Abstract
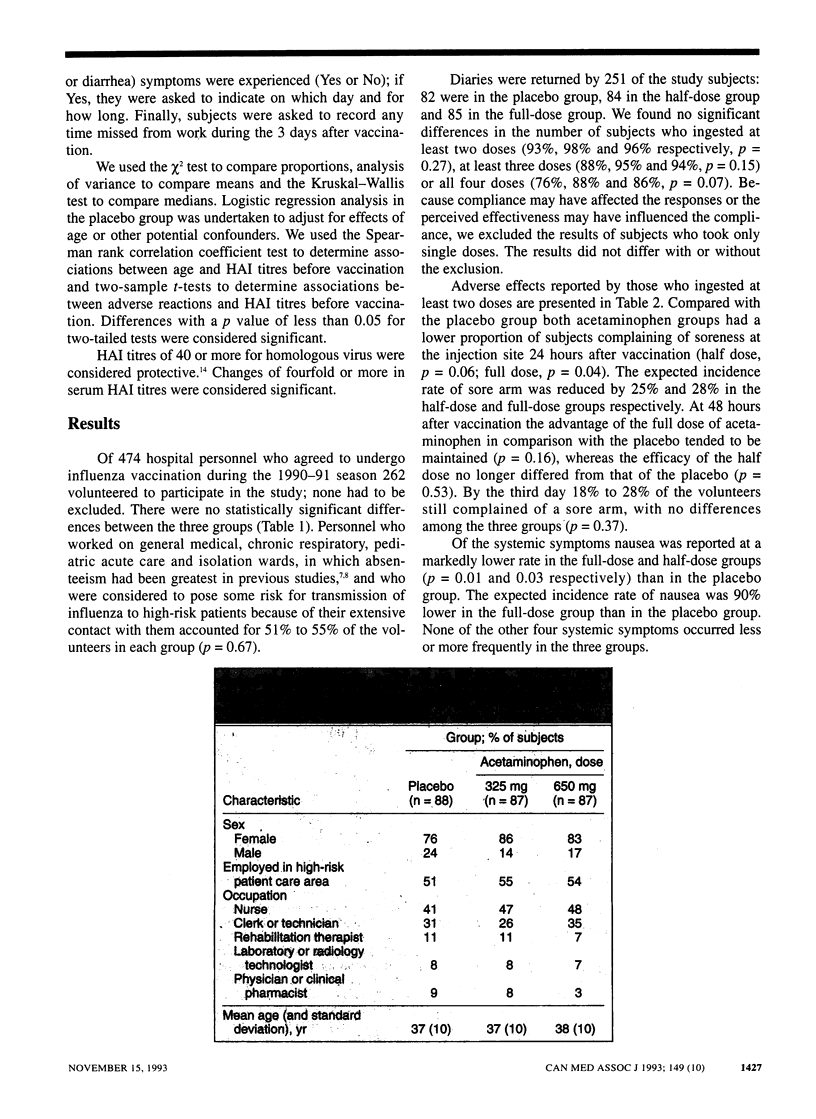
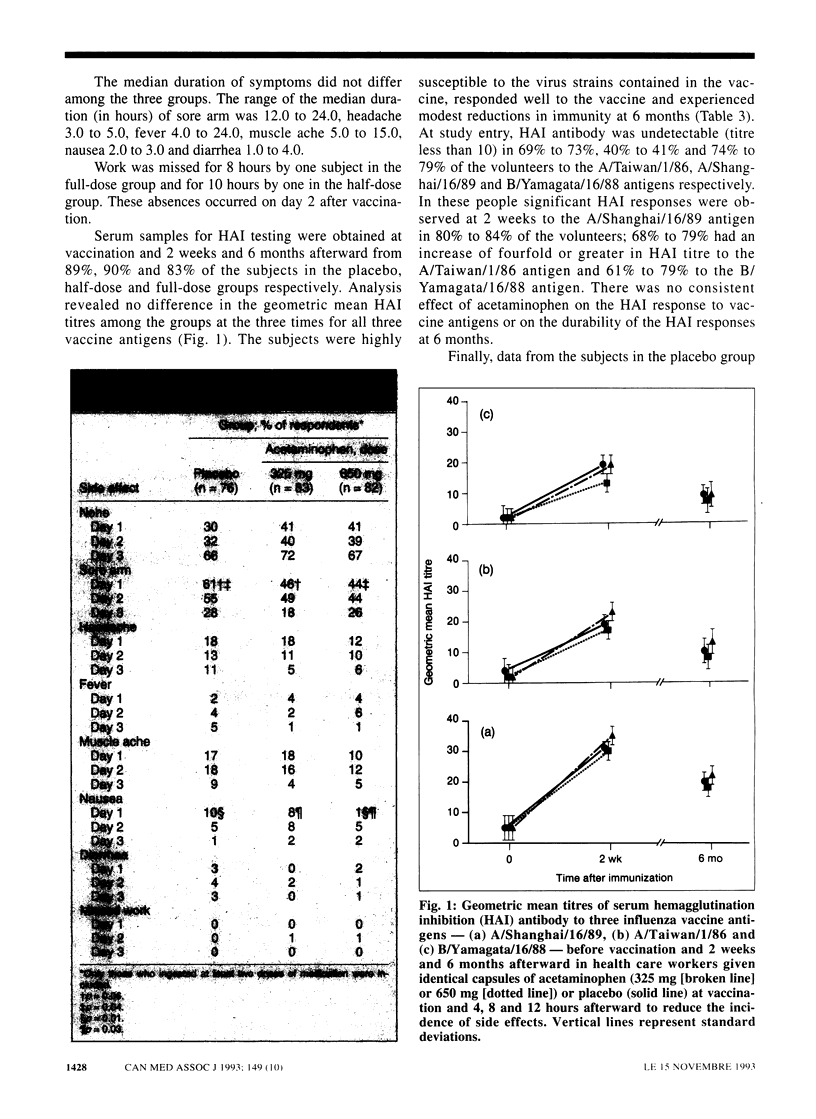
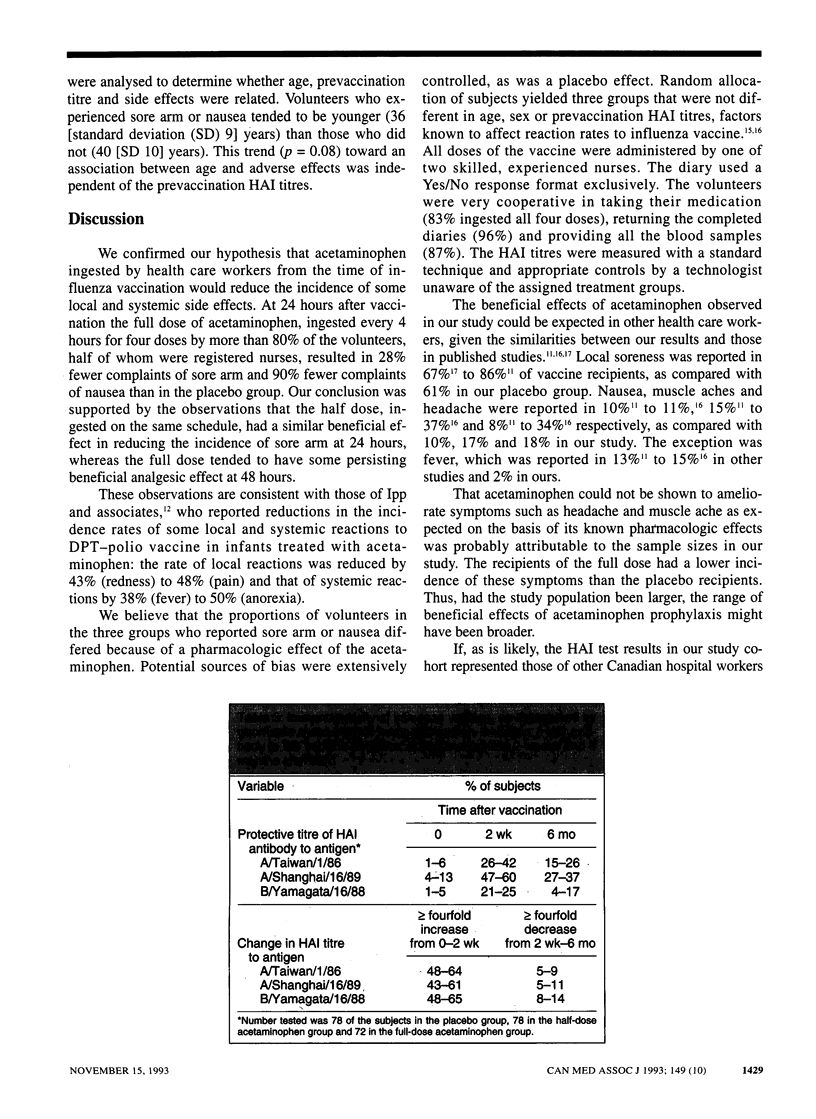
OBJECTIVE: To evaluate the effects of acetaminophen on the incidence of adverse effects to, and the immunogenicity of, whole-virus influenza vaccine in health care workers. DESIGN: Prospective, randomized, double-blind placebo-controlled trial. SETTING: Health Sciences Centre, an acute care teaching hospital in Winnipeg. PARTICIPANTS: Of 474 hospital personnel who agreed to undergo influenza vaccination during the 1990-91 season 262 volunteered to participate in the study. INTERVENTIONS: A dose of 0.5 mL of inactivated trivalent whole-virus influenza vaccine was injected into the deltoid muscle. Volunteers were randomly assigned to ingest two capsules of acetaminophen in a half dose (162.5 mg per capsule) or a full dose (325 mg per capsule) or two identical placebo capsules. Capsules were to be taken at vaccination and at 4, 8 and 12 hours afterward. Subjects were asked to answer questions regarding six symptoms in a diary for the 3 days after vaccination and to record their ingestion of the study medication. MAIN OUTCOME MEASURES: Incidence of local (sore arm) and systemic (headache, fever, muscle ache, nausea and diarrhea) side effects as well as serum titres of hemagglutination inhibition (HAI) antibody to vaccine antigens before vaccination and 2 weeks and 6 months afterward. RESULTS: A total of 87, 87 and 88 subjects received the half dose, full dose and placebo respectively; 96% returned the diaries, 83% ingested all four doses of medication, and 87% volunteered all blood samples. Compared with the placebo group the incidence of sore arm was 25% to 28% lower in the half-dose and full-dose groups respectively at 24 hours after vaccination, and the rate of nausea was 90% lower in the full-dose group. The HAI titres were similar among the groups at the three test times. CONCLUSIONS: The full dose of acetaminophen significantly reduced the incidence of sore arm and nausea without affecting the antibody response. Acetaminophen use may increase the acceptance of influenza vaccine by health care workers in whom concern about side effects is an impediment to vaccination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMENFELD H. L., KILBOURNE E. D., LOURIA D. B., ROGERS D. E. Studies on influenza in the pandemic of 1957-1958. I. An epidemiologic, clinical and serologic investigation of an intrahospital epidemic, with a note on vaccination efficacy. J Clin Invest. 1959 Jan;38(1 Pt 2):199–212. doi: 10.1172/JCI103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Cheang M. Absenteeism among hospital staff during an influenza epidemic: implications for immunoprophylaxis. Can Med Assoc J. 1984 Sep 1;131(5):449–452. [PMC free article] [PubMed] [Google Scholar]
- Ipp M. M., Gold R., Greenberg S., Goldbach M., Kupfert B. B., Lloyd D. D., Maresky D. C., Saunders N., Wise S. A. Acetaminophen prophylaxis of adverse reactions following vaccination of infants with diphtheria-pertussis-tetanus toxoids-polio vaccine. Pediatr Infect Dis J. 1987 Aug;6(8):721–725. doi: 10.1097/00006454-198708000-00005. [DOI] [PubMed] [Google Scholar]
- Kapila R., Lintz D. I., Tecson F. T., Ziskin L., Louria D. B. A nosocomial outbreak of influenza A. Chest. 1977 May;71(5):576–579. doi: 10.1378/chest.71.5.576. [DOI] [PubMed] [Google Scholar]
- Pachucki C. T., Lentino J. R., Jackson G. G. Attitudes and behavior of health care personnel regarding the use and efficacy of influenza vaccine. J Infect Dis. 1985 Jun;151(6):1170–1171. doi: 10.1093/infdis/151.6.1170. [DOI] [PubMed] [Google Scholar]
- Scheifele D. W., Bjornson G., Johnston J. Evaluation of adverse events after influenza vaccination in hospital personnel. CMAJ. 1990 Jan 15;142(2):127–130. [PMC free article] [PubMed] [Google Scholar]
- Van Voris L. P., Belshe R. B., Shaffer J. L. Nosocomial influenza B virus infection in the elderly. Ann Intern Med. 1982 Feb;96(2):153–158. doi: 10.7326/0003-4819-96-2-153. [DOI] [PubMed] [Google Scholar]
- Weingarten S., Riedinger M., Bolton L. B., Miles P., Ault M. Barriers to influenza vaccine acceptance. A survey of physicians and nurses. Am J Infect Control. 1989 Aug;17(4):202–207. doi: 10.1016/0196-6553(89)90129-6. [DOI] [PubMed] [Google Scholar]
- Wise T. G., Dolin R., Mazur M. H., Top F. H., Jr, Edelman R., Ennis F. A. Serologic responses and systemic reactions in adults after vaccination with bivalent A/Victoria/75-A/New Jersey/76 and monovalent B/Hong Kong/72 influenza vaccines. J Infect Dis. 1977 Dec;136 (Suppl):S507–S517. doi: 10.1093/infdis/136.supplement_3.s507. [DOI] [PubMed] [Google Scholar]


