Abstract
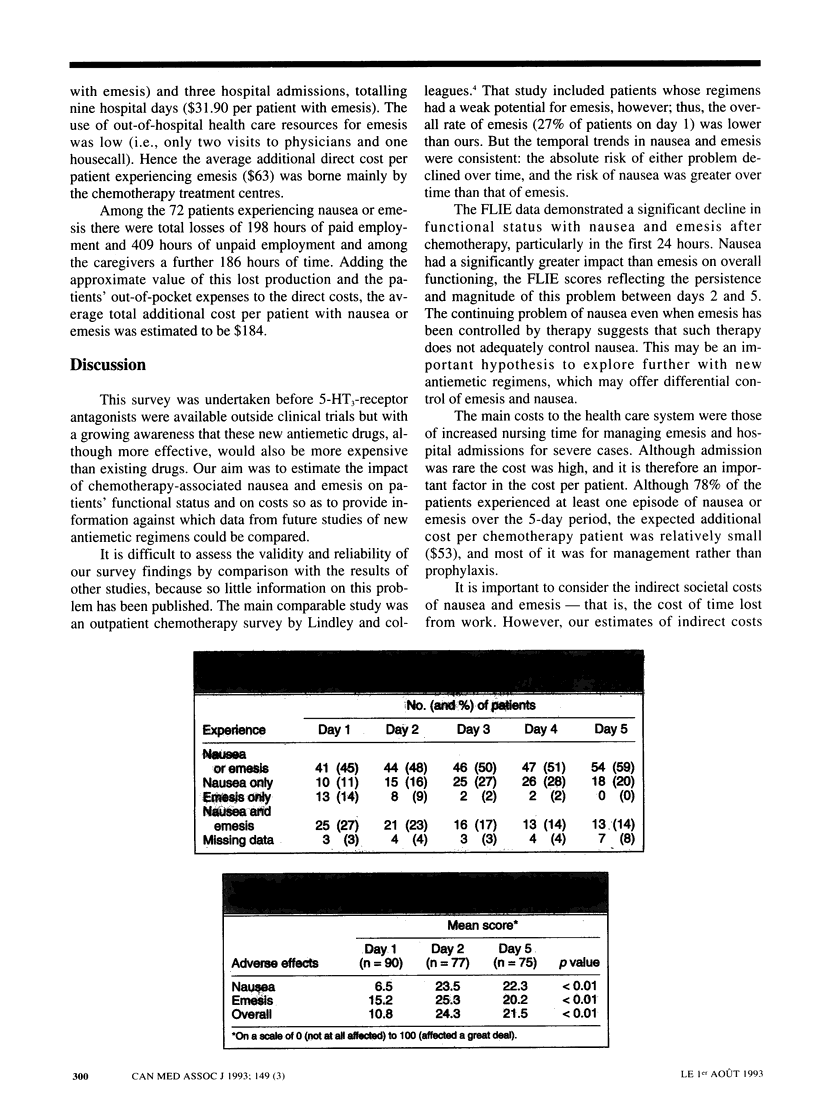
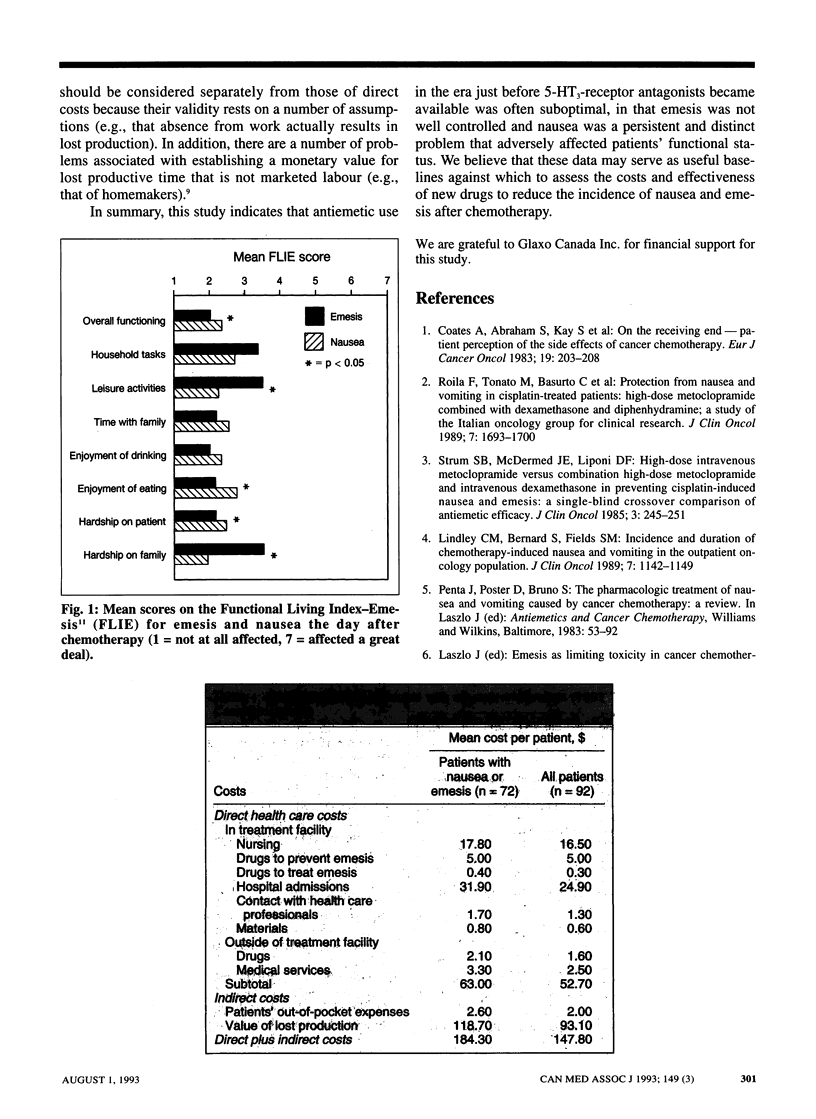
OBJECTIVE: To estimate the effect of chemotherapy-associated nausea and emesis on patients' functional status and on costs to the health care system, the patients and society before antagonists to the serotonin (5-hydroxytryptamine) receptor subtype 5-HT3 became available. DESIGN: A 5-day prospective survey between February and May 1991 of patients receiving chemotherapy for cancer. Data were obtained from questionnaires completed by nurses and patients. SETTING: Five Canadian cancer treatment centres in Ontario (three) and Quebec (two). PATIENTS: Outpatients and inpatients 18 years of age and older who were scheduled to receive chemotherapy with a moderate to high potential for emesis as defined by standardized criteria. Patients were excluded if they were scheduled to receive an investigational antiemetic or had received chemotherapy within the previous 7 days. Of the 128 who were eligible, 112 agreed to participate; 107 returned the completed questionnaire, but the data for 15 were excluded because the patients received multiple-day chemotherapy. MAIN OUTCOME MEASURES: The degree of nausea (on a seven-point scale) and the frequency of emesis (vomiting or retching) were recorded for each day of the survey. Functional status was assessed before and after chemotherapy by means of the Functional Living Index-Emesis (FLIE). The direct health care costs and the indirect costs (e.g., of time off work) associated with nausea and emesis were estimated from the survey responses and secondary data sources. RESULTS: On the day of chemotherapy 38 of the 92 patients (41%) experienced emesis with or without nausea, and over the 5 days of the survey 72 patients (78%) reported at least one episode of nausea or emesis. The absolute risk of either problem decreased over time, but the risk of nausea relative to emesis increased over time. The FLIE scores indicated significant worsening of functional status after chemotherapy. On the day after treatment the main impact was from emesis, particularly with regard to leisure activities, household tasks and hardship to the family. Nausea had a significantly greater impact than emesis on overall functioning. The additional direct health care cost for managing emesis was estimated to be $63 and the indirect cost $121. CONCLUSIONS: Despite prophylaxis with antiemetic drugs, nausea and emesis were significant problems in this population receiving chemotherapy. The management of emesis consumed relatively small amounts of health care resources, but there were costs outside the hospital for patients and others.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coates A., Abraham S., Kaye S. B., Sowerbutts T., Frewin C., Fox R. M., Tattersall M. H. On the receiving end--patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983 Feb;19(2):203–208. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- Einhorn L. H., Nagy C., Werner K., Finn A. L. Ondansetron: a new antiemetic for patients receiving cisplatin chemotherapy. J Clin Oncol. 1990 Apr;8(4):731–735. doi: 10.1200/JCO.1990.8.4.731. [DOI] [PubMed] [Google Scholar]
- Lindley C. M., Bernard S., Fields S. M. Incidence and duration of chemotherapy-induced nausea and vomiting in the outpatient oncology population. J Clin Oncol. 1989 Aug;7(8):1142–1149. doi: 10.1200/JCO.1989.7.8.1142. [DOI] [PubMed] [Google Scholar]
- Roila F., Tonato M., Basurto C., Picciafuoco M., Bracarda S., Donati D., Malacarne P., Monici L., Di Costanzo F., Patoia L. Protection from nausea and vomiting in cisplatin-treated patients: high-dose metoclopramide combined with methylprednisolone versus metoclopramide combined with dexamethasone and diphenhydramine: a study of the Italian Oncology Group for Clinical Research. J Clin Oncol. 1989 Nov;7(11):1693–1700. doi: 10.1200/JCO.1989.7.11.1693. [DOI] [PubMed] [Google Scholar]
- Schipper H., Clinch J., McMurray A., Levitt M. Measuring the quality of life of cancer patients: the Functional Living Index-Cancer: development and validation. J Clin Oncol. 1984 May;2(5):472–483. doi: 10.1200/JCO.1984.2.5.472. [DOI] [PubMed] [Google Scholar]
- Strum S. B., McDermed J. E., Liponi D. F. High-dose intravenous metoclopramide versus combination high-dose metoclopramide and intravenous dexamethasone in preventing cisplatin-induced nausea and emesis: a single-blind crossover comparison of antiemetic efficacy. J Clin Oncol. 1985 Feb;3(2):245–251. doi: 10.1200/JCO.1985.3.2.245. [DOI] [PubMed] [Google Scholar]
- Warr D., Willan A., Fine S., Wilson K., Davis A., Erlichman C., Rusthoven J., Lofters W., Osoba D., Laberge F. Superiority of granisetron to dexamethasone plus prochlorperazine in the prevention of chemotherapy-induced emesis. J Natl Cancer Inst. 1991 Aug 21;83(16):1169–1173. doi: 10.1093/jnci/83.16.1169. [DOI] [PubMed] [Google Scholar]


