method described in Longtine et al. (1998).
Cell Synchronization
Cells were arrested in G1 in YPD medium containing 5 μg/ml α-factor and subsequently released into α-factor-free YPD medium at 37°C. To release cells from a nocodazole block, cells were first arrested by addition of 15 μg/ml nocodazole (1% dimethyl sulfoxide [DMSO]) in YPD medium for 2–3 h and then washed and released into fresh YPD containing 1% DMSO. To avoid activation of the HOG pathway by heat shock, the following regimen was used to shift temperature-sensitive mutants to the restrictive temperature. Cells were grown at 25°C and then washed and resuspended in medium and placed into a water-bath shaker that has been set to 30°C. The temperature of the water bath was then raised gradually to reach 37°C within a 10- to 15-min time interval. Sorbitol was prewarmed to 37°C before adding to cells.
Microscopy
Immunofluorescence (tubulin, Myc-tagged Dbf2, and hemagglutinin [HA]-tagged Cdc14), fluorescence (SPC42-cyan fluorescent protein [CFP]), and light microscopy techniques were as described previously in Visintin et al. (1999) and Visintin and Amon (2001). Images were captured using an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) and analyzed using the Openlab software package (Improvision, Lexington, MA). Cell viability was determined using the LIVE/DEAD yeast viability assay manufactured by Invitrogen (Carlsbad, CA).
Protein Techniques
Western blot analysis and Dbf2 and Clb2 kinase assays were performed as described previously (Visintin and Amon, 2001). The following antibodies were used: anti-HA (HA-11; BAbCO, Richmond, CA), anti-myc (9E10; BAbCO), anti-α-tubulin (Oxford Biotechnology, Kidlington, United Kingdom), and anti-Hog1 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Hog1 phosphorylation was measured using an anti-phospho-p38 antibody (Cell Signaling Technology, Beverly, MA).
RESULTS
A Hypertonic Environment Suppresses the Proliferation Defect of Temperature-sensitive MEN Mutants
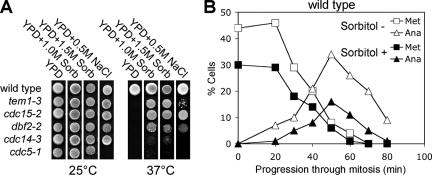
Temperature-sensitive mutants in the MEN components CDC15, TEM1, and DBF2 arrest in anaphase due to a failure to inactivate Clb-CDKs, and as a consequence, cells do not exit from mitosis. However, when grown in hypertonic medium, such as YPD supplemented with sorbitol or sodium chloride, temperature-sensitive MEN mutants were able to proliferate (Figure 1A;Grandin et al., 1998). A hypertonic environment did not allow cells deleted for MEN components to proliferate (our unpublished data), indicating that some MEN activity was necessary for high osmolarity to bring about exit from mitosis. The suppression of the temperature-sensitive lethality by high osmolarity was nevertheless specific to MEN mutants because the proliferation defect of other temperature-sensitive cell cycle mutants was not suppressed by a hypertonic environment (Table 1).
Figure 1.
A hypertonic environment rescues the mitotic exit defect of MEN mutants. (A) Strains carrying temperature-sensitive alleles of genes encoding components of the MEN were spotted on solid media supplemented with sorbitol or NaCl. Strains used (from top) are A1411, A1740, A1674, A851, A5321, and A859. Note that the cdc5-1 strain was sensitive to 0.5 M NaCl. (B) A hypertonic environment does not accelerate exit from mitosis: wild-type cells were arrested with 15 μg/ml nocodazole followed by release from the block in the presence or absence of 1.5 M sorbitol. The percentage of cells with metaphase and anaphase spindles was determined at the indicated times. Note that all cells were arrested by the nocodazole treatment. However, upon washout of the drug (0 time point), repolymerization of metaphase spindles is sluggish leading to only 30–50% of cells having reformed metaphase spindles at the beginning of the time course.
Table 1.
Suppression of various temperature-sensitive cell cycle mutants by high osmolarity at restrictive temperature
| Strain | Growth on YPD + 1.5 M sorbitol at 37°C |
|---|---|
| cdc13-1 | − − − |
| cdc28-4 | − − − |
| cdc23-1 | − − − |
| cdc27-1 | − − + |
| cdc15-2 | + + + |
| cdc14-3 | − − − |
| cdc14-1 | − − − |
| cdc9-1 | − − − |
| cdc7-1 | − − − |
| cdc20-1 | − − − |
| cdc5-1 | − − − |
| cdc5-2 | − − − |
| cdc15-2 sic1Δ | − − − |
| cdc15-2 cdh1Δ | − − − |
In contrast to MEN mutants, strains carrying temperature-sensitive mutations in the MEN effector CDC14 (Shou et al., 1999; Visintin et al., 1999) were not able to grow under hypertonic culture conditions. Cells bearing either the cdc14-1 or cdc14-3 allele failed to proliferate in sorbitol-containing medium at 37°C (Figure 1A and Table 1). A previous report (Grandin et al., 1998) mentioned that high osmolarity suppressed the temperature-sensitive growth defect of cdc14-1 mutants. The reason for this discrepancy is at present unclear.
SIC1, which encodes an inhibitor of Clb-CDKs, and CDH1, which functions as an activator of Clb cyclin degradation, are critical targets of CDC14 in promoting exit from mitosis (reviewed in Stegmeier and Amon, 2004). Deletion of either gene prevented cdc15-2 mutants to proliferate at 37°C in the presence of sorbitol (Table 1). Furthermore the lethality associated with the expression of a Clb2 mutant that cannot be degraded was not suppressed by hypertonic growth conditions (our unpublished data). Together, these results indicate that CDK inactivation is required for high osmolarity to promote proliferation of MEN mutants. Our results suggest that a hypertonic environment either restores signaling through the MEN or promotes Cdc14 activity and Clb-CDK inactivation by a parallel mechanism.
To examine whether a hypertonic environment also affects the kinetics of exit from mitosis during an unperturbed cell cycle, we analyzed the effects of 1.5 M sorbitol on wild-type cells exiting mitosis upon release from a nocodazole-induced metaphase arrest. Cells treated with sorbitol progressed through mitosis less efficiently than untreated cells, but the kinetics of exit from mitosis was similar under both culture conditions (Figure 1B). Furthermore, exit from mitosis upon release from a MEN mutant block was also not accelerated by addition of sorbitol to the medium (our unpublished data). These results indicate that a hypertonic environment cannot accelerate exit from mitosis when this cell cycle transition occurs with wild-type kinetics. Thus, the exit from mitosis promoting function of a hypertonic environment is only revealed when cell cycle progression is halted or delayed in anaphase.
The Suppression of MEN Mutants by High Osmolarity Is Not Due to Increased Glycerol Production
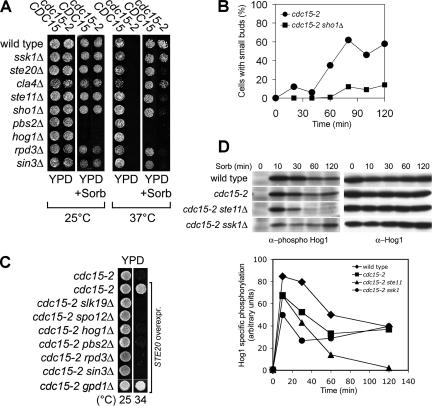
To investigate whether the rescue of the temperature-sensitive growth defect of MEN mutants by high osmolarity was due to an increase in intracellular glycerol levels that occurs in response to a high osmotic environment (reviewed in Hohmann, 2002), we examined the consequences of deleting GPD1. GPD1 encodes the major NAD-dependent glycerol-3-phosphate dehydrogenase that is responsible for glycerol accumulation in response to an increase in the osmotic environment (Albertyn et al., 1994). Deletion of GPD1 reduced the ability of MEN mutants to grow on medium containing sorbitol to the same extent as that of wild-type cells (our unpublished data). Several other observations further argue against the idea that an increase in intracellular glycerol is responsible for the suppression of the temperature-sensitive lethality of MEN mutants by high osmolarity. First, overexpression of GPD1 did not promote proliferation of MEN mutants that were defective in the HOG pathway component PBS2 (our unpublished data). Second, overexpression of the HOG pathway component STE20 rescued the temperature-sensitive growth defect of cdc15-2 mutants in a GPD1-independent manner (Figure 7C). Finally, inactivation of the osmosensor SHO1 prevents cdc15-2 mutants to exit from mitosis and to grow at 37°C in the presence of sorbitol (Figure 7, A and B), yet GPD1 induction and osmoresistance are not affected by deleting SHO1 (Figure 7A; Maeda et al., 1995; O’Rourke and Herskowitz, 2004). Together, these results indicate that an increase in intracellular glycerol levels is neither required nor sufficient for the rescue of MEN mutants by high osmolarity.
Figure 7.
Suppression of the temperature-sensitive growth defect of MEN mutants by high osmolarity depends on the Sho1 signaling branch of the HOG pathway. (A) The Sho1 branch, but not the Sln1 branch of the HOG pathway, is necessary for the high osmolarity-induced suppression of MEN mutants. Strains were spotted onto media with or without 1.5 M sorbitol. Strains used (from the top, the CDC15+ strain is noted first) are A2587, A1674, A11678, A11681, A11812, A11813, A5816, A8353, A11679, A11682, A11680, A11683, A9610, A10710, A9608, A10707, A11572, A11573, A11576, and A11577. (B) cdc15-2 (A1674) and cdc15-2 sho1Δ (11683) cells were arrested at 37°C for 2 h. When the arrest was complete, cells were transferred into medium containing 1.5 M sorbitol. At the indicated times, samples were taken to determine the percentage of cells with small buds. (C) Cells carrying a multicopy plasmid containing STE20 were grown on YPD plates at the indicated temperatures. Strains used (from the top) are A1674, A12227, A12228, A12229, A12230, A12231, A12232, A12233, A12227, and A14535. (D) Activation of Hog1 in cdc15-2 strains in response to osmotic stress. cdc15-2 cells were arrested at 37°C. After 2 h (0-time point), 1.5 M sorbitol was added. Samples were taken at the indicated times to determine Hog1 activity using an antibody that recognizes double phosphorylation of the TGY amino acid motif in Hog1. The total amount of Hog1 was determined using an anti-Hog1 antibody. Signals were quantified and normalized to protein levels of Hog1 (graph). Strains used (from the top) are A1411, A1674, A11682, and A11681.
The Suppression of MEN Mutants by Hypertonic Treatment Depends on HOG Pathway Function
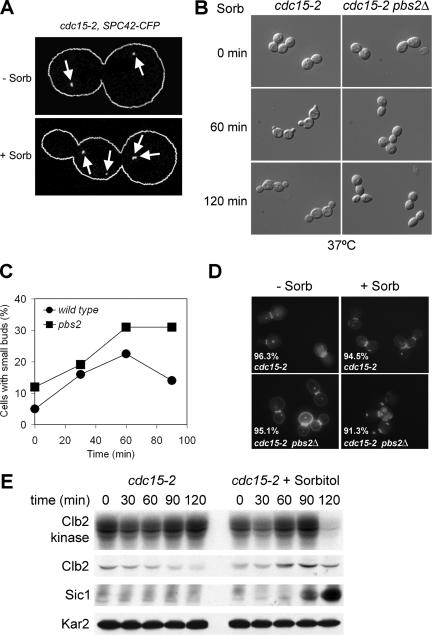
In budding yeast, hypertonic stress activates the HOG pathway (reviewed in Hohmann, 2002). We found that suppression of the proliferation defect of MEN mutants by a hypertonic environment depended on this MAP kinase signaling cascade. Transfer to a hypertonic environment allowed cdc15-2 cells to exit from mitosis and enter the next cell cycle as judged by their ability to duplicate spindle pole bodies (Figure 2A) and to form new buds (Figure 2B).
Figure 2.
The HOG pathway drives exit from mitosis in MEN mutants in response to hypertonic stress. (A) Budding pattern as well as SPB number and distribution were analyzed in cdc15-2 (A9943) cells arrested in anaphase at restrictive temperature before (−Sorb) and 60 min after (+Sorb) addition of 1.5 M sorbitol. SPB number and distribution was monitored using CFP fused to the SPB protein Spc42 (localization indicated by white arrows). (B) cdc15-2 (A1674) and cdc15-2 pbs2Δ (A10710) cells were arrested in anaphase at 37°C (0-min time point), treated with 1.5 M sorbitol and then examined at the indicated time points by light microscopy using a DIC filter. (C) Wild-type (A1411) and pbs2Δ (A9610) cells were arrested in mitosis with nocodazole at 37°C. When the arrest was complete (after 150 min), cells were released from the block in the presence of 1.5 M sorbitol at 37°C, and the percentage of cells with small buds was determined. (D) cdc15-2 (A1674) and cdc15-2 pbs2Δ (A10710) cells were analyzed for their metabolic activity and cell wall integrity before and 150 min after osmotic stress (1.5 M sorbitol) at 37°C. The percentage of viable cells is indicated for each strain and conditions. (E) cdc15-2 (A1674) cells were arrested at 37°C for two hours. When the arrest was complete the culture was split, and one-half of the culture was maintained at 37°C and the other half was transferred into medium containing 1.5 M sorbitol. At the same time, 5 μg/ml α-factor was added to prevent sorbitol-treated cells from entering the next cell cycle. At the indicated times, samples were taken to determine the total amount of Clb2 and Sic1 protein as well as Clb2 kinase activity.
To determine whether the exit from mitosis observed in cdc15-2 mutants in the presence of sorbitol was associated with the inactivation of Clb-CDKs, we measured Clb2-associated kinases activity as well as Clb2 cyclin and Sic1 levels, all markers for exit from mitosis (reviewed in Stegmeier and Amon, 2004). Because exit from mitosis brought about by sorbitol does not occur very synchronously α-factor pheromone was added at the time of sorbitol addition to arrest cells in G1 when Clb-CDK activity is low. Sic1 accumulation and Clb2-CDK inactivation was observable 90 and 120 min after sorbitol addition, respectively (Figure 2E). Clb2 cyclin degradation was not as obvious and only started to occur at the last time point. Why Clb2 degradation is not occurring earlier is at present unclear. Our results nevertheless indicate that the exit from mitosis in cdc15-2 mutants caused by sorbitol is accompanied by Clb-CDK inactivation.
Next, we determined whether an intact HOG pathway was required for a hypertonic environment to induce exit from mitosis in MEN mutants. cdc15-2 mutants lacking the HOG pathway MAPKK encoding gene PBS2 failed to exit from mitosis and to form new buds (Figure 2B). This failure to exit from mitosis was not simply due to cells lacking PBS2 to proliferate in a high osmotic environment. pbs2Δ cells formed new buds when released from a mitotic arrest induced by nocodazole in the presence of 1.5 M sorbitol (Figure 2C), and cells retained high viability (Figure 2D). Furthermore, deletion of SHO1, which does not impair proliferation under hypertonic conditions (Maeda et al., 1995), also prevented cdc15-2 mutants to exit from mitosis in the presence of sorbitol (Figure 7B). We conclude that an intact HOG pathway is necessary for hypertonic stress to bring about exit from mitosis in cdc15-2 mutants.
The Hog1 MAP kinase is activated only transiently in response to hypertonic stress and its activity returns to prestress levels within 20 min after exposure to hypertonic conditions at 30°C (Brewster et al., 1993; Maeda et al., 1995). How is it possible that this transient activation supports continuous proliferation of MEN mutants? We observed that conditions that rescued MEN mutants (1.5 M sorbitol at 37°C) significantly lengthened HOG pathway activation as judged by the persistence of dual T174, Y176 phosphorylation on Hog1 in both wild-type and cdc15-2 strains (Figure 7D). Our results indicate that sustained activation of the HOG pathway allows MEN mutants to proliferate at the restrictive temperature.
Hypertonic Conditions Promote Cdc14 Release from the Nucleolus in MEN Mutants
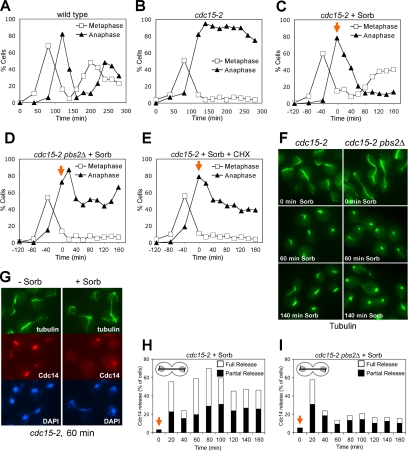
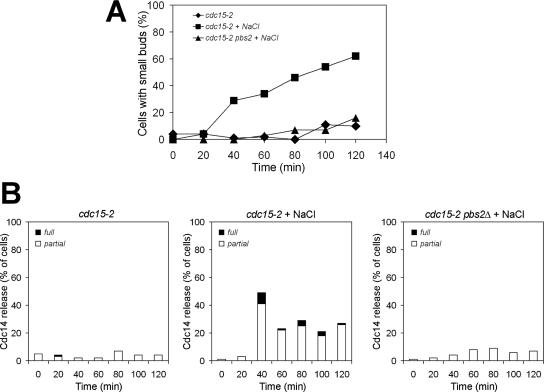
The MEN stimulates exit from mitosis by promoting the dissociation of the protein phosphatase Cdc14 from its inhibitor Cfi1/Net1 in the nucleolus (Shou et al., 1999; Visintin et al., 1999). In the absence of MEN function, cells arrest in anaphase with Cdc14 sequestered in the nucleolus. To determine whether exit from mitosis in MEN mutants brought about by HOG pathway activation was associated with the release of Cdc14 from the nucleolus, we analyzed cell cycle progression in cdc15-2 and cdc15-2 pbs2Δ mutants in detail. Cells were synchronized in G1 and released into the cell cycle at the restrictive temperature. When 80% of cdc15-2 mutants were arrested in anaphase, sorbitol (1.5 M) was added. Cdc14 seemed released from the nucleolus immediately after sorbitol addition in cdc15-2 cells (Figure 3, G and H) and anaphase spindle disassembly occurred (Figure 3, B, C, and F). Cells then entered the next cell cycle as judged by their ability to form new buds and metaphase spindles (Figures 2B and 3, C and F). Cdc14 was also initially released from the nucleolus in 60% of cdc15-2 pbs2Δ cells but was then found resequestered in the nucleolus within 20 min (Figure 3I). Anaphase spindles also disassembled in 40% of cdc15-2 pbs2Δ cells (Figure 3, D and F), but cells never exited mitosis and entered the next cell cycle as judged by their inability to form new buds and metaphase spindles (Figures 2B and 3D). Curiously, after prolonged incubation periods (260-min time point) anaphase spindles seemed to reform in cdc15-2 pbs2Δ cells (Figure 3, E and I). Cdc14 was also released from the nucleolus and exit from mitosis occurred in cdc15-2 mutants treated with NaCl (Figure 4). However, in contrast to sorbitol treatment addition of NaCl did not promote the dissociation of Cdc14 from the nucleolus in cdc15-2 pbs2Δ cells (Figure 4) further indicating that the transient release of Cdc14 from the nucleolus observed in sorbitol treated cdc15-2 pbs2Δ cells is due to a transient disruption of the nucleolus.
Figure 3.
The HOG pathway promotes Cdc14 release from the nucleolus in MEN mutants treated with sorbitol. cdc15-2 (A1674) and cdc15-2 pbs2Δ (A10710) cells were arrested in G1 with α-factor and released from the block at 37°C. The percentage of cells with metaphfase and anaphase spindles (A–E) or Cdc14 release from the nucleolus in anaphase cells (H and I) was scored at the indicated times. Sorbitol (C, D, H, and I) or sorbitol and cycloheximide (1 mg/ml) (E) was added 120 min after release from the G1 arrest (0-time point, indicated by the orange arrow) with the exception of the control strains (A and B). Hyperosmotic stress stimulates the appearance of metaphase spindles (tubulin staining) in cdc15-2, but not in cdc15-2 pbs2Δ mutants (F) and release of Cdc14 from the nucleolus (G) at the restrictive temperature. Note that in contrast to anaphase spindles, metaphase spindles do not seem to be sensitive to sorbitol treatment. Spindle depolymerization upon sorbitol addition did not occur in cdc13-1 (A2598; our unpublished data).
Figure 4.
The HOG pathway promotes Cdc14 release from the nucleolus in MEN mutants treated with NaCl. cdc15-2 (A1674) and cdc15-2 pbs2Δ (A10710) cells were arrested at 37°C for 2 h. When the arrest was complete, the cdc15-2 culture was split, and one-half of the culture was maintained at 37°C and the other half was transferred into medium containing 0.5 M NaCl. The cdc15-2 pbs2Δ culture was transferred to 37°C medium containing 0.5 M NaCl. At the indicated times, samples were taken to determine the percentage of cells with small buds (A) and the release of Cdc14 from the nucleolus (B). The graph on the left in B shows the percentage of cdc15-2 cells with Cdc14 released from the nucleolus in medium lacking NaCl. The graph in the middle shows the percentage of cdc15-2 cells with Cdc14 released from the nucleolus in medium containing NaCl. The graph on the right shows the percentage of cdc15-2 pbs2Δ cells with Cdc14 released from the nucleolus in medium containing NaCl. “Partial release” is defined as some Cdc14 being diffuse throughout the cell, but some enrichment of Cdc14 being detectable in the nucleolus.
Our results indicate that in the presence of sorbitol, release of Cdc14 from the nucleolus is biphasic in cdc15-2 cells with no significant difference between the cdc15-2 and the cdc15-2 pbs2Δ strain during the initial phase of the Cdc14 release (Figure 3, H and I; 140-min time point). However, the second wave of Cdc14 release from the nucleolus depends on the HOG pathway. We believe that the first HOG pathway-independent wave of Cdc14 loss from the nucleolus reflects high osmolarity-induced disturbances of the nucleolar compartment and in consequence redistribution of Cdc14 bound to its inhibitor, rather than the dissociation of Cdc14 from its inhibitor and hence its activation. This conclusion is supported by two findings. First, cdc15-2 pbs2Δ cells do not exit from mitosis (Figure 3E). Second, in agreement with previous findings (Nanduri et al., 2001), we also observed that the nucleolar protein Nop1 (Schimmang et al., 1989) temporarily diffused (for up to 60 min) throughout the cell after sorbitol addition at 37°C (our unpublished data). The disassembly of the mitotic spindle in 40% of cdc15-2 pbs2Δ cells also did not reflect exit from mitosis, as entry into a new cell cycle did not occur (Figure 3, A and E). Instead, spindle disassembly is likely to be caused by a sensitivity of anaphase spindles to mechanical stress imposed by cell volume shrinkage (Reiser et al., 2003) during the initial exposure to hypertonic conditions. Consistent with this idea is the observation that a similar collapse of anaphase spindles occurred in the cdc14-3 mutant, whose proliferation defect was not rescued by a hypertonic environment (our unpublished data). The disassembly of the mitotic spindle in 40% of cdc15-2 pbs2Δ cells also shows that anaphase spindle disassembly cannot be used as a marker for exit from mitosis when examining the effects of osmotic stress on MEN mutants. Rather, the formation of new buds and of metaphase spindles must be used as an indicator as to whether or not exit from mitosis had occurred.
In summary, our results revealed two responses of MEN mutants to sorbitol treatment. A transient HOG pathway-independent response that leads to a temporary disruption of nuclear and nucleolar structures and a sustained, HOG pathway-dependent response that leads to the suppression of the mitotic exit defect of MEN mutants. Our results further indicate that the HOG pathway promotes exit from mitosis by inducing Cdc14 release from the nucleolus. This release likely requires the transcriptional response induced by the HOG pathway, because it did not occur in the presence of the translation inhibitor cycloheximide (Figure 3E) and depended on the chromatin remodeling factors Rpd3 and Sin3, which are necessary for the transcriptional induction of HOG pathway-responsive genes (de Nadal et al., 2004; Figure 7A).
The FEAR Network Is Required for Hypertonic Treatment to Promote Exit from Mitosis in MEN Mutants
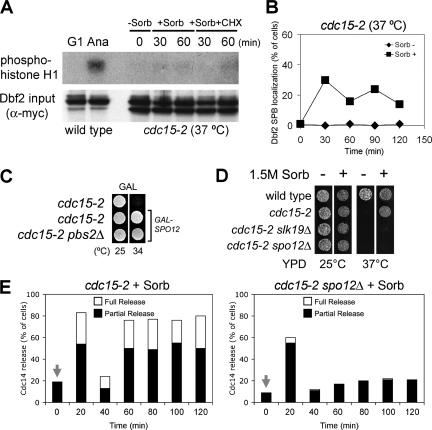
How does activation of the HOG pathway promote Cdc14 release from the nucleolus and exit from mitosis in MEN mutants? We know of two pathways, the mitotic exit and FEAR networks that promote the dissociation of Cdc14 from its inhibitor in the nucleolus during anaphase. To test whether high osmolarity restores signaling to MEN mutants, we first examined the localization and activity of Dbf2 kinase in cdc15-2 mutants treated with sorbitol. DBF2 is the most downstream component of the MEN identified to date. Upon MEN activation Dbf2 associates with spindle pole bodies (SPBs) and becomes active as a kinase (Visintin and Amon, 2001). As reported previously (Visintin and Amon, 2001), Dbf2 did not localize to SPBs, and kinase activity was low in cdc15-2 mutants (Figure 5, A and B). Addition of sorbitol to cdc15-2 mutants allowed Dbf2 to associate with SPBs in 25% of cells (Figure 5B) but did not lead to detectable amounts of Dbf2 kinase activity (Figure 5A). This result together with the finding that the HOG pathway cannot promote exit from mitosis in cells deleted for MEN components suggests that some level of MEN signaling, though too low to detect by in vitro Dbf2 kinase assays, is important for the HOG pathway to promote exit from mitosis.
Figure 5.
The FEAR network is required for the suppression of MEN mutants by high osmolarity. (A) A hypertonic environment does not increase the activity of the MEN kinase Dbf2. cdc15-2 (A2096) cells were arrested at the nonpermissive temperature (0 min), treated with sorbitol (1.5 M) with or without cycloheximide (1 mg/ml), and then assayed for Dbf2 kinase activity at indicated times using histone H1 as a substrate. As a control, Dbf2 kinase activity was measured in wild-type cells (A2747 arrested in G1 [G1] and in wild-type cells that were in anaphase [Ana]). Anaphase cells were obtained by first synchronizing cells in G1 and then releasing cells from the G1 block. Cells were harvested when >90% of cells were in anaphase. (B) cdc15-2 cells carrying a Dbf2-Myc fusion (A2096) were arrested at 37°C for 2 h. When the arrest was complete the culture was split, and one-half of the culture was maintained at 37°C and the other half was transferred into medium containing 1.5 M sorbitol. At the indicated times samples were taken to determine the localization of Dbf2 at SPBs. (C) Cells (from top: A1674, A6895, and A12435) all carrying SPO12 under the control of the GAL1-10 promoter were spotted onto plates containing galactose at either 25 or 37°C. (D) cdc15-2 mutants carrying mutations in FEAR network components fail to grow on plates containing 1.5 M sorbitol. Strains were spotted on plates with or without sorbitol. Strains used (from top) are A1411, A1674, A4168, and A10010. (E) cdc15-2 mutants (A1674) and cdc15-2 spo12Δ mutants (A10408) were arrested in G1 with α-factor and released from the block at 37°C. Sorbitol was added 120 min after release from the G1 arrest (0-time point), and the localization of Cdc14 in anaphase cells was examined.
Next, we tested whether Cdc14 release from the nucleolus and exit from mitosis in MEN mutants brought about by the HOG pathway required the FEAR network. Components of the FEAR network such as Spo12 or Cdc5 can, when overproduced, suppress the temperature-sensitive lethality of MEN mutants (Parkes and Johnston, 1992; Jaspersen et al., 1998; Stegmeier et al., 2002). We found that overexpression of SPO12 suppressed the proliferation defect of cdc15-2 mutants as well as of cdc15-2 pbs2Δ mutants (Figure 5C). Furthermore, cdc15-2 mutants lacking the FEAR network components SPO12 or SLK19 were no longer able to proliferate under hypertonic culture conditions at 37°C (Figure 5D), and Cdc14 release induced by high osmolarity was lost in the cdc15-2 spo12Δ double mutant (Figure 5E). These results indicate that the HOG pathway requires the FEAR network to suppress the mitotic exit defect of MEN mutants. Thus, the HOG pathway either functions upstream of or in parallel to the FEAR network to promote exit from mitosis when the MEN is impaired.
The HOG Pathway Contributes to Exit from Mitosis under Nonstress Conditions
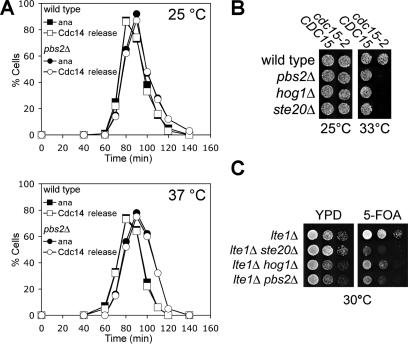
The HOG pathway could promote exit from mitosis only in response to hypertonic conditions or function during every cell cycle under nonstress conditions to promote exit from mitosis. To distinguish between these possibilities, we analyzed the localization of Cdc14 in cells lacking HOG1 or PBS2 during an unperturbed cell cycle. Deletion of either MAP kinase cascade component had no effect on Cdc14 localization either at 25 or 37°C (Figure 6A). However, deletion of the HOG pathway components HOG1, PBS2, or STE20 reduced the restrictive temperature of cdc15-2 mutants (Figure 6B) and led to a synthetic growth defect when combined with a deletion in the MEN activator LTE1 (Figure 6C; Hofken and Schiebel, 2002; note that the growth defect of lte1Δ ste20Δ double mutants is more severe than that of lte1Δ pbs2Δ or lte1Δ hog1Δ mutants, which indicates that STE20 has functions in addition to HOG pathway signaling during exit from mitosis). Our results indicate that the HOG pathway contributes to the regulation of exit from mitosis under nonstress conditions.
Figure 6.
The HOG pathway promotes exit from mitosis under nonstress conditions. (A) Wild-type (A1411) and pbs2Δ (A9610) strains were released from a pheromone-induced G1 arrest and the percentage of cells with anaphase spindles (ana) and Cdc14 released from nucleolus (Cdc14) was determined at the indicated times. (B) Mutations in the HOG pathway enhance the temperature sensitivity of cdc15-2 mutants. Strains were spotted on YPD plates and cultured at the indicated temperatures. Strains used (from the top left, CDC15) are A1674, A9610, A9608, and A11812 (CDC15) and (from the top right, cdc15-2) A1674, A10710, A10707, and A11813 (cdc15-2). (C) Mutations in the HOG pathway enhance the proliferation defect of lte1Δ cells. Serial dilutions of strains with the indicated genotype and carrying an LTE1-URA3 plasmid were spotted on YPD and on plates containing 5′-fluoroorotic acid (5-FOA). Strains used (from the top) are A4101, A6307, A12253, and A12254.
The Sho1 Branch of the HOG Pathway Promotes Exit from Mitosis in MEN Mutants
The HOG pathway consists of two branches, each defined by a transmembrane protein at the top of the pathway. Although inactivation of both the Sln1 and Sho1 branch is necessary to eliminate the response to osmotic stress, it is thought that the two branches of the pathway respond to different signals. Turgor pressure is thought to activate the Sln1 branch (Reiser et al., 2003). An osmotic signal controlling the Sho1 branch of the pathway has not been identified.
We examined the ability of hypertonic conditions to suppress the proliferation defect of cdc15-2 mutants in the absence of components of the two HOG pathway branches. Whereas the cdc15-2 ssk1Δ strain (the SLN1 branch is inactive) displayed a similar proliferation rate as a cdc15-2 strain in the presence of sorbitol at the restrictive temperature (Figure 7A), the cdc15-2 sho1Δ mutant neither exited mitosis in the presence of sorbitol as judged by the formation of small buds (Figure 7B), nor did it form colonies on plates containing sorbitol (Figure 7A). cdc15-2 cells deleted for either STE20 or STE11, both of which encode protein kinases acting in the Sho1 branch of the HOG pathway did not proliferate either (Figure 7A). Furthermore, we found that overexpression of STE20 was able to alleviate the growth defect of cdc15-2 mutants (Figure 7C). This suppression depended on downstream components in the HOG pathway (PBS2, HOG1, RPD3, and SIN3) as well as the FEAR network components SLK19 and SPO12 (Figure 7C). These data indicate that the Sho1 branch of the HOG pathway is responsible for promoting exit from mitosis in MEN mutants. Consistent with this idea was the observation that activation of the SHO1 branch in cdc15-2 mutants in response to hypertonic stress occurred over an extended period, which was in stark contrast to the transient activation of the SLN1 branch (Figure 7D). Our results demonstrate an essential role for the Sho1 branch of the HOG pathway in promoting mitotic exit in MEN mutants after osmotic stress and advocate for a functional diversification between individual signaling branches of the HOG pathway in osmoprotection.
DISCUSSION
Here, we show that hypertonic stress allows mutants defective or compromised in exit from mitosis to traverse this cell cycle transition. Mutants compromised in other cell cycle transitions are not rescued by a hypertonic environment, suggesting a specific effect of high osmolarity on mutants defective in exit from mitosis. The suppression of the mitotic exit defects of MEN mutants by high osmolarity is accompanied by the release of Cdc14 from the nucleolus and is mediated by the HOG pathway, indicating that the HOG pathway brings about exit from mitosis in MEN mutants by promoting the release of Cdc14 from its inhibitor in the nucleolus. Finally, we find that only one of the two branches of the HOG pathway, the Sho1 branch of the pathway, is required for promoting exit from mitosis in MEN mutants, which, to our knowledge, represents the first specific function for the Sho1 branch of the HOG pathway.
The Effects of High Osmolarity and Its MAP Kinase Pathway on Exit from Mitosis
The effects of osmotic stress on exit from mitosis only become apparent in cells with reduced MEN activity, i.e., in temperature-sensitive MEN mutants or cells defective for the MEN activator LTE1. The mitotic exit promoting activities of a hypertonic environment were not observed in an unperturbed cell cycle indicating that high osmolarity does not accelerate exit from mitosis when this cell cycle transition occurs with wild-type kinetics. Does this mean that the suppression of MEN mutants by the HOG pathway is the result of pleiotropic nonspecific effects of HOG pathway stimulation? We think it does not. Our results indicate that an increase in intracellular glycerol levels, which could lead to suppression of temperature-sensitive mutants, was not responsible for the suppression of MEN mutants. Furthermore, it is important to note that it is not trivial to accelerate exit from mitosis in wild-type cells. Only severe overproduction of Cdc5 (Visintin et al., 2003) or of a dominant active form of Cdc15 (Bardin et al., 2003) can accomplish this. This is probably not surprising because multiple layers of control govern this cell cycle transition, and other pathways are likely to prevent the HOG pathway from accelerating exit from mitosis. Only when MEN activity is compromised is the positive regulatory role of the HOG pathway revealed.
Another key question is whether the HOG pathway promotes exit from mitosis only in response to hypertonic conditions or whether it also functions under nonstress conditions to induce exit from mitosis. Although inactivation of HOG1 or PBS2 did not affect exit from mitosis or Cdc14 localization during an unperturbed cell cycle, two observations point to the MAP kinase pathway contributing to promoting exit from mitosis under nonstress conditions. Deletion of HOG pathway components reduces the restrictive temperature of MEN mutants and leads to a synthetic growth defect when combined with a deletion of LTE1. Thus, our results suggest that—although not essential for promoting exit from mitosis—the HOG pathway contributes to exit from mitosis under nonstress conditions, which becomes apparent when MEN activity is compromised.
The HOG Pathway Regulates Cdc14 Localization
The HOG pathway seems to bring about exit from mitosis in MEN mutants by activating Cdc14. The mitotic exit observed in MEN mutants upon transfer of cells to a hypertonic environment is accompanied by the release of Cdc14 from the nucleolus. This release solely depends on the HOG pathway when cells are challenged with NaCl and is biphasic when cells are challenged with sorbitol. In the latter case, we observe that the initial wave of Cdc14 release is independent of the HOG pathway and not capable of promoting exit from mitosis. We therefore think that this release of Cdc14 from the nucleolus does not actually reflect the dissociation of Cdc14 from its inhibitor and thus Cdc14 activation but rather a transient disruption of nucleolar structures due to the sudden changes in osmolarity (Nanduri and Tartakoff, 2001). The second mode by which Cdc14 is released from the nucleolus in response to sorbitol addition depends on the HOG pathway and reflects genuine activation of Cdc14. It is accompanied by exit from mitosis and is followed by entry into the next cell cycle.
Which aspects of HOG pathway signaling are important for its role in releasing Cdc14 from the nucleolus and in promoting exit from mitosis? Suppression of MEN mutants by high osmolarity requires the transcriptional program induced by the HOG pathway. The expression of many genes is modulated upon HOG pathway activation (O’Rourke and Herskowitz, 2004), making the identification of effector(s) that regulate mitotic exit in response to HOG pathway activation difficult. However, clear predictions can be made concerning their characteristics. First, genes that bring about exit from mitosis in MEN mutants ought to be induced (in case they are activators of mitotic exit) or inhibited (in case they are inhibitors of exit from mitosis) by high osmolarity in MEN mutants at 37°C. Second, transcription of this factor(s) should be affected by the deletion of SHO1 but not by deletion of components of the Sln1 branch of the pathway.
The transcriptional response induced by the HOG pathway ultimately impinges on regulators of Cdc14 localization. Our results show that suppression of the MEN mutants by high osmolarity not only requires the HOG pathway but also MEN activity. Although we do not detect stimulation of Dbf2 kinase activity by high osmolarity, sorbitol addition cannot promote exit from mitosis in cells deleted for MEN components. Do these findings then mean that high osmolarity directly affects MEN signaling? Although possible we favor the idea that the HOG pathway functions in parallel to the MEN, through the FEAR network to promote exit from mitosis because exit from mitosis brought about by the HOG pathway depends on the FEAR network. Consistent with this idea is our observation that Cdc5 activity is modestly (2-fold) stimulated by high osmolarity (our unpublished data). Although this twofold increase is not sufficient to bring about exit from mitosis in MEN mutants (our unpublished data), the increase in Cdc5 activity is likely to contribute to the rescue of the proliferation defect brought about by high osmolarity. Finally, we note that it is of course also possible that the HOG pathway stimulates exit from mitosis by a novel yet to be identified pathway that functions in parallel to, both, the FEAR network and the MEN.
An Extracellular Signal Controlling Exit from Mitosis
Several intracellular events, including onset of chromosome segregation and nuclear position, have been described to regulate exit from mitosis through controlling Cdc14 activity (reviewed in D’Amours and Amon, 2004). High osmolarity is, to our knowledge, the first extracellular signal shown to affect this key phosphatase. Why would high osmolarity promote exit from mitosis? Perhaps stress survival is higher in G1 than during mitosis and one function of the HOG pathway could be to push cells that are delayed in anaphase into G1 by promoting the activation of Cdc14 (this study) and stabilization of the CDK inhibitor Sic1 (Escote et al., 2004) to increase their survival chance.
It is also possible that the HOG pathway is involved in coupling exit from mitosis to events occurring at the bud neck. Previous studies showed that Sho1 localizes to the bud neck (Raitt et al., 2000; Reiser et al., 2000), a region of the cell where osmotic stress could occur due to cell wall remodeling and/or contraction of the acto-myosin ring during cytokinesis. We speculate that osmotic imbalances in this region signal through the HOG pathway to the mitotic exit machinery to promote exit from mitosis.
Several studies point to a considerable role for MAP kinase pathways in regulating different cell cycle stages in response to environmental stress (reviewed in Pearce and Humphrey, 2001). Recently, a role for the stress MAP kinase Spc1/Sty1 in mitotic commitment and recovery from stress has been described in fission yeast (Petersen and Hagan, 2005) MKK7, an activator of stress-activated protein kinase stress-activated protein kinase in mammals, has been shown to couple stress signaling to progression into M phase and cellular senescence (Wada et al., 2004). Our data now show that osmotic balance regulates mitotic exit and points to a new mechanism in regulation of the final stages of mitosis by stress-activated MAP kinase signaling cascade.
ACKNOWLEDGMENTS
We thank F. Solomon and members of the Amon laboratory for critical reading of manuscript and for support; and Gustav Ammerer, R. Visintin, and F. Stegmeier for advice and discussions. This research was supported by a National Institutes of Health Grant GM-56800 to A. A., who is also an investigator of the Howard Hughes Medical Institute.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-11-1067) on April 26, 2006.
REFERENCES
- Albertyn J., Hohmann S., Thevelein J. M., Prior B. A. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 1994;14:4135–4144. doi: 10.1128/mcb.14.6.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alepuz P. M., Jovanovic A., Reiser V., Ammerer G. Stress-induced MAP kinase Hog1 is part of transcription activation complexes. Mol. Cell. 2001;7:767–777. doi: 10.1016/s1097-2765(01)00221-0. [DOI] [PubMed] [Google Scholar]
- Alexander M. R., Tyers M., Perret M., Craig B. M., Fang K. S., Gustin M. C. Regulation of cell cycle progression by Swe1p and Hog1p following hypertonic stress. Mol. Biol. Cell. 2001;12:53–62. doi: 10.1091/mbc.12.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin A. J., Boselli M. G., Amon A. Mitotic exit regulation through distinct domains within the protein kinase Cdc15. Mol. Cell Biol. 2003;23:5018–5030. doi: 10.1128/MCB.23.14.5018-5030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- D’Amours D., Amon A. At the interface between signaling and executing anaphase–Cdc14 and the FEAR network. Genes Dev. 2004. 2004;18:2581–2595. doi: 10.1101/gad.1247304. [DOI] [PubMed] [Google Scholar]
- de Nadal E., Casadome L., Posas F. Targeting the MEF2-like transcription factor Smp1 by the stress-activated Hog1 mitogen-activated protein kinase. Mol. Cell Biol. 2003;23:229–237. doi: 10.1128/MCB.23.1.229-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadal E., Zapater M., Alepuz P. M., Sumoy L., Mas G., Posas F. The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes. Nature. 2004;427:370–374. doi: 10.1038/nature02258. [DOI] [PubMed] [Google Scholar]
- Escote X., Zapater M., Clotet J., Posas F. Hog1 mediates cell-cycle arrest in G1 phase by the dual targeting of Sic1. Nat. Cell Biol. 2004;6:997–1002. doi: 10.1038/ncb1174. [DOI] [PubMed] [Google Scholar]
- Grandin N., de Almeida A., Charbonneau M. The Cdc14 phosphatase is functionally associated with the Dbf2 protein kinase in Saccharomyces cerevisiae. Mol. Gen. Genet. 1998;258:104–116. doi: 10.1007/s004380050712. [DOI] [PubMed] [Google Scholar]
- Hofken T., Schiebel E. A role for cell polarity proteins in mitotic exit. EMBO J. 2002;21:4851–4862. doi: 10.1093/emboj/cdf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen S. L., Charles J. F., Tinker-Kulberg R. L., Morgan D. O. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol. Biol. Cell. 1998;9:2803–2817. doi: 10.1091/mbc.9.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P, Pringle J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Maeda T., Takekawa M., Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Nanduri J., Tartakoff A. M. Perturbation of the nucleus: a novel Hog1p-independent, Pkc1p-dependent consequence of hypertonic shock in yeast. Mol. Biol. Cell. 2001;12:1835–1841. doi: 10.1091/mbc.12.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke S. M., Herskowitz I. Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol. Biol. Cell. 2004;15:532–542. doi: 10.1091/mbc.E03-07-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes V., Johnston L. H. SPO12 and SIT4 suppress mutations in DBF2, which encodes a cell cycle protein kinase that is periodically expressed. Nucleic Acids Res. 1992;20:5617–5623. doi: 10.1093/nar/20.21.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce A. K., Humphrey T. C. Integrating stress-response and cell-cycle checkpoint pathways. Trends Cell Biol. 2001;11:426–433. doi: 10.1016/s0962-8924(01)02119-5. [DOI] [PubMed] [Google Scholar]
- Petersen J., Hagan I. M. Polo kinase links the stress pathway to cell cycle control and tip growth in fission yeast. Nature. 2005;435:507–512. doi: 10.1038/nature03590. [DOI] [PubMed] [Google Scholar]
- Posas F., Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Posas F., Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F., Wurgler-Murphy S. M., Maeda T., Witten E. A., Thai T. C., Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Proft M., Struhl K. Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol. Cell. 2002;9:1307–1317. doi: 10.1016/s1097-2765(02)00557-9. [DOI] [PubMed] [Google Scholar]
- Raitt D. C., Posas F., Saito H. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 2000;19:4623–4631. doi: 10.1093/emboj/19.17.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V., Raitt D. C., Saito H. Yeast osmosensor Sln1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J. Cell Biol. 2003;161:1035–1040. doi: 10.1083/jcb.200301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V., Ruis H., Ammerer G. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell. 1999;10:1147–1161. doi: 10.1091/mbc.10.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V., Salah S. M., Ammerer G. Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1 and Cdc42. Nat. Cell Biol. 2000;2:620–627. doi: 10.1038/35023568. [DOI] [PubMed] [Google Scholar]
- Rep M., Reiser V., Gartner U., Thevelein J. M., Hohmann S., Ammerer G., Ruis H. Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell. Biol. 1999;19:5474–5485. doi: 10.1128/mcb.19.8.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T., Tollervey D., Kern H., Frank R., Hurt E. C. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and is essential for viability. EMBO J. 1989;8:4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou W., Seol J. H., Shevchenko A., Baskerville C., Moazed D., Chen Z. W., Jang J., Shevchenko A., Charbonneau H., Deshaies R. J. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell. 1999;97:233–244. doi: 10.1016/s0092-8674(00)80733-3. [DOI] [PubMed] [Google Scholar]
- Stegmeier F., Amon A. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 2004;38:203–232. doi: 10.1146/annurev.genet.38.072902.093051. [DOI] [PubMed] [Google Scholar]
- Stegmeier F., Visintin R., Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–220. doi: 10.1016/s0092-8674(02)00618-9. [DOI] [PubMed] [Google Scholar]
- Visintin R., Amon A. Regulation of the mitotic exit protein kinases Cdc15 and Dbf2. Mol. Biol. Cell. 2001;12:2961–2974. doi: 10.1091/mbc.12.10.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R., Hwang E. S., Amon A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 1999;398:818–823. doi: 10.1038/19775. [DOI] [PubMed] [Google Scholar]
- Visintin R., Stegmeier F., Amon A. The role of the polo kinase Cdc5 in controlling Cdc14 localization. Mol. Biol. Cell. 2003;14:4486–4498. doi: 10.1091/mbc.E03-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T., Joza N., Cheng H. Y., Sasaki T., Kozieradzki I., Bachmaier K., Katada T., Schreiber M., Wagner E. F., Nishina H., Penninger J. M. MKK7 couples stress signalling to G2/M cell-cycle progression and cellular senescence. Nat. Cell Biol. 2004;6:215–226. doi: 10.1038/ncb1098. [DOI] [PubMed] [Google Scholar]