Abstract
The immunogenicity and efficacy of nucleic acid vaccines can be greatly enhanced when antigen production is under the control of an alphaviral replicase enzyme. However, replicase-mediated mRNA overproduction does not necessarily result in enhanced antigen level. Instead, the strong adaptive immune response of alphavirus replicon-based vectors is due to their production of double-stranded RNA (dsRNA) intermediates, which trigger innate immunity. Because viral infections are known to trigger innate immune responses that lead to the rapid production of Type I Interferons (IFNs), namely IFN-α and IFN-β, we investigated the role of Type I IFNs in the enhanced immunogenicity of replicase-based DNA vaccines. In vitro, cells transfected with replicase-based plasmids produce significantly more Type I IFNs than cells transfected with a conventional DNA plasmid. In vivo, replicase-based DNA vaccines yield stronger humoral responses in the absence of Type I IFN signaling but the lack of this signaling pathway in IFN-αβ receptor-/- (knockout) mice abolishes T cell mediated efficacy against tumors of both conventional and alphavirus replicase-based DNA vaccines. Moreover, the co-delivery of an IFNα-encoding plasmid significantly improved the efficacy of a weakly immunogenic conventional plasmid. These results suggest a central role for Type I IFNs in the mechanism of replicase-based DNA vaccines and indicate that vaccines can be enhanced by enabling their capacity to triggering innate anti-viral defense pathways.
Keywords: DNA vaccines, Alphavirus, Type I IFN, Tumor therapy
1. Introduction
Numerous strategies have been employed in an attempt to improve the immunogenicity and efficacy of nucleic acid vaccines, i.e., RNA and DNA vaccines. In most DNA vaccines, the expression of the antigen is under the control of strong viral promotors such as the cytomegalovirus (CMV)-derived promotor in order to achieve high-level antigen expression (reviewed in [1]). The immunogenicity of DNA vaccines is influenced by various factors such as the delivery method (e.g., injection versus delivery by gene gun or in liposomes) or the level of antigen expression. Modifying these parameters can significantly boost vaccine efficacy [1]. To this end, a new generation of RNA [2] and DNA vaccines [3] was created in which antigen expression is controlled by an alphaviral replicase–enzyme complex with the objective of amplifying RNA production and to obtain high levels of antigen expression [4,5]. Despite surprisingly low antigen expression compared to conventional DNA plasmids, the replicase-based constructs were very immunogenic [6]. The goal of our studies is to identify and characterize the mechanisms responsible for the superior efficacy of replicase-based nucleic acid vaccines. This knowledge can in turn be used for the improvement of other types of vaccines.
Replicase-based nucleic acid vaccines encode the replicase enzyme complex together with the gene of interest. The replicase acts as an RNA polymerase amplifying mRNA that encodes the antigen of interest [1]. We have previously demonstrated that the generation of double-stranded RNA (dsRNA) species resulting from the RNA amplification triggers anti-viral defense pathways in transfected cells thus mimicking the effects of a viral infection [7,8]. These replicase-based DNA vaccines activate dsRNA-sentinel molecules such as the double-stranded RNA-dependent protein kinase R (PKR) [9], which ultimately results in the apoptotic death of the transfected host cell. This apoptotic death is responsible for the increased immunogenicity of the replicase-based vaccines [10] most likely because of increased uptake by dendritic cells [11]. In the current study we sought to identify the signal emitted from the transfected host cells, which accounts for the adjuvant effect of the alphaviral replicase. We hypothesized that the activity of the replicase is interpreted by host cells as a viral infection and would thus result in “distress signals” similar to those emitted by virus-infected cells. Type I Interferons (IFN, namely IFN-α and -β) are the main cytokines involved in innate immune responses against viral infections [12,13]. They belong to the Th1-associated cytokines [14], and – in case of a viral infection – they are produced in response to the presence of dsRNA in cells [15,16]. Type I IFNs have a number of effects, including inhibition of translation, and enhancement of immune responses (reviewed in [13,17]). Importantly, Type I IFNs are involved in the differentiation/maturation of dendritic cells [18]. IFN-α therapy is widely used either alone (reviewed in [19]) or – to a limited extent – in the form of an adjuvant. The severe side effects of these potent cytokines (reviewed in [20]) can be potentially eliminated by using plasmid-encoded INF-α as a molecular adjuvant [21].
Although only one type of IFN-β is produced, there are several variants of IFN-α thus making it virtually impossible to generate a knockout animal model. Therefore, we employed a mouse model in which the common Type-I IFN receptor had been knocked out [22,23] and studied humoral, cellular and anti-tumor responses after immunization with a conventional (i.e., CMV-promoter driven) or a Sindbis virus replicase-based DNA plasmid. As a disease model, we chose B16 melanoma and targeted two melanocyte/melanoma-associated antigens, tyrosinase-related protein 1 (TRP1) and gp100 (pmel17). Both, TRP1 and gp100 have previously been described as melanocyte/melanoma-associated tumor-rejection antigens [24,25].
2. Materials and methods
2.1. Plasmid- and recombinant virus vaccines
Sindbis replicase-based plasmid vectors encoding enhanced Green Fluorescence Protein (pSin-EGFP) and the tyrosinase-related protein 1 (pSin-TRP1) have been described [6] as well as pSport-βgal (designated pCMV-βgal)[9]. pEGFP-C1 (designated pCMV-EGFP) is from Clontech Laboratories (Palo Alto, CA). The IFN-α1 plasmid on the pkCMV backbone (designated pCMV-IFNα)[26] was a gift from Dr. Daniel Carr (The University of Oklahoma Health Sciences Center). Plasmids were purified using EndoFree purification columns (Qiagen, Hilden, Germany) and stored at 4 °C in TE-buffer. Plasmid pWRG-hgp100 was generated by blunt-end cloning of the PCR-amplified full-length gp100 sequence into the blunted BlnI site of the pWRG-7077 vector (Powderject Vaccines, Middletown, WI) [27]. Fowlpox virus encoding β-galactosidase [28] was injected intravenously (1 × 107 PFU/injection) twice with 3 weeks between immunizations (see Fig. 2(A) for immunization regimens). All plasmids in which antigen expression is under the control of a CMV-promotor are considered “conventional” plasmids (pCMV-βgal, pCMV-EGFP, pCMV-TRP1, pWRG-gp100). Plasmids that encode an alphaviral (in this study Sindbis virus) replicase for the control of antigen expression are designated “replicase-based” DNA plasmids (pSin-EGFP, pSin-TRP1, pSin-gp100, pSin-βgal).
Fig. 2.
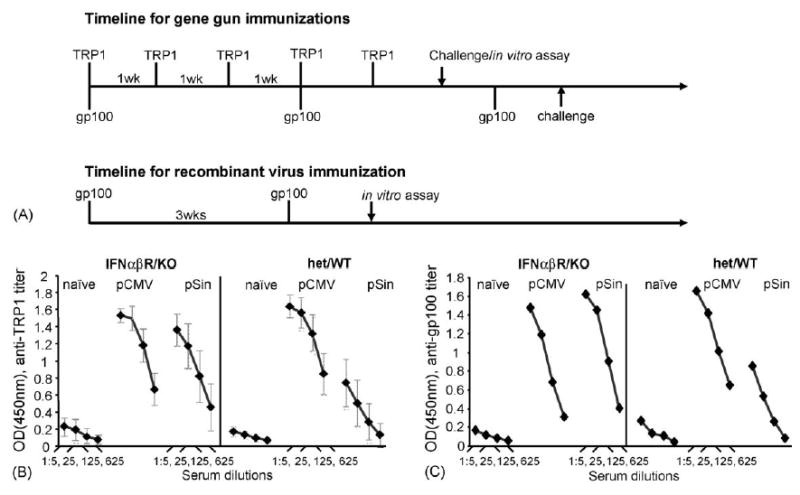
(A) Timeline of in vivo experiments. Immunization regimens had previously been optimized for the different vaccines and antigen. (B and C) Mean serum antibody titer of individual IFN-αβ R KO or WT/het C57Bl/6 littermates (n = 5) after immunization with a conventional (pCMV or pWRG) or a replicase-based (pSin) plasmid encoding mTRP1 or hTRP1 (Panel B) or hgp100 (Panel C). ELISA plates are coated with recombinant mTRP1 (B) or recombinant hgp100 (C). Shown are data (with S.E.M.) from a representative experiment. The result was confirmed in two independent repeat experiments.
2.2. In vitro Type I IFN production
Murine cell lines (C2C12, MC205, B16.F10 cells (ATCC, Manassas VA)) were grown in 6-well plates to approximately 50% confluency and transfected with plasmid using lipofectamine 2000 (Invitrogen, Carlsbad, CA). Lower transfection efficiency and IFN-production was achieved with Superfect (Qiagen, Valencia, CA), but increased Type I IFN production after transfection with the pSin-plasmid was still apparent (data not shown). Transfection conditions had been optimized for both cell lines and plasmids (C2C12: 1:5 ratio (DNA/lipofectamine) for pCMV-EGFP (2 μg), transfection efficiency = 83.4%, 1:2 ratio for pSin-EGFP (1 μg), transfection efficiency = 25.2%; MC205: 1:5 ratio for pCMV-EGFP (1 μg), transfection efficiency = 81.7%, 1:2.5 ratio for pSin-EGFP (1 μg), transfection efficiency = 38.9%; B16.F10: 1:5 ratio for CMV-EGFP (0.5 μg), transfection efficiency = 60.3%, 1:2 ratio for pSin-EGFP (2 μg), transfection efficiency = 42.2%). Transfection efficiencies indicated above are from the representative experiment shown in Fig. 1.
Fig. 1.
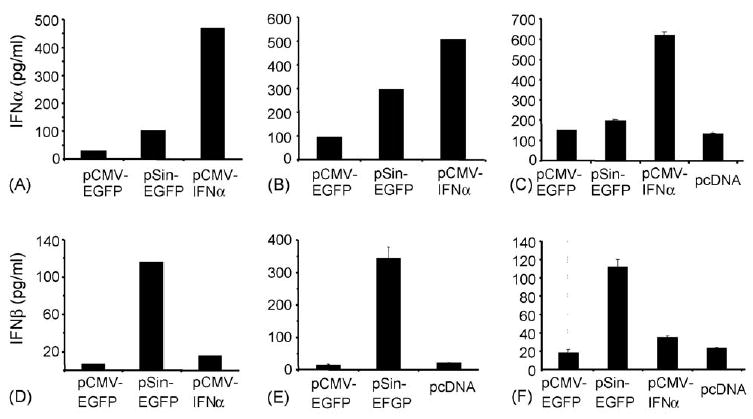
Cells transfected with a replicase-based plasmid produce higher levels of Type I IFNs than cells transfected with a conventional DNA vaccine. C2C12 myoblast cells (A and D), MC205 colon carcinoma (B and E) and B16.F10 melanoma cells (C and F) were transfected and supernatants were collected after 24 h for the IFN-β ELISA (D, E, F) whereas cells were collected for the IFN-α ELISA (A, B, C). pcDNA3 without insert served as a negative control. Data from one experiment (duplicate wells for each condition) are shown with standard deviations and the results were confirmed in an independent transfection experiment. Data are from transfections with lipofectamine 2000.
Cell supernatants and pellets were harvested 24 h later and frozen until analysis for IFN production using IFN-α and -β ELISA kits (Research Diagnostics Inc., Flanders NJ). Cell pellets were resuspended at 5 × 105 cells/ml in ice-cold PBS, sonicated for 5 s on ice and then immediately used in the ELISA according to the manufacturer’s instructions.
2.3. Mice and immunizations
All animal experiments were conducted according to protocols approved by the Animal Care and Use Committee of the National Cancer Institute, NIH. IFN-αβ-receptor (IFNαβR) knockout mice on a 129 background were obtained from Dr. Polly Matzinger (NIAID/NIH, Bethesda, MD) and fully backcrossed with C57BL/6 mice (National Cancer Institute/FCRDC, Frederick, MD) using a speed-congenic breeding protocol based on 80 simple sequence length polymorphism (SSLP) markers (Biocon Inc., Rockville, MD). As controls for the IFN-αβR knockout mice, heterozygous or wildtype littermates were used since no difference between them in response to DNA vaccines was observed (data not shown).
Plasmids were delivered using the Helios gene gun (Bio-Rad, Hercules, CA) [29]. For experiments in which TRP1 plasmids were delivered, mice were immunized five times at weekly intervals with three shots/immunization (calculated amount of DNA/immunization = 3 μg) [9] (Fig. 2(A)). Seven to 10 days after the last immunization mice were challenged subcutaneously with 1 × 105 B16.F10 (Tumor Repository of the National Cancer Institute/FCRDC, Frederick, MD). Tumor growth in five to eight mice/group was determined with calipers (two dimensions) for at least 3 weeks after challenge in a blinded fashion. The gp100 plasmids were delivered three times at 3-week intervals with three shots/immunization (Fig. 2(A)). Serum and splenocytes for in vitro assays were obtained 1 week after the last immunization.
For the co-immunization experiments, gold particles were coated with a mixture of pCMV-mTRP1 and pCMV-IFNα1 (or control plasmid) at a ratio of 2:1 or 10:1. Intramuscular plasmid DNA injection was previously described [29].
2.4. Serology
Mice immunized with TRP1 DNA were bled 2 days before challenge. Mice immunized with gp100 DNA were bled 3 weeks after the third immunization. Serum ELISAs were conducted as described [9,30]. Mouse-IgG1 and IgG2-specific secondary antibody were from Southern Biotechnology Associates Inc. (Birmingham, AL).
2.5. IFN-γ-release assay
CD8+ T cell function was assessed in pooled spleen cells (four mice per group cultured in the presence of 1 μg/ml human gp100 peptide (gp10025–33)[31] for 9 days as previously described [6]. The H-2Ld-restricted βgal peptide 876–884 has been described [6]. The sequence of the control NP peptide corresponded with NP366–374 H-2Db (ASNEN-METM).
2.6. Statistical analysis
Statistical differences in tumor growth and serum antibody titers were first determined by ANOVA using Minitab software (Minitab Inc., State College, PA). Groups that differed significantly were identified using the Tukey post test (error rate 5%).
3. Results
3.1. In vitro IFN expression
Based on our hypothesis, cells transfected with a conventional plasmid should produce little or no Type I IFNs while replicase-based plasmids should trigger significantly higher production of IFN-α or -β. To test this, we transfected three cell lines representing different types of tissue with a conventional plasmid (pCMV-EGFP) or a replicase-based plasmid (pSin-EGFP)[6,9] and measured the production of Type I IFNs. Transfection with pCMV-IFNα1 was used as a positive control for IFN-α production (Fig. 1). Panels (A–C) (Fig. 1) show the levels of intracellular IFN-α after lysis of 50,000 cells 24 h after transfection. IFN-α could only be measured in lysates of cells transfected with the replicase-based pSin and the positive control plasmid, but not the conventional DNA plasmid (data not shown). In contrast, significant amounts of IFN-β were detected only in culture supernatants and not cell lysates. Although the absolute levels of either IFN depended on the cell line used, cells transfected with the replicase-based plasmid produced significantly more Type I IFNs than cells transfected with the conventional plasmid. This was clearly evident despite the much lower transfection efficiency with the replicase-based plasmid (18–42%) compared to the smaller conventional plasmid (62–83%). Therefore, we proceeded to investigate in vivo the possible role these cytokines may play in the immunogenicity of the two types of DNA vaccines.
3.2. Humoral responses after DNA vaccination in the presence or absence of IFNαβR
Mice lacking a functional Type I IFN-receptor show no overt anomalies but are unable to cope with viral infections, despite otherwise normal immune responses [23]. To examine the involvement of IFN-α or -β on the humoral immune response induced by DNA immunization, we vaccinated WT and KO mice with conventional or replicase-based plasmids encoding TRP1 or gp100. Although humoral responses to these melanoma-associated antigens are not believed to be involved in tumor rejection, the antibody responses are a useful indicator for the immunogenicity of the vaccines (Fig. 2(B and C)). The results obtained with the melanocyte antigens were confirmed using the model antigen β-galactosidase (Fig. 3). Sera from TRP1-plasmid immunized mice were obtained 1 week after the last boost and analyzed by ELISA. The absence of the IFN-αβ-signaling pathway had no significant impact on antibody titers induced with the conventional pCMV-hTRP1 DNA plasmid (Fig. 2(B)). When immunized with the replicase-based plasmid (pSin-mTRP1), the IFNαβR KO mice produced strongly increased anti-TRP1 antibody titers comparable to those observed after immunization with the conventional plasmid. Statistical analysis using ANOVA analysis followed by Tukey post test confirms that there is no significant difference in the antibody titer obtained with pCMV-plasmids in IFNαβR knockout and control mice or when immunizing IFNαβR knockout mice with the conventional versus the replicase-based plasmid. In contrast, antibody titers induced by immunization with the pSin plasmid are significantly higher in IFNαβR knockout mice than in wildtype or heterozygous mice at all serum dilutions (p < 0.0001).
Fig. 3.
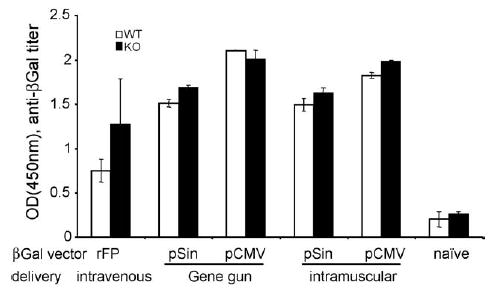
Serum ELISA of IFNαβR KO (black) or wildtype/heterozygous (white). C57Bl/6 littermates (mean of individual mice, n = 2) after three immunizations (3-week interval) with various vectors encoding βGal (3 μg/gene gun immunization, 50 μg/intramuscular injection). Shown are titers (±S.D.) at a serum dilution of 1:20.
Cells from IFNαβR knockout mice lack the ability to initiate a rapid Type I IFN-dependent anti-viral response. These cytokines otherwise mediate a shutdown of antigen production or destruction of the infected/transfected cells. Our results suggest prolonged in vivo survival of transfected cells in the absence of Type I IFN-signaling and thus increased antigen production in vivo. In the presence of the Type I IFN pathway, anti-TRP1 antibody titers induced by the replicase-based plasmid were significantly lower than those induced by the conventional DNA vaccine (p = 0.02). A parallel result was obtained after immunization with gp100 encoding plasmids (Fig. 3(C)). There, mice were immunized three times at 3-week intervals. As in the case of TRP1, the inactivation of the IFNαβR pathway (Fig. 2(B)) allowed the replicase-based plasmid to induce antibody levels comparable to those achieved with the conventional DNA vaccine.
To exclude that these results are (a) restricted to melanocyte/melanoma-associated antigens or (b) an artifact of the route of plasmid delivery (gene gun), we used β-galactosidase-encoding conventional (pCMV) and replicase-based plasmids (pSin) for gene gun versus intramuscular injections (Fig. 3). Additionally, we examined the humoral immune responses induced in the presence or absence of Type I IFN signaling after immunization with a recombinant fowlpox virus vaccine encoding β-galactosidase (rFP-βGal) administered intravenously. Regardless of the vaccine vector (recombinant virus, conventional DNA vaccine or replicase-based DNA vaccine) or route of immunization (intramuscular, epidermal gene gun delivery of DNA vaccines), no differences in the magnitude of the humoral response were found between IFNαβR knockout mice and wildtype mice (Fig. 3). These results firmly establish that the lack of the IFNαβR does not impair the mice’s ability to mount a humoral immune response. Therefore, reduced or absent vaccine efficacy in IFNαβR knockout mice is not due to an immunocompromised phenotype of these animals or the nature of the antigen.
We next sought to determine whether the lack of the Type I IFN signaling pathway may have an effect on the type of the immune response induced [32]. For this purpose, we analyzed the sera from mice immunized with the TRP1-plasmids in an antibody-isotype specific serum ELISA. Based on the antibody profile, both IFNαβR knockout and control mice mounted a Th2 type response characterized by a predominance of IgG1 antibodies with no detectable IgG2a response against TRP1 (Fig. 4) typical for immune responses induced by gene gun [33].
Fig. 4.
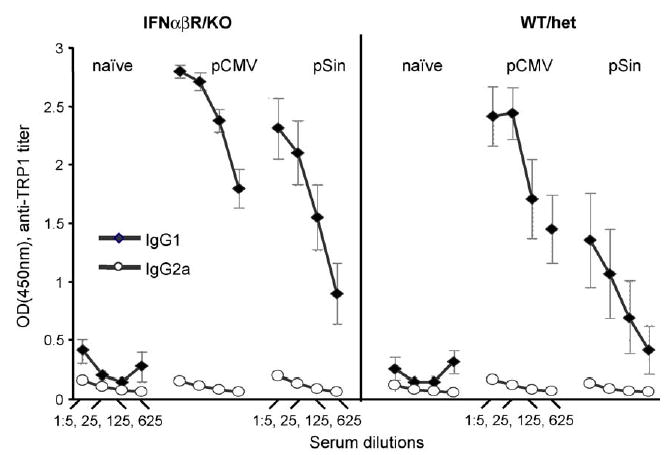
Isotype profile of antibodies induced by conventional and replicase-based plasmids. Mean of individual antibody titers of wildtype/heterozygous and IFNαβR-knockout mice immunized with TRP1 encoding plasmids were determined as in Fig. 2. Anti-TRP1 antibodies were detected using IgG1 (full diamonds) or IgG2a (open diamonds)-specific HRP labeled secondary antibodies. Data (with S.E.M.) are averages of two independent experiments (n = 10). Sera were diluted 1:5, 1:25, 1:125 and 1:625.
3.3. DNA vaccines loose their anti-tumor efficacy in the absence of the Type I IFN pathway
Our data demonstrate that IFNαβR KO mice mount a normal (in case of conventional plasmids, Figs. 2 and 3) or even increased humoral immune response (in case of replicase-based plasmids, Figs. 2 and 3) after DNA immunization with no apparent change in the isotype pattern caused by the lack of the IFNαβR (Fig. 4). We next investigated the consequences of the lack of a Type I IFN pathway on anti-tumor T cell responses after DNA vaccination. Mice were challenged subcutaneously with 1 × 105 B16.F10 cells following gene gun immunizations with either a conventional or the replicase-based plasmid encoding TRP1 (Fig. 5(A)). Tumor growth was accelerated in knockout mice compared to the tumor growth in non-immunized wildtype or heterozygous mice. This was also the case with non-melanoma tumor cell lines (data not shown). We and others have demonstrated that immunization with a conventional DNA plasmid encoding human TRP1 could significantly reduce the growth of B16 melanoma [9,34]. In addition, immunization with replicase-based plasmids encoding (human or murine) TRP1 protected the majority of mice from melanoma challenge [9]. In contrast, as shown in Fig. 5(A), IFNαβR KO mice showed no sign of anti-tumor response after immunization with either the conventional or the replicase-based plasmid indicating that Type I IFNs may be critically involved in the efficacy of DNA vaccines that depend on cellular immunity. ANOVA analysis followed by Tukey post test revealed a significantly faster tumor growth in IFNαβR KO mice than in controls regardless of immunization (p = 0.002). No difference was detected between immunized and not immunized knockout mice.
Fig. 5.
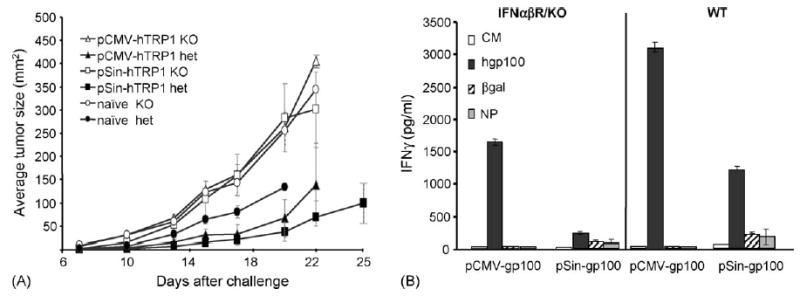
T cell responses and anti-tumor immunity are impaired in IFNαβR KO mice after immunization with conventional or replicase-based DNA vaccines. (A) Immunized IFNαβR KO or wildtype/heterozygous C57Bl/6 mice were challenged subcutaneously with 1 × 105 B16.F10 melanoma cells. Shown are the average tumor sizes from a representative experiment (n = 5). Groups are terminated when 40% of the mice in that group have died or reached maximum tumor size (2 cm in either direction). Data shown are representative of three independent experiments. (B) IFNαβR KO or wildtype/heterozygous C57Bl/6 mice were immunized with DNA vaccines encoding hgp100. Splenocytes were cultured in the presence of the gp100 H-2Db peptide and then re-stimulated with the indicated peptides. The level of IFN-γ released within 24 h was measured by cytokine ELISA using the supernatants from two independent wells.
Tumor protection against B16 melanoma is predominantly mediated by T cells and T cells have been shown to be effectors in this model [9,35,36]. Therefore, we analyzed the induction of melanoma/melanocyte specific T cells in vitro in the presence or absence of Type I IFN signaling (Fig. 5(B)). Due to the lack of a validated in vitro CD8 T cell assay for TRP1, we measured T cell activation after immunization with gp100 encoding plasmids and in vitro stimulation with the gp100 H-2Db peptide in a standard IFN-γ release assay. As predicted by the challenge results (Fig. 5(A)), T cell responses in knockout mice immunized with either the conventional or the replicase-based plasmid were strongly reduced. In summary, this indicates a deficiency in the induction of CD8 T cell (Fig. 5(B)), but not humoral immune responses (Figs. 2 and 3) when mice that lack the Type I IFN pathway were immunized with a DNA vaccine.
3.4. Combination of conventional DNA vaccine and IFN-α as a molecular adjuvant achieve similar efficacy as replicase-based vaccines
The data from this study demonstrate that (a) Type I IFNs play a crucial role in anti-tumor responses induced by DNA immunization and that (b) Type I IFNs may be part of the mechanism leading to the increased efficacy of replicase-based DNA vaccines. As proof of concept that induction of Type I IFNs is responsible for the high efficacy of replicase-based DNA vaccines, we immunized C57Bl/6 mice with conventional DNA vaccines using IFN-α as a molecular adjuvant. For this purpose, we immunized wildtype mice with pCMV-mTRP1, a plasmid that despite high-level antigen expression is not efficacious in preventing B16 melanoma [9]. This plasmid was co-precipitated onto gold particles with either pCMV-IFNα1 or a control plasmid (pcDNA3 or pKCMVintPolyIi, the control plasmid for pCMV-IFNα1, both of which yielded the same results) to assure delivery to the same cells in vivo and thus simulate the IFN-α induction by the replicase-based plasmids as had been observed in vitro (Fig. 1). C57Bl/6 mice were immunized by gene gun and challenged as above. Co-delivery of IFN-α increased the immunogenicity of pCMV-mTRP1 and in some experiments raised the antibody titer to the levels achieved with the replicase-based plasmid (data not shown). More importantly, the co-delivered IFN-α plasmid at both doses significantly increased the efficacy of the poorly immunogenic pCMV-mTRP1 (p < 0.01 by ANOVA, followed by a Tukey post test comparing pCMV-TRP1 plus pcDNA with pCMV-TRP1 plus pCMV-IFNα) (Fig. 6). ANOVA showed that all immunized groups had significantly reduced tumor growth compared to naive (p < 0.001 at all time points). Immunization with pCMV-IFNα1 alone only had a minor impact on tumor growth and provided no survival benefit, thus establishing that the effect of the co-delivered plasmids was antigen-specific (data not shown).
Fig. 6.
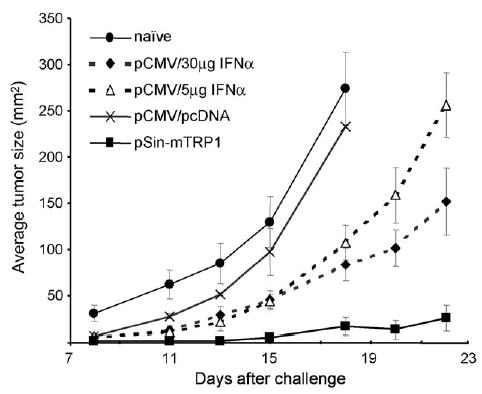
Using IFN-α as a molecular adjuvant for a poorly immunogenic melanoma DNA vaccine. Co-delivery of a plasmid encoding IFN-α with a conventional DNA vaccine encoding mTRP1 renders the plasmid immunogenic. Plasmid pCMV-mTRP1 was co-precipitated on gold particles with a control plasmid (pcDNA shown above, or pkCMV (data not shown)) or pCMV-IFNα at two different ratios. Immunized wildtype C57/Bl6 mice were challenged with melanoma as in Fig. 5(A). Shown are averages (n = 5) with S.D. The result was confirmed by an independent repeat experiment.
4. Discussion
In this study, we have demonstrated for the first time the critical role Type I IFNs play in the immunogenicity and efficacy of replicase-based nucleic acid vaccines. These RNA and DNA constructs represent the latest breakthrough in the quest to improve the efficacy of nucleic acid vaccines. We extend our previous finding that nucleic acid vaccines encoding an alphaviral replicase enzyme complex trigger aspects of anti-viral defense mechanisms such as the activation of dsRNA-triggered pro-apoptotic pathways [9]. The current study reveals that in addition to intracellular events resulting from the activity of the replicase, transfected mammalian cells also produce downstream signals associated with a viral infection, which can be exploited as molecular adjuvants.
While improving the level of antigen expression was considered the main goal when developing replicase-based DNA vaccines, a more promising approach is to deliver the antigen together with a strong immunological danger signal to initiate a powerful innate immune response. While numerous experimental adjuvants can achieve this, their side effects preclude them from clinical use thus prompting the search for “clean” and effective molecular adjuvants [37–40], which include the use of plasmid-encoded Type I IFNs [21]. We have previously demonstrated that DNA plasmids, which express antigen under the control of a replicase–enzyme complex of an alphavirus deliver a strong molecular-adjuvant signal that appears to be the reason for their high immunogenicity. Understanding what molecular pathways are triggered by replicase-based constructs could point the way for the development of highly effective but “harmless” molecular adjuvants [41–43].
Although several studies including ours have shown that replicase-based plasmids can yield significantly higher antibody responses than conventional plasmids when delivered by intramuscular or intradermal injection, we did not find this difference in the current study. The most likely explanation for this discrepancy is the method of plasmid delivery: to obtain the high level of anti-tumor protection reported in this and our previous study [9], the plasmids were delivered by gene gun. When comparing the humoral responses of conventional and replicase-based vaccines encoding βGal delivered by intramuscular or intradermal needle-and-syringe injection with gene gun delivery, we noticed that the gene gun-delivered plasmids yielded comparable antibody titers (unpublished observation) presumably due to the strong adjuvant effect the gene gun provides. Regardless of the humoral response, the cellular immune response and tumor protection induced by the gene gun-delivered replicase-based plasmid were still superior compared to the conventional DNA vaccine.
As we have previously shown, the action of the alphaviral replicase results in the production of dsRNA, which is detected by triggering intracellular pattern recognition receptors (PRR) [9]. PRR are a fast growing collection of molecules that include among others the dsRNA binding molecule PKR and various Toll-like receptors (TLRs)[44–46]. As a consequence of the dsRNA recognition, the transfected or infected host cell eventually undergoes apoptotic cell death resulting in recognition and elimination by dendritic cells [11]. This death is essential for the efficacy of the vaccine [10], but the underlying signals are unknown. We hypothesized that the same downstream signals generated in response to viral infections are responsible for the enhanced immune responses induced by replicase-based DNA vaccines, namely Type I IFN. IFN-α and -β are produced by a wide variety of cells in response to TLR engagement mediated by mitogenic, viral and microbial stimuli (reviewed in [17]) and they have been reported to sensitize cells to lysis after viral infection or in vitro exposure to dsRNA [47].
In our current study we showed for the first time that mammalian cells transfected with replicase-based DNA produced significantly higher levels of Type I IFNs than those transfected with conventional DNA plasmids. The expected difference between the two types of plasmids in vivo is even greater since the in vitro assay does not take into account the much lower in vitro transfection efficiency achieved with the large replicase-based plasmids.
The above-background levels of Type I IFNs release from cells transfected with a conventional plasmid (Fig. 1) are not surprising. Various studies have shown that plasmid DNA is capable of triggering some innate immune pathways, particularly after CpG recognition through TLR9 (reviewed in [48]). Indeed, Type I IFN production in response to CpG signaling was suggested to be involved in the adjuvant effect of DNA vaccines [49]. We previously observed that conventional DNA plasmids trigger cell death, albeit at much lower levels than replicase-based plasmids [10]. Thus, the mechanism(s) responsible for the immunogenicity of replicase-based and conventional plasmids may not be fundamentally different, but instead may simply be initiated by different stimuli with different signal strength though generating quantitatively, but not qualitatively different results. In our experimental system, the conventional DNA plasmid is unable to break tolerance to a self-antigen and protect against melanoma [9]. Consistent with our hypothesis, however, increased IFN-α production from transfected host cells significantly boosted the immunogenicity and efficacy of the otherwise weakly immunogenic plasmid. Because Type I IFNs are likely just one type of several immunostimulatory molecules produced in response to TLR engagement, a simple add-back experiment with an IFN plasmid would not boost the efficacy of a conventional DNA plasmid to the level of a replicase-based plasmid. Indeed, there is evidence for at least two separate TLR-triggered pathways, one leading to the production of inflammatory cytokines including IL-12 and one IFN-dependent pathway triggered, for example, by TLR-3 engagement responsible for regulating IFN-regulated genes [50]. Both pathways are believed to cooperate for an optimal anti-pathogen response and, thus, an optimal molecular-adjuvant strategy should also engage both pathways. Furthermore, we have only investigated the efficacy of one single IFN-α subtype while the binding of different subtypes of Type I IFNs to the Type I IFN-receptor triggers different cell signaling pathways [51]. But even if Type I IFNs are not the only downstream factors responsible for the high immunogenicity of our pSin-plasmids, we have shown that they are critical for the anti-tumor efficacy of the replicase-based DNA vaccine. In mice that lack the ability to respond to Type I IFN due to a lack of the common Type I IFN receptor, the efficacy of both the replicase-based plasmid and the conventional plasmid were severely impaired.
The results from this study further improve our understanding of alphavirus replicase “enhanced” nucleic acid vaccines and demonstrate the benefits of adding strong innate triggers such as IFN-α in the form of molecular adjuvants to conventional DNA-based vaccines.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The authors would like to thank the NCI-CCR Fellows Editorial Board for critical review and suggestions.
References
- 1.Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999;18(9–10):765–77. doi: 10.1016/s0264-410x(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X, Berglund P, Rhodes G, Parker SE, Jondal M, Liljestrom P. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine. 1994;12(16):1510–4. doi: 10.1016/0264-410x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 3.Hariharan MJ, Driver DA, Townsend K, Brumm D, Polo JM, Belli BA, et al. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol. 1998;72(2):950–8. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driver DA, Polo JM, Belli BA, Banks TA, Hariharan MJ, Dubensky TW. Plasmid DNA-based alphavirus expression vectors for nucleic acid immunization. Curr Res Mol Ther. 1998;1:510–7. [PubMed] [Google Scholar]
- 5.Tubulekas I, Berglund P, Fleeton M, Liljestrom P. Alphavirus expression vectors and their use as recombinant vaccines: a minireview. Gene. 1997;190(1):191–5. doi: 10.1016/s0378-1119(96)00679-8. [DOI] [PubMed] [Google Scholar]
- 6.Leitner WW, Ying H, Driver DA, Dubensky TW, Restifo NP. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 2000;60(1):51–5. [PMC free article] [PubMed] [Google Scholar]
- 7.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ 2006. [DOI] [PubMed]
- 8.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7(2):131–7. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 9.Leitner WW, Hwang LN, De Veer MJ, Zhou A, Silverman RH, Williams BR, et al. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat Med. 2003;9(1):33–9. doi: 10.1038/nmxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitner WW, Hwang LN, Bergmann-Leitner ES, Finkelstein SE, Frank S, Restifo NP. Apoptosis is essential for the increased efficacy of alphaviral replicase-based DNA vaccines. Vaccine. 2004;22(11–12):1537–44. doi: 10.1016/j.vaccine.2003.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying H, Zaks T, Wang RF, Irvine KR, Kammula U, Marincola FM, et al. Cancer therapy using a self-replicating RNA vaccine. Nat Med. 1999;5(7):823–7. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202(4):461–5. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tough DF. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk Lymphoma. 2004;45(2):257–64. doi: 10.1080/1042819031000149368. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G, Peritt D, Gerosa F. Acute induction and priming for cytokine production in lymphocytes. Cytokine Growth Factor Rev. 1996;7(2):123–32. doi: 10.1016/1359-6101(96)00018-4. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 16.Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424(6946):324–8. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 17.Bogdan C, Mattner J, Schleicher U. The role of type I interferons in non-viral infections. Immunol Rev. 2004;202:33–48. doi: 10.1111/j.0105-2896.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 18.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O’Garra A, Vicari A, et al. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201(7):1157–67. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 20.Sleijfer S, Bannink M, Van Gool AR, Kruit WH, Stoter G. Side effects of interferon-alpha therapy. Pharm World Sci. 2005;27(6):423–31. doi: 10.1007/s11096-005-1319-7. [DOI] [PubMed] [Google Scholar]
- 21.Cull VS, Broomfield S, Bartlett EJ, Brekalo NL, James CM. Coimmunisation with type I IFN genes enhances protective immunity against cytomegalovirus and myocarditis in gB DNA-vaccinated mice. Gene Ther. 2002;9(20):1369–78. doi: 10.1038/sj.gt.3301809. [DOI] [PubMed] [Google Scholar]
- 22.Aguet M, Grobke M, Dreiding P. Various human interferon alpha subclasses cross-react with common receptors: their binding affinities correlate with their specific biological activities. Virology. 1984;132(1):211–6. doi: 10.1016/0042-6822(84)90105-3. [DOI] [PubMed] [Google Scholar]
- 23.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91(14):6458–62. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami Y, Robbins PF, Rosenberg SA. Human melanoma antigens recognized by T lymphocytes. Keio J Med. 1996;45(2):100–8. doi: 10.2302/kjm.45.100. [DOI] [PubMed] [Google Scholar]
- 26.Carr DJ, Noisakran S. The antiviral efficacy of the murine alpha-1 interferon transgene against ocular herpes simplex virus type 1 requires the presence of CD4(+), alpha/beta T-cell receptor-positive T lymphocytes with the capacity to produce gamma interferon. J Virol. 2002;76(18):9398–406. doi: 10.1128/JVI.76.18.9398-9406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surman DR, Irvine KR, Shulman EP, Allweis TM, Rosenberg SA, Restifo NP. Generation of polyclonal rabbit antisera to mouse melanoma associated antigens using gene gun immunization. J Immunol Methods. 1998;214(1–2):51–62. doi: 10.1016/s0022-1759(98)00036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, et al. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154(9):4685–92. [PMC free article] [PubMed] [Google Scholar]
- 29.Leitner WW, Seguin MC, Ballou WR, Seitz JP, Schultz AM, Sheehy MJ, et al. Immune responses induced by intramuscular or gene gun injection of protective deoxyribonucleic acid vaccines that express the circumsporozoite protein from Plasmodium berghei malaria parasites. J Immunol. 1997;159(12):6112–9. [PubMed] [Google Scholar]
- 30.Touloukian CE, Leitner WW, Robbins PF, Li YF, Kang X, Lapointe R, et al. Expression of a “self-”antigen by human tumor cells enhances tumor antigen-specific CD4(+) T-cell function. Cancer Res. 2002;62(18):5144–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188(2):277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14(4):461–70. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 33.Weiss R, Scheiblhofer S, Freund J, Ferreira F, Livey I, Thalhamer J. Gene gun bombardment with gold particles displays a particular Th2-promoting signal that over-rules the Th1-inducing effect of immunostimulatory CpG motifs in DNA vaccines. Vaccine. 2002;20(25–26):3148–54. doi: 10.1016/s0264-410x(02)00250-5. [DOI] [PubMed] [Google Scholar]
- 34.Weber LW, Bowne WB, Wolchok JD, Srinivasan R, Qin J, Moroi Y, et al. Tumor immunity and autoimmunity induced by immunization with homologous DNA. J Clin Invest. 1998;102(6):1258–64. doi: 10.1172/JCI4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohm W, Thoma S, Leithauser F, Moller P, Schirmbeck R, Reimann J. T cell-mediated, IFN-gamma-facilitated rejection of murine B16 melanomas. J Immunol. 1998;161(2):897–908. [PubMed] [Google Scholar]
- 36.Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol. 2003;171(11):5940–7. doi: 10.4049/jimmunol.171.11.5940. [DOI] [PubMed] [Google Scholar]
- 37.Calarota SA, Weiner DB. Enhancement of human immunodeficiency virus type 1-DNA vaccine potency through incorporation of T-helper 1 molecular adjuvants. Immunol Rev. 2004;199:84–99. doi: 10.1111/j.0105-2896.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 38.Sin JI, Kim J, Chattergoon M, Ayyavoo V, McCallus D, Ugen KE, et al. Engineering of DNA vaccines using molecular adjuvant plasmids. Dev Biol (Basel) 2000;104:187–98. [PubMed] [Google Scholar]
- 39.Zhou H, Luo Y, Lo JF, Kaplan CD, Mizutani M, Mizutani N, et al. DNA-based vaccines activate innate and adaptive antitumor immunity by engaging the NKG2D receptor. Proc Natl Acad Sci USA. 2005;102(31):10846–51. doi: 10.1073/pnas.0502208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27(1):49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Claassen E, de Leeuw W, de Greeve P, Hendriksen C, Boersma W. Freund’s complete adjuvant: an effective but disagreeable formula. Res Immunol. 1992;143(5):478–83. doi: 10.1016/0923-2494(92)80057-r. [DOI] [PubMed] [Google Scholar]
- 42.Leenaars PP, Hendriksen CF, Angulo AF, Koedam MA, Claassen E. Evaluation of several adjuvants as alternatives to the use of Freund’s adjuvant in rabbits. Vet Immunol Immunopathol. 1994;40(3):225–41. doi: 10.1016/0165-2427(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 43.Brennan FR, Dougan G. Non-clinical safety evaluation of novel vaccines and adjuvants: new products, new strategies. Vaccine. 2005;23(24):3210–22. doi: 10.1016/j.vaccine.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 44.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 45.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 46.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6(15):1382–7. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, et al. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol. 2000;74(3):1513–23. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 49.Tudor D, Dubuquoy C, Gaboriau V, Lefevre F, Charley B, Riffault S. TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine. 2005;23(10):1258–64. doi: 10.1016/j.vaccine.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201(9):1435–46. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cull VS, Tilbrook PA, Bartlett EJ, Brekalo NL, James CM. Type I interferon differential therapy for erythroleukemia: specificity of STAT activation. Blood. 2003;101(7):2727–35. doi: 10.1182/blood-2002-05-1521. [DOI] [PubMed] [Google Scholar]


