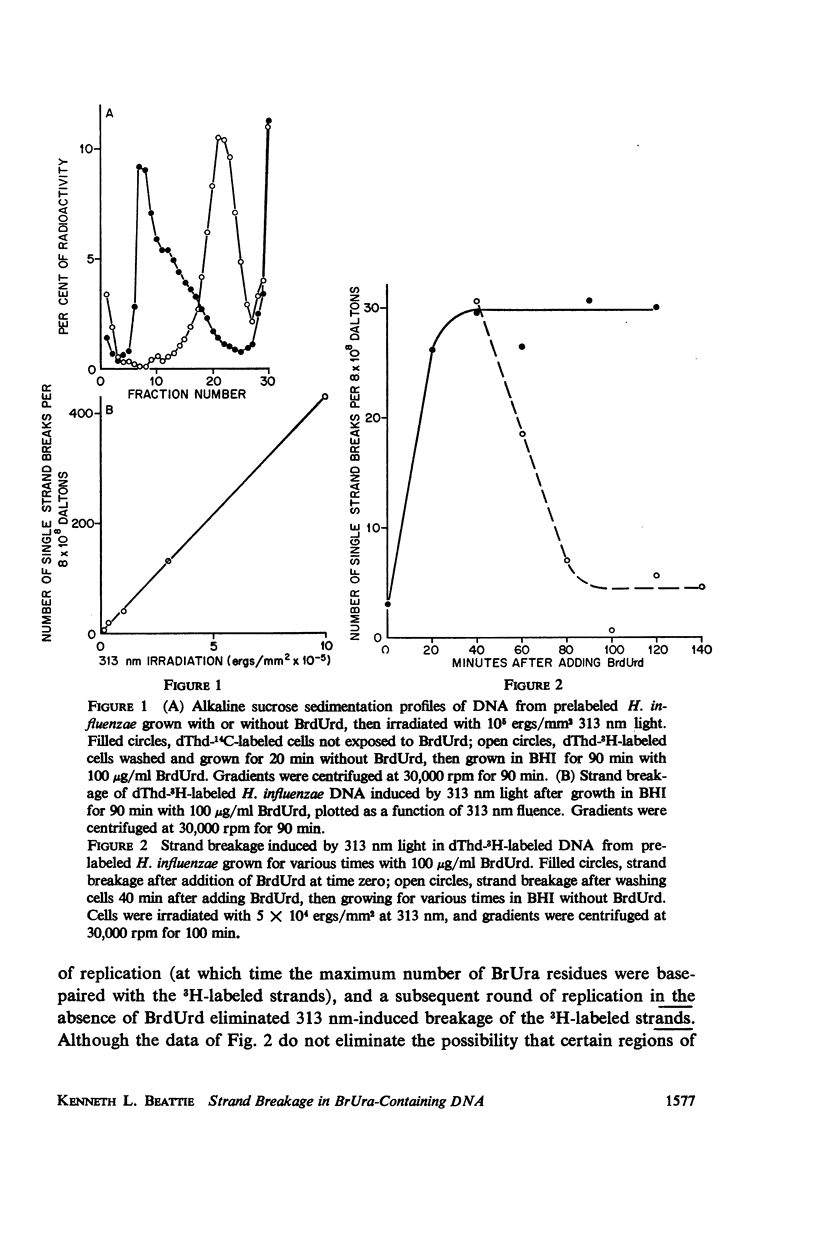
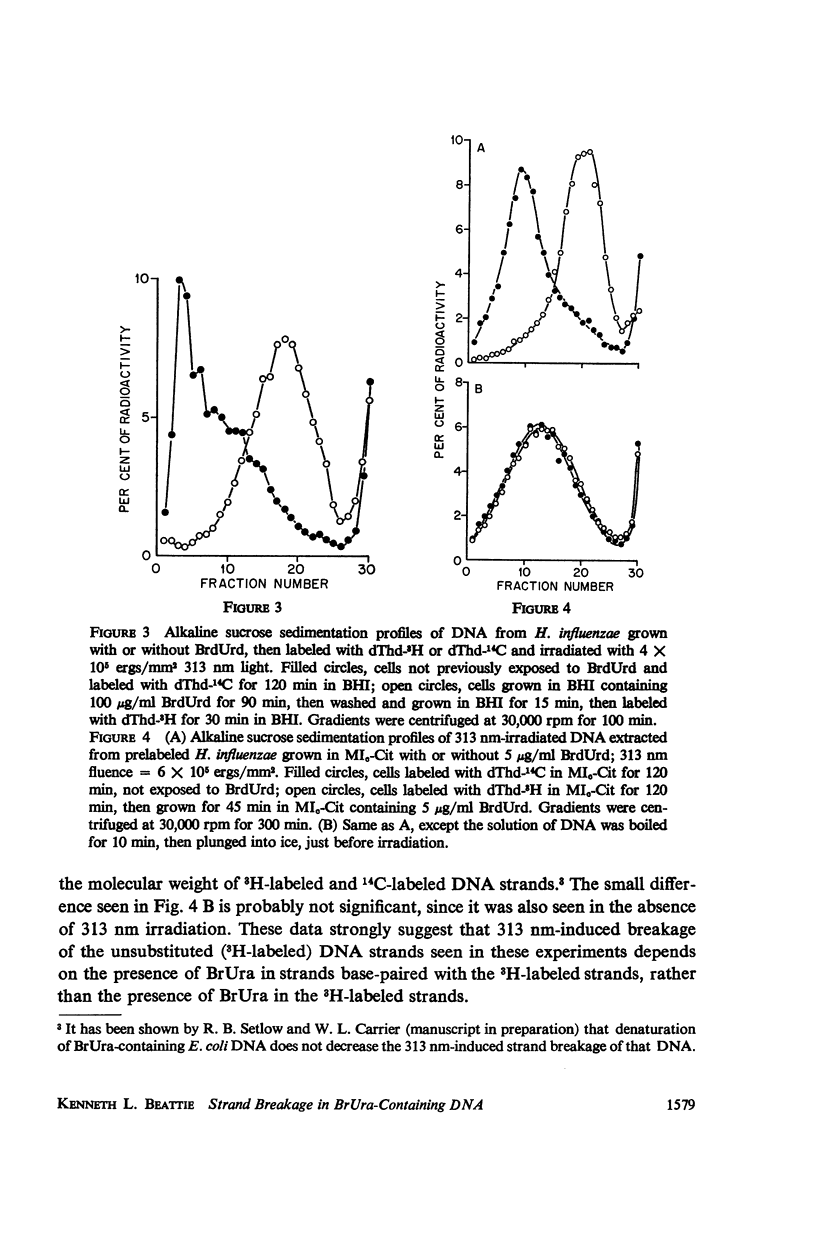
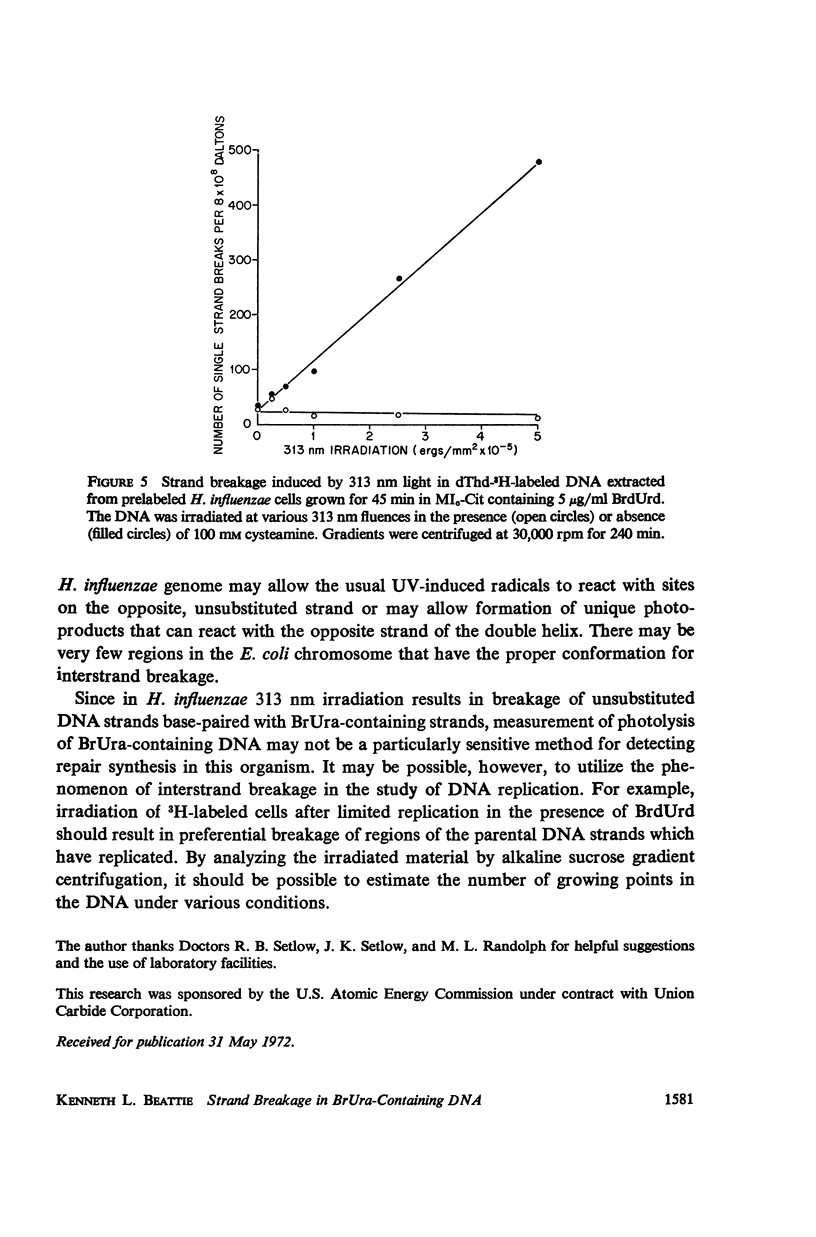
Abstract
Haemophilus influenzae was labeled with thymidine-3H (dThd), then grown in the presence of 5-bromodeoxyuridine (BrdUrd), and then irradiated with 313 nm light (a wavelength that selectively photolyzes DNA containing 5-bromouracil [BrUra]). Irradiation with 313 nm light induced breaks in the 3H-labeled strands in cells grown with BrdUrd at a much higher frequency than in 14C-labeled DNA of cells not exposed to BrdUrd. Breakage of the 3H-labeled strands was about 0.6% as efficient as that of fully BrUra-substituted DNA. During growth in the presence of BrdUrd, susceptibility to 313 nm-induced breakage of the 3H-labeled DNA strands increased, reaching a maximum in about one generation, and it decreased to zero during subsequent growth for one generation in medium containing dThd instead of BrdUrd. Heat denaturation of DNA extracted from dThd-3H-labeled cells grown in the presence of BrdUrd eliminated 313 nm-induced breakage of the 3H-labeled strands. It is concluded that breakage of the 3H-labeled DNA strands resulted from reaction with photoproducts in the base-paired, BrUra-containing strands, rather than from photolysis of BrdUrd incorporated into parental strands. It may be possible to utilize the phenomenon of interstrand breakage in physical studies of DNA replication.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie K. L., Setlow J. K. Transformation-defective strains of Haemophilus influenzae. Nat New Biol. 1971 Jun 9;231(23):177–179. doi: 10.1038/newbio231177a0. [DOI] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. Dependence of Vegetative Recombination Among Haemophilus influenzae Bacteriophage on the Host Cell. J Virol. 1969 Sep;4(3):240–243. doi: 10.1128/jvi.4.3.240-243.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram S. Secondary structure of DNA depends on base composition. Nat New Biol. 1971 Aug 11;232(2):174–176. doi: 10.1038/newbio232174a0. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. Y., Vogt M., Modan M. Defined medium for growth of Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):513–516. doi: 10.1128/jb.101.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz G., Reuschl H. Damage to deoxyribose molecules and to U-gene reactivation in UV-irradiated 5-bromouracil-DNA of phage T4 Bor as influenced by cysteamine. Mol Gen Genet. 1967;99(1):5–11. doi: 10.1007/BF00306453. [DOI] [PubMed] [Google Scholar]
- Hutchinson F., Hales H. B. Mechanism of the sensitization of bacterial transforming DNA to ultraviolet light by the incorporation of 5-bromouracil. J Mol Biol. 1970 May 28;50(1):59–69. doi: 10.1016/0022-2836(70)90103-8. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Wang S. Y. Photochemistry of 5-bromo-1,3-dimethyluracil in aqueous solution. Biochemistry. 1966 Jul;5(7):2302–2307. doi: 10.1021/bi00871a019. [DOI] [PubMed] [Google Scholar]
- Köhnlein W., Hutchinson F. ESR-studies of normal and 5-bromouracil-substituted DNA of Bacillus subtilis after irradiation with ultraviolet light. Radiat Res. 1969 Sep;39(3):745–757. [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Ley R. D., Setlow R. B. Repair replication in Escherichia coli as measured by the photolysis of bromodeoxyuridine. Biophys J. 1972 Apr;12(4):420–431. doi: 10.1016/S0006-3495(72)86094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- RUPP W. D., PRUSOFF W. H. PHOTOCHEMISTRY OF IODOURACIL. II. EFFECTS OF SULFUR COMPOUNDS, ETHANOL AND OXYGEN. Biochem Biophys Res Commun. 1965 Jan 18;18:158–164. [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B., Ley R. D. Normal and defective repair of damaged DNA in human cells: a sensitive assay utilizing the photolysis of bromodeoxyuridine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):708–712. doi: 10.1073/pnas.68.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]


