Abstract
ACTR (also called AIB1 and SRC-3) was identified as a coactivator for nuclear receptors and is linked to multiple types of human cancer due to its frequent overexpression. However, the molecular mechanism of ACTR oncogenicity and its function independent of nuclear receptors remain to be defined. We demonstrate here that ACTR is required for both normal and malignant human cells to effectively enter S phase. RNA interference-mediated depletion and chromatin immunoprecipitation assays show that endogenous ACTR directly controls the expression of genes important for initiation of DNA replication, which include cdc6, cdc25A, MCM7, cyclin E, and Cdk2. Moreover, consistent with its critical role in cell cycle control, ACTR expression appears to be cell cycle regulated, which involves E2F. Interestingly, ACTR is recruited to its own promoter at the G1/S transition and activates its own expression, suggesting a positive feedback mechanism for ACTR action in the control of cell cycle progression and for its aberrant expression in cancers. Importantly, overexpression of ACTR alone transforms human mammary epithelial cells, which requires its association with E2F. These findings reveal a novel role for ACTR in cell cycle control and support the notion that the ability of aberrant ACTR to deregulate the cell cycle through E2F underlies its oncogenicity in human cancers.
The mammalian cell cycle involves a dynamic transcriptional control program responsible for the timely expression of key cyclins, cdks, and proteins with functions in DNA synthesis and replication. This program is mediated primarily by members of the E2F family, which are encoded by eight distinct E2F genes. Recent studies have provided evidence that E2F1, -2, and -3 function primarily as transcriptional activators while E2F4 to -8 act as repressors (2, 7, 11, 30, 33, 38, 56). These transcriptional regulation activities of E2Fs are likely mediated by distinct groups of nuclear cofactors. Indeed, strong evidence suggests important roles played by enzymatic complexes of chromatin modification and remodeling in transcriptional silencing of E2F target genes (13, 20). Thus, in quiescent cells E2Fs associate with hypophosphorylated pocket proteins (pRb, p107, and p130), and together they recruit the enzymatic complexes, such as histone deacetylases, the Brg1 chromatin-remodeling complex, and histone methyltransferases, to repress certain cell cycle genes, such as cyclin E, cyclin A, and cdc2 (1, 24, 29, 39-42, 52). Once cells reenter the cell cycle, hyperphosphorylation of pocket proteins leads to their dissociation from E2Fs and disassembly of the corepressor complex.
Much less is understood about the subsequent process of transcriptional activation by E2Fs. Based on biochemical analysis and reporter gene assay, a number of cofactors, including p300, CBP, and PCAF, have been implicated in the process of E2F-mediated transactivation (34, 57). However, evidence of their direct involvement in controlling key cell cycle gene expression has not yet been presented, suggesting the existence of other E2F coactivators. In this regard, it has recently been demonstrated that components of the TRRAP (transactivation-transformation domain-associated protein)/Tip60/GCN5 histone acetyltransferase complexes are required for cell proliferation and recruited to a subset of E2F target gene promoters, supporting the notion that distinct chromatin modifying-remodeling complexes might be participating in activation of different groups of E2F target genes (26, 27, 54).
ACTR (activator of thyroid and retinoid receptors), also named AIB1 (amplified in breast cancer 1) and SRC-3 (steroid receptor coactivator 3), is a member of the p160/SRC transcriptional coregulator family (18, 35, 49). Like other p160s, ACTR was identified as a nuclear cofactor that associates with hormone-bound nuclear receptors and mediates the transcriptional activation function of the receptors. The p160s contain functional domains for interactions with receptors, the coregulator proteins CBP and p300, PCAF, and arginine methyltransferases (51). It is generally accepted that the p160s are recruited to hormone-responsive genes through their interaction with activated receptors and then nucleate the assembly of a coactivator complex, which in turn remodels chromatin through histone modifications and facilitates RNA polymerase II (Pol II) transcription.
ACTR is linked to cancer because of its frequent amplification and/or overexpression. Although the initial analysis suggested a correlation between ACTR amplification and positive estrogen receptor (ER) status, later studies found that overexpression of ACTR in breast cancers does not correlate with positive ER status (3). In fact, more clinical studies have revealed the aberration of ACTR in a broad spectrum of malignancies with high frequency, including pancreatic adenocarcinoma, hepatocellular carcinoma, gastric cancers, esophageal squamous cell carcinoma, and prostate cancer (14, 19, 21, 46, 59, 67). Despite these clinical studies, little was known about how elevated ACTR may induce tumorigenesis and/or promote tumor growth. By crossing SRC-3 (the mouse ortholog of ACTR) knockout mice with MMTV-v-Ha-ras transgenic mice, it was recently demonstrated that ACTR is required for oncogenic ras-induced mammary tumorigenesis (25). Interestingly, deletion of ACTR did not affect the promotional role of ovarian hormones in mammary tumor formation or the hormone-responsive gene expression in the mammary gland, suggesting that ACTR and estrogens contribute to mammary carcinogenesis through different mechanisms. Another study showed that overexpression of ACTR in multiple tissues of mice resulted in many types of malignancy, including tumor formation in the mammary gland, pituitary, uterus, lung, liver, and skin, indicating that aberrant ACTR can act as an oncogene in vivo (55). Although defects or abnormal activity in IGF-1-mediated signaling were observed in these studies, it is unclear whether deregulation of the IGF-1 pathway is the primary molecular underpinning of aberrant ACTR-induced tumorigenesis.
In our attempt to determine the potential role of ACTR in the control of breast cancer cell proliferation, we found that silencing ACTR expression inhibits the proliferation of ER-positive and -negative breast cancer cells and that overexpression of ACTR negates the growth-inhibitory effect of antiestrogens (32). Through functional analysis, we uncovered that ACTR directly interacts with E2F1 and that ectopically expressed ACTR up-regulates key cell cycle genes, including those for cyclin E1, cyclin A2, cdk2, and E2F1. We report here that endogenous ACTR directly controls the expression of genes critical for initiation of DNA replication and is required for effective G1-S progression of both normal and malignant human cells. Surprisingly, we found that ACTR gene transcription appears to be cell cycle regulated, which involves an ACTR-E2F complex. More importantly, we found that elevated ACTR transforms nonmalignant human breast epithelial cells independently of the ER but dependent on its ability to associate with E2Fs.
MATERIALS AND METHODS
RNA interference (RNAi), recombinant adenovirus vectors, and plasmid constructs.
Small interfering RNA (siRNA) oligonucleotides specifically targeting ACTR and control RNA oligonucleotides were synthesized by Dharmacon with the following sequences: siACTR, 5′-GGUCUUACCUGCAGUGGUGAAdTdT-3′ (sense) and 5′-UUCACCACUGCAGGUAAGACCdTdT-3′ (antisense); control siRNA, 5′-CCAUGAGUCACGCAUCCAAGCdTdT-3′(sense) and 5′-GCUUGGAUGCGUGACUCAUGGdTdT-3′ (antisense). Adenoviral vectors expressing ACTR, GFP, or ACTR-siRNA and GFP-siRNA were created as previously described (32). In brief, for RNAi vector construction, the human H1 gene promoter sequence was inserted into the pShuttle plasmid from the Adeasy system. Oligodeoxynucleotides containing coding sequences for siRNA targeting ACTR (5′GGTCTTACCTGCAGTGGTGAA) and green fluorescent protein (GFP) (5′GAACTTCAGGGTCAGCTTG) were inserted downstream of the H1 promoter. The resulting pShuttle-RNAi constructs were then used to generate recombinant adenoviruses. Viral particles were purified by centrifugation in a CsCl step gradient. Viral titers were determined by endpoint cytopathogenic effect assay and/or with the anti-hexon antibody-based Adeno-X rapid titer kit (BD Biosciences).
Based on the sequence information at the National Center for Biotechnology Information EvidenceViewer for human ACTR/NCOA3, a genomic DNA fragment (1.6 kb, HindIII-NcoI) containing the first exon of ACTR was amplified by Pfu polymerase from human prostate cancer cell line LNCaP cells and inserted into vector pGL3-basic (Promega) to generate ACTR promoter luciferase reporter pGL3-ACTR-1.6kb. The pGL3-ACTR-0.6kb plasmid was constructed by inserting into pGL3 the 0.6-kb BamHI-BamHI fragment contained in the 1.6-kb genomic DNA. Wild-type and mutant pSh-HCMV-ACTR plasmids were described before (32).
Cell proliferation, anti-BrdU (bromodeoxyuridine) staining, and fluorescence-activated cell sorter (FACS) analysis of the cell cycle.
Human diploid fibroblasts (IMR-90 and WI38) were obtained from the American Type Culture Collection (ATCC) and maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gemini) and antibiotics. Early passages of the fibroblasts (105) were plated in six-well plates and infected 24 h later with adenovirus vectors expressing ACTR-siRNA or GFP-siRNA at an MOI (multiplicity of infection) of 50. Cells were refed with fresh medium every 2 days. Cell proliferation was measured every 2 days by cell numeration of triplicates of coded samples. Parallel samples were also harvested for analysis of cell cycle distribution by staining with propidium iodide and flow cytometry by FACScan (Coulter). For anti-BrdU staining, IMR-90 cells (105) were plated in six-well plates containing untreated glass slides (Fisher) and infected 24 h later as described above. Cells were then maintained in medium containing 0.1% FBS for 3 days, replenished with 10% FBS, and maintained for another 24 or 48 h. One hour before harvesting, cells were pulse-labeled for 60 min with 10 μM BrdU (Roche). Cells were fixed in 70% ethanol and then treated with 2 N HCl and 0.5% Triton X-100 for 10 min. After washing with phosphate-buffered saline, the slides were incubated with a 1:50 dilution of anti-BrdU-fluorescein isothiocyanate (FITC) antibody (Roche) for 1 h. Cells were washed in phosphate-buffered saline and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) for 3 min. FITC and DAPI signals were detected by fluorescence microscopy, and digital images were recorded. The percentage of BrdU-positive cells was determined by counting the BrdU-positive cells in five different frames and dividing by the total number of DAPI-positive cells. Results represent averages of two independent experiments.
MCF10A cells were obtained from the ATCC and maintained in mammary epithelial growth medium with supplements from BioWhittaker or in DMEM/F12 supplemented with 2% horse serum (Invitrogen), 1 μg of insulin per ml, 1 ng of cholera toxin per ml, 100 μg of hydrocortisone per ml, and 10 ng of human epidermal growth factor. For cell proliferation assay, MCF10A cells maintained in six-well plates in DMEM/F12 with the supplements described above (as full supplements) were infected with the ACTR- or GFP-adenovirus vectors at equal MOIs. Four hours later, the medium was changed to DMEM/F12 with 0.1% of the full supplements. On different days after infection, cells were harvested for proliferation analysis by cell enumeration in triplicate.
Soft-agar colony formation assay.
MCF10A cells maintained in mammary epithelial growth medium with full supplementation were infected at 70% confluence with the adenovirus vectors as described above. Twenty-four hours after infection, cells were detached from the plates by trypsinization. Cells were resuspended as individuals in DMEM/F12 growth medium with the full supplements described above mixed at a 3:1 ratio with 1.6% agarose (SeaPlaque; BioWhittaker). The mixture was then plated onto six-well plates at 5 × 104 cells/well over a bottom layer of 0.8% agarose in DMEM/F12 with the supplements. Cells were maintained at 37°C with a medium change every 3 days. Two weeks later, cell aggregates with diameters of 0.2 mm or larger (containing approximately 50 or more cells) were counted as colonies. The entire experiment was repeated once.
Transfection and reporter gene assay.
HeLa and human glioblastoma T98G cells were obtained from the ATCC and maintained in DMEM plus 10% FBS and antibiotics. For transfection of synthetic siRNA, HeLa or T98G cells were plated into six-well plates and transfected at 30 to 40% confluence with 250 pmol (2 μg) of duplex siRNA with Oligofectamine (Invitrogen) following the manufacturers' protocol. Cells were then maintained in DMEM plus 5% charcoal-stripped FBS and harvested at different time points for cell proliferation (cell enumeration of triplicate coded samples), Western blotting, or RNA extraction. Reporter gene assay was performed with adenovirus E1A-transformed human embryonic retina (HER) cells as described previously (32), except that firefly luciferase reporter activity was normalized with β-galactosidase activity via cotransfection of plasmid pCMX-β-gal (8). HER cells can be readily transfected with Lipofectamine (Invitrogen), achieving 80 to 90% efficiency. Each transfection was performed in triplicate.
Western blotting and quantitative reverse transcription (RT)-PCR analysis.
Whole-cell lysates were prepared from cells treated and harvested as indicated in buffer containing 50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 10% glycerol, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 1× protease inhibitor cocktail (Promega). Western blotting analysis of proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed with specific antibodies as described previously (32) and monoclonal antibodies from Santa Cruz against Cdc6 (180.2), Cdc25A (F-6), PCNA (PC10), and MCM7 (141.2). For semiquantitative RT-PCR analysis of gene expression, 3 μg of total RNA was used for an RT reaction with Moloney murine leukemia virus reverse transcriptase and oligo(dT)18 primers. cDNAs were diluted 10-fold, and 5 μl was used for the PCRs (25 to 28 cycles). The sequences of the primers used for RT-PCR are listed in Table 1. Quantitative RT-PCR was performed as previously described (32).
TABLE 1.
Nucleotide sequences of the PCR primers used in this study
| Primer | Forward sequence | Reverse sequence |
|---|---|---|
| ChIP primers | ||
| ACTR-A | TTTTCCTGCCTTAGCCTCCAG | TCACAGAGAATGGGGTTCAGACAC |
| ACTR-B | GCTTCAGCCTCCCAAGTTGC | TTGCTTCTCTCCCTTTCATCCTC |
| ACTR-C | ACCCCGTCCCTACTAAAAATGC | TTCTTTCCTCCTGGCTTGCTC |
| ACTR-D | TCTTGAACTCCCGACCTCAGG | TGCCCTTCCCTCTCTAAACTCATC |
| ACTR-E | ACAACAGTCCTGACCCATTATTGG | GTGGAGCGTTTCTGGTATGCC |
| ACTR-F | CCAGAAACGCTCCACAAATGTTAG | GCCTCCTCCTTTATCTCCACTCAC |
| ACTR-G | GCGGGAGGTGAGTGGAGATAAAG | TAGGGACAGAAGGGGAAAGTGGG |
| ACTR-H | ATGGGGATTGTGGACTAAGCG | TTTATTTGCTCGGGAGGTGC |
| Cdc25A | AAGAAAGGGGTCCACAATACTGC | CAATGAGAGGGGAAGAAGAAAGC |
| Cdc6 | CACAGAACCCAAAACGGTAGGTATC | CCAGTGGAAGAGTTACAGCCAATG |
| Cdk2 | GATGGAACGCAGTATACCTCTC | AAAGCAGGTACTTGGGAAGAGTG |
| E2F1 | GCAAGTTGAGGATGGAAGAGGTG | TGGGGACACGGGAACATAGG |
| Mcm7 | AGCCCGAATGACAGCAACAG | AAAGTGACAGCATAAGCCCCG |
| TopBP1 | GCAGAGAAAGAGAACGGCGATGTCC | AGGACCACAGGCCAGGAATACTCC |
| RT-PCR primers | ||
| ACTR-3′UTRa | CTGCTGTTGCCTCTCTTTGACAC | TGGTGGGTCTGGAAATAATCAGG |
| CycE2 | ACTGACTGCTGCTGCCTTGTGC | TCGGTGGTGTCATAATGCCTCC |
| GAPDHb | GAAATCCCATCACCATCTTCCAG | ATGAGTCCTTCCACGATACCAAAG |
| Mcm7 | AAGCCAGGAGTGCCAAACCAAC | GCAGCAGTGCCTTCTTCACATC |
| pCAF | CAAATGAGCAAGTCAAGGGCTATG | CTTTTCCACTCGGTTTCCAGC |
UTR, untranslated region.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Cell cycle synchronization and chromatin immunoprecipitation (ChIP).
T98G cells grown to 60% confluence were serum starved for 3 days in DMEM containing 0.1% FBS. Cells were then released to the cell cycle by changing to medium containing 20% FBS. Alternatively, overnight after plating in regular growth medium (DMEM containing 10% FBS), cells were treated with 2 mM hydroxyurea for 18 h and then washed three times with the regular growth medium containing 2 μM nocodazole and incubated in the same medium for 12 h. Cells were released to the cell cycle by washing three times with drug-free growth medium. Cells were harvested at specified time points after the medium change for flow cytometric analysis of cell cycle distribution, gene expression analysis, or ChIP assay by fixing in 1% formaldehyde for 8 min. ChIP assays were performed essentially as described previously (31). The crude chromatin solution was diluted and incubated overnight at 4°C with specific antibodies (25 μl of anti-ACTR [9], 5 μg of anti-E2F1 [1:1 mixture of C-20 and KH95; Santa Cruz], 5 μg of anti-rabbit immunoglobulin G [Santa Cruz], and 35 μl of anti-RNA Pol II (8WG16; Covance). PCR with 28 cycles was performed with 5 to 10 μl of purified ChIP DNA with promoter-specific primers. The sequences of the primers used are shown in Table 1. Representative gel pictures or quantified PCR products from three independent experiments are presented.
RESULTS
ACTR is required for proliferation of both normal and malignant human cells.
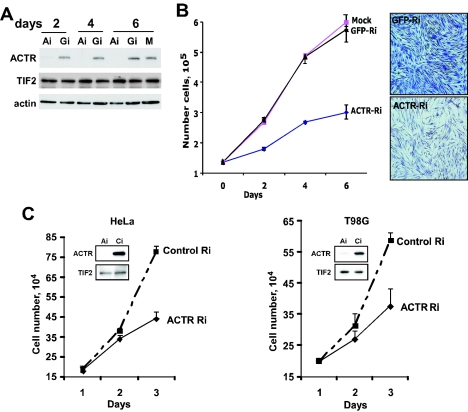
Our previous study with breast cancer cells demonstrated that ACTR overexpression abrogates the growth-inhibitory effect of antiestrogens. To determine whether endogenous ACTR functions similarly in other cell types, including nonmalignant cells, we examined the effect of acute ACTR depletion on the proliferation of a panel of different cell types. As shown in Fig. 1A, adenoviral vector-mediated RNAi specifically prevented expression of the ACTR protein, but not the other p160 member TIF2, in IMR-90 diploid human fibroblasts 2 days after viral vector treatment. Examination of IMR-90 cells treated with the vectors indicated that depletion of ACTR resulted in a marked inhibition of cell proliferation, which lasted for a week (Fig. 1B), while no effect was observed with cells treated with RNAi-GFP, compared to mock-treated cells. Similar results were obtained with diploid fibroblast WI-38 cells (data not shown). To further extend the analysis, we examined the effect of ACTR knockdown on the proliferation of malignant cells such as HeLa and T98G. Proliferation of these cells is not responsive to stimulation by hormones such as estrogen and androgen. Knocking down the high endogenous levels of ACTR strongly reduced the proliferation rate of both HeLa and T98G cells (Fig. 1C). Together, these results indicate that ACTR is required for the proliferation of both normal and malignant human cells that are nonresponsive to hormonal stimulation and are derived from different tissues.
FIG. 1.
ACTR is required for the proliferation of normal and cancerous human cells. (A and B) Depletion of ACTR inhibits the proliferation of diploid human fibroblast cells. IMR-90 cells were infected 24 h after plating with equal MOIs of adenoviral vectors expressing ACTR siRNA (Ai) or GFP siRNA (Gi) or mock infected (M). Cells were harvested 2, 4, and 6 days after infection for Western blotting (A) or cell proliferation (B). Four days after infection, cells were fixed with 70% ethanol and stained with crystal violet before digital images of representative fields of cells were taken. (C) Depletion of ACTR inhibits the proliferation of HeLa and T98G cells. Cells growing in six-well plates were transfected with synthetic siRNA targeting ACTR (ACTR-Ri) or control siRNA (Control-Ri). On different days after transfection, cells were harvested and counted in triplicates. Western analysis was performed with cell lysates harvested 2 days after transfection.
ACTR depletion strongly impedes cell entry into S phase.
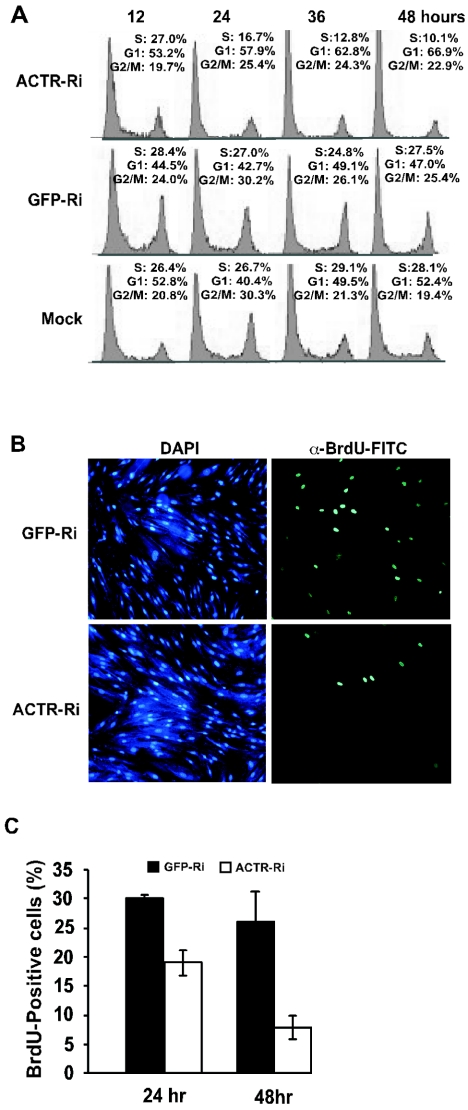
Next, we wish to understand whether ACTR is involved in promoting cell proliferation by controlling cell cycle progression. Asynchronously proliferating IMR-90 cells were infected with the adenoviral vector for ACTR depletion and harvested at different times for analysis of cell cycle distribution by flow cytometry. As shown in Fig. 2A, 48h after infection, cells treated with adenovirus ACTR RNAi showed a significant decrease in the S-phase cell population (from 27% at 12 h to 10% at 48 h) with a concomitant increase in the number of G1-phase cells (from 53% at 12 h to 67% at 48 h), while cells mock treated or treated with adenovirus GFP-RNAi displayed no significant change in cell cycle distribution between the two points. Interestingly, a slight increase in the percentage of cells in G2/M phase was also observed in adenovirus ACTR RNAi-treated cells (from 19.7% at 12 h to 22.9% at 48 h), suggesting that ACTR may also play a role in G2/M. To directly assess the function of ACTR in G1-to-S progression, we examined the effect of ACTR depletion on DNA synthesis in synchronized cells. Cells were first deprived of serum for arrest at G1/G0 and infected with the adenovirus RNAi, released to reenter the cell cycle, and pulse-labeled with BrdU. Figure 2B and C show that at 48 h after adenovirus RNAi treatment, while cells treated with the control GFP-RNAi adenovirus vector had about 26% BrdU-positive cells, cells treated with the ACTR-RNAi adenovirus vector had only about 7% BrdU-positive cells. Taken together, these results suggest that ACTR depletion strongly impedes the DNA synthesis of IMR-90 cells.
FIG. 2.
ACTR depletion blocks cell entry into S phase. (A) IMR-90 cells were infected with RNAi adenovirus vectors targeting ACTR (ACTR-Ri) or GFP (GFP-Ri) or mock infected and harvested 12, 24, 36, and 48 h after infection. Fixed cells were stained with propidium iodide. Cell cycle profiles were determined by flow cytometry. (B and C) IMR-90 cells were infected with RNAi adenovirus vectors, deprived of serum for arrest at G1/G0, released to reenter the cell cycle, and then pulse-labeled with BrdU for 1 h before being harvested. Cells were first stained with anti-BrdU-FITC and then counterstained with DAPI. Results in panel B represent the 48-h time point, showing the same field of cells stained with DAPI or anti-BrdU-FITC. The percentage of BrdU-positive cells in panel C was determined by counting the BrdU-positive cells in five frames and dividing by the total number of DAPI-positive cells. Results represent averages of two independent experiments.
ACTR is required for the expression of genes with critical functions in DNA replication.
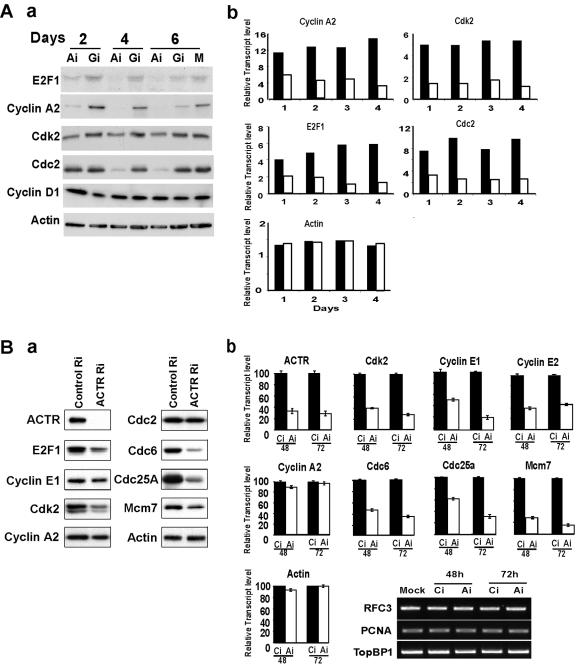
The above finding that ACTR is required for G1-to-S progression prompted us to examine whether ACTR is required for expression of genes that are crucial for cell cycle progression. Given the observation we made previously that ACTR associates with activator E2Fs, we focused our analysis on key cell cycle genes with expression regulated by E2Fs. As shown in Fig. 3A, depletion of ACTR in IMR-90 cells resulted in a significant reduction of cyclin A2, cdk2, cdc2, and E2F1 expression at both the mRNA (part b) and protein (part a) levels. The effect on the mRNA level can be observed as early as 24 h after adenovirus ACTR RNAi treatment. Notably, cyclin D1 was not affected by ACTR depletion. We then extended our study to HeLa and T98G cells and observed a similar effect of ACTR depletion on the expression of cyclin E, cdk2, and E2F1, but not on that of cyclin A2 (Fig. 3B and data not shown).
FIG. 3.
Depletion of ACTR inhibits the expression of cdc25A, cdc6, and MCMs, as well as cyclins and Cdk. (A) IMR-90 cells were infected with RNAi adenovirus vectors as in Fig. 1A and harvested at different times after infection for Western blotting (part a) or semiquantitative RT-PCR analysis (part b). The relative transcript levels are presented as arbitrary units of PCR products quantified by the Fluorchem software for cells treated with adenovirus vectors of ACTR-RNAi (open bars) or GFP-RNAi (filled bars). (B) HeLa cells were transfected with synthetic siRNA targeting either ACTR (ACTR-Ri) or a control RNAi oligonucleotide (Control-Ri) and harvested 48 later for Western blotting (part a) or 48 and 72 h later for quantitative RT-PCR, with the value from control RNAi set as 100 (part b). No significant difference in expression is illustrated by the gel image of semiquantitative RT-PCR products.
DNA replication in eukaryotic cells involves timely expression and assembly of multiple protein complexes. The expression of a number of genes, including those for cdc6, cdc25A, and MCMs, is induced at late G1 and is regulated by E2Fs. We thus wished to determine whether inhibition of DNA synthesis by ACTR depletion resulted from suppressed gene expressions critical for initiation of DNA replication. Results in Fig. 3B indicate that, indeed, depletion of ACTR caused a two- to fivefold decrease in cdc6, cdc25A, and MCM7 transcripts and their proteins. Interestingly, the expression of RFC3, PCNA, MCM10, cdc45, and TopBP1 was not affected under the same conditions (Fig. 3B and data not shown), suggesting that ACTR is required for a subset of E2F target genes involved in DNA replication of HeLa cells.
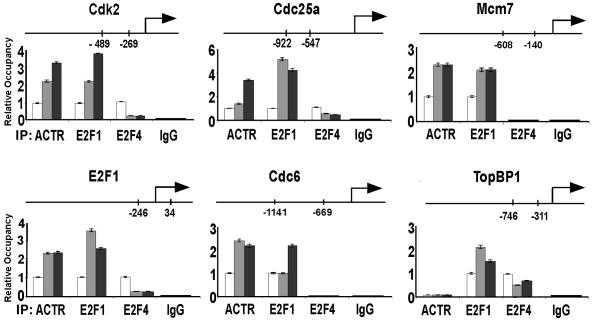
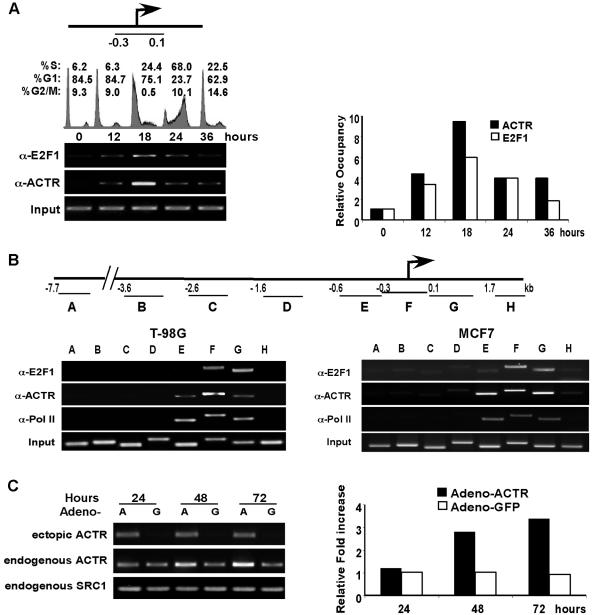
Endogenous ACTR is recruited to a subset of E2F target genes at G1/S.
Having found that endogenous ACTR is required for the expression of a subgroup of cell cycle genes, we sought to determine whether ACTR is directly involved in transcriptional control of their expression by examining the promoter occupancy of ACTR. We thus performed ChIP assays with T98G cells that can be readily rendered quiescent and progress synchronously through the cell cycle (53). In agreement with the results from ACTR depletion, we found that endogenous ACTR protein is recruited to a region containing E2F binding sites on the promoters of cdk2, E2F1, cdc6, cdc25A, and MCM7 (Fig. 4). Importantly, the recruitment is increased significantly (three- to fourfold, depending on the target genes) as the cells enter S phase. Similar induction of recruitment is observed with E2F1. Consistent with the findings that E2F4 primarily plays a repressor role at early G1 (53), the occupancy of E2F4 decreases as cells start to proliferate. The fact that ACTR does not occupy promoters of genes with expression unaffected by ACTR depletion, such as TopBP1 and caspase 9 (Fig. 4 and data not shown), indicates that the observed induction of ACTR recruitment is promoter specific and not a simple reflection of its elevated expression during cell cycle progression (details are described below).
FIG. 4.
ACTR is recruited to the promoters of E2F target genes at G1/S. T98G cells were synchronized by serum starvation for 3 days in DMEM containing 0.1% FBS and then released to the cell cycle by changing to medium containing 20% FBS. Cells were harvested for ChIP assay with the indicated antibodies at 0 (open bars), 12 (gray bars), and 18 (black bars) h after serum readdition. The relative occupancy at the specific promoters was determined by quantifying the ChIP PCR products obtained from three experiments. The location of the sequence analyzed at each promoter is indicated. IgG, immunoglobulin G.
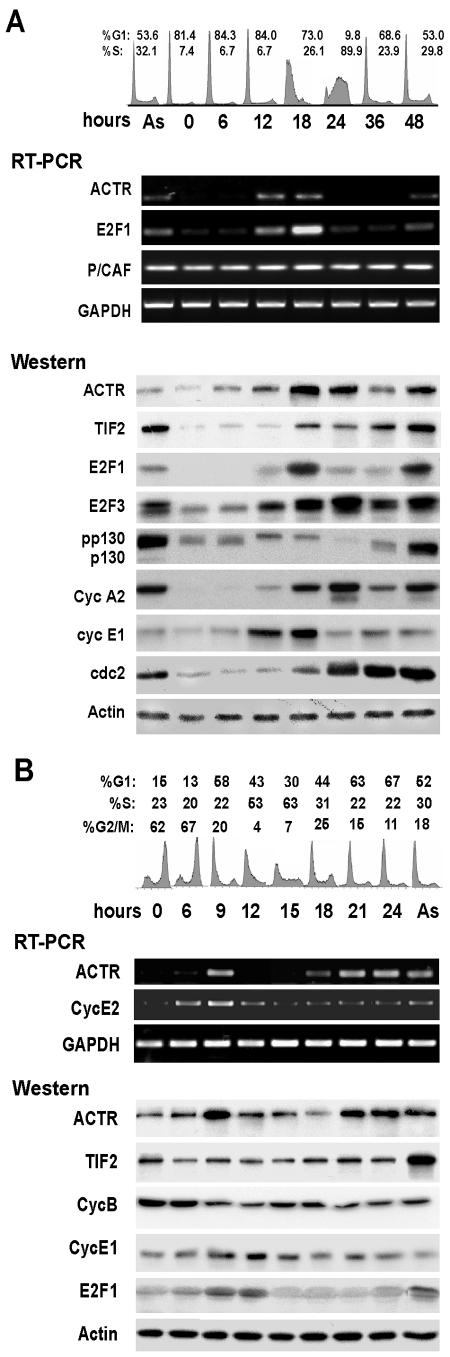
ACTR expression is cell cycle regulated.
Overexpression of ACTR has been detected in many types of human cancers. However, how ACTR expression is regulated remains unknown. In our examination of gene expression with cells synchronized in cell cycle progression, we noticed that ACTR expression fluctuates. We therefore set up experiments to determine whether ACTR gene expression is regulated in a cell cycle-dependent manner. First, we examined ACTR expression in T98G cells during their synchronous cell cycle progression. After being released from serum deprivation, fractions of cells were analyzed for cell cycle progression and for gene expression by semiquantitative RT-PCR and Western analysis. Data from flow cytometry analysis (Fig. 5A, top part) show that cells began to enter S phase around 18 h after being released from G1/G0 and progressed through S phase during the next 6 h, as indicated by an increase in the S-phase cell population from 6.7% to 26% at the 18-h point and a further increase to nearly 90% at the 24-h point. The synchronous cell cycle progression is also evidenced by the oscillation of cyclin E1 and A2 levels, as well as the timely hyperphosphorylation of pocket protein p130 (indicated by the band upshifts at the 12- and 18-h points, bottom part), as p130 is usually hyperphosphorylated by cyclin/Cdk complexes at late G1. Strikingly, ACTR expression was strongly up-regulated at mRNA level in late G1 at the 12-h point (middle part), with its protein level peaking at G1/S around the 18-h point (bottom part). Interestingly, ACTR expression was sharply attenuated after cells entered S phase. Such induction kinetics of ACTR was also observed with IMR-90 cells and closely mimics that of its interacting proteins, such as E2F1 and E2F3, but not that of TIF2, P/CAF, and CBP (Fig. 5A and data not shown).
FIG. 5.
Expression of ACTR is induced at late G1 and attenuated during S phase. (A) T98G cells were synchronized by serum deprivation as for Fig. 4 and harvested at the indicated times after serum stimulation for flow cytometry, semiquantitative RT-PCR, and Western blot analysis. (B) T98G cells were synchronized in mitosis by treatment with hydroxyurea and then with nocodazole. Cells were released to reenter the cell cycle by switching to nocodazole-free growth medium. Cells were harvested at the indicated times after release for analyses as in panel A. As, asynchronous proliferating cells.
To rule out the possibility that the change in ACTR expression is due to the effect of growth factors in the serum and to confirm that ACTR is indeed cell cycle regulated, we synchronized cells for cell cycle progression by a protocol without altering the growth factor or serum in the medium. Thus, we treated proliferating T98G cells first with hydroxyurea (which arrests the cell cycle by inhibiting DNA synthesis) to partially synchronize T98G cells at G1/S and then released them from the block in the presence of nocodazole, which in turn blocks cells at metaphase. At different points after the drug was removed, cells were harvested for flow cytometry and gene expression analysis. As shown in Fig. 5B, top part, 9 to 12 h after being released from nocodazole treatment, cells exited mitosis and entered G1-S. At the same time, the expression of ACTR, like that of E2F1 and cyclin E, was induced to a peak level (middle and bottom parts), in agreement with the expression kinetics shown in Fig. 5A. Unlike that of ACTR, the TIF2 protein level did not appear to change. Taken together, these results suggest that ACTR expression is cell cycle regulated at the transcript level, peaking at the G1-S boundary.
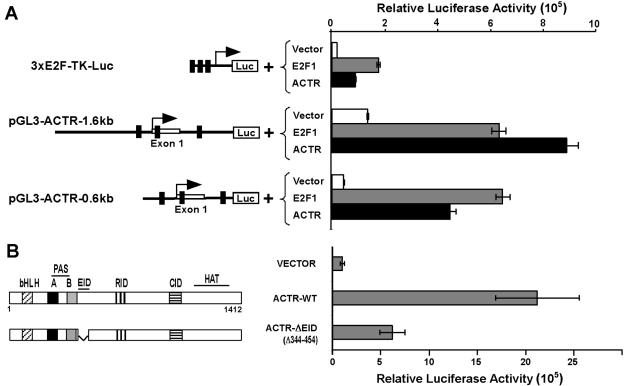
ACTR promoter is activated by E2F and ACTR itself.
To understand the molecular mechanism underlying cell cycle regulation of ACTR expression, we first examined its promoter response to E2F stimulation. Thus, a 1.6-kb genomic DNA fragment encompassing the first exon of ACTR was isolated and subcloned upstream of the luciferase reporter gene in vector pGL3. As shown in Fig. 6A, the resulting construct displayed strong promoter activity when transfected into proliferating HER cells (compare the reporter activities driven by pGL3-ACTR-1.6kb with that of 3XE2F-TK-Luc). Similar results were obtained with a shorter version of the construct, pGL3-ACTR-0.6kb. Importantly, this ACTR promoter activity can be markedly stimulated by E2F1 (about 5-fold or 15-fold, respectively, for the two promoter reporters). Since the 0.6-kb fragment confers most or all of the responsiveness to E2F stimulation, we analyzed its sequence with the PROMO database and identified multiple putative, noncanonical E2F1 binding sites (5′-CCGCC/G-3′) located in the region between −150 and +360. Similar sequences have recently been demonstrated to directly bind to E2F1 and mediate its transcriptional response (58). In light of our recent finding that ACTR can act as an E2F coactivator to control E2F target gene expression, we speculated that ACTR might be involved in the control of its own promoter via E2F. Results in Fig. 6A show that, indeed, ACTR significantly enhanced the transcription of its own promoter when tested on either the 0.6-kb or the 1.6-kb reporter construct. To determine whether ACTR acts on its own promoter through association with E2Fs, we tested the activity of a mutant form of ACTR that lacks the E2F interaction domain (ΔEID) in the reporter gene assay. Strikingly, eliminating its interaction with E2Fs severely diminished the ability of ACTR to activate its own promoter (Fig. 6B). The mutant ACTR is expressed at a level similar to that of the wild type (see Fig. 8E). These results suggest that ACTR controls its own promoter through association with E2Fs such as E2F1.
FIG. 6.
ACTR controls its own promoter through E2F1. (A) HER cells were cotransfected with the indicated reporter plasmids, expression constructs for E2F1 or ACTR, and β-galactosidase. Luciferase activities were normalized with the β-galactosidase activities. The putative E2F binding sites were identified with the PROMO database (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) and are indicated by small bars on the schematics of the pGL3-ACTR-1.6kb and -0.6kb constructs. (B) Cells were cotransfected with the pGL3-ACTR-0.6kb reporter and an empty vector or an expression plasmid for mutant or wild-type ACTR. The structure-function domain of ACTR is shown in the schematic, which includes moderate sequence homology to bHLH and PAS domains, protein interaction domains for E2F (EID), nuclear hormone receptors (RID), and CBP/p300 (CID) and a region with histone acetyltransferase (HAT) activity. WT, wild type.
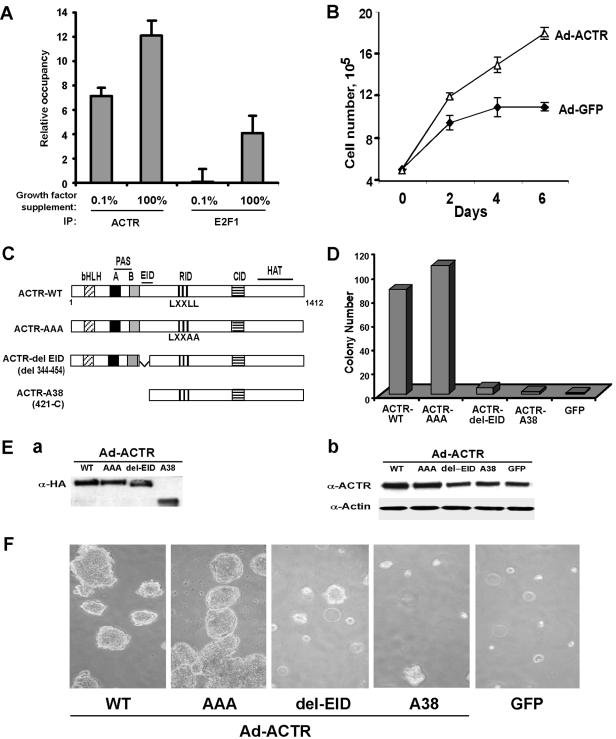
FIG. 8.
Transformation of human mammary epithelial cells by overexpressed ACTR requires its association with E2F. (A) Recruitment of ACTR and E2F1 to the ACTR promoter in MCF10A cells is stimulated by growth factors. Proliferating MCF10A cells maintained in DMEM/F12 with the full supplements (100%; details are in Materials and Methods) or changed to medium with 0.1% of the full supplements for 6 h (0.1%) were harvested for ChIP assay with anti-ACTR or -E2F1 antibodies. ChIP DNA was analyzed for ACTR and E2F1 occupancy at the ACTR promoter with PCR primers amplifying the F fragment as shown in Fig. 7B. (B) ACTR overexpression stimulates growth factor-independent cell proliferation. MCF10A cells maintained in six-well plates in DMEM/F12 with the full supplementation were infected with the ACTR- or GFP-adenovirus (Ad) vector at equal MOIs. Four hours later, the medium was changed to DMEM/F12 with 0.1% of the full supplements. On different days after infection, cells were harvested for proliferation analysis by cell enumeration in triplicate. (D to F) Wild-type ACTR, but not E2F association-defective mutant forms of ACTR, transforms MCF10A cells. MCF10A cells were infected with equal MOIs of adenovirus vectors for expression of GFP or different forms of ACTR as indicated in the diagram (C), harvested 24 h after infection, resuspended as individual cells mixed with soft agar, and plated for colony formation (D and F). Two weeks later, colonies (with diameters of 0.2 mm or larger) were counted and digital images of representative fields were captured. (Note that the one colony containing about 50 cells present in the images for del-EID and A38, respectively, was counted as a colony for the results shown in the bar graph in panel D.) The number of colonies presented was from 5 × 104 plated cells infected by each of the adenovirus vectors. The experiment was repeated once, and essentially identical results were obtained. For protein analysis (E), infected cells were harvested 10 days after infection for Western blotting with anti-HA antibody or anti-ACTR antibody. WT, wild type; IP, immunoprecipitation.
ACTR-E2F complex is recruited to ACTR promoter at late G1 to stimulate its expression.
To address whether the endogenous ACTR-E2F complex controls ACTR transcription in a cell cycle-dependent manner, we examined its occupancy at the ACTR promoter in cells synchronously progressing through the cell cycle. T98G cells released from serum starvation were harvested for ChIP at different times after the release. As shown in Fig. 7A, quiescent cells (at the 0-h point) had little E2F or ACTR occupancy of the ACTR promoter. At 12 h after cells reentered the cell cycle, the occupancy of the ACTR promoter by the ACTR-E2F complex was significantly induced. By 18 h, when the majority of cells were at late G1 and a fraction of cells (24.4%) entered S phase, much stronger recruitment of the complex was observed. Strikingly, when most of the cells were in S phase (at 24 h), the ACTR-E2F occupancy was markedly decreased. This dynamic occupancy of the ACTR promoter by the ACTR-E2F complex correlates closely with the kinetics of ACTR gene expression during cell cycle progression shown in Fig. 5, supporting the notion that the E2F-ACTR complex plays an important role in cell cycle regulation of ACTR expression. To confirm that the cell cycle-regulated recruitment of the E2F-ACTR complex is specific to the proximal promoter, we examined the occupancy of E2F1, ACTR, and RNA Pol II over a 10-kb genomic DNA sequence around the promoter. We found that when T98G cells were at the G1-S boundary (the18-h point), both E2F1 and ACTR were recruited primarily to the 1-kb promoter sequence (covered by amplicons E, F, and G), which encompasses the 0.6-kb sequence used in the reporter assays (Fig. 7B, left part). Consistent with its strong promoter activity, this 1-kb region displayed a robust Pol II occupancy in the proliferating cells, as detected by the anti-Pol II antibody. Importantly, similar occupancy by E2F1, ACTR, and Pol II was observed in asynchronously proliferating MCF7 human breast cancer cells (Fig. 7B, right part), suggesting that ACTR expression is also regulated by the E2F-ACTR complex in human breast cancer cells. To examine whether ectopic expression of ACTR can stimulate endogenous ACTR gene expression in T98G cells, we infected the cells with an adenovirus vector that expressed only the coding sequence of ACTR with a hemagglutinin (HA) tag at its 3′ end. As shown in Fig. 7C, at 48 h after infection, we observed a significant increase in the expression of the endogenous ACTR gene (detected by its 3′ untranslated region sequence) in cells infected with the ACTR adenovirus vector (A), but not with the control GFP-adenovirus vector (G). Interestingly, we did not observe any significant effect of ectopic ACTR expression on the expression of SRC-1, the other member of the p160 coactivator family, suggesting that SRC-1 is not subject to regulation by ACTR under these conditions.
FIG. 7.
The ACTR-E2F complex is recruited to the ACTR promoter at late G1 to stimulate its expression. (A) T98G cells were synchronized by serum derivation and harvested at the indicated times after serum readdition for FACS and ChIP analyses. The relative occupancy at the indicated region of the ACTR promoter was determined by quantifying the PCR products of coprecipitated genomic DNA by E2F1 and ACTR antibodies. (B, left part) A ChIP assay was performed as for panel A with T98G cells. Coprecipitated genomic DNA from cells harvested at 18 h after serum readdition was analyzed for occupancy by E2F1, ACTR, and RNA Pol II over the 10-kb region containing the ACTR promoter. The location of each amplicon (A to H) is shown in the diagram above, with the number indicating the position relative to the transcription initiation site of the 5′ primers used. (B, right part) A ChIP assay was performed with asynchronously proliferating MCF7 cells, and precipitated genomic DNA was analyzed as in the left part of panel B. (C) T98G cells were infected with adenovirus vectors to mediate the ectopic expression of ACTR (lanes A) or GFP (lanes G). Cells were harvested at the indicated times after infection and analyzed by semiquantitative RT-PCR for ectopic or endogenous ACTR expression with specific primers (for ectopic expression, a primer annealed to the 3× HA tag sequence 3′ of the ACTR cDNA coding sequence, together with a primer to the coding region, was used; for endogenous expression, a primer pair annealed to the cDNA sequence of the 3′ untranslated region of the endogenous ACTR transcripts was used). The relative increase shown in the right part of panel C was determined by quantifying the RT-PCR products of endogenous ACTR with the digital number of the product from adenovirus-GFP-infected cells at 24 h set as 1 U.
Overexpression of ACTR alone transforms human mammary epithelial cells, which requires its association with E2F.
Having demonstrated that ACTR plays an important role in the proliferation of a number of cell types and that ACTR autoregulation may contribute to its overexpression, a critical question to be addressed is whether ACTR overexpression alone can elicit any oncogenic effect on nonmalignant human cells. MCF10A, an ER-negative, spontaneously immortalized, nontransformed human mammary epithelial cell line, has been widely used to assess the oncogenic activity of many oncogenes in in vitro cell transformation assays (10, 37, 43, 45). First, to determine whether ACTR regulates its own expression in MCF10A cells, we performed a ChIP assay to examine the occupancy by ACTR and E2F1 at the ACTR promoter with proliferating MCF10A cells maintained either in medium with the full (100%) growth factor supplements or changed to medium with 0.1% of the full supplements and maintained for 6 h. As shown in Fig. 8A, strong occupancy by ACTR and E2F1 was detected at the ACTR promoter when cells were proliferating in the medium with full supplements, indicating that ACTR and E2F1 are indeed involved in the control of ACTR expression in MCF10A cells. Interestingly, deprivation of the growth factors reduced their occupancy, suggesting that the recruitment of ACTR and E2F1 to the ACTR promoter in MCF10A cells is stimulated by growth factors. Next, we examined whether ACTR overexpression stimulates growth factor-independent cell proliferation. MCF10A cells maintained in medium with full supplements were infected with the ACTR- or GFP-adenovirus vector. After infection, cells were essentially deprived of growth factors (maintained in 0.1% of the full supplements). As shown in Fig. 8B, at 2 days after growth factor deprivation, proliferation of cells infected with the control GFP-adenovirus vector was essentially stopped. In contrast, cells infected with the ACTR-adenovirus vector continued to proliferate. It is worth noting that when MCF10A cells were maintained in the medium with full supplements, infection with the ACTR- or GFP-adenovirus vector did not have any significant effect on their proliferation (data not show). These results suggest that high levels of ACTR can stimulate growth factor-independent proliferation of MCF10A cells.
To examine the activity of ACTR in cellular transformation, MCF10A cells infected with adenovirus vectors were plated in soft agar and observed for colony formation. As shown in Fig. 8E, recombinant adenovirus vectors mediates the expression in MCF10A cells of the wild-type and mutant forms of ACTR (as diagramed in panel C) at a similar level, when analyzed by Western blotting with an anti-HA antibody (part a). The level of ectopically expressed ACTR proteins was about threefold higher over the endogenous ACTR, judged by the results of Western blotting with an anti-ACTR antibody (part b; note that the monoclonal antibody recognizes an epitope within the E2F interaction domain [EID] and therefore does not detect ectopically expressed ACTR-ΔEID or -A38). As shown in Fig. 8D and F, at 2 weeks after plating in soft agar, cells overexpressing wild-type ACTR displayed a high frequency of colony formation, while no colony was observed with control cells expressing GFP. Strikingly, cells expressing ACTR mutant forms that lack the E2F-interaction domain (ACTR-ΔEID or ACTR-A38 containing an N-terminal truncation) showed severely diminished colony formation activity. In fact, the few colonies formed contained much fewer cells and thus were smaller than the ones formed with wild-type ACTR (Fig. 8F). Consistent with our previous finding that the receptor interaction activity of ACTR is not involved in mediating E2F transcription (32), abolishing ACTR association with the receptors by changing the three LXXLL motifs to LXXAA in the ACTR-AAA mutant appeared not to have any negative effect on colony formation in either number or size (Fig. 8D and F). These results suggest that overexpression of ACTR alone can transform human mammary epithelial cells and that the transforming activity requires the association of ACTR with E2F. Given that MCF10A cells lack ER expression and that mutant ACTR lacking receptor interaction possesses the same neoplastic transformation activity as the wild type, these results also suggest that the transforming activity of ACTR does not involve the ER.
DISCUSSION
Aberrant ACTR has been linked to multiple types of human cancer. However, the function of ACTR in the control of cell proliferation has not been well understood. In this report, we demonstrated that ACTR is required for both normal and malignant human cells to effectively enter S phase and that the underlying mechanism involves the direct control of gene expressions by ACTR that are critical for initiation of DNA replication.
ACTR functions as an important cell cycle regulator.
The E2F transcription factor family plays a key role in mammalian cell cycle progression by controlling gene expression critical for G1/S and G2/M transitions (22, 62, 68). Different E2Fs, in conjunction with members of the pocket proteins, likely control a specific target gene network in a cell and tissue context-dependent manner (6, 23, 36, 53, 61). Although it is well understood how mitogenic signaling initiated by growth factors impinges on the E2F-pocket protein complex to release the transcriptional repression mediated by pRb and its family members, little is known about the process of E2F-mediated activation of gene expression. In this regard, our present study, together with our previous work, strongly supports the notion that the coactivator ACTR serves as a key mediator of the transcriptional activation by E2Fs. We found that endogenous ACTR associates with activators E2F1 and E2F3 but not with repressor E2Fs such as E2F4 and is recruited to the same region occupied by E2Fs on the E2F target genes. Its ectopic expression drives quiescent cells to reenter the cell cycle (32). As demonstrated in this study, the induction of ACTR recruitment occurs primarily at late G1. Consistent with its timely physical presence, we found that ACTR is required for the expression of E2F target genes that are normally induced at late G1 or early S phase and have important functions in the G1-S transition (such as cyclin E and cdk2) and DNA replication (such as cdc6, cdc25A, and MCM7). Together, these findings provide a mechanistic insight into the role of ACTR in the control of the cell cycle.
Since ACTR was linked to cancer due to its overexpression, it is possible that its function to promote cell proliferation is manifested primarily by the aberrantly high levels of ACTR protein in cancer cells. Indeed, Zhou et al. recently reported that knockdown of ACTR in prostate cancer cells slowed down cell cycle progression and decreased their proliferation (67). Previous studies by others and us (28, 32) and the results reported here also indicate that high levels of ACTR are required for the proliferation of malignant cells from multiple different human cancers. Interestingly, however, when we depleted the relatively low level of ACTR in normal human fibroblast cells, we observed striking inhibitory effects on their S-phase entry and cell cycle gene expression, indicating that ACTR plays a critical role in the control of the cell cycle not only in malignant cells but also in normal human cells. Lending further support to this notion, we found that the ACTR gene itself is cell cycle regulated, with a peak induction at the G1-S boundary in both normal and cancerous human cells. Although it has been shown that several transcription cofactor proteins, including p300/CBP and PCAF, can interact with E2F, this is the first demonstration that a cofactor can play such a unique role in directly controlling cell cycle progression. Moreover, consistent with our assertion that ACTR is an important cell cycle regulator, a mouse knockout study revealed that deletion of ACTR severely impairs the ability of mouse embryo fibroblasts to proliferate in response to growth factor stimulation (60, 64).
Given the enormous complexity of cellular signaling in the control of cell growth and differentiation, it is conceivable that multiple sequence-specific transcription factors and cofactors are involved in the regulation of cell cycle gene expression. Indeed, eight members of the E2F family have been identified. Recent studies have quickly expanded the repertoire of E2F targets (6, 44). Interestingly, our analysis here suggests that only a subset of the E2F targets is under the regulation of ACTR. Thus, we found that knocking down ACTR affects the expression of cyclins E1 and E2, cdk2, cdc6, cdc25A, and MCM7 but not that of PCNA, RFC3, cdc45, and TopBP1 in HeLa cells. Likewise, our recent microarray analysis revealed that the primary effect of ACTR overexpression is on the expression of a subset of E2F target genes involved in the G1/S transition and DNA replication but not on genes involved in DNA damage/checkpoint and apoptosis (unpublished data). Since Cdc6, MCM7, and Cdc25A are required for prereplicative complex formation while PCNA, RFC, and cdc45 are involved in the transition to replication, our results imply that ACTR is required specifically for the expression of genes critical for prereplication complex formation.
There can be many explanations for the cell- and promoter-specific control of E2F target genes by ACTR. One of them could be that another nuclear protein(s) modulates the association of ACTR with E2F and/or the assembly of E2F-ACTR complexes at target gene loci. Several transcription factors, including TFE3, YY1, and TopBP1, have been shown to play a role in the choice of target genes by E2F (16, 17, 29, 47). Alternatively, the cellular context dependency of ACTR may be contributed by the multiple signal input on ACTR through its posttranslational modifications such as phosphorylation and acetylation (9, 12, 63). It is also possible that ACTR acts in combination with other enzymatic complexes that modify or remodel chromatin structure, such as CBP/p300 and the TRRAP/Tip60 complex, to coordinate the expression of cell cycle gene expression.
ACTR autoregulation as a mechanism of its aberrant expression or function in cancers.
Elevated levels of ACTR gene expression have been detected in an increasing number of human cancers. Although gene amplification may account for a small fraction of aberrant ACTR, the underlying mechanism for ACTR overexpression in the majority of cancers has been poorly understood. Our finding that ACTR expression is cell cycle regulated led to our investigation of its gene regulation. Remarkably, we found that ACTR expression is controlled not only by E2F but also by its own protein. These results suggest the possibility that cell cycle deregulation in the early lesion of tumorigenesis elevates ACTR expression, which in turn enhances its own gene expression and accelerates cell cycle progression. This positive feedback loop could contribute to the complete subversion of a normal cell cycle control mechanism through the selective deregulation of a subset of E2F target genes by elevated levels of ACTR. Indeed, the majority of ACTR target genes we identified, including cyclin E (both E1 and E2), cyclin A2, E2F1, MCM7, and cdc25A, are often overexpressed in many types of cancers (4, 5, 15, 50, 66). Coelevation of ACTR with E2F1 has been found in esophageal squamous cell carcinoma (14). Conceivably, similar findings will be made with other types of cancer when both genes are examined in tumor samples. As most cancer cells have accelerated proliferation, it is not surprising that ACTR overexpression is prevalent in a broad spectrum of human cancers. Therefore, the positive feedback mechanism of ACTR expression may represent a major loop that is amplified in many types of cancers. On the other hand, the findings reported here do not rule out other possible mechanisms, such as altered protein stability (48, 65), that may contribute to the aberrant level of ACTR protein found in multiple types of human cancer.
We demonstrated for the first time that overexpression of ACTR alone can transform normal mammary epithelial cells, suggesting that overexpressed ACTR is sufficient to trigger events crucial for anchorage-independent growth, such as enhanced cell cycle progression and protection from apoptosis. Further study is needed to understand the exact downstream events of ACTR-mediated neoplastic transformation. In this respect, our data are in line with the findings from animal studies which suggest an ER-independent mechanism of ACTR action (25, 55). Importantly, our observation that the full transforming activity of overexpressed ACTR requires its association with E2F directly points to the involvement of E2F. Thus, it is plausible that aberrant ACTR up-regulates a subset of target genes of the ACTR-E2F complex to accelerate the cell cycle. Whether elevation of IGF-1 signaling by aberrant ACTR, as suggested in the animal studies, or other events are responsible for averting cells from apoptosis remains to be determined. In any event, these results support the notion that quantitative alteration of ACTR can result in a unique functional integration of distinct transcriptional control programs, which is ultimately responsible for the tumorigenesis associated with aberrant ACTR.
Acknowledgments
We are grateful to J. Nevins, K. Helin, G. Leone, Y. Nakatani, and W. Lin for providing reagents. We thank H. Kung and R. Wisdom for constructive discussions. We appreciate the technical help from P. Mack, D. Hsu, and A. Rabinovich and the University of California at Davis Flow Cytometry Core Facility for FACS analysis.
This study was supported by grants from the National Institutes of Health (DK60019) and the California Breast Cancer Research Program and Cancer Research Coordinating Committee programs to H.W.C. M.C.L. was a trainee of an National Institutes of Health molecular and cellular biology training grant.
REFERENCES
- 1.Ait-Si-Ali, S., V. Guasconi, L. Fritsch, H. Yahi, R. Sekhri, I. Naguibneva, P. Robin, F. Cabon, A. Polesskaya, and A. Harel-Bellan. 2004. A Suv39h-dependent mechanism for silencing S-phase genes in differentiating but not in cycling cells. EMBO J. 23:605-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attwooll, C., E. Lazzerini Denchi, and K. Helin. 2004. The E2F family: specific functions and overlapping interests. EMBO J. 23:4709-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouras, T., M. C. Southey, and D. J. Venter. 2001. Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res. 61:903-907. [PubMed] [Google Scholar]
- 4.Brake, T., J. P. Connor, D. G. Petereit, and P. F. Lambert. 2003. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 63:8173-8180. [PubMed] [Google Scholar]
- 5.Bukholm, I. R., G. Bukholm, and J. M. Nesland. 2001. Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int. J. Cancer 93:283-287. [DOI] [PubMed] [Google Scholar]
- 6.Cam, H., E. Balciunaite, A. Blais, A. Spektor, R. C. Scarpulla, R. Young, Y. Kluger, and B. D. Dynlacht. 2004. A common set of gene regulatory networks links metabolism and growth inhibition. Mol. Cell 16:399-411. [DOI] [PubMed] [Google Scholar]
- 7.Cam, H., and B. D. Dynlacht. 2003. Emerging roles for E2F: beyond the G1/S transition and DNA replication. Cancer Cell 3:311-316. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H., R. J. Lin, R. L. Schiltz, D. Chakravarti, A. Nash, L. Nagy, M. L. Privalsky, Y. Nakatani, and R. M. Evans. 1997. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569-580. [DOI] [PubMed] [Google Scholar]
- 9.Chen, H., R. J. Lin, W. Xie, D. Wilpitz, and R. M. Evans. 1999. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell 98:675-686. [DOI] [PubMed] [Google Scholar]
- 10.Dechow, T. N., L. Pedranzini, A. Leitch, K. Leslie, W. L. Gerald, I. Linkov, and J. F. Bromberg. 2004. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc. Natl. Acad. Sci. USA 101:10602-10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimova, D. K., and N. J. Dyson. 2005. The E2F transcriptional network: old acquaintances with new faces. Oncogene 24:2810-2826. [DOI] [PubMed] [Google Scholar]
- 12.Font de Mora, J., and M. Brown. 2000. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol. 20:5041-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frolov, M. V., and N. J. Dyson. 2004. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J. Cell Sci. 117:2173-2181. [DOI] [PubMed] [Google Scholar]
- 14.Fujita, Y., C. Sakakura, K. Shimomura, M. Nakanishi, R. Yasuoka, H. Aragane, A. Hagiwara, T. Abe, J. Inazawa, and H. Yamagishi. 2003. Chromosome arm 20q gains and other genomic alterations in esophageal squamous cell carcinoma, as analyzed by comparative genomic hybridization and fluorescence in situ hybridization. Hepatogastroenterology 50:1857-1863. [PubMed] [Google Scholar]
- 15.Galaktionov, K., A. K. Lee, J. Eckstein, G. Draetta, J. Meckler, M. Loda, and D. Beach. 1995. CDC25 phosphatases as potential human oncogenes. Science 269:1575-1577. [DOI] [PubMed] [Google Scholar]
- 16.Giangrande, P. H., T. C. Hallstrom, C. Tunyaplin, K. Calame, and J. R. Nevins. 2003. Identification of E-box factor TFE3 as a functional partner for the E2F3 transcription factor. Mol. Cell. Biol. 23:3707-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giangrande, P. H., W. Zhu, R. E. Rempel, N. Laakso, and J. R. Nevins. 2004. Combinatorial gene control involving E2F and E Box family members. EMBO J. 23:1336-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 19.Gnanapragasam, V. J., H. Y. Leung, A. S. Pulimood, D. E. Neal, and C. N. Robson. 2001. Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer. Br. J. Cancer 85:1928-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harbour, J. W., and D. C. Dean. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14:2393-2409. [DOI] [PubMed] [Google Scholar]
- 21.Henke, R. T., B. R. Haddad, S. E. Kim, J. D. Rone, A. Mani, J. M. Jessup, A. Wellstein, A. Maitra, and A. T. Riegel. 2004. Overexpression of the nuclear receptor coactivator AIB1 (SRC-3) during progression of pancreatic adenocarcinoma. Clin. Cancer Res. 10:6134-6142. [DOI] [PubMed] [Google Scholar]
- 22.Humbert, P. O., R. Verona, J. M. Trimarchi, C. Rogers, S. Dandapani, and J. A. Lees. 2000. E2f3 is critical for normal cellular proliferation. Genes Dev. 14:690-703. [PMC free article] [PubMed] [Google Scholar]
- 23.Ishida, S., E. Huang, H. Zuzan, R. Spang, G. Leone, M. West, and J. R. Nevins. 2001. Role for e2f in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol. Cell. Biol. 21:4684-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang, H., K. Cui, and K. Zhao. 2004. BRG1 controls the activity of the retinoblastoma protein via regulation of p21CIP1/WAF1/SDI. Mol. Cell. Biol. 24:1188-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuang, S. Q., L. Liao, H. Zhang, A. V. Lee, B. W. O'Malley, and J. Xu. 2004. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 64:1875-1885. [DOI] [PubMed] [Google Scholar]
- 26.Lang, S. E., S. B. McMahon, M. D. Cole, and P. Hearing. 2001. E2F transcriptional activation requires TRRAP and GCN5 cofactors. J. Biol. Chem. 276:32627-32634. [DOI] [PubMed] [Google Scholar]
- 27.Li, H., C. Cuenin, R. Murr, Z. Q. Wang, and Z. Herceg. 2004. HAT cofactor Trrap regulates the mitotic checkpoint by modulation of Mad1 and Mad2 expression. EMBO J. 23:4824-4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.List, H. J., K. J. Lauritsen, R. Reiter, C. Powers, A. Wellstein, and A. T. Riegel. 2001. Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J. Biol. Chem. 276:23763-23768. [DOI] [PubMed] [Google Scholar]
- 29.Liu, K., Y. Luo, F. T. Lin, and W. C. Lin. 2004. TopBP1 recruits Brg1/Brm to repress E2F1-induced apoptosis, a novel pRb-independent and E2F1-specific control for cell survival. Genes Dev. 18:673-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan, N., A. Graham, X. Zhao, R. Fisher, B. Maiti, G. Leone, and N. B. La Thangue. 2005. E2F-8: an E2F family member with a similar organization of DNA-binding domains to E2F-7. Oncogene 24:5000-5004. [DOI] [PubMed] [Google Scholar]
- 31.Louie, M. C., H. Q. Yang, A. H. Ma, W. Xu, J. X. Zou, H. J. Kung, and H. W. Chen. 2003. Androgen-induced recruitment of RNA polymerase II to a nuclear receptor-p160 coactivator complex. Proc. Natl. Acad. Sci. USA 100:2226-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louie, M. C., J. X. Zou, A. Rabinovich, and H. W. Chen. 2004. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol. Cell. Biol. 24:5157-5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiti, B., J. Li, A. de Bruin, F. Gordon, C. Timmers, R. Opavsky, K. Patil, J. Tuttle, W. Cleghorn, and G. Leone. 2005. Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J. Biol. Chem. 280:18211-18220. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Balbas, M. A., U. M. Bauer, S. J. Nielsen, A. Brehm, and T. Kouzarides. 2000. Regulation of E2F1 activity by acetylation. EMBO J. 19:662-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 36.Muller, H., A. P. Bracken, R. Vernell, M. C. Moroni, F. Christians, E. Grassilli, E. Prosperini, E. Vigo, J. D. Oliner, and K. Helin. 2001. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15:267-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthuswamy, S. K., D. Li, S. Lelievre, M. J. Bissell, and J. S. Brugge. 2001. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3:785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nevins, J. R. 2001. The Rb/E2F pathway and cancer. Hum. Mol. Genet. 10:699-703. [DOI] [PubMed] [Google Scholar]
- 39.Nicolas, E., C. Roumillac, and D. Trouche. 2003. Balance between acetylation and methylation of histone H3 lysine 9 on the E2F-responsive dihydrofolate reductase promoter. Mol. Cell. Biol. 23:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen, S. J., R. Schneider, U. M. Bauer, A. J. Bannister, A. Morrison, D. O'Carroll, R. Firestein, M. Cleary, T. Jenuwein, R. E. Herrera, and T. Kouzarides. 2001. Rb targets histone H3 methylation and HP1 to promoters. Nature 412:561-565. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa, H., K. Ishiguro, S. Gaubatz, D. M. Livingston, and Y. Nakatani. 2002. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296:1132-1136. [DOI] [PubMed] [Google Scholar]
- 42.Rayman, J. B., Y. Takahashi, V. B. Indjeian, J. H. Dannenberg, S. Catchpole, R. J. Watson, H. te Riele, and B. D. Dynlacht. 2002. E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex. Genes Dev. 16:933-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reginato, M. J., K. R. Mills, E. B. Becker, D. K. Lynch, A. Bonni, S. K. Muthuswamy, and J. S. Brugge. 2005. Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes. Mol. Cell. Biol. 25:4591-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren, B., H. Cam, Y. Takahashi, T. Volkert, J. Terragni, R. A. Young, and B. D. Dynlacht. 2002. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev. 16:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryo, A., Y. C. Liou, G. Wulf, M. Nakamura, S. W. Lee, and K. P. Lu. 2002. PIN1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Mol. Cell. Biol. 22:5281-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakakura, C., A. Hagiwara, R. Yasuoka, Y. Fujita, M. Nakanishi, K. Masuda, A. Kimura, Y. Nakamura, J. Inazawa, T. Abe, and H. Yamagishi. 2000. Amplification and over-expression of the AIB1 nuclear receptor co-activator gene in primary gastric cancers. Int. J. Cancer 89:217-223. [PubMed] [Google Scholar]
- 47.Schlisio, S., T. Halperin, M. Vidal, and J. R. Nevins. 2002. Interaction of YY1 with E2Fs, mediated by RYBP, provides a mechanism for specificity of E2F function. EMBO J. 21:5775-5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao, W., E. K. Keeton, D. P. McDonnell, and M. Brown. 2004. Coactivator AIB1 links estrogen receptor transcriptional activity and stability. Proc. Natl. Acad. Sci. USA 101:11599-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, C. L., and B. W. O'Malley. 2004. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 25:45-71. [DOI] [PubMed] [Google Scholar]
- 50.Span, P. N., V. C. Tjan-Heijnen, P. Manders, L. V. Beex, and C. G. Sweep. 2003. Cyclin-E is a strong predictor of endocrine therapy failure in human breast cancer. Oncogene 22:4898-4904. [DOI] [PubMed] [Google Scholar]
- 51.Stallcup, M. R. 2001. Role of protein methylation in chromatin remodeling and transcriptional regulation. Oncogene 20:3014-3020. [DOI] [PubMed] [Google Scholar]
- 52.Strobeck, M. W., K. E. Knudsen, A. F. Fribourg, M. F. DeCristofaro, B. E. Weissman, A. N. Imbalzano, and E. S. Knudsen. 2000. BRG-1 is required for RB-mediated cell cycle arrest. Proc. Natl. Acad. Sci. USA 97:7748-7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi, Y., J. B. Rayman, and B. D. Dynlacht. 2000. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14:804-816. [PMC free article] [PubMed] [Google Scholar]
- 54.Taubert, S., C. Gorrini, S. R. Frank, T. Parisi, M. Fuchs, H. M. Chan, D. M. Livingston, and B. Amati. 2004. E2F-dependent histone acetylation and recruitment of the Tip60 acetyltransferase complex to chromatin in late G1. Mol. Cell. Biol. 24:4546-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torres-Arzayus, M. I., J. F. De Mora, J. Yuan, F. Vazquez, R. Bronson, M. Rue, W. R. Sellers, and M. Brown. 2004. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6:263-274. [DOI] [PubMed] [Google Scholar]
- 56.Trimarchi, J. M., and J. A. Lees. 2002. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 3:11-20. [DOI] [PubMed] [Google Scholar]
- 57.Trouche, D., A. Cook, and T. Kouzarides. 1996. The CBP co-activator stimulates E2F1/DP1 activity. Nucleic Acids Res. 24:4139-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuge, M., R. Hamamoto, F. P. Silva, Y. Ohnishi, K. Chayama, N. Kamatani, Y. Furukawa, and Y. Nakamura. 2005. A variable number of tandem repeats polymorphism in an E2F-1 binding element in the 5′ flanking region of SMYD3 is a risk factor for human cancers. Nat. Genet. 37:1104-1107. [DOI] [PubMed] [Google Scholar]
- 59.Wang, Y., M. C. Wu, J. S. Sham, W. Zhang, W. Q. Wu, and X. Y. Guan. 2002. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer 95:2346-2352. [DOI] [PubMed] [Google Scholar]
- 60.Wang, Z., D. W. Rose, O. Hermanson, F. Liu, T. Herman, W. Wu, D. Szeto, A. Gleiberman, A. Krones, K. Pratt, R. Rosenfeld, C. K. Glass, and M. G. Rosenfeld. 2000. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. USA 97:13549-13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinmann, A. S., P. S. Yan, M. J. Oberley, T. H. Huang, and P. J. Farnham. 2002. Isolating human transcription factor targets by coupling chromatin immunoprecipitation and CpG island microarray analysis. Genes Dev. 16:235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu, L., C. Timmers, B. Maiti, H. I. Saavedra, L. Sang, G. T. Chong, F. Nuckolls, P. Giangrande, F. A. Wright, S. J. Field, M. E. Greenberg, S. Orkin, J. R. Nevins, M. L. Robinson, and G. Leone. 2001. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 414:457-462. [DOI] [PubMed] [Google Scholar]
- 63.Wu, R. C., J. Qin, P. Yi, J. Wong, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 2004. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic responses to multiple cellular signaling pathways. Mol. Cell 15:937-949. [DOI] [PubMed] [Google Scholar]
- 64.Xu, J., L. Liao, G. Ning, H. Yoshida-Komiya, C. Deng, and B. W. O'Malley. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad. Sci. USA 97:6379-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yi, P., R. C. Wu, J. Sandquist, J. Wong, S. Y. Tsai, M. J. Tsai, A. R. Means, and B. W. O'Malley. 2005. Peptidyl-prolyl isomerase 1 (Pin1) serves as a coactivator of steroid receptor by regulating the activity of phosphorylated steroid receptor coactivator 3 (SRC-3/AIB1). Mol. Cell. Biol. 25:9687-9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, S. Y., S. C. Liu, L. F. Al-Saleem, D. Holloran, J. Babb, X. Guo, and A. J. Klein-Szanto. 2000. E2F-1: a proliferative marker of breast neoplasia. Cancer Epidemiol. Biomarkers Prev. 9:395-401. [PubMed] [Google Scholar]
- 67.Zhou, H. J., J. Yan, W. Luo, G. Ayala, S. H. Lin, H. Erdem, M. Ittmann, S. Y. Tsai, and M. J. Tsai. 2005. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 65:7976-7983. [DOI] [PubMed] [Google Scholar]
- 68.Zhu, W., P. H. Giangrande, and J. R. Nevins. 2004. E2Fs link the control of G1/S and G2/M transcription. EMBO J. 23:4615-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]