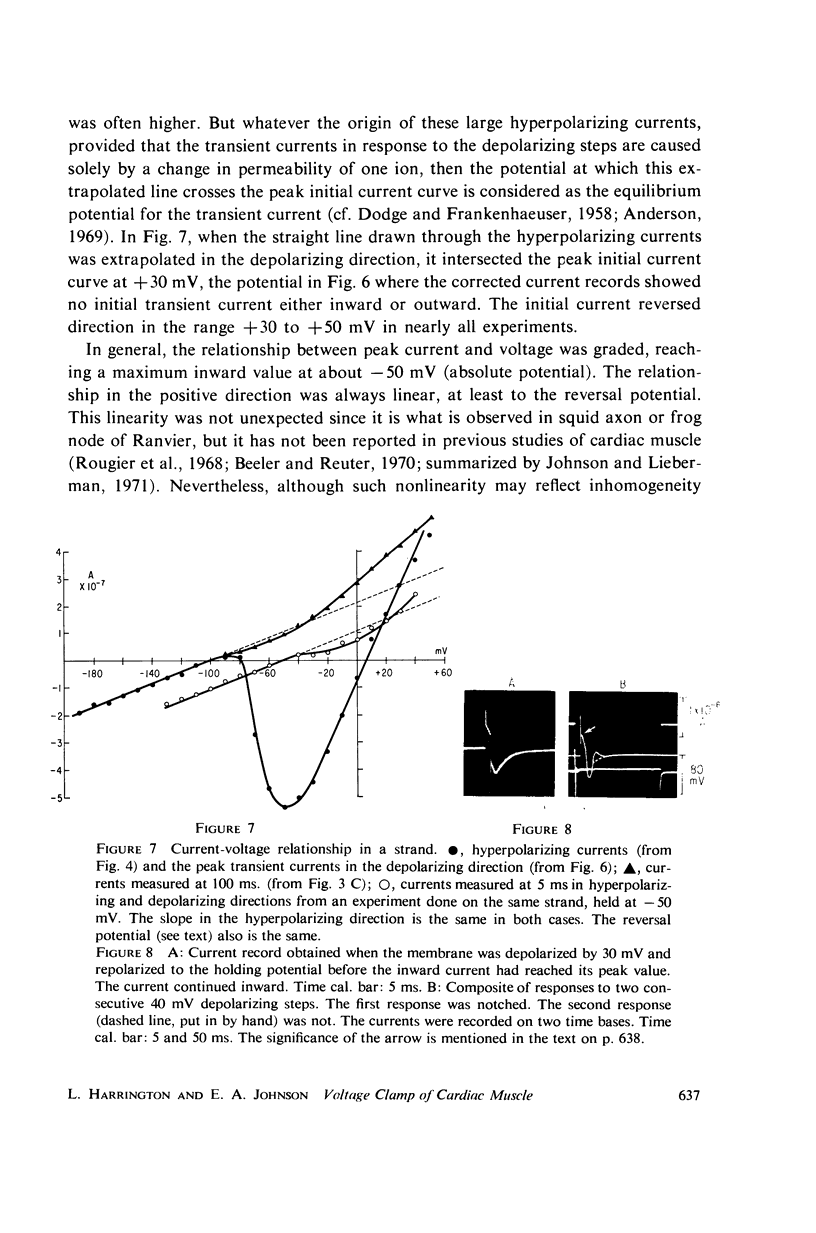
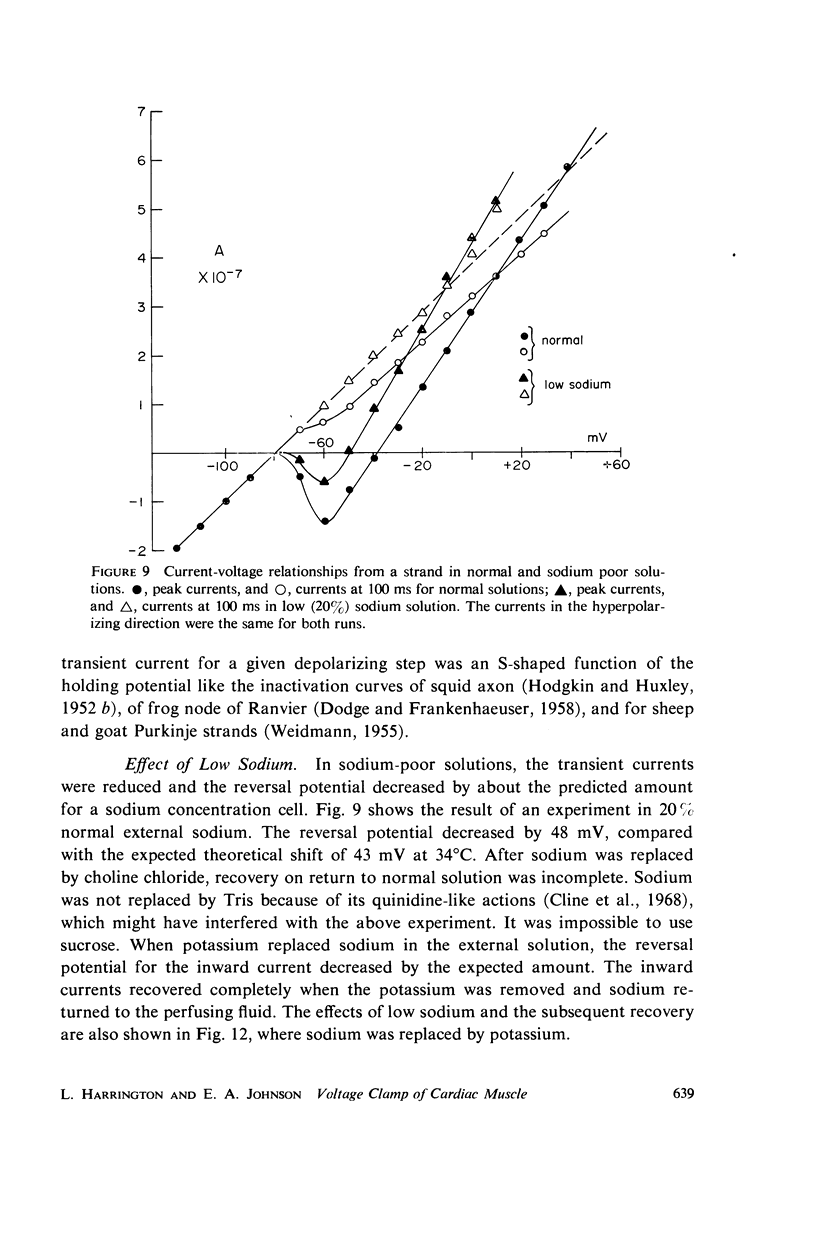
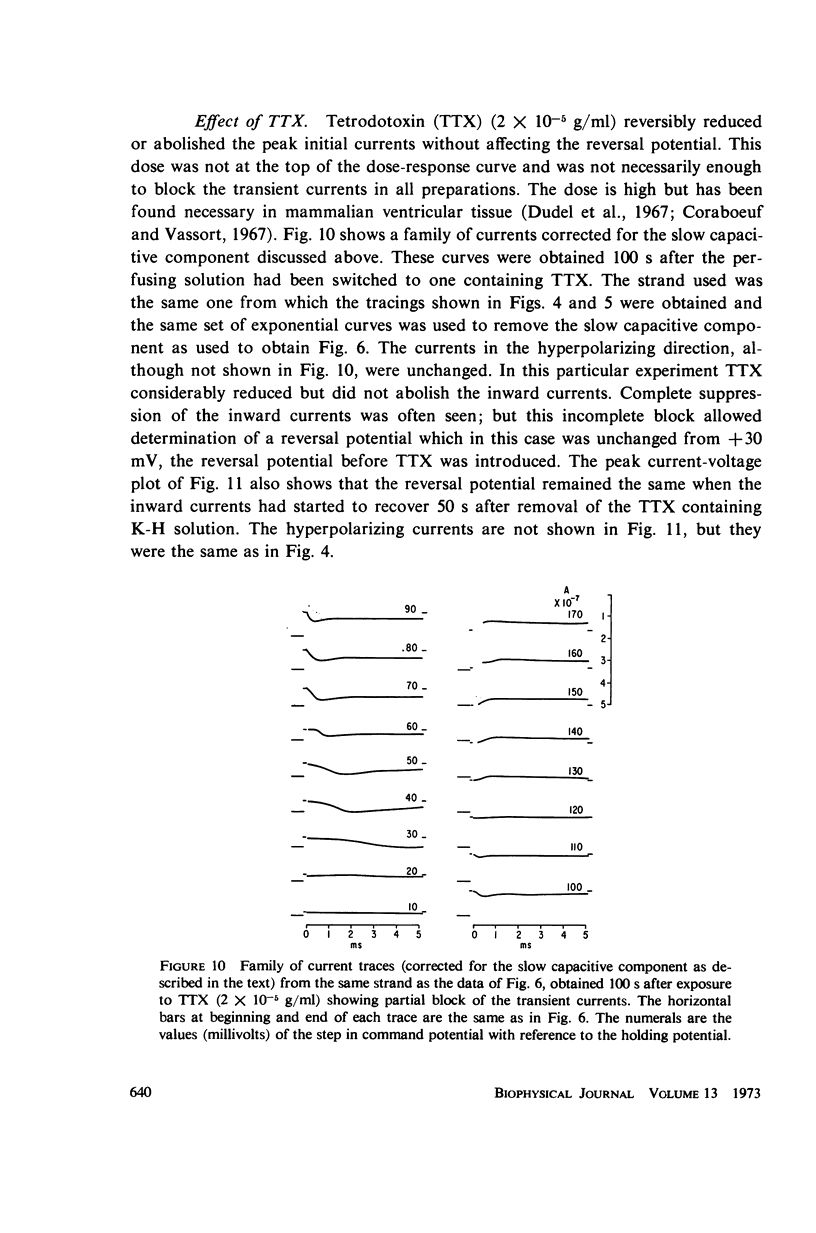
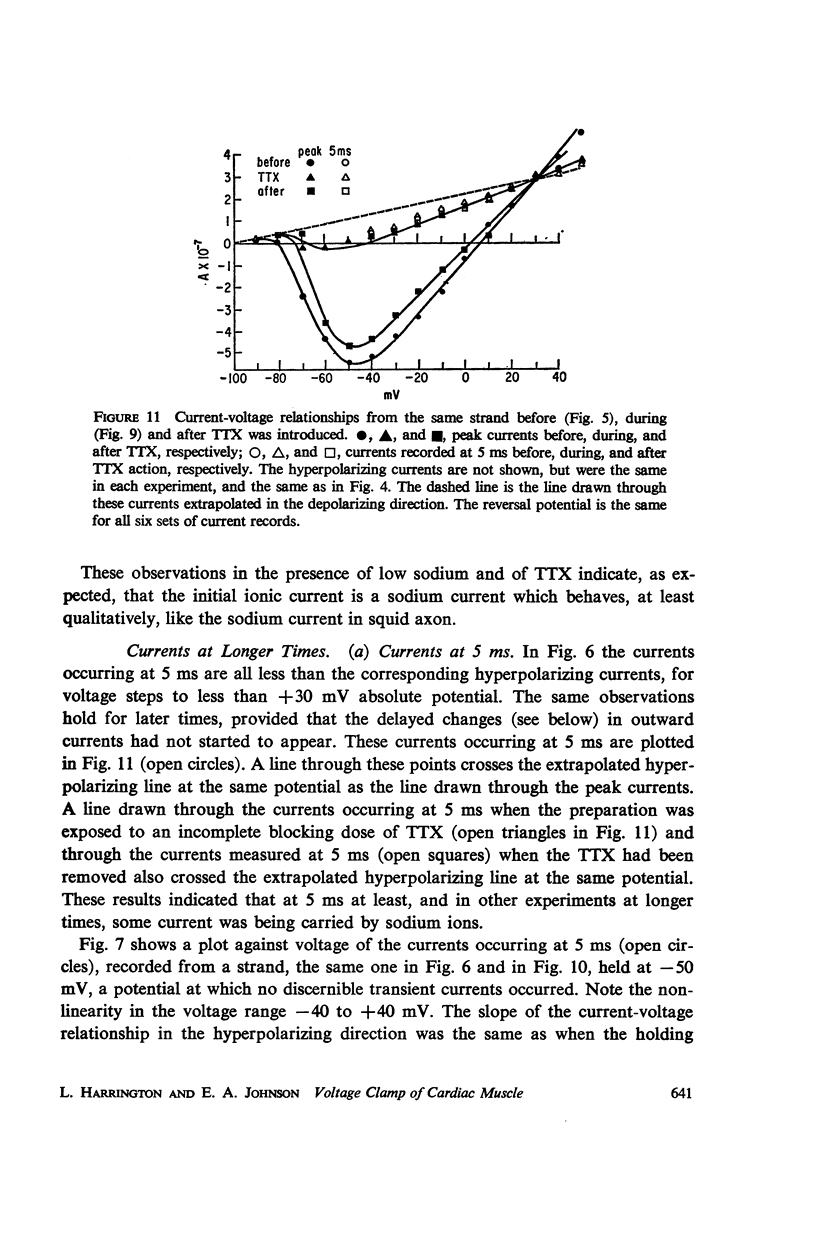
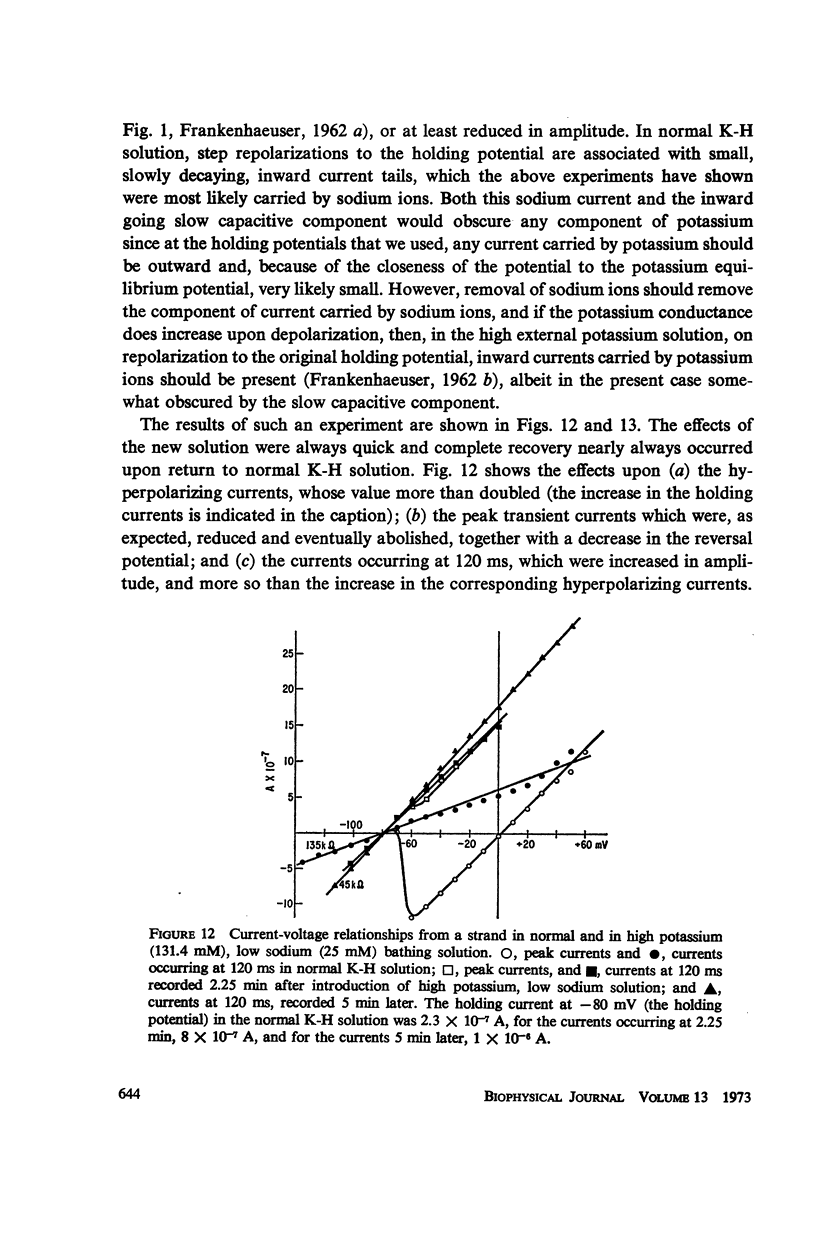
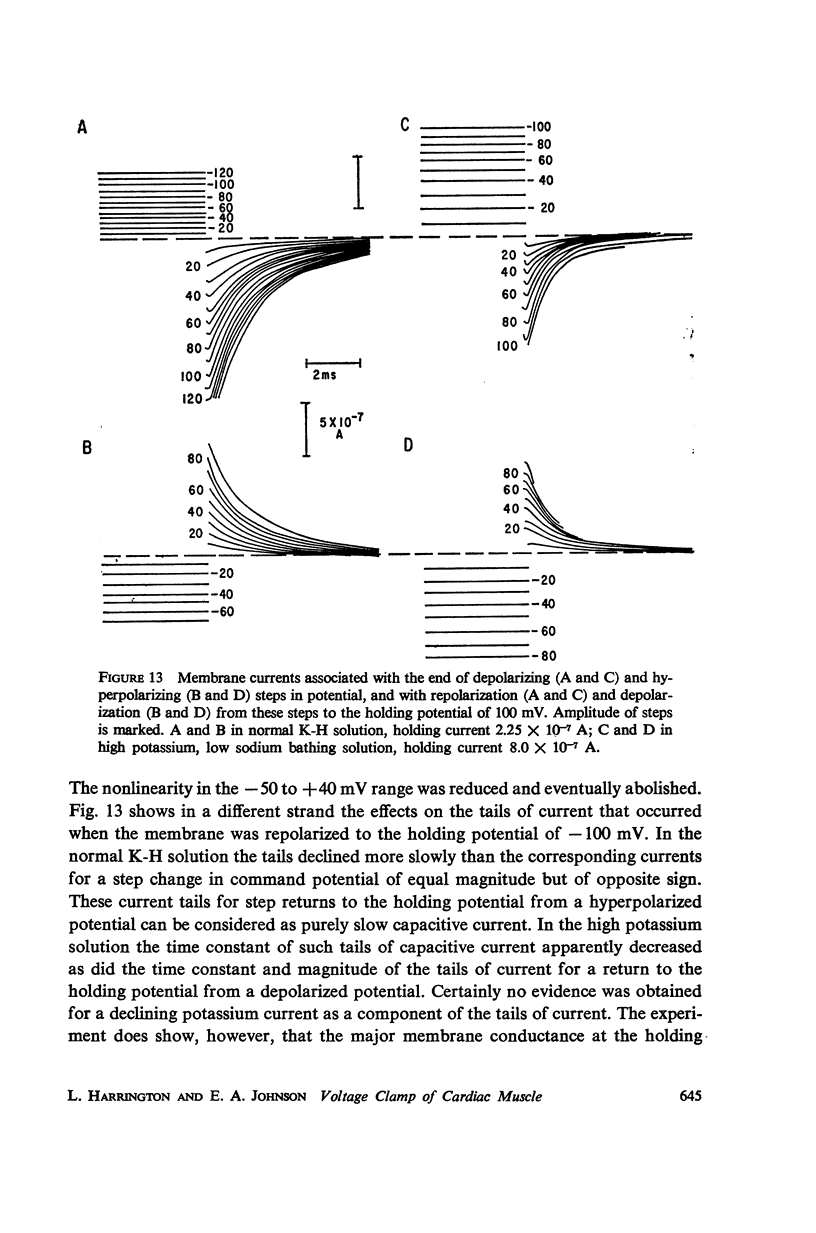
Abstract
A method of stabilizing the membrane potential of a small area of cardiac muscle membrane and the limitations of this method are described. Tiny bundles or strands, approximately 80 μm in diameter, of electrically interconnected fibers from the ventricles of rabbit hearts were used in a double sucrose gap. Current records associated with step changes in voltage were complicated by two capacitive surges of current of nodal and nonnodal origin and large “leakage” currents of nonnodal origin resulting mainly from the multifibered nature of the preparation and emphasized by the method. The transient, inward membrane currents in response to moderate depolarizing steps in command potential had the same duration as the upstroke of the action potential. In good runs, currents were smooth and free from notches. These initial currents behaved qualitatively like the initial sodium currents in squid axon and in other excitable membranes. A fraction of the initial sodium current persisted at least as long as 300 ms. The relationship between peak initial current and voltage was graded and linear in the positive direction. In the negative region the relationship was often very steep, indicating insufficient voltage control of all the membranes despite the squareness of the voltage record. Other indications of inadequacy of control could occur and thus even with this optimum preparation of cardiac muscle it was not feasible to analyze quantitatively either the initial or the prolonged sodium currents.
Full text
PDF



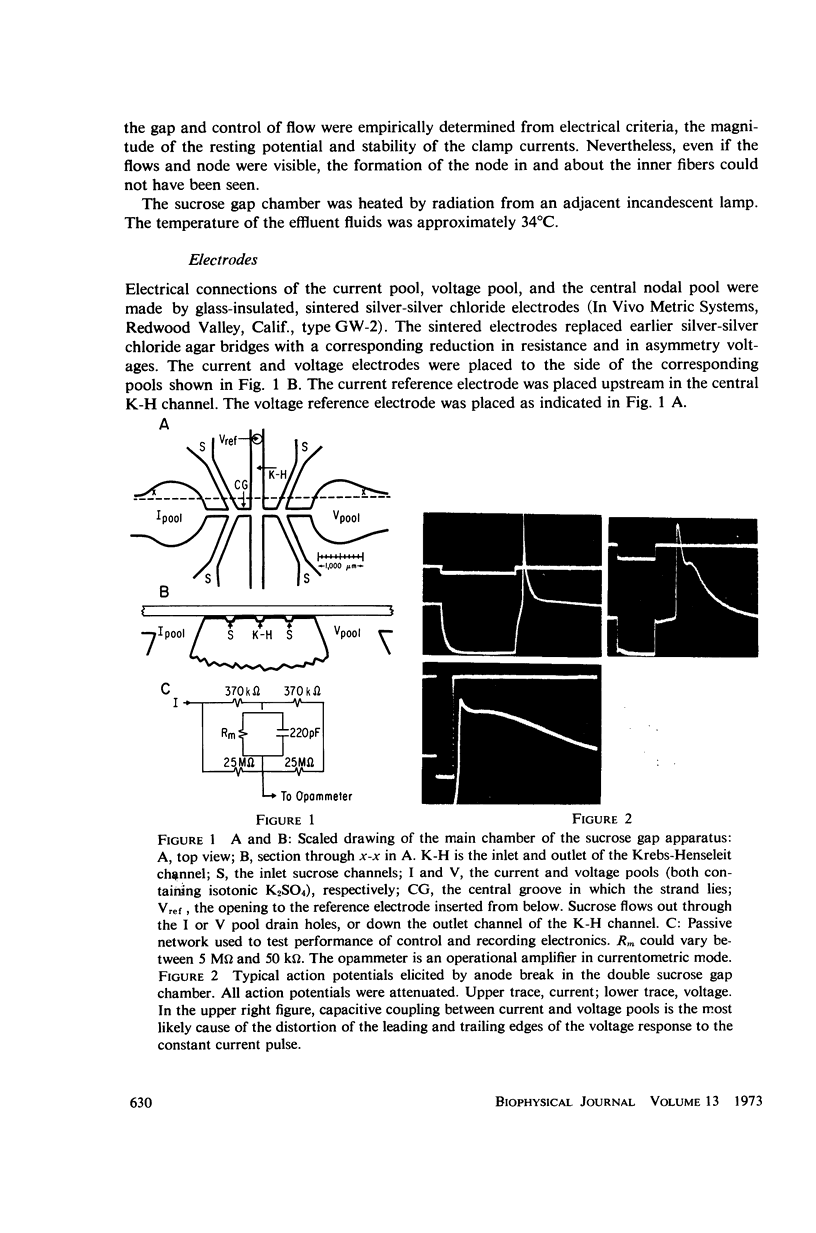


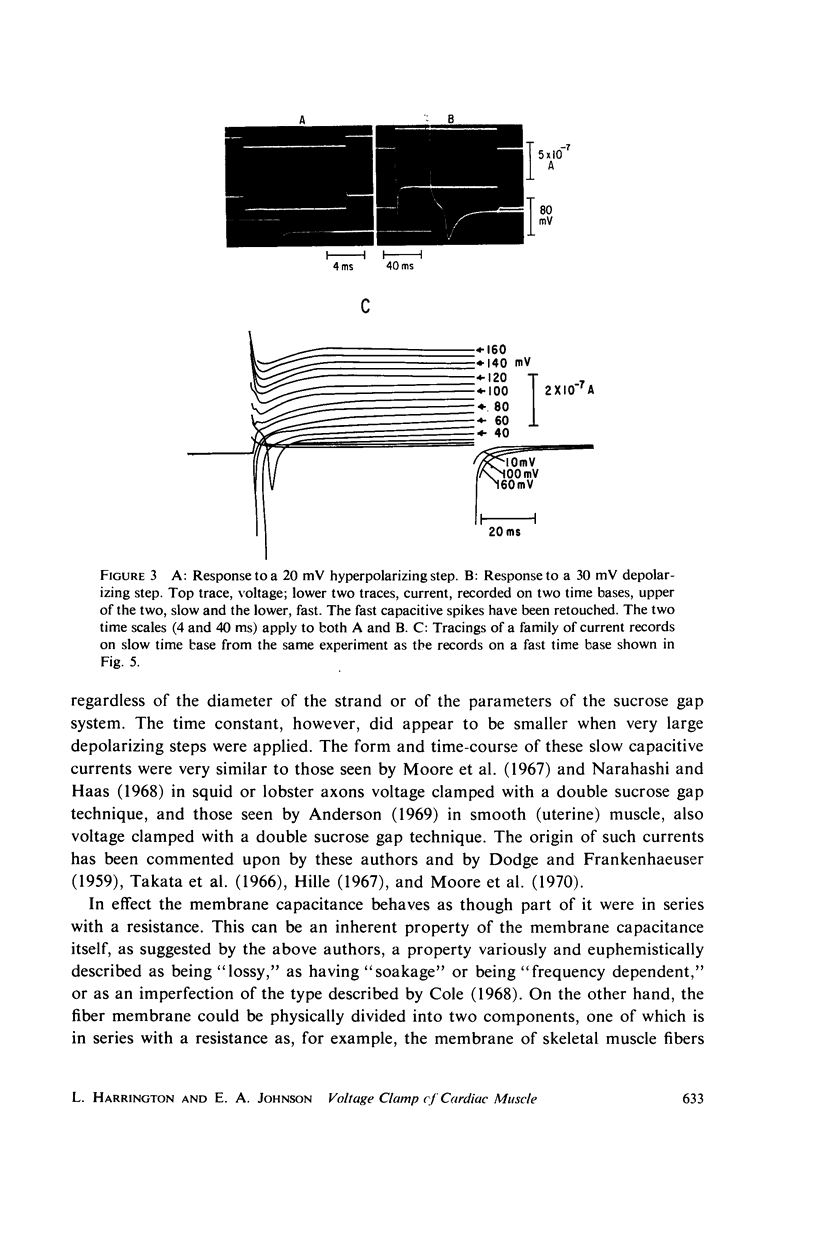
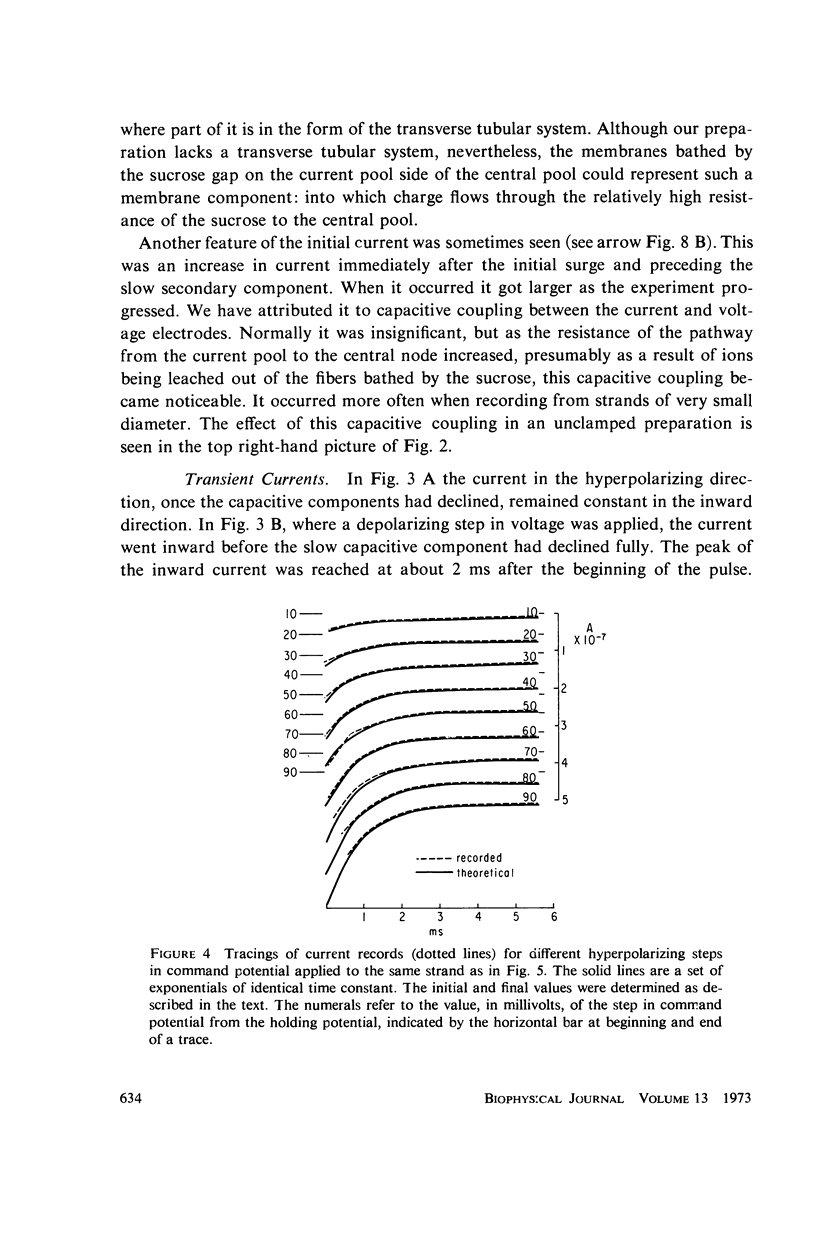
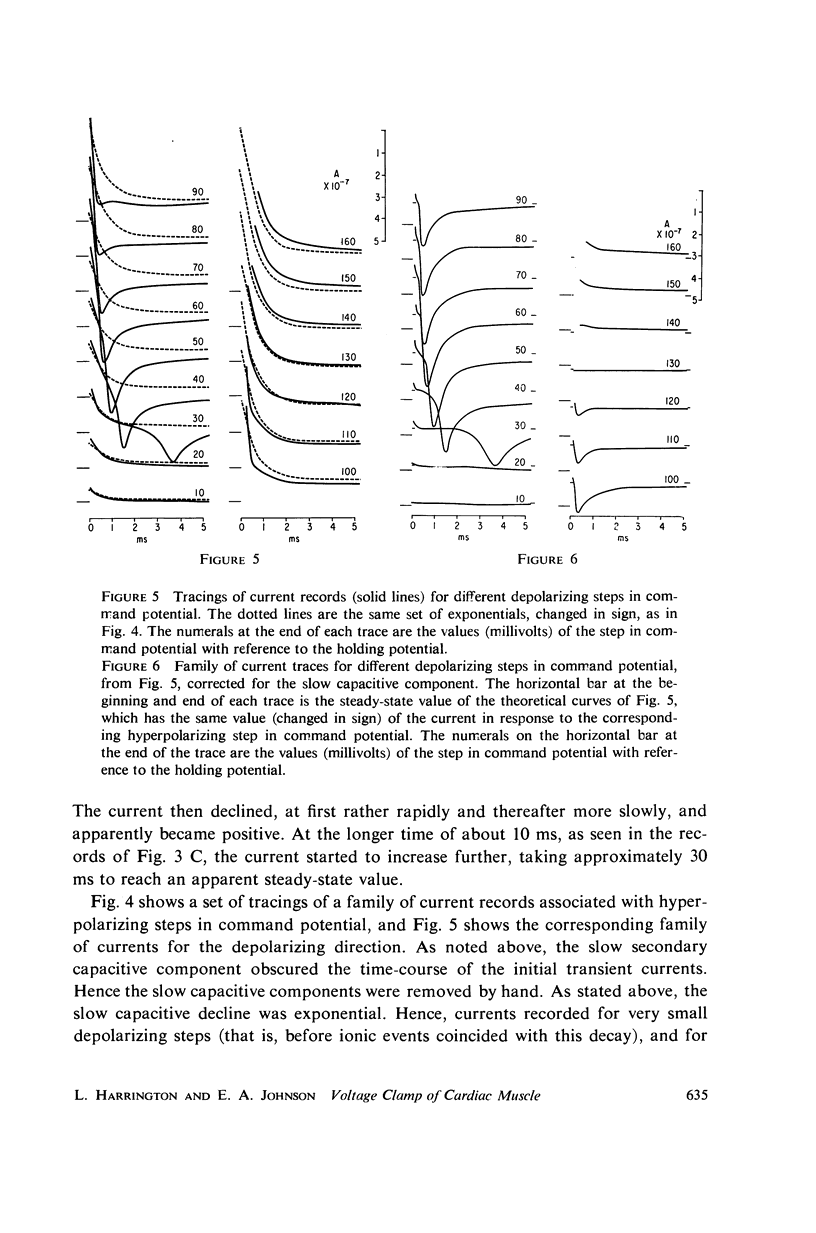






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. Competitive action of calcium and procaine on lobster axon. A study of the mechanism of action of certain local anesthetics. J Gen Physiol. 1966 May;49(5):1043–1063. doi: 10.1085/jgp.49.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline R. E., Wallace A. G., Sealy W. C., Young W. G. Antiarrhythmic properties of Tris (hydroxymethyl aminomethane). Am J Cardiol. 1968 Jan;21(1):38–43. doi: 10.1016/0002-9149(68)90011-8. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E., Vassort G. Effets de la tétrodotoxine, du tétraéthylammonium et du manganèse sur l'activité du myocarde de rat et de cobaye. C R Acad Sci Hebd Seances Acad Sci D. 1967 Feb 20;264(8):1072–1075. [PubMed] [Google Scholar]
- DECK K. A., KERN R., TRAUTWEIN W. VOLTAGE CLAMP TECHNIQUE IN MAMMALIAN CARDIAC FIBRES. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:50–62. doi: 10.1007/BF00412615. [DOI] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. The effect of tetrodotoxin on the membrane current in cardiac muscle (Purkinje fibers). Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;295(3):213–226. doi: 10.1007/BF01844101. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Sommer J. R. A strand of cardiac muscle. Its ultrastructure and the electrophysiological implications of its geometry. J Cell Biol. 1967 Apr;33(1):103–129. doi: 10.1083/jcb.33.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher D., Peper K. Two components of inward current in myocardial muscle fibers. Pflugers Arch. 1969;307(3):190–203. doi: 10.1007/BF00592084. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The time and voltage dependence of the slow outward current in cardiac Purkinje fibres. J Physiol. 1966 Oct;186(3):632–662. doi: 10.1113/jphysiol.1966.sp008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Blaustein M. P., Anderson N. C., Narahashi T. Basis of tetrodotoxin's selectivity in blockage of squid axons. J Gen Physiol. 1967 May;50(5):1401–1411. doi: 10.1085/jgp.50.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Trautwein W. The effect of the duration of the action potential on contraction in the mammalian heart muscle. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299(1):66–82. doi: 10.1007/BF00362542. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Haas H. G. Interaction of DDT with the components of lobster nerve membrane conductance. J Gen Physiol. 1968 Feb;51(2):177–198. doi: 10.1085/jgp.51.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi R. The slow inward current and the action of manganese ions in guinea-pig's myocardium. Pflugers Arch. 1970;316(1):81–94. doi: 10.1007/BF00587898. [DOI] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Stämpfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. doi: 10.1007/BF00362729. [DOI] [PubMed] [Google Scholar]
- STAMPFLI R. A new method for measuring membrane potentials with external electrodes. Experientia. 1954 Dec 15;10(12):508–509. doi: 10.1007/BF02166189. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative ultrastructural study with special reference to frog and chicken hearts. Z Zellforsch Mikrosk Anat. 1969;98(3):437–468. [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Pickard W. F., Lettvin J. Y., Moore J. W. Ionic conductance changes in lobster axon membrane when lanthanum is substituted for calcium. J Gen Physiol. 1966 Nov;50(2):461–471. doi: 10.1085/jgp.50.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. The effect of the cardiac membrane potential on the rapid availability of the sodium-carrying system. J Physiol. 1955 Jan 28;127(1):213–224. doi: 10.1113/jphysiol.1955.sp005250. [DOI] [PMC free article] [PubMed] [Google Scholar]


