Abstract
Alphaviral replicons can increase the efficacy and immunogenicity of naked nucleic acid vaccines. To study the impact of apoptosis on this increased effectiveness, we co-delivered an anti-apoptotic gene (Bcl-XL) with the melanocyte/melanoma differentiation antigen TRP-1. Although cells co-transfected with Bcl-XL lived longer, produced more antigen and elicited increased antibody production in vivo, co-delivery of pro-survival Bcl-XL with antigen significantly reduced the ability of the replicase-based vaccine to protect against an aggressive tumor challenge. These data show for the first time that the induction of apoptotic cell death of transfected cells in vivo is required for the increased effectiveness of replicase-based vaccines. Our findings also provide an explanation for the paradoxical observation that replicase-based DNA vaccines are much more immunogenic than conventional constructs despite reduced antigen production.
Keywords: DNA vaccines, Alphavirus, Apoptosis, Bcl-XL
1. Introduction
The role of apoptosis in the induction or prevention of an immune response has recently been debated. While some groups have shown that the uptake of apoptotic cells by dendritic cell results in T-cell tolerance [1–3], others have demonstrated that antigen produced by apoptotic cells increased the immunogenicity of the antigen [4–6]. The reason for this apparent controversy may be due to artifacts such as induction of heat shock proteins in vitro due to cell culture or assay conditions, which can stimulate dendritic cells [7,8]. In addition, it is certainly misleading to view apoptosis as a single, defined pathway by which cells undergo (programmed) cell death. A wide variety of stimuli induce apoptosis. While all of them eventually result in the death of the cell, the context of the death process varies widely [9]. Therefore, a distinction can be made between bland apoptosis, inflammatory apoptosis, and immune apoptosis [10,11]. This classification provides an explanation for how programmed cell death can result in ignorance of antigen by the immune system (e.g., when it occurs as part of a developmental process), tolerance (e.g., when self-antigen from naturally dying host cells are presented to naïve T cells), or immunity (e.g., when cells die as a consequence of infection).
Mammalian cells have developed a variety of mechanisms to recognize and respond to viral infections. When RNA viruses replicate in a host cell they produce dsRNA, which acts as a very strong inducer of anti-viral pathways. The key molecules in this process are RNase L and the RNA-dependent protein kinase PKR, which are responsible for degradation of mRNA and inhibition of protein translation, respectively, which leads to the apoptotic death of the virus infected cell [12–15]. Recognition of a viral infection by a mammalian cell induces apoptosis to limit or prevent virus propagation. During this process, various factors are released and molecules expressed that attract and activate antigen-presenting cells thus initiating an immune response to viral antigens.
We and others have previously described a new generation of nucleic acid vaccines where expression of the encoded antigen is under the control of an alphaviral RNA replicase enzyme [16–21]. While these replicase-based DNA vectors were originally developed with the goal to produce more antigen, we have consistently found that the increased efficacy of these vaccines may not simply be based on increased antigen production [22,23]. Instead, cells trans-fected with replicase-based nucleic acid vaccines appear to respond to the transfection by activating the same anti-viral defense pathways (including the PKR and RNase L pathway) that are triggered by the infection with an alphavirus. We hypothesize that the “stress”-response of the cell, including the eventual apoptotic death, provides a strong adjuvant-type signal that enhances the immune response to the plasmid-encoded antigen. In this model, induction of apoptosis is crucial for the increased immunogenicity of replicase-based nucleic acid vaccines. Others have previously demonstrated that the induction of apoptosis by co-delivery of either the genes for CD95/Fas or caspases enhances the immunogenicity and efficacy of conventional DNA vaccines [24,25] establishing a link between apoptosis and enhanced immune responses. In our study, we asked the question whether apoptosis induced by replicase-based DNA vaccines plays a significant role in the efficacy of these vectors. We used Bcl-XL [26] to interfere with vaccine-induced apoptosis [6,23], since this member of the Bcl-2 family of anti-apoptotic proteins has previously been shown to prolong the survival of alphavirus-infected cells [27]. Here, we demonstrate for the first time that interfering with apoptosis in cells transfected with a replicase-based DNA vaccine significantly reduces the efficacy of these vaccines. Thus, these data validate our hypothesis that “inflammatory apoptosis”, induced by alphavirus replicase-based vaccines, is critical for the immunogenicity of this new generation of nucleic acid vaccines.
2. Materials and methods
2.1. Plasmids and in vitro expression
Sindbis replicase-based plasmid vectors encoding EGFP and human or mouse TRP-1 have been described previously [23] as well as pSport-hTRP1 (here designated pCMV-hTRP1) [22]. pEGFP-C1 (here designated pCMV-EGFP) was obtained from Clontech Laboratories (Palo Alto, CA). pcDNA-hBcl-XL has previously been described [28]. Plasmids were purified using EndoFree purification columns (Qiagen, Hilden, Germany) and stored at 4 °C in TE-buffer. Transfection of BHK-21 (ATCC, Manassas, Virginia) cells with Lipofectamine PLUS (Invitrogen, Carlsbad, CA) was performed according to manufacturer’s instructions. Briefly, 1 μg DNA (single plasmid or 1:1 (w/w) plasmid mix for co-transfection experiments) was incubated with 15 μl PLUS-reagent for 15 min in 150 μl OptiMEM (Gibco/Invitrogen, Grand Island, NY) and subsequently with 15 μl Lipofectamine in 150 μl OptiMEM for 15 min. The resulting complexes were added to cells grown overnight in 6 well plates. In vitro expression of Bcl-XL was determined by Western blotting (NuPage system, Novex, San Diego, CA) followed by immunostaining of BHK-21 cell lysates. As a positive control, blots were double-stained with anti-GAPDH (clone 6C5, Research Diagnostics, Flanders, NJ). Antibody binding was detected using a biotin conjugated secondary antibody (goat anti-mouse/HRP, Amersham, Piscataway, NJ) and ECL-reagent (Pierce, Rockford, IL). The anti-Bcl-XL antibody (anti-human Bcl-XL clone 2H12) [29] was a kind gift of Dr. Yi-Te Hsu (University of Charleston, SC). In vitro expression of EGFP in transfected BHK-21 cells was determined by flow cytometry (FACScan, Becton Dickinson, San Jose, CA).
2.2. In vitro cell survival assay
BHK cells (1 × 105 cells per well, 6-well plate) were cultured for 24 h and transfected with 1 μg of DNA/well (mix of two plasmids—1:1, w/w) using LipofectAMINE PLUS. Cells were harvested 24 h after transfection and seeded in quadruplicate at approximately 50 cells per well in 96 well plates. Living EGFP+ cells were counted daily in a blinded fashion using a fluorescence microscopy [23]. For the apoptosis-inhibition assays, cells were kept in the presence of 20 μM/ml caspase inhibitors (peptides zVAD-fmk, BD-fmk and zFA-fmk; Enzyme Systems Products, Livermore, CA). Fresh caspase inhibitor was added every 24 h.
2.3. Cell sorting
BHK-21 cells were transfected with pCMV-EGFP using LipofectAMINE PLUS. After 24 h, cells were harvested with trypsin and double-sorted (Clinical Services Program, SAIC-Frederick, Maryland) using a FACStar Plus (Becton Dickinson).
2.4. Mice and immunization
C57BL/6 mice (National Cancer Institute/FCRDC, Frederick, MD) were used at 6–10 weeks of age. Plasmid-mixtures (1:1, w/w) were coated onto gold particles as described previously [30] and shot into the skin of the shaved abdomen using the Helios gene gun (BioRad, Hercules, CA) with helium at a pressure of 400 psi. Mice were immunized five times at weekly intervals with three shots per immunization (estimated amount of DNA is 3 μg). Seven to 10 days after the last immunization, mice were challenged subcutaneously with 1 × 105 B16.F10 (FCRDC, Frederick, MD). Tumor growth was monitored for at least 3 weeks after challenge in a blinded fashion. Five to eight mice per group were immunized and challenged in each experiment.
2.5. Serology
Mice immunized with TRP-1 DNA were bled 3 days before challenge. Serum was diluted in PBS/1% BSA and tested by ELISA. Plates (Nunc, Roskilde, Denmark) were coated with 50 ng recombinant mTRP-1 [31] per well. Secondary goat anti-mouse/HRP antibody (Amersham) was used at 1:3000. The color reaction after addition of TMB substrate solution (Endogen, Woburn, MA) was measured at 450 nm.
3. Results
3.1. Bcl-XL expression increases survival of cells co-transfected with either conventional or replicase-based DNA
We have previously shown the induction of caspase-dependent apoptosis in cells transfected with replicase-based DNA or RNA [23]. In addition, we have found that trans-fection with conventional, CMV-promoter driven DNA plasmids induces apoptosis but the rate of cell death induced by conventional DNA is much lower than that by replicase-based DNA. The survival of transfected cells can significantly be increased with a caspase-inhibitor demonstrating that the cell death appears to be caspase-dependent apoptosis (Fig. 1A). Furthermore, the cell death induced by the conventional DNA plasmid is dependent on the expression level of the transgene in individual transfected cells as demonstrated by differences in the survival curves of trans-fected cells sorted based on antigen expression (Fig. 1B). The high-expressor cells, which died most rapidly after transfection with the conventional plasmid, could be rescued by the addition of two different caspase inhibitors (Fig. 1C).
Fig. 1.
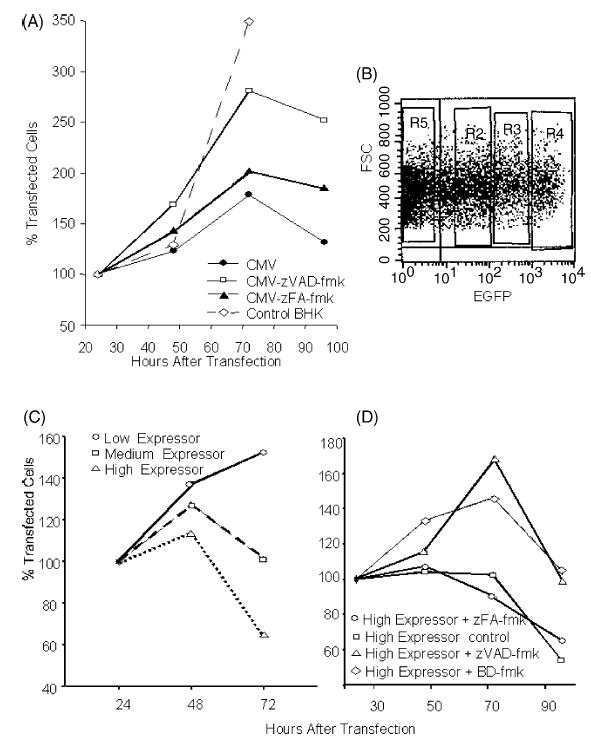
Induction of apoptosis by a conventional DNA vaccine: (A) Reduced cell proliferation after transfection with a conventional DNA plasmid is—at least in part—due to caspase-dependent apoptosis and cell survival can be prolonged with a caspase-inhibitor (zVAD-fmk). (B) Sorting of BHK-21 cells transfected with a conventional, EGFP expressing plasmid into three groups 24 h after transfection. Shown is the setting of the sorting windows in an FSC-FL1 scattergram. Medium-expressor cells are defined as cells expressing EGFP levels comparable to cells transfected with the replicase-based EGFP plasmid [23]. (C) Sorted cells are cultured and their survival is monitored. (D) Cell survival in the high-expressor group can be significantly prolonged by the addition of two different caspase inhibitors (zVAD-fmk, BD-fmk). Shown are the average values of quadruplicate wells (96-well plate) based on the number of cells seeded (set to 100%).
In contrast to the conventional plasmid, the survival of BHK-21 cells transfected with the replicase-based plasmid (pSin) is still significantly lower than of cells trans-fected with the conventional plasmid (P = 0.0001 for co-transfection with empty pcDNA plasmid, by StatView) (Fig. 2A). To determine the functionality of the Bcl-XL construct and its impact on cell death triggered by the two types of plasmids, BHK-21 cells were co-transfected with Bcl-XL and either replicase-based or conventional plasmid and counted daily. Bcl-XL overexpression significantly improved survival of cells transfected with either EGFP-construct (P = 0.017 for pCMV-EGFP and p < 0.0001 for pSin-EGFP using StatView) (Fig. 2A); however, the survival benefit was much more pronounced with the replicase-based plasmid. There was nearly a doubling of the survival time of Bcl-XL/pSin-co-transfected cells (Fig. 2A). In addition, the level of EGFP expression, as measured by flow cytometry, was elevated in cells co-transfected with Bcl-XL compared to empty plasmid all time points after transfection (not shown). Again, the effect was much more pronounced with the replicase-based plasmid than the conventional plasmid (5% increase for pCMV-EGFP versus 30% increased antigen production for pSin 48 h after transfection). Thus, Bcl-XL co-delivery increased antigen production in individual cells and increased the apparent number of cells transfected. These findings raised the question of how prolonged survival and increased antigen production associated with Bcl-XL co-transfection affected the in vivo immunogenicity of the antigens encoded on the co-delivered plasmids.
Fig. 2.
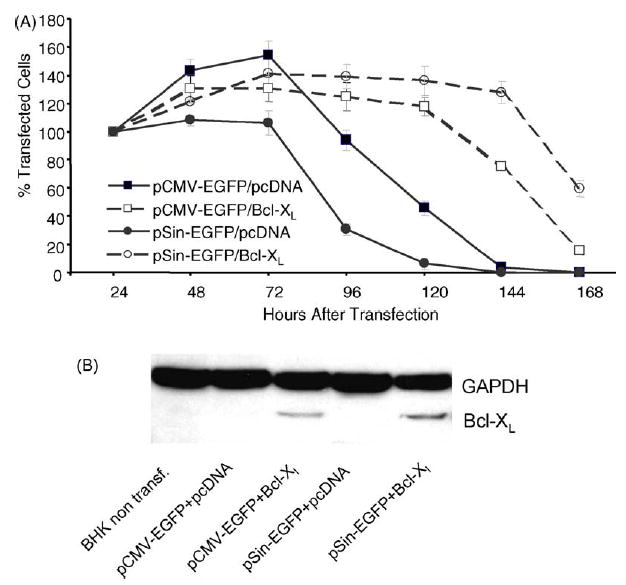
Effect of Bcl-XL co-expression in vitro: (A) The biological effect of Bcl-XL expression on cells co-transfected with a conventional plasmid (pCMV-EGFP) or replicase-based plasmid (pSin-EGFP) was measured by counting transfected cells using a fluorescence microscope daily for 1 week. Quadruplicate wells were counted in a blinded fashion, and the experiment was repeated with similar results. Data are shown in percent (with SE) based on the cell count 24 h after transfection, which was set to 100%. (B) Bcl-XL expression is not suppressed by co-transfection of cells with a replicase-based plasmid: BHK-21 cells were transfected with pcDNA-Bcl-XL and either a conventional DNA plasmid (pCMV-EGFP) or replicase-based plasmid (pSin-EGFP). To determine whether co-delivery with another plasmid in vivo could affect Bcl-XL expression, Bcl-XL was detected by immunostaining of cell lysates after PAGE and Western blotting. The experiment was repeated yielding the same result.
3.2. Bcl-XL is expressed in cells co-transfected with a conventional or a replicase-based plasmid
To achieve elevated production of alphaviral progenitors in an infected cell, the alphaviral replicase enzyme complex must take control of the antigen expression of the host cell. Therefore, it is possible that antigen expression from a conventional plasmid (in this case encoding Bcl-XL) is affected by the activity of the replicase in cells double co-transfected with a replicase-based plasmid. To assess production of Bcl-XL from a conventional pcDNA-based plasmid we analyzed lysates of cells transfected with either a conventional or a replicase-based plasmid encoding EGFP by immunostaining of Western blots with an anti-Bcl-XL antibody. Although Bcl-XL expression was reduced under conditions of co-transfection and co-expression, the level of Bcl-XL expression in cells co-transfected with the conventional or replicase-based EGFP plasmid were comparable (Fig. 2B).
3.3. Co-immunization with Bcl-XL increases antibody titers to the co-delivered antigen
To test the in vivo impact of Bcl-XL expression on the immunogenicity of DNA vaccines we used a previously described B16 murine melanoma model [22]. DNA plasmids encoding the melanoma/melanocyte associated tyrosinase-related protein-1 (TRP-1) were delivered by gene gun into the epidermis of mice. Mice are protected from subcutaneous challenge with B16 melanoma by repeated immunization with a conventional (pCMV-hTRP1) or a replicase-based plasmid (pSin-hTRP1) encoding the xenogeneic, cross-reactive variant of the murine TRP-1 antigen (human TRP-1) [22,32]. When delivering the non-mutated murine TRP-1 antigen, only the replicase-based plasmid (pSin-mTRP1) was effective. In the current study, gold particles were coated with the TRP-1 plasmids (pCMV-hTRP1 or pSin-hTRP-1) and either empty (pcDNA) plasmid or the Bcl-XL plasmid to ensure co-delivery into the same cells [33]. Mice were immunized five times weekly and bled one week after the last boost. When TRP-1 plasmids were co-delivered with Bcl-XL, serum antibody titers to TRP-1 were increased suggesting prolonged survival of the transfected cells and/or increased antigen production in vivo (Fig. 3). This raised the question of whether the co-immunization with the Bcl-XL gene would improve or impair tumor rejection induced with the two types of DNA vectors encoding a melanoma antigen.
Fig. 3.
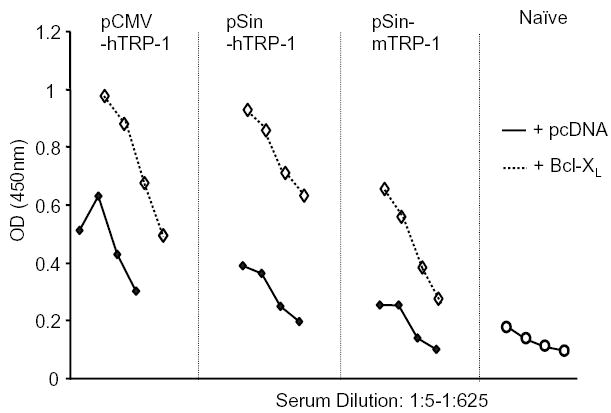
Co-immunization with Bcl-XL increases antibody titers to the co-delivered antigen: Serum antibody titers after DNA co-immunization of TRP-1 with Bcl-XL. Mice were immunized five times by gene gun using gold particles that carried both TRP-1 and Bcl-XL plasmid. The serum was diluted 1:5, 1:25, 1:125, 1:625, and analyzed by ELISA using recombinant mTRP-1 as plate-antigen. Shown is one representative experiment of four.
3.4. Co-immunization with Bcl-XL decreases the efficacy of a replicase-based but not a conventional melanoma DNA vaccine
Previous reports have demonstrated a significant role for T cells in the prevention of B16 melanoma, and we have shown a dominant role of CD8+ cells induced by a replicase-based DNA plasmid encoding TRP-1 in the rejection of melanoma [22,34,35]. Similarly, combined DNA immunization against h-gp100 and mTRP-2 yielded a protective response against B16 dependent on CD8+ cells [36]. Therefore, the increased antibody titers after co-immunization with Bcl-XL (Fig. 3) would not be expected to directly correlate with tumor protection. Mice immunized as described above were challenged with B16 subcutaneously, and tumor growth was monitored for 3 weeks. Tumor growth in mice immunized with a conventional DNA vector (pCMV-hTRP1) together with the plasmid control did not significantly differ from tumor growth after co-immunization with a Bcl-XL plasmid (Fig. 4A). This indicates that the efficacy of this pCMV-promoter driven vector does not depend on the induction of apoptosis in the transfected cells. In contrast, co-delivery of the anti-apoptotic gene significantly reduced the efficacy of the replicase-based plasmid (pSin-hTRP1) thus demonstrating an important role of vaccine-induced apoptosis in the superior efficacy of these vaccines (Fig. 4B).
Fig. 4.
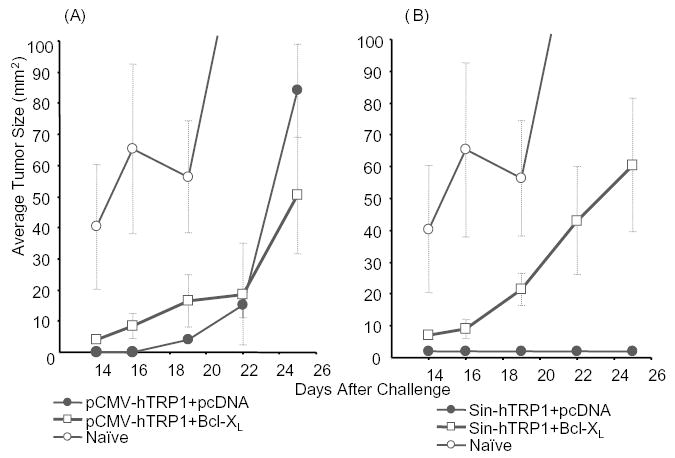
Interfering with apoptosis reduces the efficacy of a replicase-based, but not a conventional melanoma DNA vaccine encoding TRP-1: Animals were immunized weekly by gene gun five times with either (A) conventional (pCMV-hTRP1) or (B) replicase-based (pSin-hTRP1) plasmid. Seven to 10 days after the last immunization mice (n = 5 per group) were challenged subcutaneously with 1 × 105 B16 melanoma cells. Shown are averages and SE of tumor sizes from a representative experiment (the experiment was repeated three times).
4. Discussion
DNA vaccines employing an alphaviral replicase (“replicase-based DNA vaccines”) were developed to improve the immunogenicity and efficacy of conventional DNA vaccines. Alphaviruses are among the most efficient replication systems in nature. Since they rapidly amplify their genome in infected cells using an RNA polymerase (replicase–enzyme complex) [37], the expression of an antigen under the control of this enzyme was thought to improve the immunogenicity of a replicase-based DNA vaccine. Although replicase-based DNA vaccines have proven to be clearly superior to conventional DNA vaccines in pre-clinical murine models of infectious disease and cancer [16,20,21,23,38–40], the underlying mechanisms have remained less clear. For instance, the increased immunogenicity is not always associated with increased antigen production. Furthermore, in reports using replicase-based vectors to produce more antigen, the increase is considerably less than would be expected based on the observations made in alphavirus infected cells [17,41,42]. Therefore, we sought to identify the molecular mechanism, which allows replicase-based DNA or RNA vaccines to stimulate the immune system more effectively.
Like many viruses, alphaviruses trigger the apoptotic death of the infected host cell [27,43,44]. Although the induction of Sindbis virus-induced host cell death had been reported to occur during virus entry [45], we and others have previously demonstrated that “naked” replicase-based nucleic acid vaccines also cause caspase-dependent apoptotic death of transfected cells [6,23,39]. The molecular “switch” for the programmed cell death appears to be double-stranded RNA produced by alphaviral replicase as an intermediary molecule during RNA replication. Consequently, we have been able to demonstrate that the immunogenicity of replicase-based DNA vaccines is significantly impaired in mice that lack a dsRNA-activated pathway (RNase L-pathway) [22]. Nevertheless, the link between the induction of apoptosis by replicase-based nucleic acid constructs and increased immunogenicity has so far only been hypothetical.
Inducing apoptosis by co-delivering a pro-apoptotic signal (overexpression of caspase 3, or CD95/Fas) with a conventional DNA vaccine has been reported to yield enhanced immune responses [24,25]. In this study, we used the reverse approach of interfering with the induction of apoptosis in transfected cells by co-delivering an anti-apoptotic signal. This was accomplished by using gene gun immunization to introduce two independent plasmids into the same cell. To impede apoptosis, we used an anti-apoptotic member of the Bcl-2 family, Bcl-XL, which together with other molecules in this family, has been shown to inhibit alphavirus-induced apoptosis [46]. In vitro, the co-transfection yielded increased antigen production, which was reflected by increased antibody production in vivo. However, interfering with apoptosis in vivo significantly decreased the efficacy of a replicase-based DNA vaccine encoding mTRP-1. The efficacy of conventional DNA, however, was not significantly affected by Bcl-XL, suggesting that induction of apoptosis was a major reason for the increased immunogenicity of replicase-based vaccines, but does not play a significant role after immunization with conventional DNA constructs. Co-immunizing with a replicase-based plasmid and a Bcl-XL plasmid did not prevent the induction of a protective immune response. However, it suggested that, without the ability to trigger host cell apoptosis, the replicase-based vaccine would still be able to deliver the same immunostimulatory signals as a conventional DNA vaccine. Alternatively, the prevention of apoptosis by Bcl-XL in vivo may not be complete, thus only delaying or reducing the extent of replicase-induced programmed cell death. This may reduce, but not abrogate the apoptosis-mediated adjuvant-type signal delivered to the immune system as observed in Bcl-overexpressing cells infected with alphaviral vectors [27]. Similarly, treatment of transfected cells in vitro with caspase inhibitors does not prevent cell death but only prolongs survival. As demonstrated in a different model system, the effect of Bcl-XL co-immunization is not due to a significant change in the T helper cell-profile or the immune response that is induced. Due to the use of gene gun delivery, the immune response can be characterized as predominantly Th2 (Bergmann-Leitner, unpublished observation).
Our findings indicate that both conventional and replicase-based DNA vaccines induce apoptosis in trans-fected cells. These data raise the question: how do these vectors differentially stimulate the immune system? First, all cells transfected with the replicase-based vector are affected and die rapidly from apoptosis. Independent of the amount of plasmid (or self-replicating RNA) they produce comparable amounts of antigen and presumably also comparable amounts of dsRNA identified as the most likely trigger of the apoptotic cascade [22,23]. In contrast, the antigen expression from cells transfected with conventional plasmids likely depends on the amount of plasmid taken up [23]. The most striking finding in this report is that plasmid uptake and antigen expression correlate with the level of apoptosis induced. Second, the mechanism of apoptosis induced by replicase-based vectors appears to be primarily mediated through the production of dsRNA by the alphaviral replicase while the precise mechanism responsible for apoptosis after transfection with conventional DNA plasmids is not clear. Although Bcl-XL can interfere with both types of apoptotic pathways as indicated by increased antigen production, only the efficacy induced by the replicase-based vectors is affected.
This study shows for the first time that apoptosis of cells transfected with replicase-based nucleic acid is directly responsible for increased immunogenicity and efficacy of this new generation of vaccines. Besides the elucidation of the underlying mechanism, our findings also underscore the potential of modulating host cell apoptosis as a means of delivering a strong adjuvant signal together with a vaccine. Triggering pro-inflammatory apoptotic pathways with molecular adjuvants would allow the induction of powerful immune responses while avoiding the harmful side effects associated with strong conventional adjuvants.
Acknowledgments
The authors would like to thank Douglas Palmer (Surgery Branch, NCI) for assistance with the data analysis, Ms. Louis Finch (NCI Frederick, Maryland) for FACS sorting and the NCI-CCR Fellows Editorial Board (NIH, Bethesda, MD) for critical review and suggestions.
References
- 1.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191(3):411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verbovetski I, Bychkov H, Trahtemberg U, Shapira I, Hareuveni M, Ben-Tal O, et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196(12):1553–61. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392(6671):86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 5.Shaif-Muthana M, McIntyre C, Sisley K, Rennie I, Murray A. Dead or alive: immunogenicity of human melanoma cells when presented by dendritic cells. Cancer Res. 2000;60(22):6441–7. [PubMed] [Google Scholar]
- 6.Ying H, Zaks T, Wang RF, Irvine KR, Kammula U, Marincola FM, et al. Cancer therapy using a self-replicating RNA vaccine. Nat Med. 1999;5(7):823–7. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167(9):4844–52. doi: 10.4049/jimmunol.167.9.4844. [DOI] [PubMed] [Google Scholar]
- 8.Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood. 2003;101(1):245–52. doi: 10.1182/blood-2002-05-1580. [DOI] [PubMed] [Google Scholar]
- 9.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166(11):6847–54. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 10.Restifo NP. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Curr Opin Immunol. 2000;12(5):597–603. doi: 10.1016/s0952-7915(00)00148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Restifo NP. Vaccines to die for. Nat Biotechnol. 2001;19(6):527–8. doi: 10.1038/89255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryman KD, White LJ, Johnston RE, Klimstra WB. Effects of PKR/RNase L-dependent and alternative antiviral pathways on alphavirus replication and pathogenesis. Viral Immunol. 2002;15(1):53–76. doi: 10.1089/088282402317340233. [DOI] [PubMed] [Google Scholar]
- 13.Terenzi F, deVeer MJ, Ying H, Restifo NP, Williams BR, Silverman RH. The antiviral enzymes PKR and RNase L suppress gene expression from viral and non-viral based vectors. Nucleic Acids Res. 1999;27(22):4369–75. doi: 10.1093/nar/27.22.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams BR. PKR: a sentinel kinase for cellular stress. Oncogene. 1999;18(45):6112–20. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Haque J, Lacoste J, Hiscott J, Williams BR. Double-stranded RNA-dependent protein kinase activates transcription factor NF-kappa B by phosphorylating I kappa B. Proc Natl Acad Sci USA. 1994;91(14):6288–92. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berglund P, Tubulekas I, Liljestrom P. Alphaviruses as vectors for gene delivery. Trends Biotechnol. 1996;14(4):130–4. doi: 10.1016/0167-7799(96)10019-6. [DOI] [PubMed] [Google Scholar]
- 17.Dubensky TW, Jr, Driver DA, Polo JM, et al. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J Virol. 1996;70(1):508–19. doi: 10.1128/jvi.70.1.508-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frolov I, Hoffman TA, Pragai BM, et al. Alphavirus-based expression vectors: strategies and applications. Proc Natl Acad Sci USA. 1996;93(21):11371–7. doi: 10.1073/pnas.93.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999;18(9–10):765–77. doi: 10.1016/s0264-410x(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlesinger S. Alphavirus vectors: development and potential therapeutic applications. Exp Opin Biol Ther. 2001;1(2):177–91. doi: 10.1517/14712598.1.2.177. [DOI] [PubMed] [Google Scholar]
- 21.Hariharan MJ, Driver DA, Townsend K, et al. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol. 1998;72(2):950–8. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitner WW, Hwang LN, De Veer MJ, Zhou A, Silverman RH, Williams BR, et al. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat Med. 2003;9(1):33–9. doi: 10.1038/nmxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leitner WW, Ying H, Driver DA, Dubensky TW, Restifo NP. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 2000;60(1):51–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki S, Amara RR, Oran AE, Smith JM, Robinson HL. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat Biotechnol. 2001;19(6):543–7. doi: 10.1038/89289. [DOI] [PubMed] [Google Scholar]
- 25.Chattergoon MA, Kim JJ, Yang JS, Robinson TM, Lee DJ, Dentchev T, et al. Targeted antigen delivery to antigen-presenting cells including dendritic cells by engineered Fas-mediated apoptosis. Nat Biotechnol. 2000;18(9):974–9. doi: 10.1038/79470. [DOI] [PubMed] [Google Scholar]
- 26.Werner MH. Stopping death cold. Structure. 1996;4(8):879–83. doi: 10.1016/s0969-2126(96)00094-9. [DOI] [PubMed] [Google Scholar]
- 27.Mastrangelo AJ, Hardwick JM, Bex F, Betenbaugh MJ. Part I. Bcl-2 and Bcl-x(L) limit apoptosis upon infection with alphavirus vectors. Biotechnol Bioeng. 2000;67(5):544–54. [PubMed] [Google Scholar]
- 28.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139(5):1281–92. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA. 1997;94(8):3668–72. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leitner WW, Seguin MC, Ballou WR, et al. Immune responses induced by intramuscular or gene gun injection of protective deoxyribonucleic acid vaccines that express the circumsporozoite protein from Plasmodium berghei malaria parasites. J Immunol. 1997;159(12):6112–9. [PubMed] [Google Scholar]
- 31.Touloukian CE, Leitner WW, Robbins PF, Li YF, Kang X, Lapointe R, et al. Expression of a “self-”antigen by human tumor cells enhances tumor antigen-specific CD4(+) T-cell function. Cancer Res. 2002;62(18):5144–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci USA. 1999;96(6):2982–7. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albertini MR, Emler CA, Schell K, Tans KJ, King DM, Sheehy MJ. Dual expression of human leukocyte antigen molecules and the B7-1 costimulatory molecule (CD80) on human melanoma cells after particle-mediated gene transfer. Cancer Gene Ther. 1996;3(3):192–201. [PubMed] [Google Scholar]
- 34.Bowne WB, Srinivasan R, Wolchok JD, Hawkins WG, Blachere NE, Dyall R, et al. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190(11):1717–22. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronte V, Apolloni E, Ronca R, Zamboni P, Overwijk WW, Surman DR, et al. Genetic vaccination with “self” tyrosinase-related protein 2 causes melanoma eradication but not vitiligo. Cancer Res. 2000;60(2):253–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Mendiratta SK, Thai G, Eslahi NK, Thull NM, Matar M, Bronte V, et al. Therapeutic tumor immunity induced by polyimmunization with melanoma antigens gp100 and TRP-2. Cancer Res. 2001;61(3):859–63. [PubMed] [Google Scholar]
- 37.Schlesinger RW. Effect of alphaviruses on host cell macromolecular synthesis. In: Wengler G, editor. The togaviruses. New York: Academic Press; 1980. p. 459.
- 38.Driver DA, Polo JM, Belli BA, Banks TA, Hariharan MJ, Dubensky TW. Plasmid DNA-based alphavirus expression vectors for nucleic acid immunization. Curr Res Mol Ther. 1998;1:510–7. [PubMed] [Google Scholar]
- 39.Cheng WF, Hung CH, Chai CY, Hsu KF, He L, Ling M, et al. Enhancement of sindbis virus self-replicating RNA vaccine potency by linkage of herpes simplex virus type 1 VP22 protein to antigen. J Virol. 2001;75(5):2368–76. doi: 10.1128/JVI.75.5.2368-2376.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schirrmacher V, Forg P, Dalemans W, Chlichlia K, Zeng Y, Fournier P, et al. Intra-pinna anti-tumor vaccination with self-replicating infectious RNA or with DNA encoding a model tumor antigen and a cytokine. Gene Ther. 2000;7(13):1137–47. doi: 10.1038/sj.gt.3301220. [DOI] [PubMed] [Google Scholar]
- 41.Herweijer H, Latendresse JS, Williams P, Zhang G, Danko I, Schlesinger S, et al. A plasmid-based self-amplifying Sindbis virus vector. Hum Gene Ther. 1995;6(9):1161–7. doi: 10.1089/hum.1995.6.9-1161. [DOI] [PubMed] [Google Scholar]
- 42.Johanning FW, Conry RM, LoBuglio AF, Wright M, Sumerel LA, Pike MJ, et al. A Sindbis virus mRNA polynucleotide vector achieves prolonged and high level heterologous gene expression in vivo. Nucleic Acids Res. 1995;23(9):1495–501. doi: 10.1093/nar/23.9.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nava VE, Rosen A, Veliuona MA, Clem RJ, Levine B, Hardwick JM. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72(1):452–9. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glasgow GM, McGee MM, Sheahan BJ, Atkins GJ. Death mechanisms in cultured cells infected by Semliki Forest virus. J Gen Virol. 1997;78:1559–63. doi: 10.1099/0022-1317-78-7-1559. [DOI] [PubMed] [Google Scholar]
- 45.Jan JT, Griffin DE. Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J Virol. 1999;73(12):10296–302. doi: 10.1128/jvi.73.12.10296-10302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriishi K, Koura M, Matsuura Y. Induction of Bad-mediated apoptosis by Sindbis virus infection: involvement of pro-survival members of the Bcl-2 family. Virology. 2002;292(2):258–71. doi: 10.1006/viro.2001.1206. [DOI] [PubMed] [Google Scholar]


