Abstract
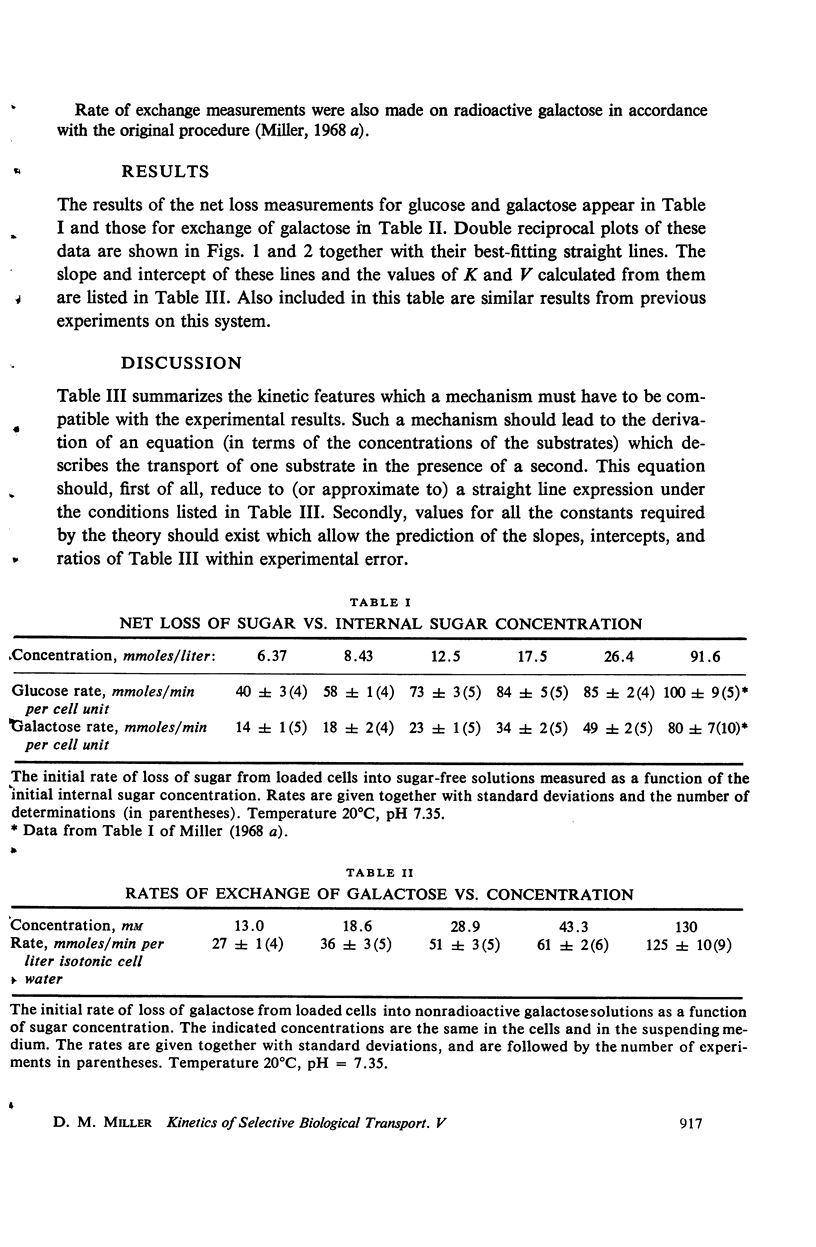
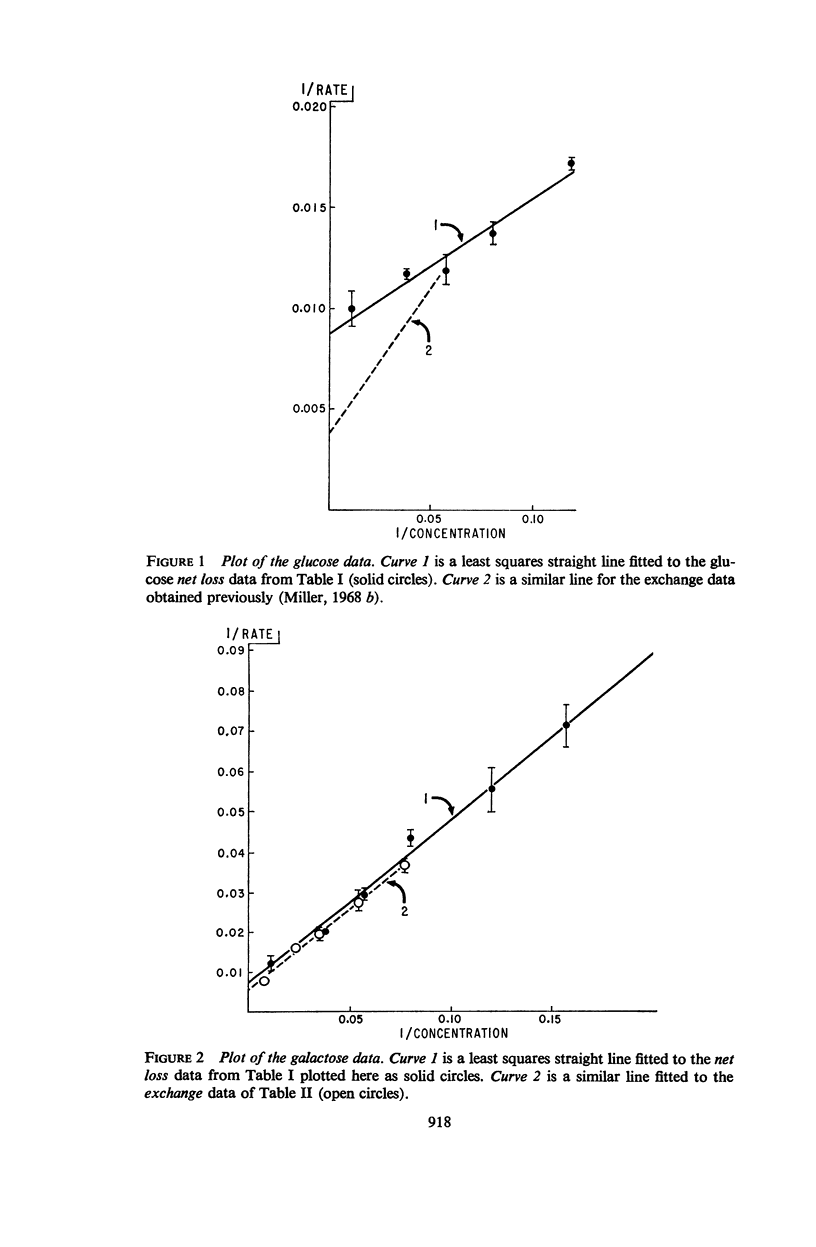
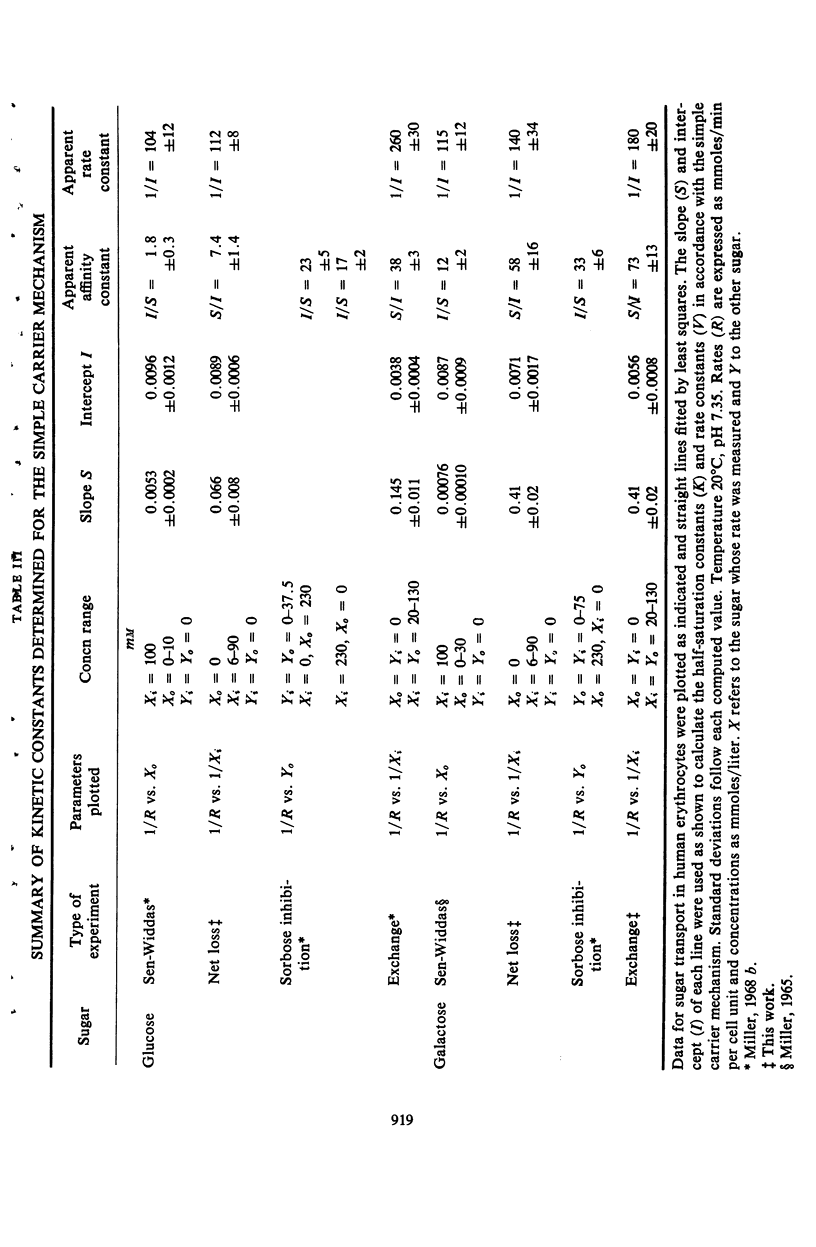
Measurements of the rate of loss of sugar from human erythrocytes into sugar-free solutions were made as a function of sugar concentrations. The half-saturation concentration of this process was found to be different from those half-saturation concentrations previously measured by other methods. These data, together with a number of similar data from former publications, are summarized in tabular form and their use in assessing postulated transport mechanisms is illustrated by consideration and rejection of a mechanism in which the transport process is assumed to be the result of a protein conformational change.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- KOSHLAND D. E., Jr, YANKEELOV J. A., Jr, THOMA J. A. Specificity and catalytic power in enzyme action. Fed Proc. 1962 Nov-Dec;21:1031–1038. [PubMed] [Google Scholar]
- Krupka R. M. Evidence for a carrier conformational change associated with sugar transport in erythrocytes. Biochemistry. 1971 Mar 30;10(7):1143–1148. doi: 10.1021/bi00783a007. [DOI] [PubMed] [Google Scholar]
- SEN A. K., WIDDAS W. F. Determination of the temperature and pH dependence of glucose transfer across the human erythrocyte membrane measured by glucose exit. J Physiol. 1962 Mar;160:392–403. doi: 10.1113/jphysiol.1962.sp006854. [DOI] [PMC free article] [PubMed] [Google Scholar]


