Abstract
Many receptors coupled to the pertussis toxin-sensitive Gi/o proteins stimulate the mitogen-activated protein kinase (MAPK) pathway. The role of the α chains of these G proteins in MAPK activation is poorly understood. We investigated the ability of Gαo to regulate MAPK activity by transient expression of the activated mutant Gαo-Q205L in Chinese hamster ovary cells. Gαo-Q205L was not sufficient to activate MAPK but greatly enhanced the response to the epidermal growth factor (EGF) receptor. This effect was not associated with changes in the state of tyrosine phosphorylation of the EGF receptor. Gαo-Q205L also potentiated MAPK stimulation by activated Ras. In Chinese hamster ovary cells, EGF receptors activate B-Raf but not Raf-1 or A-Raf. We found that expression of activated Gαo stimulated B-Raf activity independently of the activation of the EGF receptor or Ras. Inactivation of protein kinase C and inhibition of phosphatidylinositol-3 kinase abolished both B-Raf activation and EGF receptor-dependent MAPK stimulation by Gαo. Moreover, Gαo-Q205L failed to affect MAPK activation by fibroblast growth factor receptors, which stimulate Raf-1 and A-Raf but not B-Raf activity. These results suggest that Gαo can regulate the MAPK pathway by activating B-Raf through a mechanism that requires a concomitant signal from tyrosine kinase receptors or Ras to efficiently stimulate MAPK activity. Further experiments showed that receptor-mediated activation of Gαo caused a B-Raf response similar to that observed after expression of the mutant subunit. The finding that Gαo induces Ras-independent and protein kinase C- and phosphatidylinositol-3 kinase-dependent activation of B-Raf and conditionally stimulates MAPK activity provides direct evidence for intracellular signals connecting this G protein subunit to the MAPK pathway.
INTRODUCTION
The mitogen-activated protein kinase (MAPK) pathway plays a central role in the stimulation of cell growth by cell surface receptors (Marshall, 1994; Cobb and Goldsmith, 1995). Both tyrosine kinase receptors and G protein-coupled receptors lead to the activation of the serine/threonine kinases known as p44 MAPK and p42 MAPK or extracellular signal-regulated kinases 1 and 2, which phosphorylate and regulate a large array of substrates, including nuclear transcription factors that control genes essential for cell proliferation (Davis, 1993). A well-characterized signaling pathway links tyrosine kinase receptors to MAPK activation. Growth factor-induced tyrosine phosphorylation of these receptors, and the subsequent recruitment of the adaptor molecules Shc and Grb2 bring to the plasma membrane the Sos protein, which acts as a guanine nucleotide exchange factor for Ras (Boguski and McCormick, 1993; Schlessinger, 1994). Ras activation is followed by a kinase cascade in which one or more of the proteins referred as Raf-1, A-Raf, and B-Raf phosphorylate and activate the MAPK/extracellular signal-regulated kinase kinases (MEK), which in turn phosphorylate and activate p44 and p42 MAPK (Marshall, 1994; Cobb and Goldsmith, 1995; Marais and Marshall, 1996; Campbell et al., 1998). G protein-coupled receptors appear to initiate a variety of pathways that mediate Ras-dependent or Ras-independent stimulation of the MAPK cascade (van Biesen et al., 1996b; Gutkind, 1998; Luttrell et al., 1999). These pathways, which involve various G protein subunits and downstream signaling molecules, have been only partially elucidated.
A large group of G protein-coupled receptors induce mitogenic responses by activating members of the pertussis toxin (PTX)-sensitive family of G proteins (Pouyssegur and Seuwen, 1992; van Biesen et al., 1996b). This family includes four G proteins potentially involved in mitogenic signaling pathways: Gi1, Gi2, Gi3, and Go (Neer, 1995). Microinjection of inhibitory antibodies has shown that both Gi2 and Go heterotrimers can exert a positive effect on cell proliferation (LaMorte et al., 1992; Baffy et al., 1994). Evidence for a mitogenic action of the α chains of these G proteins comes from studies using constitutively activated mutants. Transfection of activated Gαi2 causes transformation of Rat-1 fibroblasts and enhances the proliferation of other cell lines (Hermouet et al., 1991; Pace et al., 1991; Gupta et al., 1992). Expression of activated Gαo can induce transformation of NIH 3T3 cells (Kroll et al., 1992). Furthermore, mutations that activate Gαi2 have been identified in a limited subset of human tumors (Lyons et al., 1990). The ability of Gi/o-coupled receptors to activate the MAPK pathway is well documented. A variety of these receptors have been shown to stimulate MAPK activity in fibroblasts and other cell types (van Biesen et al., 1996b; Gutkind, 1998). Several lines of evidence indicate that the Gβγ complex plays an important role in PTX-sensitive MAPK activation (van Biesen et al., 1996b; Gutkind, 1998). Cells expressing free Gβγ heterodimers have revealed that these subunits initiate a phosphatidylinositol-3 kinase (PI3K)- and Src-dependent pathway that leads to tyrosine phosphorylation of Shc, formation of a Shc–Grb2 complex, and Ras-dependent activation of the MAPK cascade (van Biesen et al., 1996b; Gutkind, 1998). Although both Gi2 and Go appear to be involved in the MAPK responses induced by receptors coupled to PTX-sensitive G proteins (Pace et al., 1995; van Biesen et al., 1996a), a role of Gαi2 and Gαo in regulation of the MAPK pathway has not been clearly established. In fact, prolonged expression of activated Gαi2 is accompanied by increased MAPK activity in Rat-1 fibroblasts, but stable or transient transfection of the same subunit in other cell types does not induce MAPK activation (Gallego et al., 1992; van Biesen et al., 1996b; Gutkind, 1998). It has been proposed that in Chinese hamster ovary (CHO) cells the muscarinic M1 receptor, which couples to both Gq and Go, activates a Gαo-dependent pathway leading to MAPK stimulation (van Biesen et al., 1996a). However, direct evidence for regulation of the MAPK pathway by this Gα subunit has not been provided yet.
Here we report that expression of the constitutively activated mutant Gαo-Q205L (Kroll et al., 1992; Wong et al., 1992) in CHO cells is not sufficient to induce MAPK activation but strongly potentiates the stimulatory effects of the epidermal growth factor (EGF) receptor and Ras. We show that Gαo can regulate the activity of the B-Raf kinase through a Ras-independent and protein kinase C (PKC)- and PI3K-dependent mechanism. Our results suggest that B-Raf regulation mediates, at least in part, the action of Gαo on the MAPK pathway.
MATERIALS AND METHODS
Materials
The Gαo antiserum was generated by immunization of rabbits with a synthetic peptide corresponding to the C-terminal sequence ANNLRGCGLY (Baffy et al., 1994). Mouse monoclonal antibodies against phosphotyrosine and human EGF receptor (LA1) were from Upstate Biotechnology (Lake Placid, NY); the anti-hemagglutinin (HA) mouse monoclonal antibody 12CA5 was from Boehringer Mannheim (Mannheim, Germany); the anti-phospho-Akt (Ser-473) mouse monoclonal antibody 4E2 was from New England BioLabs (Beverly, MA); and rabbit polyclonal antibodies against p42 MAPK (C-14), Raf-1 (C-20), A-Raf (C-20), B-Raf (C-19), and human EGF receptor (1005) were from Santa Cruz Biotechnology (Santa Cruz, CA). ATP, myelin basic protein (MBP), phorbol 12-myristate 13-acetate (PMA), LY 294002, and dopamine were purchased from Sigma (St. Louis, MO). Human EGF was from Boehringer Mannheim; wortmannin, GF 109203X, and human fibroblast growth factor (FGF) were from Calbiochem (La Jolla, CA), and PTX was from List Biological Laboratories (Campbell, CA). Recombinant glutathione S-transferase (GST)-p42 MAPK and GST-MEK1 were from Upstate Biotechnology; protein A-Sepharose CL-4B and GammaBind G-Sepharose were from Amersham Pharmacia Biotech (Uppsala, Sweden); and [γ-32P]ATP was from DuPont New England Nuclear (Boston, MA). All other chemicals were reagent grade.
DNA Constructs
The cDNAs encoding wild-type Gα, Gβ, and Gγ subunits, constitutively activated Gα chains (Gαo-Q205L, Gαi2-Q205L, Gαi3-Q204L, and Gαq-R183C) (Conklin et al., 1992; Wong et al., 1992), and the human dopaminergic D2L receptor (Wong et al., 1992) were in the pcDNAI expression vector. L. Beguinot (Scientific Institute San Raffaele, Milan, Italy) provided the pCO11 plasmid containing humam EGF receptor cDNA. HA-p44 MAPK (Meloche et al., 1992a) in pcDNAI and pRSV vectors containing cDNAs for the activated mutant Ras-Q61L and the dominant negative mutant N17-Ras were gifts from E.P. Sturani and R. Zippel (University of Milan, Milan, Italy).
Cell Culture, DNA Transfection, and Preparation of Lysates
CHO cells were maintained in culture in Dulbecco's modified Eagle's medium-Ham's F-12 (1:1) supplemented with 10% FCS. Transient transfections were performed using the Transfectam reagent (Promega, Madison, WI). Cells were grown to 60–70% confluence in 6- or 10-cm dishes, washed with serum-free medium, and incubated in the same medium (1.5 ml per 6-cm dish and 4 ml per 10-cm dish) containing DNA:Transfectam (1 μg:2 μl) complexes. In all experiments, the total amount of transfected DNA was kept constant by addition of empty vector. After 5 h at 37°C, dishes were washed and incubated in 10% FCS medium for 24 h. Transfection efficiencies were determined by transfection of a plasmid encoding a mutant green fluorescent protein (pEGFP-N1; Clontech, Cambridge, United Kingdom). Expression was detected by fluorescence microscopy and was consistently observed in ∼35% of the transfected cells. For the various assays, cells were serum starved for 18 h in medium containing 0.5% FCS and, where indicated, pretreated with the appropriate agents and/or incubated in serum-free medium in the absence or presence of EGF or dopamine. The cells were then washed once with ice-cold Ca2+- and Mg2+-free PBS and incubated on ice for 20 min with the lysis buffer specified for each assay (0.6 ml per 6-cm dish and 1 ml per 10-cm dish). Cell lysates were cleared by centrifugation at 13,000 rpm for 15 min at 4°C.
Measurement of MAPK Activity
The activities of endogenous p42 MAPK and HA-p44 MAPK were determined by an immune complex kinase assay. Cells in 6-cm dishes were lysed in buffer containing 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM EDTA, 40 mM Na4P2O7, 200 μM Na3VO4, 1% Triton X-100, 1 mM dithiothreitol, 1 mM PMSF, and 10 μg/ml chymostatin, leupeptin, antipain, and pepstatin (CLAP). Lysates were incubated at 4°C with 1 μg of anti-p42 MAPK antibodies for 1 h or with 3 μg of anti-HA antibodies for 2 h. Immune complexes were collected by incubation with 50 μl of protein A-Sepharose (50% slurry) for 1 h at 4°C, centrifuged (2 min at 3000 rpm at 4°C), and washed three times in lysis buffer and twice in kinase buffer (40 mM HEPES-NaOH, pH 7.5, 10 mM MgCl2, 1 mM EGTA, 200 μM Na3VO4, 2 mM dithiothreitol, 1 mM PMSF, 10 μg/ml CLAP, and 3 mM benzamidine). The final pellets were resuspendend in 50 μl of kinase buffer supplemented with 250 μg/ml MBP as a substrate. The kinase reaction was started by addition of 5 μl of 500 μM ATP containing 4 μCi of [γ-32P]ATP, incubated at 30°C for 30 min, and stopped with 5 μl of 88% formic acid. After a brief centrifugation, 35 μl of the supernatant fraction were spotted on squares of Whatman (Maidstone, United Kingdom) P81 paper. Free [γ-32P]ATP was eluted by four washes in 150 mM phosphoric acid, and the amount of radioactivity incorporated into MBP was measured by scintillation counting. Blank kinase reactions, carried out after incubation of antibodies with lysis buffer and protein A-Sepharose, were always performed. The radioactivity of these reactions (∼500 cpm) was subtracted from the result of each sample.
Measurement of Raf Activity
The ability of Raf-1, A-Raf, and B-Raf to activate MEK was measured by an immune complex “coupled” assay in which recombinant GST-MEK1 activates recombinant GST-p42 MAPK (Alessi et al., 1995). Cells in 6- or 10-cm dishes were lysed in buffer containing 20 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 10 mM EGTA, 50 mM NaF, 40 mM Na4P2O7, 200 μM Na3VO4, 1% Triton X-100, 20 mM N-octyl-β-d-glucopyranoside, 1 mM PMSF, 10 μg/ml CLAP, and 20 μg/ml aprotinin. Lysates were incubated with antibodies against Raf-1, A-Raf, or B-Raf (1.5 μg/ml) for 2 h at 4°C. Immune complexes were collected with protein A-Sepharose (50 μl of 50% slurry) for 1 h at 4°C, centrifuged, and washed three times in lysis buffer without PMSF and twice in washing buffer (50 mM Tris-HCl, pH 7.5, 0.1 mM EGTA, 0.5 mM Na3VO4, and 0.1% 2-mercaptoethanol). For the first step of the kinase reaction, pellets were resuspended in 30 μl of kinase buffer (30 mM Tris-HCl, pH 7.5, 10 mM magnesium acetate, 0.1 mM EGTA, 0.1% 2-mercaptoethanol, 0.03% Brij 35, 20 mM N-octyl-β-d-glucopyranoside, and 200 μM ATP) supplemented with 3 μg/ml GST-MEK1 and 30 μg/ml GST-p42 MAPK. After 30 min at 30°C, 15 μl of the supernatant were mixed with 15 μl of washing buffer containing 1 mg/ml BSA. Activation of GST-p42 MAPK was assayed by incubating 10 μl of this mixture with 40 μl of kinase buffer (50 mM Tris-HCl, pH 7.4, 12.5 mM magnesium acetate, 0.1 mM EGTA, 50 μM ATP, and 2 μCi [γ-32P]ATP) supplemented with 400 μg/ml MBP. The reaction (15 min at 30°C) was terminated by spotting 40 μl on squares of Whatman P81 paper, and radioactivity incorporated into MBP was determined as described for p42 MAPK and HA-p44 MAPK assays. The values of blank reactions performed after incubation of antibodies with lysis buffer and protein A-Sepharose (∼4000 cpm) were subtracted from the result of each sample. Preliminary experiments showed that no activity was associated with protein A-Sepharose incubated with lysates in the absence of antibodies. The activity detected in reactions performed without GST-MEK1 was ∼15% (for Raf-1 and A-Raf) and <0.01% (for B-Raf) of that observed in the presence of the recombinant kinase. The presence of comparable amounts of Raf in the immunoprecipitates used in the kinase assay was verified by immunoblot analysis of the protein A-Sepharose pellets as described below.
Analysis of Protein Expression and Tyrosine Phosphorylation
Expression and tyrosine phosphorylation of transfected EGF receptors were evaluated by immunoprecipitation and subsequent immunoblotting. Cells in 10-cm dishes were lysed in buffer containing 20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM EDTA, 40 mM Na4P2O7, 1 mM Na3VO4, 1% Triton X-100, 10% glycerol, 1 mM PMSF, and 10 μg/ml CLAP. Lysates were precleared with protein G-Sepharose (40 μl of 50% slurry) for 45 min at 4°C and incubated with 5 μg of anti-EGF receptor LA1 antibody overnight at 4°C. Immune complexes were collected with protein G-Sepharose (60 μl of 50% slurry) for 2 h at 4°C, centrifuged, and washed three times in lysis buffer. Two equal parts of each immunoprecipitate were separated by 7.5% SDS-PAGE and transferred to nitrocellulose filters (Schleicher & Schuell, Keene, NH). For detection of the EGF receptor, filters were blocked for 1 h in Blotto (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20, 0.01% sodium azide, and 5% nonfat dry milk), incubated overnight at 4°C in Blotto containing 0.4 μg/ml anti-EGF receptor 1005 antibody, incubated for 2 h in Blotto with horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA), and finally processed for enhanced chemiluminescence (Amersham Pharmacia Biotech). For analysis of tyrosine phosphorylation, filters were kept for 2 h in BSA blocking solution (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.02% sodium azide, and 5% BSA) and incubated in the same solution with anti-phosphotyrosine antibodies (1 μg/ml) overnight at 4°C. Detection was performed with 125I-labeled secondary antibodies (Amersham Pharmacia Biotech). Preliminary experiments showed that both the LA1 and 1005 antibodies used in these studies recognize equally well the nonphosphorylated and phosphorylated forms of the EGF receptor. For detection of Akt phosphorylation, cells were lysed in buffer containing 20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM EDTA, 20 mM NaF, 40 mM Na4P2O7, 1 mM Na3VO4, 1% Triton X-100, 10% glycerol, 1 mM PMSF, 10 μg/ml CLAP, and 20 μg/ml aprotinin. Lysates (150 μg of protein) were separated by 7.5% SDS-PAGE and immunoblotted using anti-phospho-Akt antibodies. The expression of transfected Gαo and HA-p44 MAPK was assessed by immunoblotting of cell lysates (100 μg of protein) prepared as described for MAPK assays and separated by 10% SDS PAGE. Immunoblot analysis of Raf proteins was performed on immunoprecipitates prepared as described for kinase assays and resolved by 7.5% SDS-PAGE. Filters were incubated with Gαo antiserum (1:200), anti-HA antibody (2.5 μg/ml), anti-phospho-Akt antibody (0.5 μg/ml), or anti-Raf-1, A-Raf, and B-Raf antibodies (0.3 μg/ml) overnight at 4°C in Blotto. The Gαo antiserum was detected with 125I-labeled protein A (Amersham Pharmacia Biotech), and all other antibodies were detected with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence.
RESULTS
Activated Gαo Potentiates EGF Receptor-dependent MAPK Stimulation
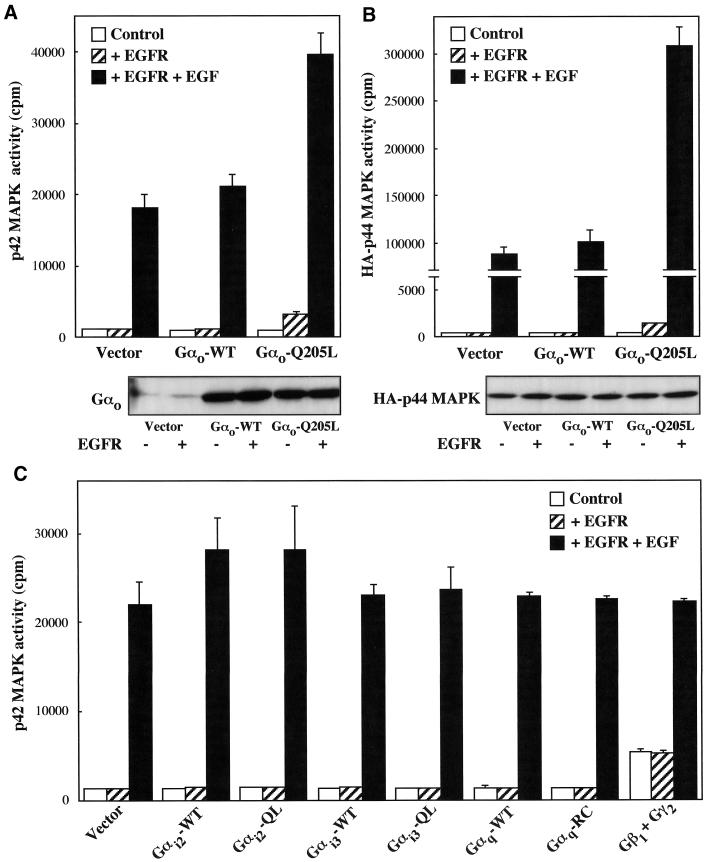
Transient expression of wild-type or mutationally activated Gαo in CHO cells did not lead to a significant activation of endogenous p42 MAPK (Figure 1A) or cotransfected HA-p44 MAPK (Figure 1B). Previous studies have detected stimulation of the MAPK pathway by certain PTX-insensitive Gα chains only in the presence of growth factors acting through tyrosine kinase receptors (De Vivo and Iyengar, 1994; Voyno-Yasenetskaya et al., 1994). The responses induced by various receptors coupled to Gi/o proteins suggest that specific subunits of these heterotrimers can also mediate signals that potentiate tyrosine kinase receptor-dependent MAPK activation (Meloche et al., 1992b; Vouret-Craviari et al., 1993; Fujitani and Bertrand, 1997). We therefore investigated the ability of Gαo to stimulate endogenous p42 MAPK in CHO cells transiently expressing the EGF receptor. In nontransfected CHO cells, EGF had no significant effect on p42 MAPK activity (see legend to Figure 1). In the presence of EGF receptors, basal p42 MAPK activity was not significantly different from that detected in control cells and increased ∼15-fold upon stimulation with 100 ng/ml EGF for 5 min (Figure 1A). Coexpression of Gαo-Q205L induced a modest elevation of unstimulated p42 MAPK activity and a twofold enhancement of the response induced by EGF. In contrast, no significant changes in the p42 MAPK response to the EGF receptor were observed in cells coexpressing wild-type Gαo. These results could not be accounted for by variable levels of expression of the Gαo subunit (Figure 1A) or the EGF receptor (see Figure 3) in cells transfected with different cDNA combinations. When coexpressed with the EGF receptor, activated Gαo significantly increased unstimulated activity and greatly amplified the effect of EGF also in HA-p44 MAPK assays (Figure 1B). These responses were not accompanied by detectable variations of HA-p44 MAPK expression. As shown in Figure 1C, measurements of p42 MAPK activity revealed that the stimulatory effect of Gαo-Q205L on the MAPK pathway was not mimicked by mutationally activated Gαi2, Gαi3, or Gαq subunits. Expression of Gβ1γ2 chains induced per se a fourfold activation of p42 MAPK, as expected from previous results obtained in CHO cells and other systems (Hawes et al., 1995; van Biesen et al., 1996b; Gutkind, 1998), but failed to potentiate the response to the EGF receptor (Figure 1C). Similar results were obtained when the effects of activated Gαi2, Gαi3, and Gαq subunits and Gβ1γ2 complexes were tested in HA-p44 MAPK assays (our unpublished results).
Figure 1.
Effect of activated Gαo on MAPK activity. CHO cells transfected in 6-cm dishes were placed for 18 h in 0.5% FCS medium and incubated for 5 min in the absence or presence of 100 ng/ml EGF. MAPK activity was determined as described in MATERIALS AND METHODS. (A) Endogenous p42 MAPK activity in cells transfected with 1.8 μg of Gαo-wild-type (WT) or Gαo-Q205L and, where indicated, 1.8 μg of EGF receptor (EGFR). The data represent means ± SE of two to four independent experiments performed in triplicate. (B) HA-p44 MAPK activity in cells transfected with 1.4 μg of HA-p44 MAPK, 1.4 μg of Gαo-WT or Gαo-Q205L and, where indicated, 1.4 μg of EGFR. The data are means ± SE of two independent experiments performed in duplicate. (C) Endogenous p42 MAPK activity in cells transfected with 1.8 μg of wild-type (WT) or activated (QL and RC) Gαi2, Gαi3, and Gαq subunits, 1.4 μg of Gβ1 and Gγ2 chains, and, where indicated, 1.8 μg of EGFR. Means ± SE from two independent experiments performed in duplicate are given. When nontransfected CHO cells were incubated with 100 ng/ml EGF for 5 min, the values of endogenous p42 MAPK activity in the absence and presence of the growth factor were 4097 ± 429 and 4278 ± 620 cpm, respectively (n = 4). Immunoblots show the expression of wild-type or activated Gαo (A) and HA-p44 MAPK (B) in cells transfected with different cDNAs. Similar results were obtained in two additional experiments.
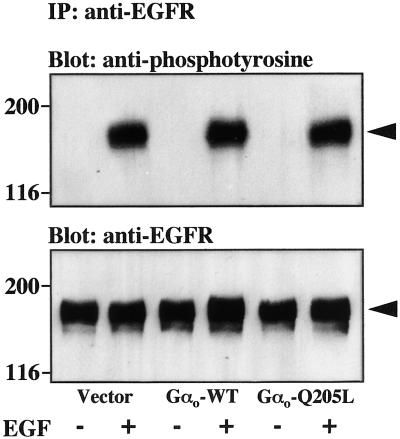
Figure 3.
Effect of activated Gαo on EGF receptor tyrosine phosphorylation. CHO cells in 10-cm dishes were transfected with 5 μg of EGFR and 5 μg of empty vector, Gαo-WT, or Gαo-Q205L, serum starved for 18 h, and stimulated with 100 ng/ml EGF for 5 min. Immunoprecipitation (IP) with anti-EGFR antibodies and immunoblot analysis of the immunoprecipitates with anti-phosphotyrosine or anti-EGFR antibodies were performed as described in MATERIALS AND METHODS. The molecular masses (kilodaltons) of marker proteins are shown to the left, and the position of the EGFR is indicated by arrows. Data are representative of three similar experiments.
Regulation of the MAPK Pathway by Gαo Involves PKC and PI3K
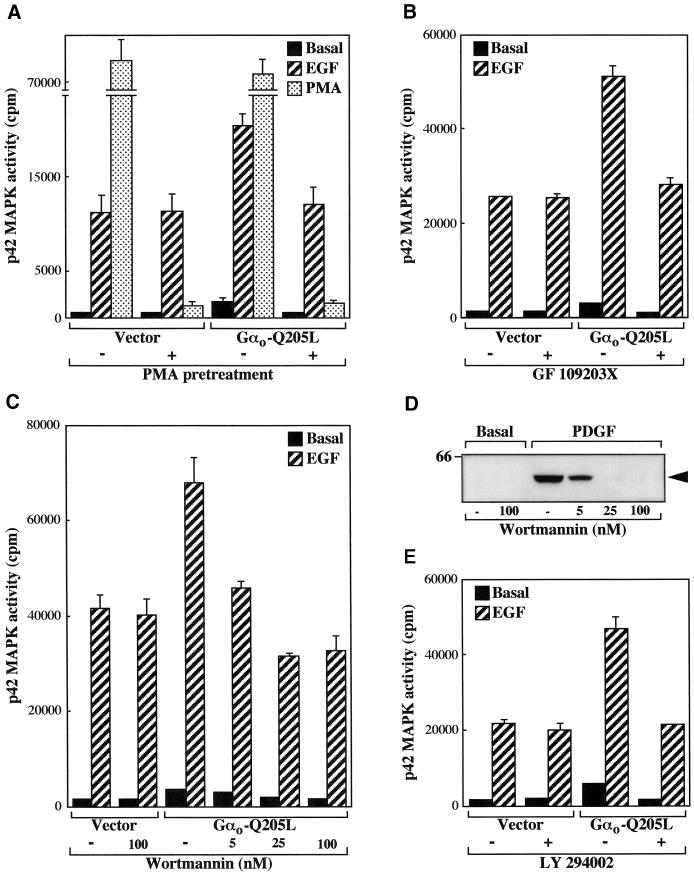
It has been reported that MAPK activation by some receptors capable of coupling to the Go protein is dependent on PKC activity (van Biesen et al., 1996a; Wylie et al., 1999). We therefore investigated the role of PKC in the regulation of the MAPK pathway mediated by Gαo. Cells transfected with the EGF receptor in the absence or presence of Gαo-Q205L were incubated with PMA for 18 h to down-regulate the expression of phorbol ester-sensitive PKC isozymes. As shown in Figure 2A, this procedure abolished the stimulation of p42 MAPK activity induced by short exposure to PMA. PKC down-regulation did not affect the p42 MAPK response to EGF in cells transfected with the EGF receptor but completely inhibited the enhancement of the stimulatory effect of the growth factor observed after coexpression of Gαo-Q205L. Pretreatment with PMA also prevented the increase in unstimulated p42 MAPK activity observed in cells expressing activated Gαo. Entirely comparable results were obtained using the PKC inhibitor GF 109203X (Toullec et al., 1991) (Figure 2B).
Figure 2.
MAPK stimulation by activated Gαo involves PKC and PI3K. (A–C and E) CHO cells in 6-cm dishes were transfected with 1.8 μg of EGFR and 1.8 μg of either empty vector or Gαo-Q205L and assayed for endogenous p42 MAPK activity as described in the legend to Figure 1. (A) Effect of PMA pretreatment on MAPK activity. Cells were incubated for 18 h in 0.5% FCS medium containing 1 μM PMA before stimulation with 100 ng/ml EGF or 1 μM PMA for 5 min. The data represent means ± SE of two independent experiments performed in duplicate. (B, C, and E) Effects of PKC or PI3K inhibitors on MAPK activity. Cells were serum starved for 18 h and incubated for 15 min with 1 μM GF 109203X (B), the indicated concentrations of wortmannin (C), or 20 μM LY 294002 (E) before stimulation with 100 ng/ml EGF for 5 min. The data are means ± SE of four to nine observations. Additional assays showed that GF 109203X completely abolished the increase in p42 MAPK activity induced by short exposure to PMA. (D) Effect of wortmannin on Akt phosphorylation. Nontransfected CHO cells grown in 6-cm dishes were serum starved for 18 h, incubated with the indicated concentrations of wortmannin for 15 min, and stimulated with 20 ng/ml PDGF for 10 min. Phosphorylated Akt was detected by immunoblot analysis of cell lysates with specific antibodies as described in MATERIALS AND METHODS. The molecular mass (kilodaltons) of a marker protein is shown to the left, and the position of phosphorylated Akt is indicated by an arrow. Data are representative of two similar experiments.
Activation of PI3K has been identified as a major signaling pathway leading to MAPK stimulation (Toker and Cantley, 1997). Upon activation of Gi/o-coupled receptors, PI3K is believed to mediate stimulation of MAPK activity by the G protein βγ subunits (van Biesen et al., 1996b; Gutkind, 1998). To determine whether the action of Gαo on MAPK also involves any of the PI3K isoforms, we used the inhibitors wortmannin (Ui et al., 1995) and LY 294002 (Vlahos et al., 1994). As shown in Figure 2C and previously described in other systems (Wennstrom and Downward, 1999), no significant effect of 100 nM wortmannin was detected in cells transfected with the EGF receptor. In contrast, a concentration of wortmannin as low as 5 nM efficiently reduced the high p42 MAPK activity displayed by cells coexpressing Gαo-Q205L. Complete inhibition was achieved in the presence of 25 nM wortmannin. We compared this effect with the inhibition of Akt phosphorylation, a well-established PI3K-dependent event (Toker and Cantley, 1997). In our experiments, endogenous platelet-derived growth factor (PDGF) receptors, but not transfected EGF receptors or endogenous FGF receptors (our unpublished results), induced a detectable phosphorylation of Akt. As shown in Figure 2D, the concentrations of wortmannin that inhibited Gαo-mediated p42 MAPK activation also blocked Akt phosphorylation by PDGF. As shown in Figure 2E, LY 294002 mimicked the effect of wortmannin on p42 MAPK activity. Taken together, these results indicate that the Gαo subunit potentiates MAPK activation by the EGF receptor through a pathway that involves both PKC and PI3K.
Gαo Potentiates Ras Activation of the MAPK Pathway
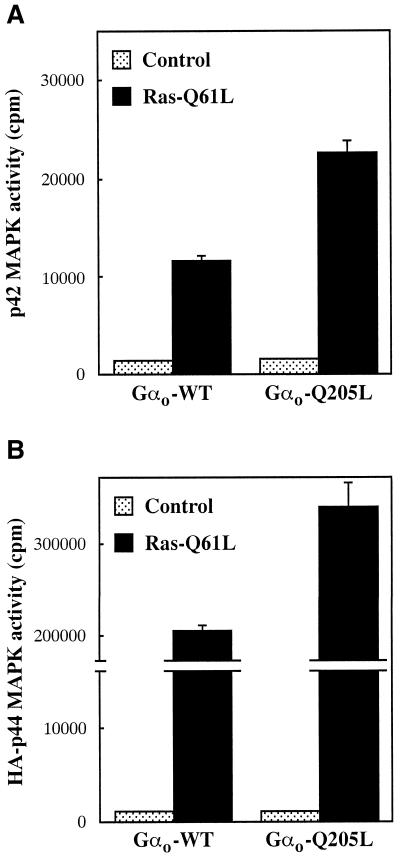
The effect of Gαo on MAPK activity implies that this subunit is able to modulate the function of at least one component of the signaling pathway connecting the EGF receptor to MAPK. We first checked whether, under the conditions used in MAPK assays, Gαo affects the state of tyrosine phosphorylation of the EGF receptor itself. As shown in Figure 3, immunoprecipitation of lysates from transfected cells with anti-EGF receptor antibodies followed by immunoblot analysis with anti-phoshotyrosine antibodies failed to reveal a significant effect of Gαo-Q205L on basal or EGF-induced tyrosine phosphorylation of the EGF receptor. To identify possible sites of action of Gαo downstream of the EGF receptor, we asked whether Gαo-Q205L can potentiate the stimulation of the MAPK pathway induced by constitutively activated Ras. In control cells, expression of activated Ras increased the activity of endogenous p42 MAPK (Figure 4A) and cotransfected HA-p44 MAPK (Figure 4B) ∼8- and 200-fold, respectively. The responses of both forms of MAPK to Ras were markedly enhanced by coexpression of Gαo-Q205L. These results indicate that Gαo potentiates EGF receptor-dependent activation of the MAPK pathway at the level or downstream of Ras.
Figure 4.
Effect of activated Gαo on Ras-stimulated MAPK activity. CHO cells transfected in 6-cm dishes were serum starved for 18 h and assayed for MAPK activity as described in the legend to Figure 1. (A) Endogenous p42 MAPK activity in cells transfected with Gαo-WT or Gαo-Q205L (1.8 μg) and either empty vector or Ras-Q61L (2.2 μg). (B) HA-p44 MAPK activity in cells transfected with HA-p44 MAPK (1.4 μg), Gαo-WT or Gαo-Q205L (1.4 μg), and either empty vector or Ras-Q61L (1.4 μg). Data represent means ± SE of two separate experiments performed in duplicate. Expression of Ras-Q61L in the absence of exogenous Gαo gave results similar to those obtained in cells transfected with the wild-type subunit.
Gαo Activates B-Raf by a Ras-independent and PKC- and PI3K-dependent Mechanism
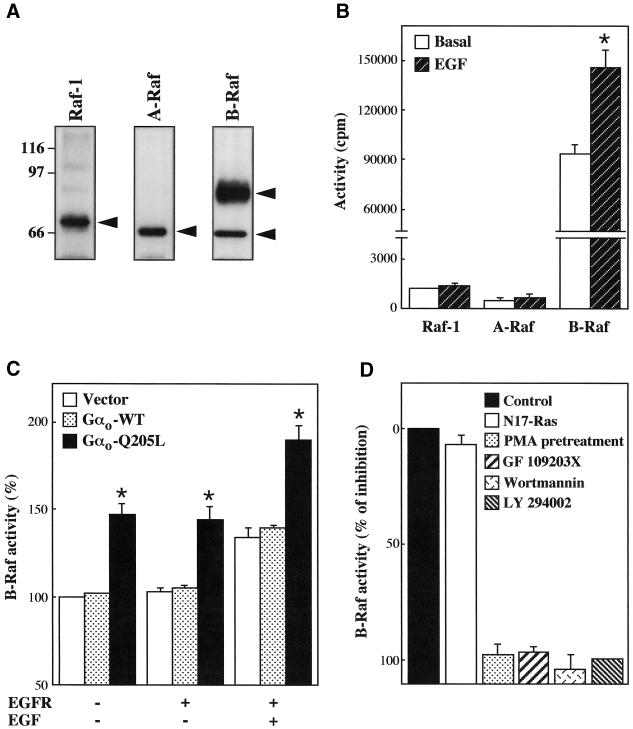
The components of the MAPK cascade located immediately downstream of Ras are the Raf kinases, Raf-1, A-Raf, and B-Raf. The central role of Ras in activation of these kinases is well established (Marais and Marshall, 1996; Campbell et al., 1998). However, increasing evidence indicates that additional signals converge on the Raf proteins and, at least in some cases, cooperate with Ras in regulating their function (Marais and Marshall, 1996; van Biesen et al., 1996b; Marais et al., 1997; Vossler et al., 1997; Campbell et al., 1998; York et al., 1998). We therefore asked whether Gαo can affect EGF receptor-dependent activation of the Raf kinases. As shown in Figure 5A, CHO cells were found to express Raf-1, A-Raf, and two B-Raf isoforms migrating with approximate molecular masses of 92 and 66 kDa. We first determined which of these kinases are activated after stimulation of EGF receptors. The activity of each type of Raf was measured by a coupled assay in which the kinase immunoprecipitated from cell lysates is used to activate sequentially recombinant forms of MEK and MAPK. As shown in Figure 5B, CHO cells transfected with the EGF receptor displayed no activation of Raf-1 and A-Raf but an ∼40% increase in B-Raf activity upon exposure to EGF. It should be noted that in these assays the basal activity of B-Raf was much higher than those of Raf-1 and A-Raf. This finding most probably reflects not only different levels of expression of the various Raf proteins in CHO cells but also the elevated rate of basal activity of B-Raf, which has been demonstrated by previous work (Marais et al., 1997). However, the lack of effect of EGF on Raf-1 and A-Raf could not be explained by a failure to detect increases in the relatively low activities of these kinases, because our assays easily revealed stimulation of Raf-1 by transfected dopaminergic D2 receptors (our unpublished results) and stimulation of both Raf-1 and A-Raf by endogenous FGF receptors (Figure 6A). We next examined the responses of the various Raf kinases to Gαo. The experiments presented in Figure 5C showed that activated but not wild-type Gαo was able to stimulate B-Raf activity. Parallel measurements of the activities of Raf-1 and A-Raf revealed no significant effect of Gαo-Q205L on these kinases (our unpublished results). In sharp contrast with the results obtained in MAPK assays, B-Raf activation by Gαo was completely independent of the EGF receptor. Gαo-Q205L caused an ∼50% increase in basal B-Raf activity both in the absence and in the presence of EGF receptors and enhanced the stimulation induced by EGF in a simply additive manner.
Figure 5.
Activated Gαo stimulates B-Raf activity. (A) Expression of Raf proteins in CHO cells. Raf-1, A-Raf, and B-Raf were immunoprecipitated from lysates of nontransfected cells and detected by immunoblotting with specific antibodies as described in MATERIALS AND METHODS. The molecular masses (kilodaltons) of marker proteins are shown to the left, and the positions of Raf-1, A-Raf, and B-Raf migration are indicated by arrows. (B–D) CHO cells transfected in 10-cm dishes were serum starved for 18 h and assayed for endogenous Raf-1, A-Raf, and B-Raf activities as described in MATERIALS AND METHODS. (B) Effect of EGFR stimulation on Raf activity. Cells were transfected with 5 μg of EGFR and incubated for 5 min in the absence or presence of 100 ng/ml EGF. Data represent means ± SE of two to three independent experiments performed in triplicate. *, Significantly higher than basal (p < 0.01 by unpaired t test). In similar experiments carried out with nontransfected CHO cells, basal B-Raf activity (106,370 ± 7071 cpm) was not significantly increased by EGF (102,468 ± 5748 cpm) (n = 4). (C) Effect of activated Gαo on B-Raf activity. Cells transfected with 5 μg of empty vector, Gαo-WT or Gαo-Q205L and, where indicated, 5 μg of EGFR were incubated for 5 min in the absence or presence of 100 ng/ml EGF. Values are means ± SE of three independent experiments performed in triplicate. EGF induced a significant increase in B-Raf activity in all transfected cells (p < 0.01 by unpaired t test). *, Significantly higher than values from cells transfected with vector or Gαo-WT (p < 0.01 by unpaired t test). (D) Effects of N17-Ras expression, PMA pretreatment, and PKC or PI3K inhibitors on Gαo-mediated B-Raf activation. Cells were transfected with 4 μg of empty vector or Gαo-Q205L, and, where indicated, 8 μg of N17-Ras. PMA (1 μM), wortmannin (25 nM), GF 109203X (1 μM), and LY 294002 (20 μM) were used as described in the legend to Figure 2. Results are expressed as percentages of inhibition of the increase induced by Gαo-Q205L and represent the mean ± SE of two experiments in duplicate determinations. Expression of N17-Ras, PMA pretreatment, and the various inhibitors did not modify B-Raf activity in cells transfected with vector. Additional assays showed that both PMA pretreatment and GF 109203X completely abolished the increase in B-Raf activity induced by 5-min stimulation with 1 μM PMA (∼50%).
Figure 6.
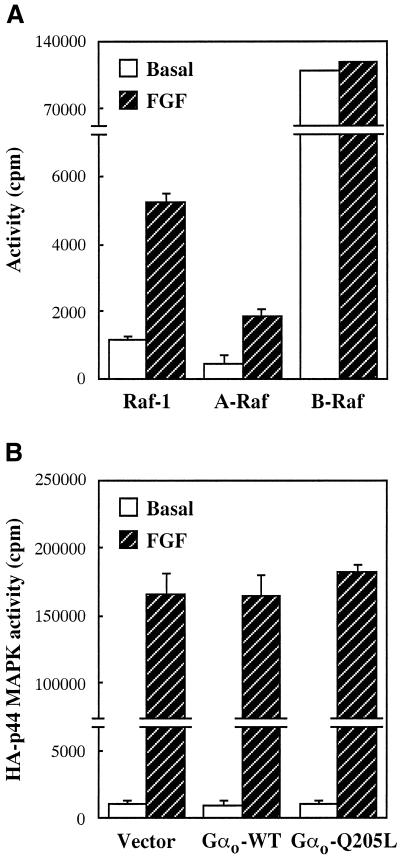
Activated Gαo does not affect MAPK stimulation by FGF receptors. (A) Effect of FGF on Raf activity in nontransfected CHO cells grown in 10-cm dishes. (B) Effect of Gαo on FGF-stimulated MAPK activity in CHO cells transfected in 6-cm dishes with 1.4 μg of HA-p44 MAPK and 1.4 μg of empty vector, Gαo-WT, or Gαo-Q205L. Cells were serum starved for 18 h before stimulation with 40 ng/ml FGF for 5 min. Raf-1, A-Raf, B-Raf, and HA-p44 MAPK activities were determined as described in the legends to Figures 1 and 5. Data are means ± SE for duplicate samples from two independent experiments.
As shown in Figure 5D, the activation of B-Raf induced by Gαo-Q205L was not inhibited when endogenous Ras function was blocked by coexpression of the dominant negative mutant N17-Ras. In contrast, prolonged PMA pretreatment and exposure to GF 109203X, wortmannin, and LY 294002, which prevent the ability of Gαo-Q205L to potentiate EGF receptor-dependent MAPK stimulation, also abolished the effect of the mutant subunit on B-Raf (Figure 5D). Taken altogether, our findings indicate that Gαo regulates the activity of B-Raf by a Ras-independent and PKC- and PI3K-dependent mechanism that enables the kinase to activate MEK and MAPK in in vitro assays but not in intact cells. The simplest hypothesis suggested by the data is that Gαo-activated B-Raf can efficiently couple to the downstream MAPK cascade when concomitantly reached by a regulatory signal provided by the EGF receptor or Ras. This model predicts that activated Gαo should fail to potentiate the stimulation of MAPK induced by B-Raf-independent pathways. We therefore tested the effect of Gαo-Q205L on the MAPK response to endogenous FGF receptors, which stimulate Raf-1 and A-Raf but not B-Raf, activity (Figure 6A). As expected, expression of Gαo-Q205L failed to affect activation of cotransfected HA-p44 MAPK by FGF (Figure 6B).
Activation of B-Raf by Receptors Coupled to Go
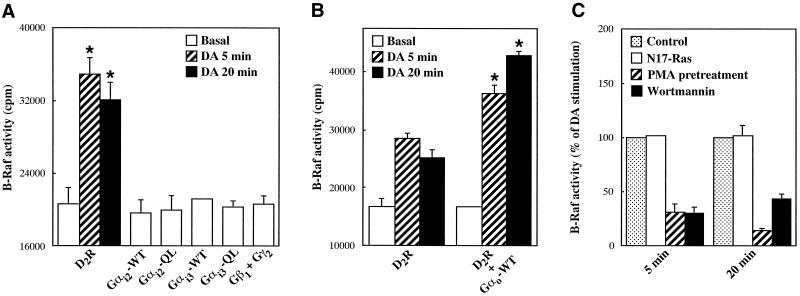
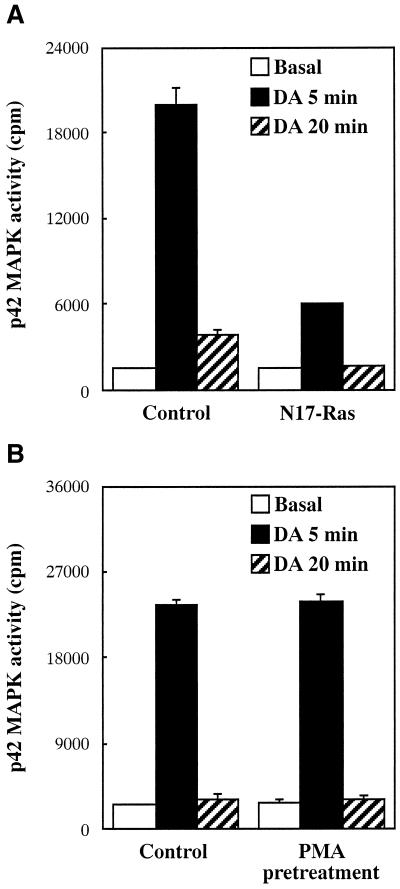
To establish whether receptor-mediated activation of Gαo induces the B-Raf response observed after expression of the constitutively active subunit, we transiently transfected into CHO cells the dopaminergic D2 receptor, which couples to both Gi and Go proteins (Liu et al., 1994; Watts et al., 1998). Exposure of D2 receptor-expressing cells to dopamine for 5 and 20 min stimulated the activity of B-Raf by ∼70 and 60%, respectively (Figure 7A). This response was completely abolished by pretreatment with 100 ng/ml PTX for 16 h (our unpublished results). Because CHO cells express Go as well as Gi2 and Gi3 (Dell'Acqua et al., 1993; van Biesen et al., 1996a), dopamine activation of B-Raf could be mediated by any of the subunits composing these heterotrimers. To determine the role of Gαo in the D2 receptor response, we first investigated the ability of Gαi2, Gαi3, and Gβγ to activate B-Raf. As shown in Figure 7A, expression of either activated Gαi2 and Gαi3 subunits or Gβ1γ2 complexes failed to significantly increase B-Raf activity. We then asked whether activation of B-Raf by D2 receptors is enhanced by cotransfection of wild-type Gαo, which, by associating with endogenous Gβγ chains, is expected to form heterotrimers available for receptor coupling. As shown in Figure 7B, expression of wild-type Gαo markedly increased dopamine-induced stimulation of B-Raf activity. Consistent with an effect mediated, at least in part, by Gαo, B-Raf activation by the D2 receptor was not affected by coexpression of N17-Ras but was largely inhibited by PMA pretreatment and wortmannin (Figure 7C). We finally investigated whether stimulation of the kinase activity of B-Raf by receptor-activated Gαo has a positive effect on the MAPK pathway. In CHO cells, D2 receptors caused a marked stimulation of endogenous p42 MAPK activity (Figure 8), which was totally blocked by pretreatment with PTX (our unpublished results). However, p42 MAPK displayed a more transient activation than B-Raf. The average 12-fold increase in p42 MAPK activity observed at 5 min was greatly reduced at 20 min (Figure 8). A complete dissociation between the effects of the D2 receptor on B-Raf and MAPK became apparent in cells cotransfected with N17-Ras or pretreated with PMA. Coexpression of N17-Ras, which has no effect on B-Raf activation, efficiently inhibited the stimulation of p42 MAPK activity (Figure 8A). Conversely, PMA pretreatment, which largely prevents activation of B-Raf, did not significantly modify the p42 MAPK response (Figure 8B). These results indicate that, upon stimulation of the D2 receptor, Gαo-activated B-Raf does not induce a MAPK response. Cumulatively, the data are consistent with the conclusion that expression of mutationally activated Gαo and receptor-dependent activation of this subunit exert a similar effect on the B-Raf kinase.
Figure 7.
B-Raf activation by dopaminergic D2 receptors. CHO cells transfected in 6-cm dishes were serum starved for 18 h and either left untreated or stimulated with 100 nM dopamine (DA) for 5 or 20 min. B-Raf activity was assayed as described in the legend to Figure 5. Data shown represent means ± SE of two independent experiments performed in duplicate. (A) Cells were transfected with 1.8 μg of dopaminergic D2 receptor (D2R), 1.8 μg of wild-type or activated Gαi2 and Gαi3 subunits, or 1.4 μg of Gβ1 and Gγ2 chains. *, Significantly higher than basal (p < 0.05 by unpaired t test). (B) Cells were transfected with 1.8 μg of D2R and, where indicated, 1.8 μg of Gαo-WT. *, Significantly higher than values obtained in the absence of Gαo-WT (p < 0.01 by unpaired t test). (C) Cells were transfected with 1.4 μg of D2R and, where indicated, 2.8 μg of N17-Ras. PMA pretreatment and incubation with wortmannin were as described in the legend to Figure 5. Results are expressed as percentages of the increase induced by DA in control cells.
Figure 8.
Effects of N17-Ras expression and PMA pretreatment on dopamine-stimulated MAPK activity. CHO cells transfected in 6-cm dishes were serum starved for 18 h and stimulated with 100 nM DA for 5 or 20 min. (A) Cells were transfected with 1.4 μg of D2R and 2.8 μg of either empty vector or N17-Ras. (B) Cells were transfected with 1.4 μg of D2R and pretreated with PMA as described in the legend to Figure 5. Endogenous p42 MAPK activity was measured as described in the legend to Figure 1. Data are means ± SE of triplicate determinations. Similar results were obtained in at least one additional experiment.
DISCUSSION
Expression of mutationally activated Gα chains or free Gβγ complexes in appropriate cell systems has greatly facilitated the understanding of the roles of individual G protein subunits in the regulation of intracellular signaling pathways. We have used this approach to investigate the effect of Gαo on the MAPK pathway. When expressed in CHO cells, activated Gαo is not sufficient to induce MAPK activation but substantially augments the stimulatory response to the EGF receptor. Previous studies have shown that other Gα chains regulate the MAPK pathway in a similar manner. Expression of activated Gα12 or Gα13 significantly increases MAPK activity when Rat-1 cells are exposed to EGF (Voyno-Yasenetskaya et al., 1994). Activated Gαq, which in certain cell types acts on its own (van Biesen et al., 1996b), requires the concomitant activation of PDGF receptors to stimulate MAPK activity in quiescent NIH 3T3 fibroblasts (De Vivo and Iyengar, 1994). However, the molecules and mechanisms involved in the interaction between these G protein subunits and the tyrosine kinase receptor signaling pathway have not been identified.
Receptors coupled to Gi/o proteins can stimulate the MAPK pathway by inducing ligand-independent tyrosine phosphorylation of the EGF receptor (Hackel et al., 1999; Luttrell et al., 1999). The fact that the MAPK response to activated Gαo is clearly dependent on the presence of EGF argues against an involvement of this mechanism. Consistently, we found that expression of Gαo-Q205L does not cause tyrosine phosphorylation of the EGF receptor. The finding that activated Gαo markedly increases MAPK activation by Ras further supports the conclusion that this subunit acts on a signaling molecule located downstream of the EGF receptor. A low “basal” activity of transfected EGF receptors therefore seems the most likely explanation of the modest activation of MAPK induced by Gαo-Q205L in the absence of EGF.
We have identified the B-Raf kinase as a site for convergence of the signaling pathways initiated by the EGF receptor and Gαo. There is increasing evidence for a differential regulation of the Raf family members by cell surface receptors (Reuter et al., 1995; Knall et al., 1996; Marais and Marshall, 1996; van Biesen et al., 1996b; York et al., 1998). Activation of the Raf kinases by growth factors varies among individual tyrosine kinase receptors and individual cell types (Reuter et al., 1995; Marais and Marshall, 1996). We found that in CHO cells the EGF receptor induces activation of B-Raf without significantly affecting Raf-1 and A-Raf activities. The results of several studies have shown that EGF activates Raf-1 in various cell types, but not in Swiss 3T3 fibroblasts, and stimulates B-Raf activity in PC12 cells (Marais and Marshall, 1996). Regulation of the Raf kinase family by receptors coupled to Gi/o proteins is less understood. Most work has concentrated on Raf-1 (van Biesen et al., 1996b), but there is evidence that Gi/o-coupled receptors also activate B-Raf (Knall et al., 1996). However, whether the subunits composing the Gi/o heterotrimers differ in their ability to regulate specific Raf kinases has not been established. Here we show that activated Gαo selectively stimulates the activity of B-Raf in CHO cells. Interestingly, our experiments did not detect any significant effect of either Gαi or Gβγ subunits on this kinase.
The lack of correlation between B-Raf and MAPK activities observed in cells expressing activated Gαo clearly indicates that the effect of this subunit on B-Raf is sufficient to promote MEK and MAPK activation in in vitro assays but not in intact cells. Such a discrepancy cannot be explained by the fact that we used MEK1, rather than MEK2, to measure B-Raf activity. The two MEK kinases appear equally responsive to B-Raf activation (Marais et al., 1997) and are expressed in comparable amounts in CHO cells (Pace et al., 1995; Xu et al., 1997) (our unpublished results). In addition, the data obtained with the dopaminergic D2 receptor seem to rule out the possibility that the “defective” response of B-Raf to Gαo is dependent on compensatory mechanisms or irrelevant effects that result from expression of the mutationally activated protein. It should also be considered that CHO cells contain at least two of the multiple B-Raf isoforms that have been identified (Barnier et al., 1995). The antibodies used in our experiments immunoprecipitate preferentially the 92-kDa form (Vaillancourt et al., 1994), and the results of kinase assays reflect mainly the activity of this protein. A differential activation of the B-Raf kinases has been suggested (Vossler et al., 1997), and it is therefore possible that only the 92-kDa form is sensitive to the regulation observed in our studies. However, because in cell lysates the 92-kDa form is only slightly less abundant than the 66-kDa form (our unpublished results), it appears unlikely that the levels of expression of the protein can limit its ability to stimulate MAPK in intact cells. Several lines of evidence suggest, however, that B-Raf regulation is involved in the ability of activated Gαo to potentiate EGF receptor-dependent MAPK activation: 1) the enhancement of Ras-stimulated MAPK activity by Gαo-Q205L is consistent with the involvement of a signaling molecule located downstream of Ras itself; 2) PKC inactivation and PI3K inhibition, which abolish B-Raf activation by Gαo-Q205L, completely prevent the effect of the mutant subunit on MAPK; and 3) activated Gαo has no significant effect on the MAPK response induced by FGF, which does not activate the B-Raf kinase in CHO cells. Although we cannot exclude other possibilities, the simplest interpretation of our results is that Gαo-activated B-Raf can efficiently stimulate the downstream MAPK cascade in the presence of a concomitant signal provided by the EGF receptor (see Figure 9).
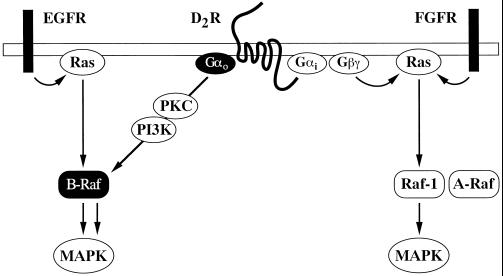
Figure 9.
Model of signaling pathways regulating Raf and MAPK in CHO cells. The EGF receptor (EGFR) activates Ras and B-Raf, whereas the FGF receptor (FGFR) activates Ras, Raf-1, and A-Raf. Activated Gαo potentiates MAPK activation by Ras and EGFR but not FGFR. The G protein subunit stimulates the kinase activity of B-Raf by a Ras-independent and PKC- and PI3K-dependent mechanism, which is insufficient to promote MAPK activation. It is proposed that this mechanism can efficiently stimulate the MAPK pathway in the presence of a B-Raf-activating signal from Ras or the EGFR. The dopaminergic D2 receptor (D2R), which couples to both Gi and Go, appears to stimulate MAPK through Ras activation of Raf-1. This effect is presumably mediated by G protein βγ complexes and, possibly, Gαi subunits. Upon stimulation of this receptor, the Ras-independent and PKC- and PI3K-dependent activation of B-Raf induced by Gαo does not produce a MAPK response.
Activation of B-Raf and the other members of the Raf family is a complex process involving mechanisms that have not been completely elucidated (Marais and Marshall, 1996; Morrison and Cutler, 1997; Campbell et al., 1998). The ability of Ras to activate B-Raf is well established. The binding of Ras induces translocation of B-Raf to the plasma membrane and potently stimulates its kinase activity (Marais et al., 1997). Recently, it has been reported that B-Raf is also directly activated by the small G proteins Rap1 and TC21 (Vossler et al., 1997; York et al., 1998; Rosario et al., 1999). The results obtained here in cells expressing Gαo-Q205L or the dopaminergic D2 receptor indicate that Gαo regulates B-Raf activity by a Ras-independent mechanism that involves both PKC and PI3K. Previous studies have reported that PKC and PI3K can lead to B-Raf activation (Peraldi et al., 1995; Reuter et al., 1995; Knall et al., 1996). The Ras-independent pathway connecting these signaling molecules to B-Raf remains to be defined. On the other hand, the fact that activated Gαo potentiates the action of Ras on the MAPK pathway would suggest that Ras activation is the EGF receptor-dependent signal that cooperates with the G protein subunit in regulating B-Raf function. We do not know how Gαo increases the kinase activity of B-Raf without allowing coupling of the kinase to its downstream effectors and how the tyrosine kinase receptor-Ras dependent pathway would promote the latter event. Mechanisms that influence the ability of B-Raf to activate MEK might involve subcellular localization, association with scaffolding proteins (Garrington and Johnson, 1999), or regulation by signaling molecules. Interestingly, signals that control the coupling of Raf-1 to the MAPK cascade have been recently reported. In NIH 3T3 cells exposed to serum, “adhesion deprivation” has no major effect on the in vitro activity of Raf-1 but strongly inhibits MAPK stimulation (Renshaw et al., 1997). Similar results have been obtained in HEK-293 cells stimulated with agonists of G protein-coupled receptors after blockade of receptor internalization (Daaka et al., 1998). Finally, it should be mentioned that during the preparation of this manuscript two reports have shown that both Gαi and Gαo can interact with Rap1GAP (Jordan et al., 1999; Mochizuki et al., 1999). It has been proposed that Rap1GAP binds more avidly to the inactive form of Gαo, and activation of the G protein subunit leads to the release of the GTPase-activating protein and the consequent inhibition of Rap1 function (Jordan et al., 1999). In cells in which Rap1 exerts a positive effect on B-Raf and MAPK, such as the PC12 line, this mechanism causes increased MAPK activity in response to wild-type but not constitutively activated Gαo (Jordan et al., 1999). Because other recent data suggest that Rap-1 induces B-Raf and MAPK activation also in CHO cells (Seidel et al., 1999), it is difficult to imagine how inhibition of this small G protein can explain our present results. However, the effects of Rap1 on the MAPK pathway are rather complex and not completely understood (Bos and Zwartkruis, 1999), and further work will be needed to elucidate the role of Rap1GAP regulation in B-Raf and MAPK stimulation by Gαo.
Different Gα chains have a well-recognized role in the control of cell growth (Landis et al., 1989; Vallar, 1996; Dhanasekaran et al., 1998). The finding that Gαo can exert a positive effect on MAPK activity reinforces the idea that this subunit is involved in mitogenic signaling pathways (Kroll et al., 1991; Kroll et al., 1992; van Biesen et al., 1996a). Our results can explain the increased proliferative response to serum previously observed in NIH 3T3 fibroblasts expressing Gαo-Q205L (Kroll et al., 1992), especially when taking into account that in these cells B-Raf has been identified as one of the major MEK activators (Reuter et al., 1995). It appears conceivable that the effect of activated Gαo identified here also represents one of the mechanisms responsible for the synergistic effects of Gi/o-coupled receptors and tyrosine kinase receptors on MAPK and cell growth observed in several systems (Meloche et al., 1992b; Pouyssegur and Seuwen, 1992; Vouret-Craviari et al., 1993; Fujitani and Bertrand, 1997). Interestingly, a synergistic stimulation of MAPK activity by dopaminergic D2 receptors and EGF receptors can be detected in CHO cells (our unpublished results). Our experiments indicate that activated Gαo requires the concomitant stimulation of the EGF receptor-Ras signaling pathway to regulate MAPK activity. However, previous results obtained in CHO cells expressing a PTX-insensitive mutant of Gαo have proposed that this subunit mediates Ras-independent and PKC-dependent MAPK activation by the muscarinic M1 receptor (van Biesen et al., 1996a). We cannot exclude the possibility that, in our experiments, desensitization or counter-regulation mechanisms prevent the detection of autonomous effects of Gαo on the MAPK pathway. However, it is also possible that upon activation of the muscarinic M1 receptor, which couples to both Go and Gq, other G protein subunits provide a Ras-independent signal that allows stimulation of the MAPK cascade by Gαo-activated B-Raf. The existence of such “cross-talk” mechanisms would be one of the possible explanations of the fact that in CHO cells certain receptors capable of coupling to the Go protein use a PKC-dependent pathway of MAPK activation (van Biesen et al., 1996a; Wylie et al., 1999), but others, like the dopaminergic D2 receptor investigated here, are clearly unable to do so (Garnovskaya et al., 1996; Hawes et al., 1998) (see Figure 9). The ability of G protein subunits to regulate the MAPK pathway by a variety of signals (van Biesen et al., 1996b; Gutkind, 1998), including transactivation of tyrosine kinase receptors (Hackel et al., 1999; Luttrell et al., 1999), suggests that there are several ways by which Gαo-activated B-Raf might participate in the MAPK responses induced by G protein-coupled receptors in specific cell types.
The effector molecules and intracellular pathways involved in signal transduction mechanisms by Gαo are poorly understood. There is evidence that Gαo can directly inhibit a specific adenylyl cyclase isoform, which is mainly expressed in brain (Sunahara et al., 1996). Other reported effects of the active form of Gαo include stimulation of polyphosphoinositide hydrolysis and PKC activity in Xenopus oocytes (Moriarty et al., 1990; Kroll et al., 1991) and activation of a Src-like kinase pathway in neurons (Diversé-Pierluissi et al., 1997). Our results indicate that in CHO cells signaling from Gαo involves PKC, as previously suggested (van Biesen et al., 1996a), as well as PI3K. The identities of the PKC and PI3K isoforms that participate in Gαo-mediated signals and their mechanisms of regulation remain to be clarified. In CHO cells, Gαo uses PKC and PI3K to induce Ras-independent activation of B-Raf and conditional stimulation of MAPK. It appears most likely that these responses occur in a set of selected cell types (van Biesen et al., 1996a,b; Gutkind, 1998). Gαo is believed to play a most important role in the nervous system (Neer, 1995; Jiang et al., 1998), where B-Raf is expressed at particularly high levels (Barnier et al., 1995). Recent data have shown that, in PC12 cells overexpressing Rap1, constitutively active Gαo has a negative rather than a positive effect on the MAPK response to NGF (Jordan et al., 1999). It will be of considerable interest to elucidate whether the signaling pathway identified here in CHO cells operates in other neuronal models and is therefore involved in intracellular networks that regulate not only growth but also differentiation and specialized cell functions.
ACKNOWLEDGMENTS
We thank A. Malgaroli, E.P. Sturani, and R. Zippel for helpful discussions and suggestions. This work was supported by grants from the Italian Association for Cancer Research and the Armenise-Harvard Foundation. Y.H.W. was supported by the Research Grants Council of Hong Kong (grant HKUST 653/96 M).
Abbreviations used:
- CHO
Chinese hamster ovary
- CLAP
chymostatin, leupeptin, antipain, and pepstatin
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- GST
glutathione S-transferase
- HA
hemagglutinin
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- MEK
MAPK/extracellular signal-regulated kinase kinase
- PDGF
platelet-derived growth factor
- PI3K
phosphatidylinositol-3 kinase
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PTX
pertussis toxin
REFERENCES
- Alessi DR, Cohen P, Ashworth A, Cowley S, Leevers SJ, Marshall CJ. Assay and expression of mitogen-activated protein kinase, MAP kinase kinase, and Raf. Methods Enzymol. 1995;255:279–290. doi: 10.1016/s0076-6879(95)55031-3. [DOI] [PubMed] [Google Scholar]
- Baffy G, Yang L, Raj S, Manning DR, Williamson JR. G protein coupling to the thrombin receptor in Chinese hamster lung fibroblasts. J Biol Chem. 1994;269:8483–8487. [PubMed] [Google Scholar]
- Barnier JV, Papin C, Eychène A, Lecoq O, Calothy G. The mouse B-raf gene encodes multiple protein isoforms with tissue-specific expression. J Biol Chem. 1995;270:23381–23389. doi: 10.1074/jbc.270.40.23381. [DOI] [PubMed] [Google Scholar]
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Bos JL, Zwartkruis FJT. Rhapsody in G proteins. Nature. 1999;400:820–821. doi: 10.1038/23594. [DOI] [PubMed] [Google Scholar]
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Conklin BR, Chabre O, Wong YH, Federman AD, Bourne HR. Recombinant Gqα mutational activation and coupling to receptors and phospholipase C. J Biol Chem. 1992;267:31–34. [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SSG, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- De Vivo M, Iyengar R. Activated Gq-α potentiates platelet-derived growth factor-stimulated mitogenesis in confluent cell cultures. J Biol Chem. 1994;269:19671–19674. [PubMed] [Google Scholar]
- Dell'Acqua ML, Carroll RC, Peralta EG. Transfected m2 muscarinic acetylcholine receptors couple to Gαi2 and Gαi3 in Chinese hamster ovary cells. J Biol Chem. 1993;268:5676–5685. [PubMed] [Google Scholar]
- Dhanasekaran N, Tsim ST, Dermott JM, Onesime D. Regulation of cell proliferation by G proteins. Oncogene. 1998;17:1383–1394. doi: 10.1038/sj.onc.1202242. [DOI] [PubMed] [Google Scholar]
- Diversé-Pierluissi M, Remmers AE, Neubig RR, Dunlap K. Novel form of crosstalk between G protein and tyrosine kinase pathways. Proc Natl Acad Sci USA. 1997;94:5417–5421. doi: 10.1073/pnas.94.10.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani Y, Bertrand C. ET-1 cooperates with EGF to induce mitogenesis via a PTX-sensitive pathway in airway smooth muscle cells. Am J Physiol. 1997;272:1492–1498. doi: 10.1152/ajpcell.1997.272.5.C1492. [DOI] [PubMed] [Google Scholar]
- Gallego C, Gupta SK, Heasley LE, Qian NX, Johnson GL. Mitogen-activated protein kinase activation resulting from selective oncogene expression in NIH 3T3 and Rat-1a cells. Proc Natl Acad Sci USA. 1992;89:7355–7359. doi: 10.1073/pnas.89.16.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnovskaya MN, van Biesen T, Hawe B, Casanas Ramos S, Lefkowitz RJ, Raymond JR. Ras-dependent activation of fibroblast mitogen-activated protein kinase by 5-HT1A receptor via a G protein βγ-subunit-initiated pathway. Biochemistry. 1996;35:13716–13722. doi: 10.1021/bi961764n. [DOI] [PubMed] [Google Scholar]
- Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Gallego C, Lowndes JM, Pleiman CM, Sable C, Eisfelder BJ, Johnson GL. Analysis of the fibroblast transformation potential of GTPase-deficient gip2 oncogenes. Mol Cell Biol. 1992;12:190–197. doi: 10.1128/mcb.12.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind JS. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184–189. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- Hawes BE, Fried S, Yao X, Weig B, Graziano MP. Nociceptin (ORL-1) and μ-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: evidence for distinct mechanisms of agonist-mediated desensitization. J Neurochem. 1998;71:1024–1033. doi: 10.1046/j.1471-4159.1998.71031024.x. [DOI] [PubMed] [Google Scholar]
- Hawes BE, van Biesen T, Koch WJ, Luttrell LM, Lefkowitz RJ. Distinct pathways of Gi- and Gq-mediated mitogen-activated protein kinase activation. J Biol Chem. 1995;270:17148–17153. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- Hermouet S, Merendino JJ, Jr, Gutkind JS, Spiegel AM. Activating and inactivating mutations of the α subunit of Gi2 protein have opposite effects on proliferation of NIH 3T3 cells. Proc Natl Acad Sci USA. 1991;88:10455–10459. doi: 10.1073/pnas.88.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Gold MS, Boulay G, Spicher K, Peyton M, Brabet P, Srinivasan Y, Rudolph U, Ellison G, Birnbaumer L. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci USA. 1998;95:3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan JD, Carey KD, Stork PJS, Iyengar R. Modulation of Rap activity by direct interaction of Gαo with Rap1 GTPase-activating protein. J Biol Chem. 1999;274:21507–21510. doi: 10.1074/jbc.274.31.21507. [DOI] [PubMed] [Google Scholar]
- Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- Kroll SD, Chen J, De Vivo M, Carty DJ, Buku A, Premont RT, Iyengar R. The Q205LGo-α subunit expressed in NIH-3T3 cells induces transformation. J Biol Chem. 1992;267:23183–23188. [PubMed] [Google Scholar]
- Kroll SD, Omri G, Landau EM, Iyengar R. Activated α subunit of Go protein induces oocyte maturation. Proc Natl Acad Sci USA. 1991;88:5182–5186. doi: 10.1073/pnas.88.12.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMorte VJ, Goldsmith PK, Spiegel AM, Meinkoth JL, Feramisco JR. Inhibition of DNA synthesis in living cells by microinjection of Gi2 antibodies. J Biol Chem. 1992;267:691–694. [PubMed] [Google Scholar]
- Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the α chain of Gs and stimulate adenylyl cyclase in human pituitary tumors. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Liu YF, Jakobs KH, Rasenick MM, Albert PR. G protein specificity in receptor-effector coupling. Analysis of the roles of Go and Gi2 in GH4C1 pituitary cells. J Biol Chem. 1994;269:13880–13886. [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- Lyons J, et al. Two G protein oncogenes in human endocrine tumors. Science. 1990;249:655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic Ras and tyrosine kinases. J Biol Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- Marais R, Marshall CJ. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Meloche S, Pagès G, Pouysségur J. Functional expression and growth factor activation of an epitope-tagged p44 mitogen-activated protein kinase, p44mapk. Mol Biol Cell. 1992a;3:63–71. doi: 10.1091/mbc.3.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche S, Seuwen K, Pages G, Pouyssegur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992b;6:845–854. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]
- Moriarty TM, Padrell E, Carty DJ, Omri G, Landau EM, Iyengar R. Go protein as signal transducer in the pertussis toxin-sensitive phosphatidylinositol pathway. Nature. 1990;343:79–82. doi: 10.1038/343079a0. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Ohba Y, Kiyokawa E, Kurata T, Murakami T, Ozaki T, Kitabatake A, Nagashima K, Matsuda M. Activation of the ERK/MAPK pathway by an isoform of rap 1 Gap associated with Gαi. Nature. 1999;400:891–894. doi: 10.1038/23738. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Cutler RE., Jr The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Pace AM, Faure M, Bourne HR. Gi2-mediated activation of the MAP kinase cascade. Mol Biol Cell. 1995;6:1685–1695. doi: 10.1091/mbc.6.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace AM, Wong YH, Bourne HR. A mutant α subunit of Gi2 induces neoplastic transformation of Rat-1 cells. Proc Natl Acad Sci USA. 1991;88:7031–7035. doi: 10.1073/pnas.88.16.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraldi P, Frodin M, Barnier JV, Calleja V, Scimeca JC, Filloux C, Calothy G, Van Obberghen E. Regulation of the MAP kinase cascade in PC12 cells: B-Raf activates MEK-1 (MAP kinase or ERK kinase) and is inhibited by cAMP. FEBS Lett. 1995;357:290–296. doi: 10.1016/0014-5793(94)01376-c. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Seuwen K. Transmembrane receptors and intracellular pathways that control cell proliferation. Annu Rev Physiol. 1992;54:195–210. doi: 10.1146/annurev.ph.54.030192.001211. [DOI] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter CWM, Catling AD, Jelinek T, Weber MJ. Biochemical analysis of MEK activation in NIH3T3 fibroblasts. J Biol Chem. 1995;270:7644–7655. doi: 10.1074/jbc.270.13.7644. [DOI] [PubMed] [Google Scholar]
- Rosario M, Paterson HF, Marshall CJ. Activation of the Raf/MAP kinase cascade by the Ras-related protein TC21 is required for the TC21-mediated transformation of NIH 3T3 cells. EMBO J. 1999;18:1270–1279. doi: 10.1093/emboj/18.5.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Seidel MG, Klinger M, Freissmuth M, Holler C. Activation of mitogen-activated protein kinase by the A2A-adenosine receptor via a rap 1-dependent and via a p21ras-dependent pathway. J Biol Chem. 1999;274:25833–25841. doi: 10.1074/jbc.274.36.25833. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- Toker A, Cantley LC. Signaling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signaling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- Vaillancourt RR, Gardner AM, Johnson GL. B-Raf-dependent regulation of the MEK-1/mitogen-activated protein kinase pathway in PC12 cells and regulation by cyclic AMP. Mol Cell Biol. 1994;14:6522–6530. doi: 10.1128/mcb.14.10.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar L. Oncogenic role of heterotrimeric G proteins. Cancer Surv. 1996;27:325–338. [PubMed] [Google Scholar]
- van Biesen T, Hawes BE, Raymond JR, Luttrell LM, Koch WJ, Lefkowitz RJ. Go-protein α-subunits activate mitogen-activated protein kinase via a novel protein kinase C-dependent mechanism. J Biol Chem. 1996a;271:1266–1269. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- van Biesen T, Luttrell LM, Hawes BE, Lefkowitz RJ. Mitogenic signaling via G protein-coupled receptors. Endocr Rev. 1996b;17:698–714. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJS. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V, Van Obberghen-Schilling E, Scimeca JC, Van Obberghen E, Pouyssegur J. Differential activation of p44mapk (ERK1) by α-thrombin and thrombin-receptor peptide agonist. Biochem J. 1993;289:209–214. doi: 10.1042/bj2890209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyno-Yasenetskaya TA, Pace AM, Bourne HR. Mutant α subunits of G12 and G13 proteins induce neoplastic transformation of Rat-1 fibroblasts. Oncogene. 1994;9:2559–2565. [PubMed] [Google Scholar]
- Watts VJ, Wiens BL, Cumbay MG, Vu MN, Neve RL, Neve KA. Selective activation of Gαo by D2L dopamine receptors in NS20Y neuroblastoma cells. J Neurosci. 1998;18:8692–8699. doi: 10.1523/JNEUROSCI.18-21-08692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennstrom S, Downward J. Role of phosphoinositide 3-kinase in activation of ras and mitogen-activated protein kinase by epidermal growth factor. Mol Cell Biol. 1999;19:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YH, Conklin BR, Bourne HR. Gz-mediated hormonal inhibition of cyclic AMP accumulation. Science. 1992;255:339–342. doi: 10.1126/science.1347957. [DOI] [PubMed] [Google Scholar]
- Wylie PG, Challiss RAJ, Blank JL. Regulation of extracellular signal-regulated kinase and c-Jun N-terminal kinase by G-protein-linked muscarinic acetylcholine receptors. Biochem J. 1999;338:619–628. [PMC free article] [PubMed] [Google Scholar]
- Xu S, Khoo S, Dang A, Witt S, Do V, Zhen E, Schaefer EM, Cobb MH. Differential regulation of mitogen-activated protein/ERK kinase (MEK)1 and MEK2 and activation by a Ras-independent mechanism. Mol Endocrinol. 1997;11:1618–1625. doi: 10.1210/mend.11.11.0010. [DOI] [PubMed] [Google Scholar]
- York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJS. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]