Abstract
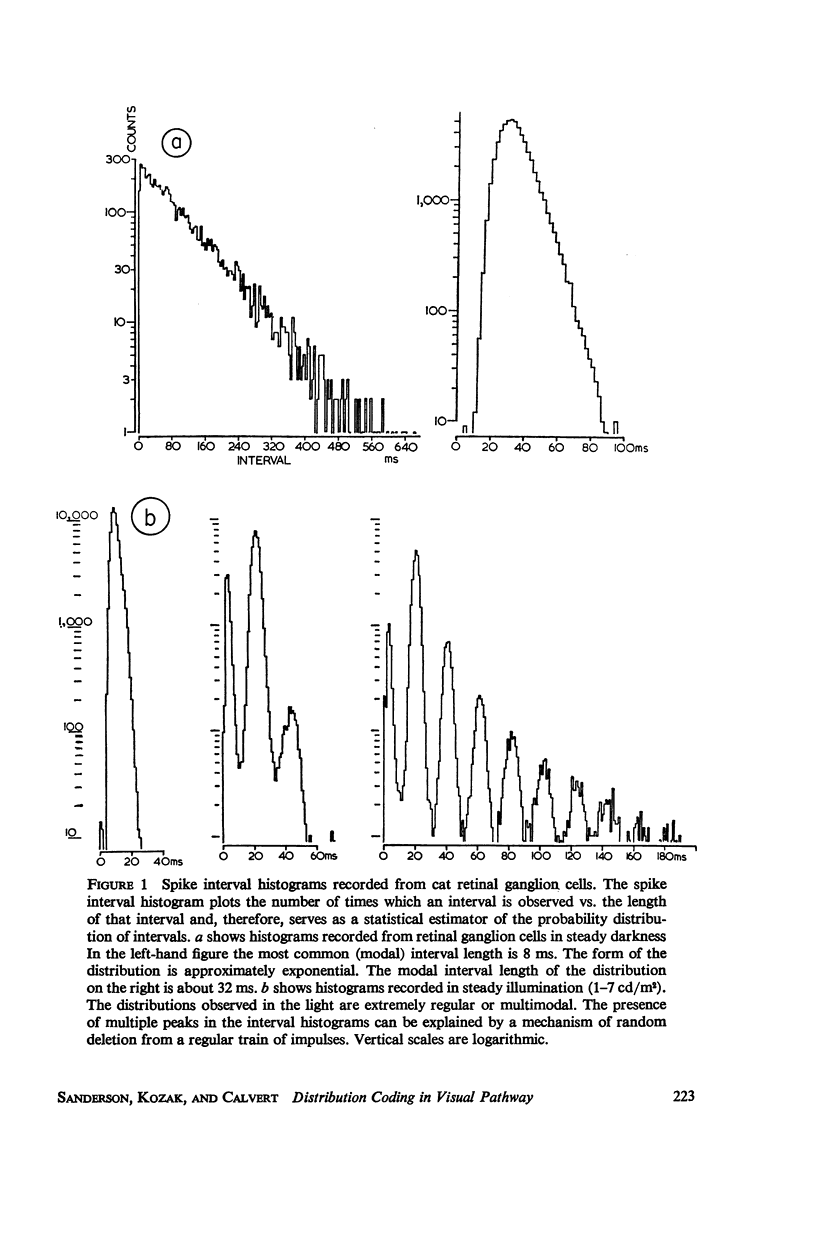
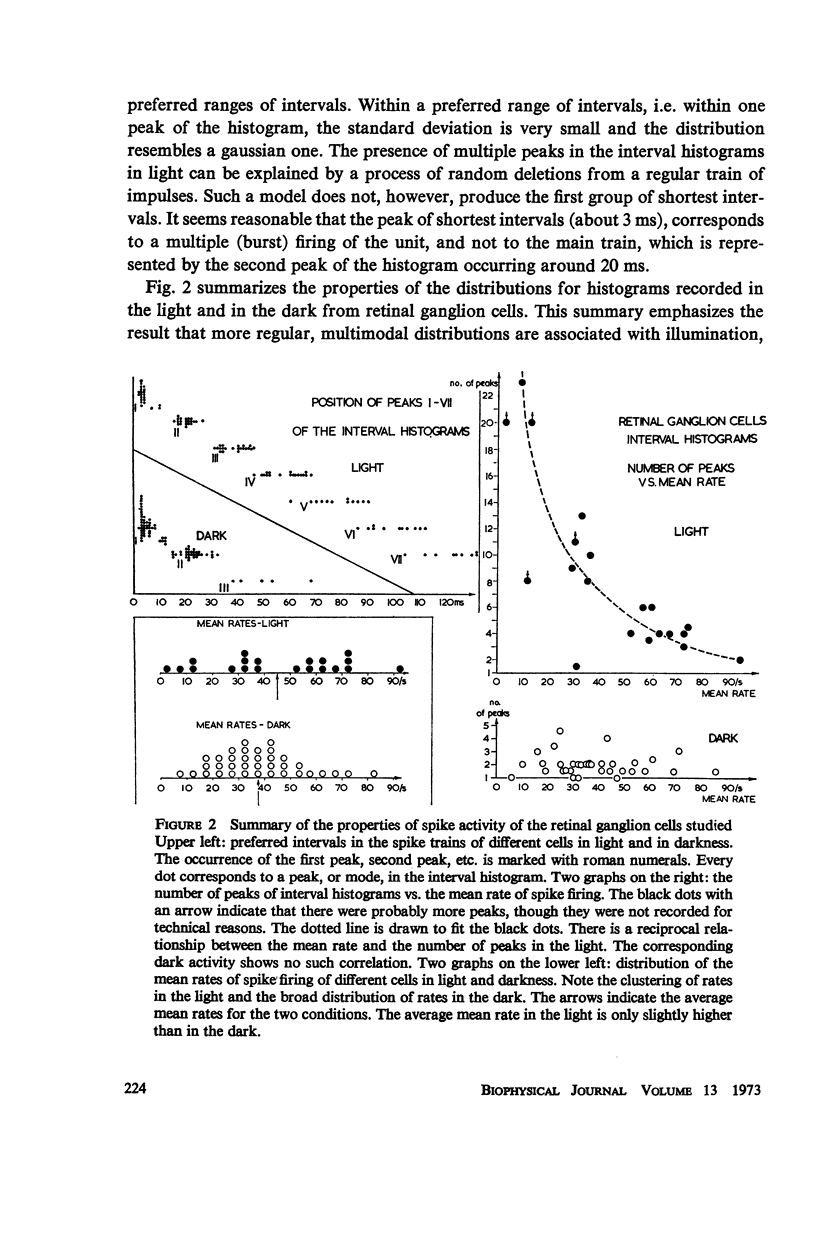
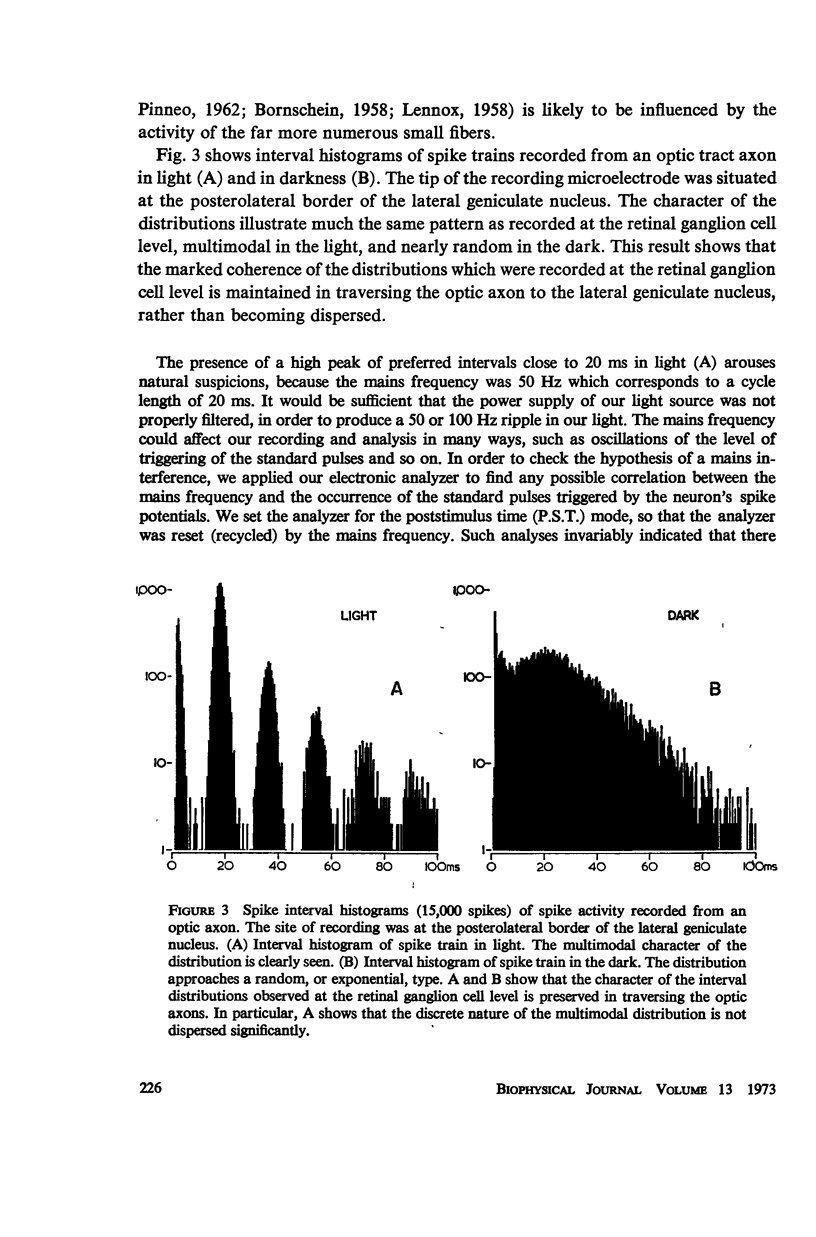
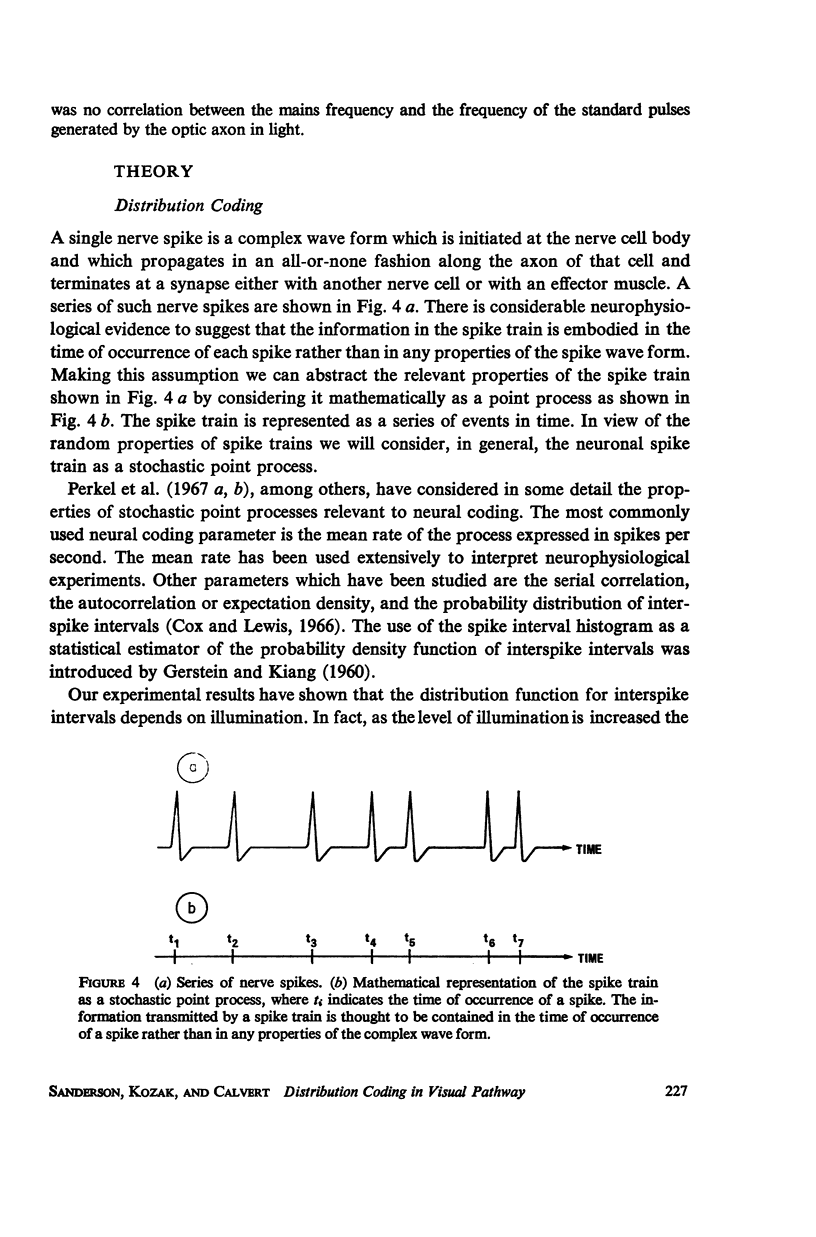
Although a variety of types of spike interval histograms have been reported, little attention has been given to the spike interval distribution as a neural code and to how different distributions are transmitted through neural networks. In this paper we present experimental results showing spike interval histograms recorded from retinal ganglion cells of the cat. These results exhibit a clear correlation between spike interval distribution and stimulus condition at the retinal ganglion cell level. The averaged mean rates of the cells studied were nearly the same in light as in darkness whereas the spike interval histograms were much more regular in light than in darkness. We present theoretical models which illustrate how such a distribution coding at the retinal level could be “interpreted” or recorded at some higher level of the nervous system such as the lateral geniculate nucleus. Interpretation is an essential requirement of a neural code which has often been overlooked in modeling studies. Analytical expressions are derived describing the role of distribution coding in determining the transfer characteristics of a simple interaction model and of a lateral inhibition network. Our work suggests that distribution coding might be interpreted by simply interconnected neural networks such as relay cell networks, in general, and the primary thalamic sensory nuclei in particular.
Full text
PDF













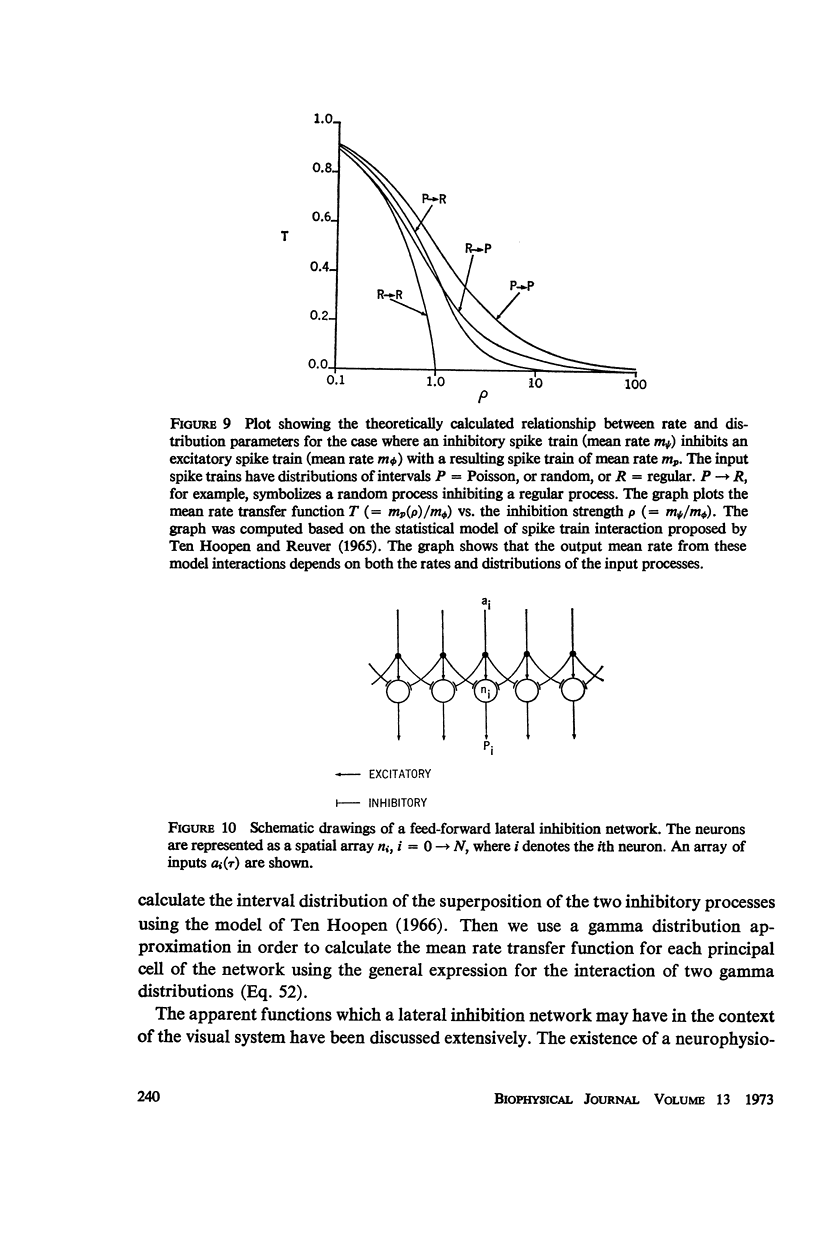
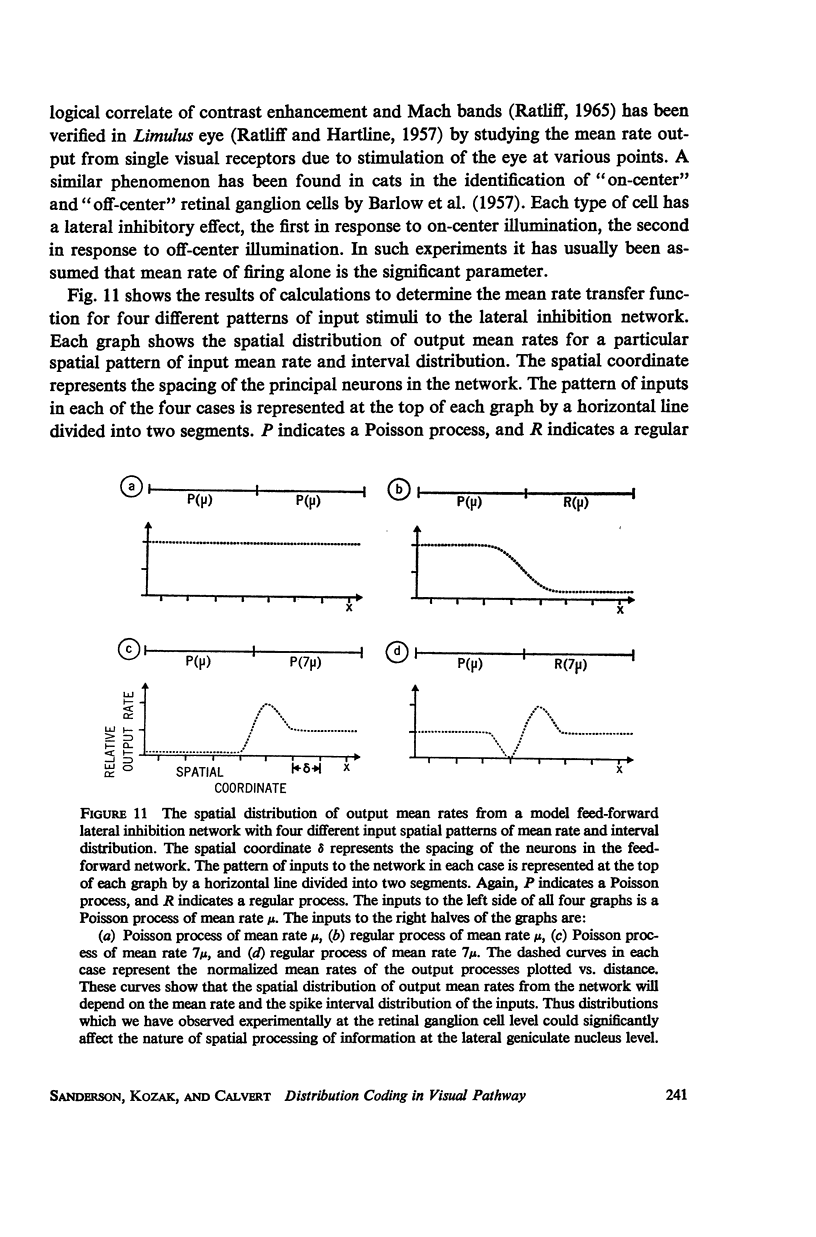



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARDUINI A., PINNEO L. R. Properties of the retina in response to steady illumination. Arch Ital Biol. 1962 Oct;100:425–448. [PubMed] [Google Scholar]
- BISHOP G. H., CLARE M. H. Organization and distribution of fibers in the optic tract of the cat. J Comp Neurol. 1955 Oct;103(2):269–304. doi: 10.1002/cne.901030204. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., JEREMY D., LANCE J. W. The optic nerve; properties of a central tract. J Physiol. 1953 Aug;121(2):415–432. doi: 10.1113/jphysiol.1953.sp004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., LEVICK W. R., VAKKUR G. J. The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol. 1962 Oct;163:503–539. doi: 10.1113/jphysiol.1962.sp006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., LEVICK W. R., WILLIAMS W. O. STATISTICAL ANALYSIS OF THE DARK DISCHARGE OF LATERAL GENICULATE NEURONES. J Physiol. 1964 Apr;170:598–612. doi: 10.1113/jphysiol.1964.sp007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORNSCHEIN H. Spontan- und Belichtungsaktivität in Einzelfasern des N. opticus der Katze. I. Der Einfluss kurzdauernder retinaler Ischämie. Z Biol. 1958 May;110(3):210–222. [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Inhibitory mechanisms in lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):231–246. doi: 10.1113/jphysiol.1966.sp008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Shape and arrangement of columns in cat's striate cortex. J Physiol. 1963 Mar;165:559–568. doi: 10.1113/jphysiol.1963.sp007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Kozak W. M. Electroretinogram and spike activity in mammalian retina. Vision Res. 1971;Suppl 3:129–149. doi: 10.1016/0042-6989(71)90035-6. [DOI] [PubMed] [Google Scholar]
- LENNOX M. A. Single fiber responses to electrical stimulation in cat's optic tract. J Neurophysiol. 1958 Jan;21(1):62–69. doi: 10.1152/jn.1958.21.1.62. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Oyster C. W., Takahashi E. Rabbit lateral geniculate nucleus: sharpener of directional information. Science. 1969 Aug 15;165(3894):712–714. doi: 10.1126/science.165.3894.712. [DOI] [PubMed] [Google Scholar]
- Srinivasan S. K., Rajamannar G., Rangan A. Stochastic models for neuronal firing. Kybernetik. 1971 May;8(5):188–193. doi: 10.1007/BF00291120. [DOI] [PubMed] [Google Scholar]
- WHITFIELD I. C., EVANS E. F. RESPONSES OF AUDITORY CORTICAL NEURONS TO STIMULI OF CHANGING FREQUENCY. J Neurophysiol. 1965 Jul;28:655–672. doi: 10.1152/jn.1965.28.4.655. [DOI] [PubMed] [Google Scholar]
- Werner G., Whitsel B. L. Topology of the body representation in somatosensory area I of primates. J Neurophysiol. 1968 Nov;31(6):856–869. doi: 10.1152/jn.1968.31.6.856. [DOI] [PubMed] [Google Scholar]


