Abstract
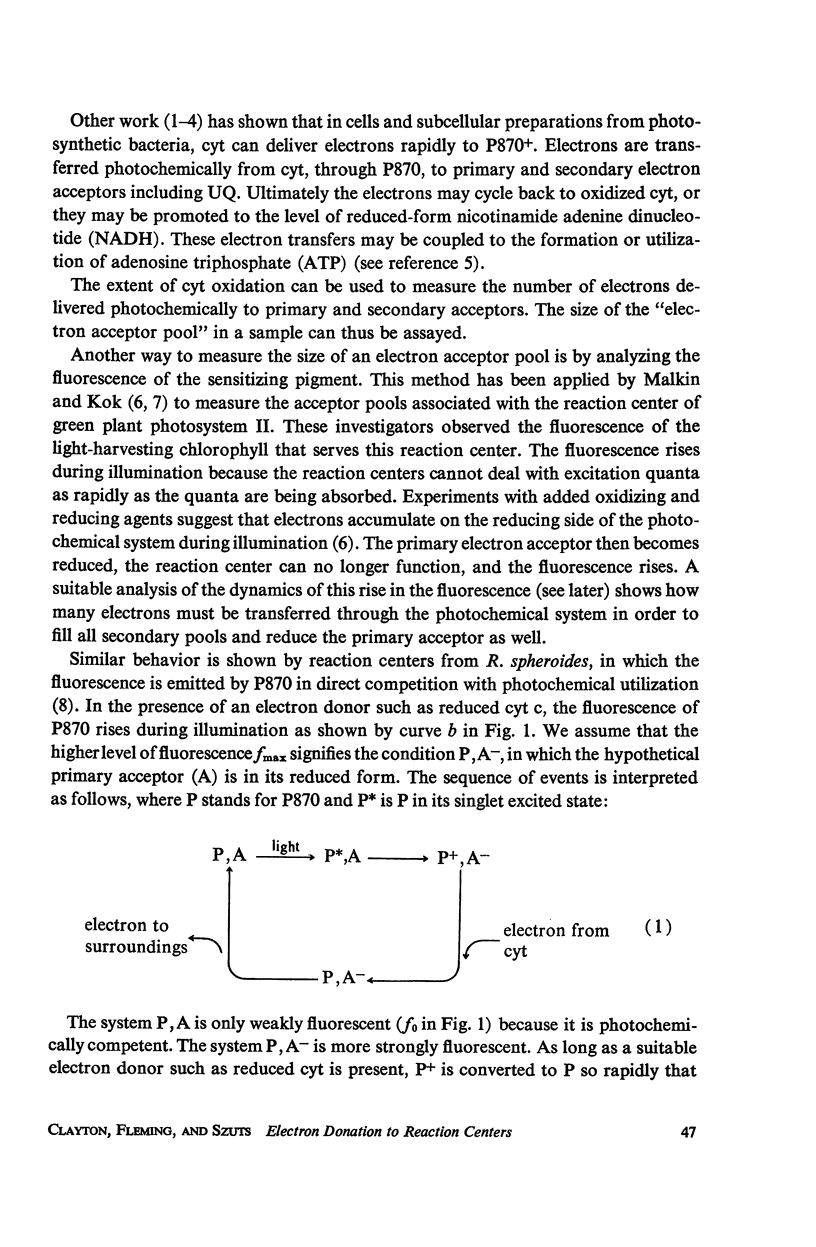
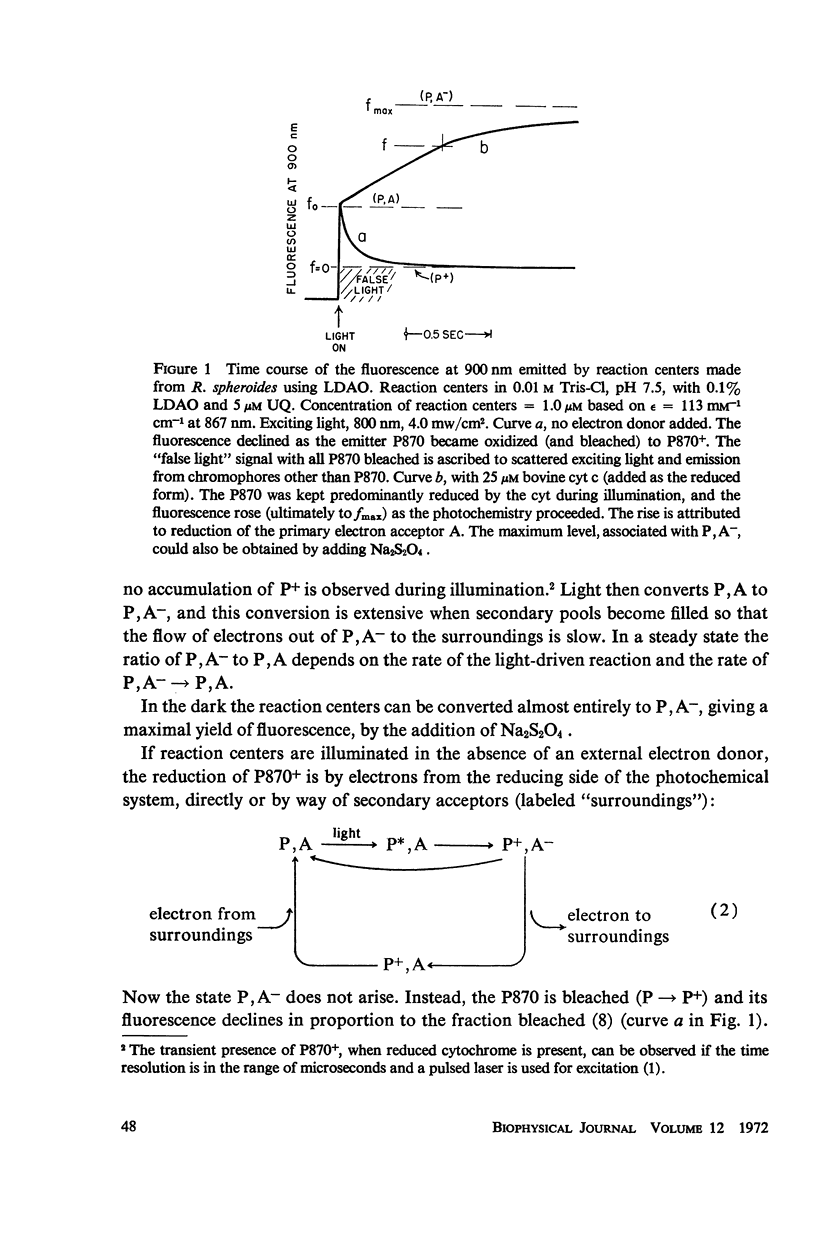
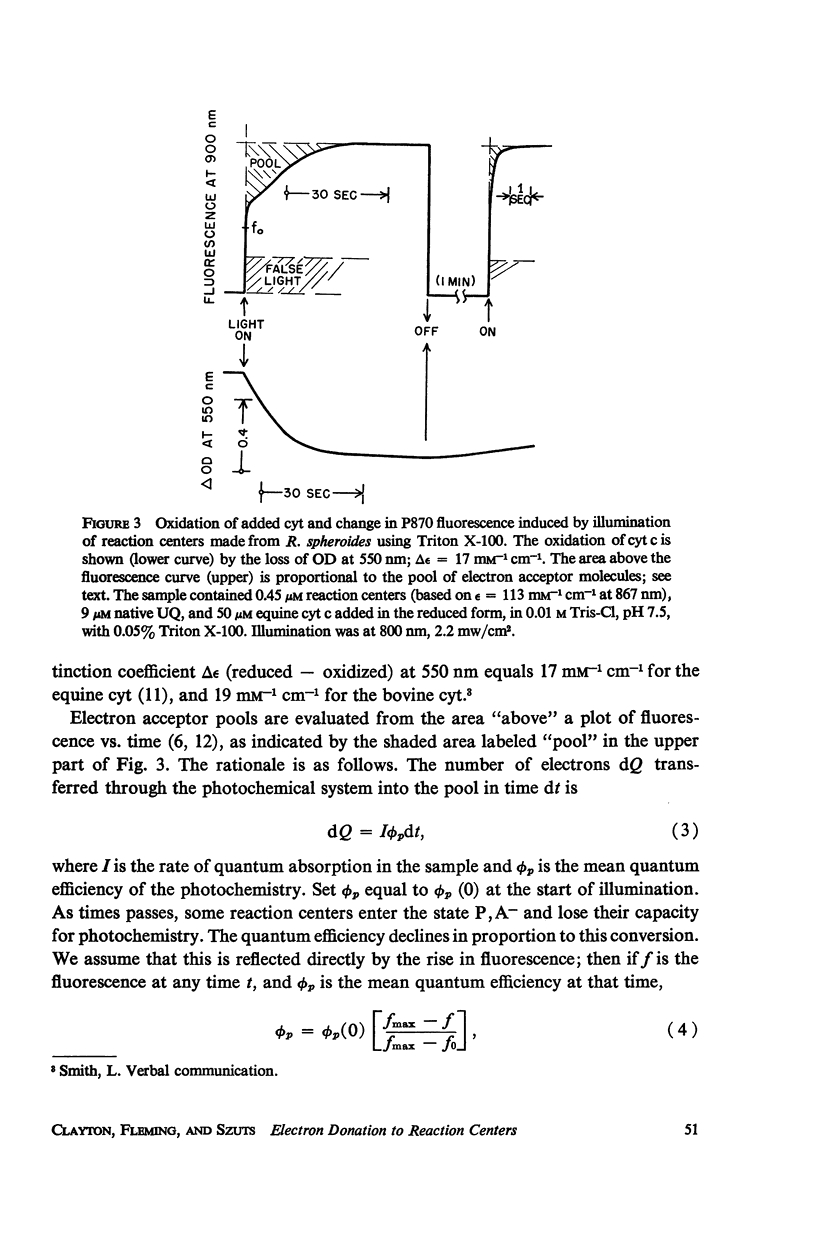
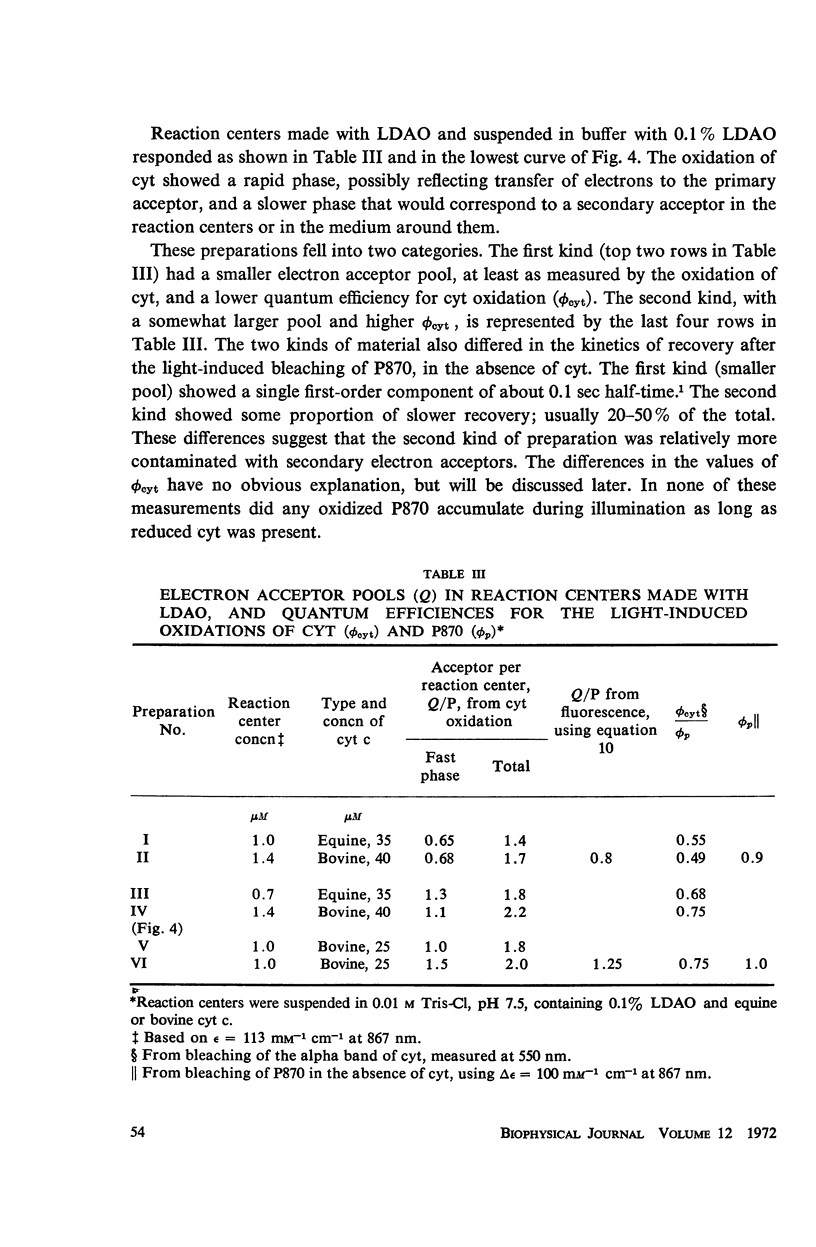
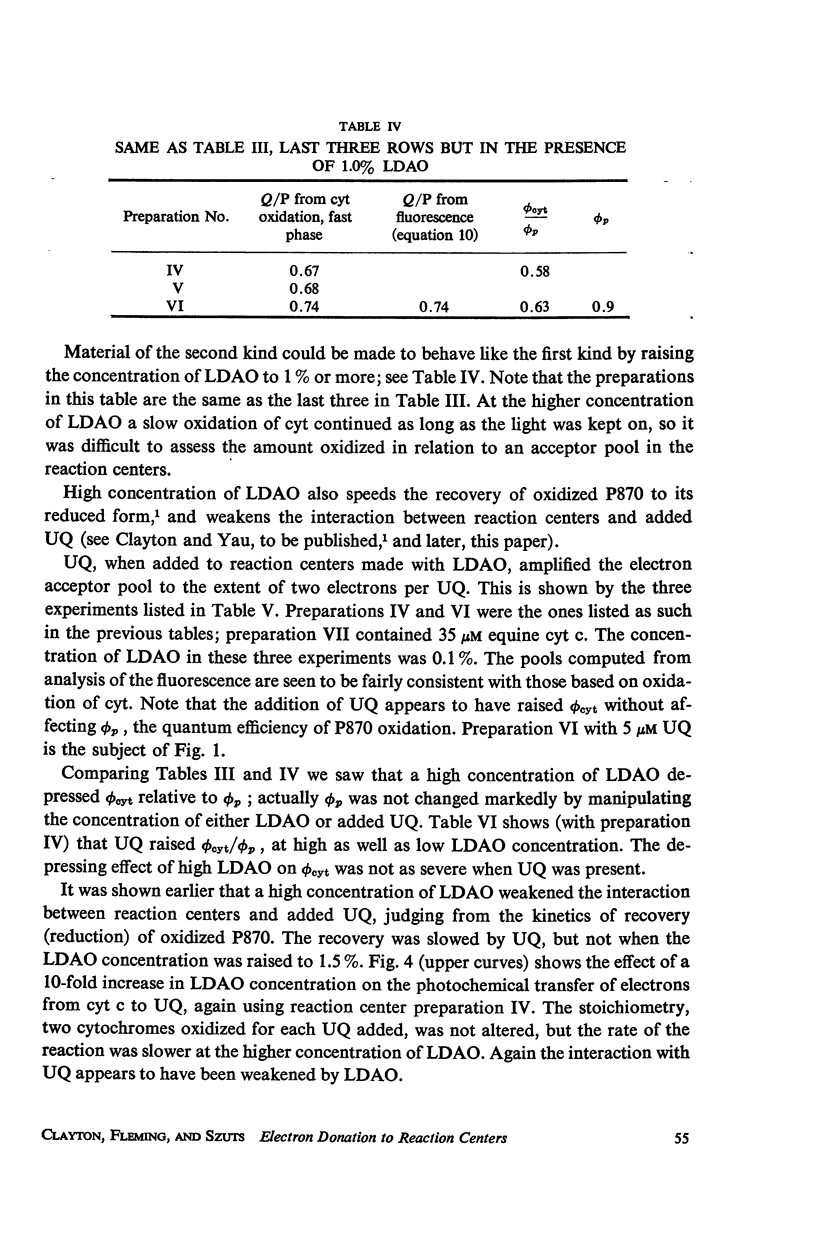
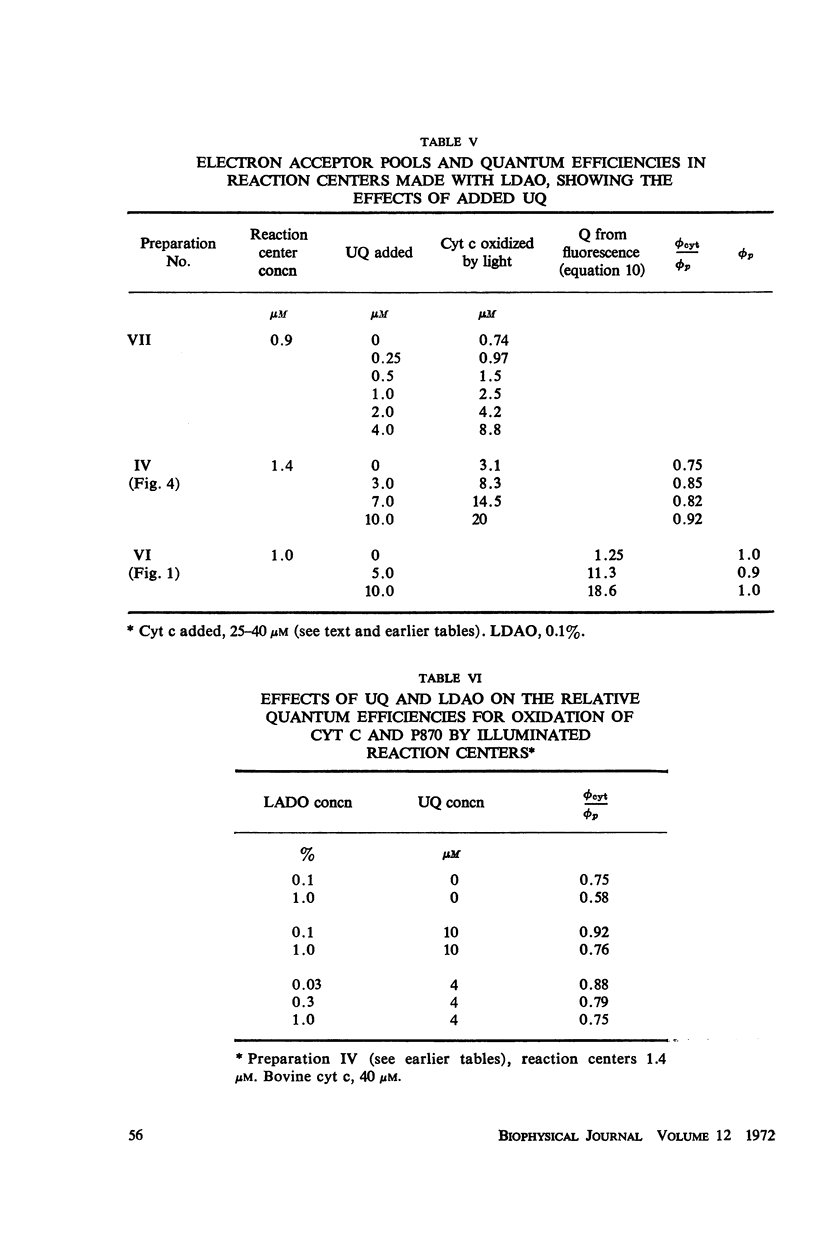
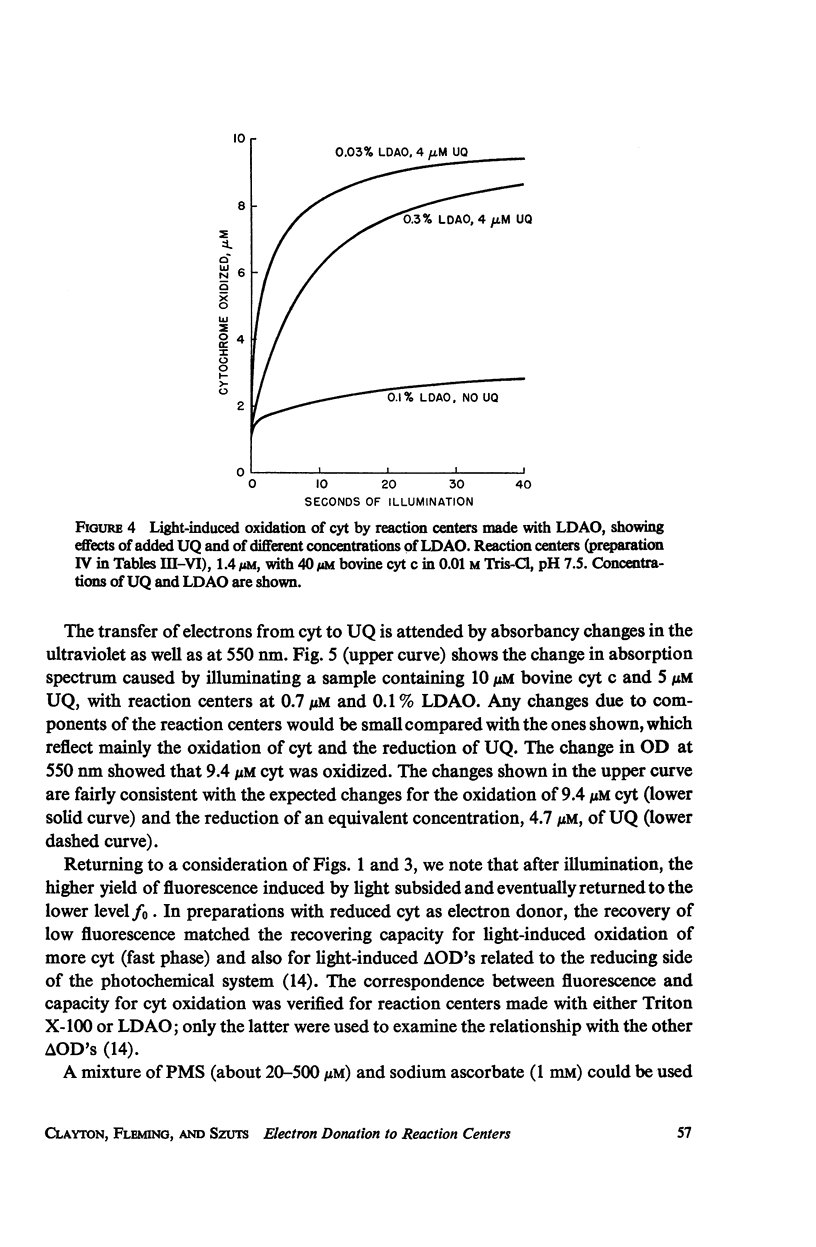
Photochemical reaction centers prepared from Rhodopseudomonas spheroides were treated with reduced cytochrome c (cyt c), and in some cases with ubiquinone (UQ), and illuminated. The light-induced oxidation of cy and reduction of UQ were observed, and also the variations in fluorescence of P870. These observations indicated that each reaction center contains a primary photochemical electron acceptor capable of holding just one electron. Depending on the method of preparation, the reaction centers may also contain secondary electron acceptor pools consisting mainly of UQ. The role of native UQ as an electron acceptor could be duplicated by added UQ. The yield of P870 fluorescence increased by a factor of 3-4, at most, during illumination of reaction centers in the presence of an electron donor such as reduced cyt. This suggests that the quantum efficiency for the primary photoact is about 0.7, rather than 0.9-1.0 as concluded in the past from optical absorption measurements. The apparent quantum efficiency for the oxidation of cyt by illuminated reaction centers can be increased by the addition of UQ and is decreased at higher concentrations of the detergent lauryl dimethylamine oxide (LDAO). These treatments do not affect the quantum efficiency of P870 oxidation, measured in the absence of cyt.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton J. R., Clayton R. K., Reed D. W. An identification of the radical giving rise to the light-induced electron spin resonance signal in photosynthetic bacteria. Photochem Photobiol. 1969 Mar;9(3):209–218. doi: 10.1111/j.1751-1097.1969.tb07285.x. [DOI] [PubMed] [Google Scholar]
- Feher G. Some chemical and physical properties of a bacterial reaction center particle and its primary photochemical reactants. Photochem Photobiol. 1971 Sep;14(3):373–387. doi: 10.1111/j.1751-1097.1971.tb06180.x. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Reynier M. Resistance of E. coli ribosomal 5S RNA to degradation by ribonuclease in reconstituted ribosomal particles. Biochem Biophys Res Commun. 1970 Apr 8;39(1):114–122. doi: 10.1016/0006-291x(70)90765-5. [DOI] [PubMed] [Google Scholar]
- GOEDHEER J. C. Spectral and redox properties of bacteriochlorophyll in its natural state. Biochim Biophys Acta. 1960 Mar 11;38:389–399. doi: 10.1016/0006-3002(60)91274-9. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Sekura D. L. Primary photochemistry and electron transport in Rhodospirillum rubrum. Biochemistry. 1968 Jul;7(7):2642–2649. doi: 10.1021/bi00847a029. [DOI] [PubMed] [Google Scholar]
- Malkin S. Fluorescence induction studies in isolated chloroplasts. II. Kinetic analysis of the fluorescence intensity dependence on time. Biochim Biophys Acta. 1966 Nov 8;126(3):433–442. doi: 10.1016/0926-6585(66)90002-1. [DOI] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Case G. D. In Chromatium, a single photochemical reaction center oxidizes both cytochrome C552 and cytochrome C555. Biochim Biophys Acta. 1970;205(2):232–245. doi: 10.1016/0005-2728(70)90253-7. [DOI] [PubMed] [Google Scholar]
- Parson W. W. The role of P870 in bacterial photosynthesis. Biochim Biophys Acta. 1968 Jan 15;153(1):248–259. doi: 10.1016/0005-2728(68)90167-9. [DOI] [PubMed] [Google Scholar]
- Reed D. W., Clayton R. K. Isolation of a reaction center fraction from Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1968 Mar 12;30(5):471–475. doi: 10.1016/0006-291x(68)90075-2. [DOI] [PubMed] [Google Scholar]
- Zankel K. L., Reed D. W., Clayton R. K. Fluorescence and photochemical quenching in photosynthetic reaction centers. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1243–1249. doi: 10.1073/pnas.61.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]


