Abstract
Kaposi's sarcoma (KS) is an inflammatory angioproliferative lesion induced by the infection of endothelial cells with the KS-associated herpesvirus (KSHV). Infected endothelial cells assume an elongated (spindle) shape that is one of the histologic signatures of KS. In vitro, latent viral infection of primary endothelial cells (but no other cell type) strikingly recapitulates these morphological findings. Here we report that the spindling phenotype involves major rearrangement of the actin cytoskeleton and can be attributed to the expression of a single viral protein, vFLIP, a known activator of NF-κB. Consistent with this, the inhibition of NF-κB activation blocks vFLIP-induced spindling in cultured endothelial cells. vFLIP expression in spindle cells also induces the production of a variety of proinflammatory cytokines and cell surface adhesion proteins that likely contribute to the inflammatory component of KS lesions.
Kaposi's sarcoma (KS) is a complex angioproliferative lesion that is the most common neoplasm in patients with untreated AIDS, though it also exists in a human immunodeficiency virus-independent form. All KS lesions have three histological components: proliferation, inflammation, and neoangiogenesis. The principal proliferating element in KS is the so-called spindle cell, so named because of its distinctive elongated, spindle-like shape. Spindle cells have long been thought to be the driving force of KS, responsible for the recruitment of the inflammatory and angiogenic components of the lesion; consistent with this, cultured spindle cells produce a number of proinflammatory and angiogenic factors (12, 34). The inflammatory infiltrate is also felt to be an important part of KS pathogenesis, since cultured spindle cells require the secreted products of activated T cells for growth (12, 28). The histogenesis of spindle cells has long been debated. Based on the expression of markers such as CD31, CD34, CD36, UEA1 lectin, and EN4 by spindle cells, it is generally believed that the cells are most likely of endothelial origin (7). However, the exact endothelial lineage from which spindle cells are derived remains unclear. A number of lines of evidence favor the notion that KS is derived from cells of lymphatic rather than vascular endothelial origin. For instance, KS tumors are never observed in tissues (e.g., brain) that are devoid of lymphatics (47). In addition, KS cells consistently stain for markers of lymphatic endothelium, e.g., VEGF-R3, LYVE-1, and podoplanin (21, 36, 46). However, the pathogenetic significance of the latter observations has been rendered ambiguous by recent findings that KS-associated herpesvirus (KSHV) infection of lymphatic or vascular endothelial cells can reprogram their expression of endothelial markers (17, 45).
Central to the pathogenesis of KS is infection by KSHV (also called human herpesvirus 8). Like all herpesviruses, KSHV has two modes of infection, latent and lytic. In KS tumors, KSHV selectively infects the spindle cells (5), most of which are latently infected (37); only a small subpopulation of spindle cells support lytic KSHV growth. Latent gene expression is therefore considered central to the development of KS. While many established cells in culture can be latently infected by KSHV, most such cells display no phenotype (2). However, recent studies from several groups show that primary vascular endothelial cells from dermal microvasculature (9) or umbilical vein (15, 30, 42) can undergo conversion from cuboidal to spindle-like morphology, strongly reminiscent of KS tumor cells, upon infection by KSHV in vitro.
Here we show that such morphological changes are due to the cell-autonomous action of latent viral gene products and occur in both lymphatic and vascular endothelium. By expressing the known KSHV latency genes individually in primary endothelial cells, we show that this morphological change can be effected by a single viral gene encoding the vFLIP protein. This protein has previously been shown to be an antiapoptotic factor (10, 16, 38) that acts by upregulating NF-κB activity (1, 8, 13, 25, 26, 39, 40). We show that NF-κB induction by vFLIP is required for spindling and, in addition, results in the upregulation of numerous proinflammatory cytokines by endothelial cells. Thus, in addition to its widely recognized antiapoptotic functions, vFLIP also induces the development of cell-autonomous morphological changes and contributes to the inflammatory microenvironment, two features that have long been recognized as signatures of Kaposi's sarcoma.
MATERIALS AND METHODS
Cells and KSHV infection.
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics and cultured in endothelial growth medium (EGM-2) supplemented with the microvascular supplement pack (Clonetics). Lymphatic endothelial cells (LECs) and blood endothelial cells (BECs) were isolated and cultured as previously described (32). BCBL-1 cells were carried in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin, streptomycin, glutamine, and β-mercaptoethanol. KSHV was concentrated from supernatants of induced BCBL-1 cells as previously described (2, 23). KSHV infections were performed in medium containing 2 μg/ml Polybrene and incubated with cells for 2 h, after which cells were rinsed and medium was added back.
Retrovirus production and infection.
Retroviruses were produced using the amphotropic Phoenix packaging cell line transfected with the Moloney murine leukemia virus-based vector pBMN (internal ribosome entry site puromycin resistance). Phoenix cells were transfected using FuGENE 6 (Roche) according to the manufacturer's specifications. Thirty-six hours after transfection, supernatants were collected and concentrated at 5,000 rpm for 16 h. Concentrated retroviruses were resuspended in EGM-2 with 6 μg/ml Polybrene and filtered through a 0.2-μm filter. Concentrated retrovirus was diluted in EGM-2 with 6 μg/ml Polybrene and applied to cells. These cultures were spun at 2,000 rpm for 1.5 h, after which virus-containing medium was removed and regular culture medium was added back. In cases were selection was employed, 24 h after transduction, medium containing the selective agent was added to cells at the stated concentration.
Immunofluorescence, cytokine array, ELISA, and flow cytometric analyses.
Immunofluorescence assays were performed as previously described using either polyclonal antibody to latency-associated nuclear antigen (LANA) (A. Polson and D. Ganem, unpublished data) or monoclonal antibody to K8.1 (gift of L. Wu and B. Forghani) or anti-p65 (Santa Cruz Biotechnology) (2). Secondary antibodies anti-rabbit and anti-mouse fluorescein isothiocyanate (FITC) and anti-mouse rhodamine (Santa Cruz Biotechnology) were used at 1:300. FITC-coupled phalloidin (Sigma) was used at 1:200. Human cytokine antibody array V was purchased from RayBiotech and used according to the manufacturer's instructions. Arrays were performed with EGM-2 basal medium plus 2% fetal bovine serum conditioned by HUVECs for 24 h. Enzyme-linked immunosorbent assays (ELISAs) were performed according to the manufacturer's instructions (BD Bioscience) using medium conditioned for 24 h. For flow cytometry, HUVECs were incubated with anti-VCAM-1 antibody at a 1:100 dilution (BD Pharmingen) for 30 min on ice. Allophycocyanin-conjugated goat anti-mouse secondary antibody (Caltag) was used at a 1:250 dilution also for 30 min on ice. Analysis was performed using a Becton Dickinson FACSCalibur.
NF-κB electrophoretic mobility shift assay.
Nuclear enriched lysates were made from cells by incubation with hypotonic buffer (20 mM HEPES [pH 7.8], 5 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol, and protease inhibitors), followed by pelleting and disruption of nuclei by incubation in high-salt buffer (0.4 M KCl, 50 mM HEPES [pH 7.9], 0.1% NP-40, 0.5 μM EDTA, 10% glycerol, and protease inhibitors). A total of 5 μg of lysate was incubated with 32P-labeled oligonucleotide encoding the NF-κB consensus sequence (Santa Cruz Biotechnology) and dI:dC DNA (Sigma) in binding buffer (20 μM HEPES [pH 7.9], 50 mM KCl, 10% glycerol, 1 mM EDTA, 1 mM MgCl2, and 1 mM dithiothreitol). In cases of competition, unlabeled wild-type or mutant oligonucleotides (Santa Cruz Biotechnology) were included in a 250-fold excess. For supershifting, 1.5 μl of anti-p65 (Santa Cruz Biotechnology) was included in the binding reaction. Binding reactions were incubated at room temperature for 30 min without the labeled probe and for an additional 30 min after the addition of the probe. Complexes were resolved in a 1× Tris-borate-EDTA-4% acrylamide gel.
Luciferase assays.
HUVECs were transduced with retroviruses encoding either vFLIP or empty vector and subjected to selection. Once the vFLIP-expressing cells had fully developed into spindle cells, the cells were transfected in OptiMEM medium (Gibco) with 0.6 μg NF-κB-luciferase vector and 0.4 μg β-galactosidase-encoding vector using 0.25 μl Jurkat transfection reagent (Mirus). The DNA transfection mixture was incubated on the cells for 4 h, after which the cells were rinsed and regular medium was added back. After 48 h, luciferase and β-galactosidase assays were carried out according to the manufacturer's specifications (Promega).
Inhibition of NF-κB.
HUVECs were treated with 4 μM Bay 11-7082 (Calbiochem) in dimethyl sulfoxide (DMSO) for 2 h before being transduced with vFLIP-encoding retrovirus as described above. After transduction, medium containing Bay 11-7082 was added back to the cells.
RESULTS
The spindle cell phenotype and its viral etiology.
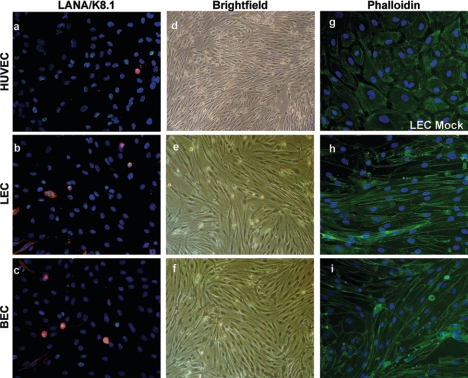
To examine the effects of KSHV on different populations of endothelial cells, we employed primary cultures of HUVECs, LECs, and BECs established by previously published methods (32). Cells were infected at high multiplicities of infection (MOIs) by KSHV virions concentrated from the medium of BCBL-1 cells, a B-lymphoma line previously shown to produce infectious KSHV virions after tetradecanoyl phorbol acetate stimulation (33). In all three lines, virus infection and spread resulted in a monolayer culture which, after a number of days, was virtually entirely latently infected, as judged by staining for the viral LANA (Fig. 1a to c); at this point, few cells (<2 to 5%) stain for the lytic marker K8.1 (Fig. 1a to c). Remarkably, all three lines display dramatic elongation to the spindle cell shape characteristic of KS tumor cells (Fig. 1d to f). Phalloidin staining (Fig. 1g, h, and i) revealed that the elongated cells had undergone a dramatic rearrangement of the actin cytoskeleton with the prominent formation of parallel arrays of actin cables. Interestingly, we did not observe immortalization of these cells, which went on to senescence with kinetics similar to those of uninfected cells (data not shown).
FIG. 1.
Infection of endothelial cells with KSHV causes the formation of spindle cells. Primary HUVECs, LECs, and BECs were obtained as described in Materials and Methods. (a to f) HUVECs, LECs, and BECs were subjected to infection with KSHV at high MOIs for 2 h and rinsed and medium was replaced. (a to c) LANA, green; K8.1, red; DAPI (4′,6′-diamidino-2-phenylindole), blue. Images were taken at 3 days postinfection. (g to i) Mock- and KSHV-infected LECs and KSHV-infected BECs were stained with FITC-coupled phalloidin.
Since latently infected cells are known to produce a number of cytokines and other paracrine factors (12), we considered the possibility that spindling might be due to extracellular signaling molecules produced in this fashion. Accordingly, we tested the effect of conditioned medium from KSHV-positive LECs and BECs on uninfected primary LEC or BEC cultures; no spindling was observed in such conditions (data not shown). While we cannot rule out the possibility that paracrine signaling might be a cofactor for spindling, it is clearly not sufficient to induce this morphology. The fact that spindle cells maintain their morphologies at even low cell densities (not shown) is more consistent with the process being largely or entirely cell autonomous.
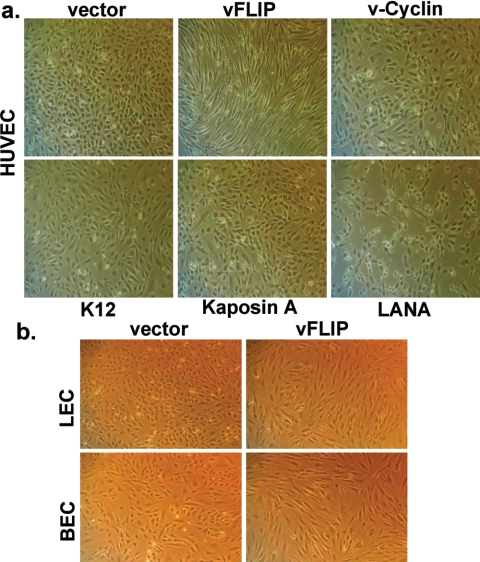
To determine what viral gene(s) is responsible for this phenotype, we tested individual KSHV latency genes for the ability to recapitulate this phenotype upon introduction into uninfected cells; HUVECs were chosen for this purpose because they are easier to prepare and maintain. Since these cells are poorly transfectable, we employed a retroviral vector to deliver each of the known KSHV latency genes: LANA, v-cyclin, vFLIP, kaposin A (open reading frame K12), and kaposins B and C (this vector may also encode small quantities of kaposin A). Early passage HUVECs were transduced at high MOIs with the individual retroviruses and subjected to selection with puromycin (0.5 μg/ml). After 3 days, cells were scored for changes in their morphologies. As shown in Fig. 2a, the expression of LANA, v-cyclin, the K12 locus, and kaposin A alone failed to induce morphological changes in the selected cell lines. However, a different result was obtained with the vFLIP vector; cells expressing this protein displayed dramatic elongation and spindling virtually identical to that observed with authentic KSHV latency. This change was also observed in LECs and BECs infected with the same vector (Fig. 2b), affirming that the results apply to those endothelial lineages as well.
FIG. 2.
vFLIP expression alone causes spindle cell formation in HUVECs, LECs, and BECs. (a) HUVECs were transduced with retroviruses encoding the individual latency-associated genes and subjected to selection with 0.5 μg/ml puromycin. Images were taken at 3 days posttransduction. (b) LECs and BECs were transduced with retroviruses encoding vFLIP or empty vector. The cells were then selected with 0.25 μg/ml puromycin. Images were taken at 2 days posttransduction.
Activation of NF-κB correlates with spindle cell development.
Several reports have established that vFLIP is a potent activator of the transcription factor NF-κB (1, 26, 38-40) vFLIP has been shown to interact with several upstream components of the NF-κB activation pathway, including TRAF 1 and 2, RIP, NIK, and IKK (13, 25), resulting in the activation of IKK. This leads to the phosphorylation and subsequent proteasome-dependent degradation of IκB, an inhibitory subunit that binds cytosolic NF-κB and inhibits its nuclear import. This degradation releases active NF-κB, allowing it to translocate into the nucleus and activate the expression of its target genes. Based on these reports, we investigated whether the development of spindle cells, as driven by vFLIP, correlated with increased activation of NF-κB.
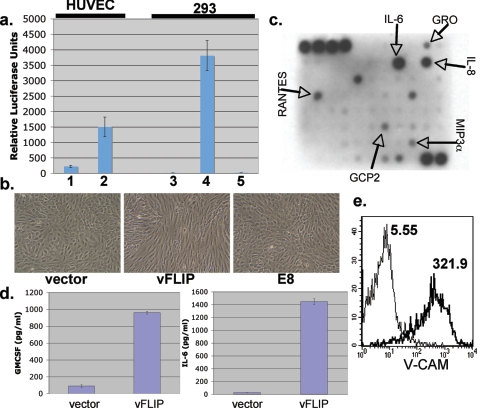
First, we affirmed that, in primary endothelium cells stably expressing v-FLIP, NF-κB was indeed activated. Spindled v-FLIP-positive HUVEC transductants were transfected with an NF-κB-dependent reporter construct; in parallel, HUVECs transduced with an empty retrovirus were similarly transfected and luciferase activity was measured in both cultures. Figure 3a, bars 1 and 2, shows that, as expected, vFLIP-expression resulted in a substantial induction of NF-κB activity. This was associated with enhanced nuclear translocation of p65 and increased binding of NF-κB to oligonucleotides bearing NF-κB recognition sites in nuclear extracts (C. Grossmann, unpublished results).
FIG. 3.
NF-κB activation correlates with spindle cell formation and upregulates inflammatory cytokines and markers of endothelial activation. (a) Bars 1 and 2, Spindled vFLIP-expressing HUVECs (1) and HUVECs expressing vector alone (2) were transfected with 0.5 μg NF-κB-luciferase reporter construct and 0.5 μg β-galactosidase-encoding plasmid to normalize for transfection efficiency. Forty-eight hours posttransfection, luciferase and β-galactosidase assays were performed; bars show normalized levels of luciferase activity. Bars 3, 4, and 5, 293 cells were transfected with equal amounts of vector (3), vFLIP (4), or E8 (5) plasmids along with an NF-κB-luciferase reporter and β-galactosidase construct. Error bars indicate standard deviations. Assays were performed at 48 h posttransfection. (b) HUVECs were transduced with retrovirus encoding either vFLIP, E8, or empty vector and selected with puromycin as described before. Images were taken 3 days posttransduction. (c) Supernatant conditioned for 24 h by fully spindled HUVECs expressing vFLIP was applied to the RayBio human cytokine antibody array V as per the manufacturer's indications. The cytokines indicated by arrows are those found to be increased over conditioned medium from vector-expressing HUVECs assayed in parallel. (d) Medium from vFLIP or empty vector-expressing HUVECs was assayed for content of IL-6 or granulocyte-macrophage colony-stimulating factor (GM-CSF) by ELISA. Error bars indicate standard deviations. (e) Spindle cells expressing vFLIP (thick line) or vector-transduced HUVECs (thin line) were stained for surface expression of VCAM-1 and examined by flow cytometry. Numbers next to histograms indicate geographic means of expression levels.
KSHV vFLIP is a member of a small family of viral FLIP proteins, all of which share partially homologous death effector domains (43). Classical viral FLIPs, like the E8 protein of equine herpesvirus, antagonize apoptosis by impairing the recruitment of caspase 8 to Fas and other death receptors, but they do not activate NF-κB (3, 8, 43). The transfection of 293 cells with an E8 expression vector did not activate LUC expression from an NF-κB-dependent reporter gene (Fig. 3a, bars 3 to 5). Importantly, the transduction of HUVECs with an E8-expressing retrovirus did not lead to cell shape changes under conditions in which the transduction of KSHV v-FLIP led to efficient spindling (Fig. 3b). Thus, endothelial spindling is closely correlated with NF-κB activation.
vFLIP-expressing spindle cells show markers of inflammatory activation.
In addition to its angioproliferative features, KS is also an inflammatory lesion. In early KS, inflammatory cells are usually a prominent feature and are a potential source of both proliferative and angiogenic signals (12). NF-κB is a central player in orchestrating the inflammatory response, and its dysregulated expression has been linked to several inflammatory disease states (41). NF-κB-regulated genes include markers of endothelial cell activation as well as several inflammatory cytokines and chemokines; many of these may play roles in KS histogenesis. Based on the potent activation of NF-κB in vFLIP-expressing spindle cells, we asked whether these cells showed evidence of upregulation of endogenous, proinflammatory NF-κB-regulated genes. Supernatant from fully spindled HUVECs expressing vFLIP was assayed for the presence of 79 cytokines by using a cytokine array bearing numerous anticytokine antibodies (Fig. 3c). Supernatant from HUVECs expressing only vFLIP was found to have increased amounts of interleukin-6 (IL-6) (in agreement with earlier data) (1), IL-8, GRO, RANTES, GCP2, and MIP3α compared to supernatant from HUVECs expressing empty vector. All of these cytokines are known to be regulated by NF-κB (24, 27, 35). ELISAs were performed on supernatant from spindled vFLIP-expressing HUVECs and confirmed elevated amounts of the proinflammatory cytokines IL-6 and granulocyte-macrophage colony-stimulating factor (Fig. 3d). Additionally, using flow cytometry, we detected upregulation of VCAM-1 on vFLIP-expressing spindle cells (Fig. 3e). VCAM-1 expression is a known marker of endothelial cell activation that is upregulated by NF-κB (18, 29) and is commonly found on KS spindle cells (12). These data suggest that the expression of vFLIP and its subsequent activation of NF-κB in endothelial cells contribute to the inflammatory microenvironment of the KS lesion.
Inhibition of NF-κB activation prevents the formation of spindle cells.
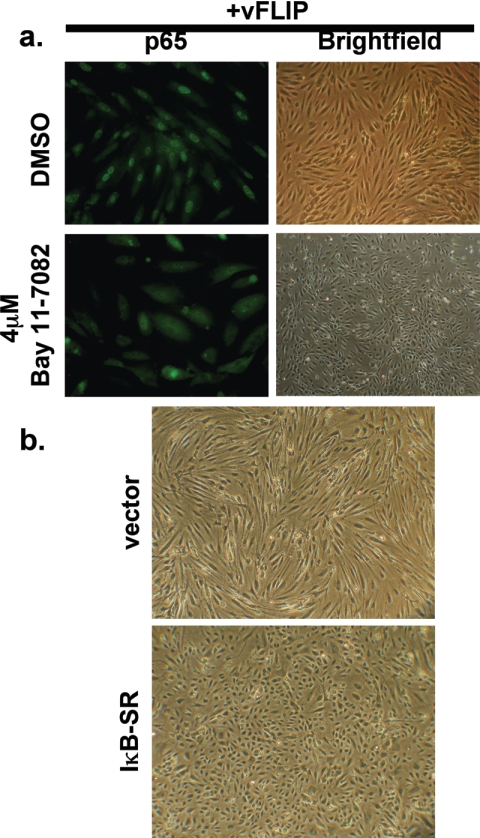
If NF-κB induction is required for the induction of the spindle cell phenotype, then inhibition of NF-κB activation should block vFLIP-mediated spindling. Accordingly, we took advantage of the availability of an NF-κB inhibitor, Bay 11-7082, to explore the importance of this transcription factor in spindling of HUVECs. Bay 11-7082 blocks NF-κB activation by inhibiting phosphorylation of IκB-α (31). HUVECs were pretreated with Bay 11-7082 for 2 h and then transduced with the retrovirus encoding vFLIP at high MOIs. After transduction, medium containing Bay 11-7082 was added back and the cells were followed for several days. As seen in Fig. 4a, treatment of HUVECs with Bay 11-7082 at 4 μM (in DMSO) prevented the activation of NF-κB in these cells, as judged by the lack of p65 staining in their nuclei. This inhibition of NF-κB coordinately inhibited the development of the spindle cell phenotype, as these cells maintained their typical cobblestone morphologies. In contrast, vFLIP-transduced cells that received only the DMSO diluent readily developed the spindle cell phenotype and showed robust NF-κB activation, as seen by the presence of p65 in their nuclei (Fig. 4a).
FIG. 4.
Inhibition of NF-κB prevents spindle cell formation. (a) HUVECs were pretreated with either 4 μM Bay 11-7082 (lower panels) or DMSO (top panels) for 2 h before being transduced at high MOIs with retroviruses encoding vFLIP. After transduction, medium containing either Bay 11-7082 or DMSO was added back. At 3 days posttransduction, the cells were photographed (right panels) or fixed and stained with a p65-specific antibody (left panels). (b) HUVECs were transduced at high MOIs with retrovirus coding for either the IκB superrepressor (lower panel) or empty vector (upper panel) and then selected in 0.5 μg/ml puromycin for several days. The cells were then superinfected at high MOIs with retrovirus encoding vFLIP, and their morphologies were assayed at 5 days posttransduction.
Bay 11-7082 is a toxic drug with a narrow therapeutic window. To further demonstrate that NF-κB activation is the central event in spindling, we took advantage of a mutant of IκB (IκB-SR) whose phosphorylation sites for IKK have been ablated. As a result, the expression of this mutant results in irreversible sequestration of NF-κB in the cytosol, since this form of IκB cannot be directed to the proteasome. Accordingly, we constructed a retroviral vector for IκB-SR and used it (or an isogenic empty-vector control virus) to transduce HUVECs; following selection for several days, cells were superinfected with the vFLIP retrovirus at high MOIs and observed for another 5 days. As shown in Fig. 4b, under conditions in which exuberant spindling occurred in the control cells, virtually no morphological change was evident in IκB-SR-expressing cells. (While it is likely that this effect results from impaired action of v-FLIP, we cannot entirely exclude the formal possibility that the inhibition of NF-κB reduces the accumulation of vFLIP protein, since levels of vFLIP are too low for routine detection by immunoblotting even in the presence of active NF-κB.)
DISCUSSION
These findings establish that expression of the latent vFLIP gene of KSHV is sufficient to cause the morphological changes that underlie the spindle cell phenotype and demonstrate that the activation of NF-κB is required for the development of this phenotype. We made concerted attempts to demonstrate that NF-κB activation is also necessary for spindling in the context of authentic viral latency by asking whether IκB-SR expression could block spindling following infection with KSHV virions. However, we found that NF-κB inhibition resulted in dramatic increases in cell death of the monolayer following KSHV infection, making scoring of spindling impossible. We believe this phenomenon to be due in part to increased lytic reactivation of the virus in cells that were unable to activate NF-κB, as has previously been suggested by Brown et al. (6) on the basis of reporter gene studies.
V-FLIP expression also upregulates genes that contribute to the inflammatory microenvironment of KS. Other described activities of vFLIP, e.g., binding of procaspase 8 or inhibition of Fas-mediated caspase 8 activation (3, 10), are unlikely to be required for spindling since selective inhibition of NF-κB activation blocks the phenotype and since other FLIPs with these activities (e.g., E8) lack the capacity to induce spindling. Interestingly, although NF-κB activation is usually an antiapoptotic signal (22), neither we nor others (9, 15, 30, 42) have observed life span prolongation of KSHV-infected HUVECs. (This is in contrast to an earlier report in which viral infection was associated with long-term outgrowth of endothelial cells; however, in that study, the clones that did emerge largely did not retain the viral genome [14], which is in keeping with the known instability of KSHV latency in vitro [15, 23]. We do not know the reason for this discrepancy.) Nonetheless, these observations do not exclude the possibility that vFLIP confers a survival advantage on spindle cells in the complex inflammatory milieu of an infection in vivo, for example, by reducing the sensitivity of cells to proapoptotic stimuli. Since KSHV-infected spindle cells accumulate progressively during the evolution of KS (11), they certainly must have a survival/growth advantage in vivo and it seems very likely that vFLIP's antiapoptotic action would play a role in this.
This is the first report suggesting a role for vFLIP in cytoskeletal rearrangement. How this cytoskeletal alteration is engendered remains unknown, but it is clear that NF-κB activation is responsible, as it is for vFLIP's known antiapoptotic effect (16). Surely, the morphological change reflects activation of a different set of NF-κB target genes from those involved in blocking programmed cell death. Since these cytoskeletal changes are specific to endothelial cells, either there are endothelium-specific NF-κB targets or the action of one or more NF-κB targets must require endothelium-specific cofactors. The linkage of NF-κB activation to cytoskeletal rearrangement was unanticipated. Our review of the literature on NF-κB and the cytoskeleton revealed no prior instances in which the activation of this factor has been linked to cytoskeletal rearrangement, although the converse has been observed; for example, NF-κB activation has been observed in response to Rac1 activation (and actin rearrangement) by endothelial shear stress and to agents that disrupt microtubules (4, 19, 20, 44).
By contrast, the antiapoptotic and proinflammatory effects of vFLIP are observed in many cell types. For example, elegant studies of B cells by Guasparri and coworkers revealed that continuous vFLIP expression is essential for B-cell survival in primary effusion lymphoma caused by KSHV (16). vFLIP also blocks apoptosis in myeloid cells deprived of growth factors (38). NF-κB induction therefore plays pivotal and overlapping, but nonidentical, roles in the lymphoid and endothelial disorders linked to KSHV.
REFERENCES
- 1.An, J., Y. Sun, R. Sun, and M. B. Rettig. 2003. Kaposi's sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-κB and JNK/AP1 pathways. Oncogene 22:3371-3385. [DOI] [PubMed] [Google Scholar]
- 2.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belanger, C., A. Gravel, A. Tomoiu, M. E. Janelle, J. Gosselin, M. J. Tremblay, and L. Flamand. 2001. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J. Hum. Virol. 4:62-73. [PubMed] [Google Scholar]
- 4.Bhullar, I. S., Y. S. Li, H. Miao, E. Zandi, M. Kim, J. Y. Shyy, and S. Chien. 1998. Fluid shear stress activation of IκB kinase is integrin-dependent. J. Biol. Chem. 273:30544-30549. [DOI] [PubMed] [Google Scholar]
- 5.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 6.Brown, H. J., M. J. Song, H. Deng, T. T. Wu, G. Cheng, and R. Sun. 2003. NF-κB inhibits gammaherpesvirus lytic replication. J. Virol. 77:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browning, P. J., J. M. Sechler, M. Kaplan, R. H. Washington, R. Gendelman, R. Yarchoan, B. Ensoli, and R. C. Gallo. 1994. Identification and culture of Kaposi's sarcoma-like spindle cells from the peripheral blood of human immunodeficiency virus-1-infected individuals and normal controls. Blood 84:2711-2720. [PubMed] [Google Scholar]
- 8.Chaudhary, P. M., A. Jasmin, M. T. Eby, and L. Hood. 1999. Modulation of the NF-κB pathway by virally encoded death effector domains-containing proteins. Oncogene 18:5738-5746. [DOI] [PubMed] [Google Scholar]
- 9.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djerbi, M., V. Screpanti, A. I. Catrina, B. Bogen, P. Biberfeld, and A. Grandien. 1999. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 190:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensoli, B., and M. Sturzl. 1998. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 9:63-83. [DOI] [PubMed] [Google Scholar]
- 13.Field, N., W. Low, M. Daniels, S. Howell, L. Daviet, C. Boshoff, and M. Collins. 2003. KSHV vFLIP binds to IKK-γ to activate IKK. J. Cell Sci. 116:3721-3728. [DOI] [PubMed] [Google Scholar]
- 14.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394:588-592. [DOI] [PubMed] [Google Scholar]
- 15.Grundhoff, A., and D. Ganem. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 113:124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guasparri, I., S. A. Keller, and E. Cesarman. 2004. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 199:993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Hong, Y. K., K. Foreman, J. W. Shin, S. Hirakawa, C. L. Curry, D. R. Sage, T. Libermann, B. J. Dezube, J. D. Fingeroth, and M. Detmar. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36:683-685. [DOI] [PubMed] [Google Scholar]
- 18.Iademarco, M. F., J. J. McQuillan, G. D. Rosen, and D. C. Dean. 1992. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J. Biol. Chem. 267:16323-16329. [PubMed] [Google Scholar]
- 19.Imberti, B., M. Morigi, C. Zoja, S. Angioletti, M. Abbate, A. Remuzzi, and G. Remuzzi. 2000. Shear stress-induced cytoskeleton rearrangement mediates NF-κB-dependent endothelial expression of ICAM-1. Microvasc. Res. 60:182-188. [DOI] [PubMed] [Google Scholar]
- 20.Jung, Y. J., J. S. Isaacs, S. Lee, J. Trepel, and L. Neckers. 2003. Microtubule disruption utilizes an NFκB-dependent pathway to stabilize HIF-1α protein. J. Biol. Chem. 278:7445-7452. [DOI] [PubMed] [Google Scholar]
- 21.Jussila, L., R. Valtola, T. A. Partanen, P. Salven, P. Heikkila, M. T. Matikainen, R. Renkonen, A. Kaipainen, M. Detmar, E. Tschachler, R. Alitalo, and K. Alitalo. 1998. Lymphatic endothelium and Kaposi's sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 58:1599-1604. [PubMed] [Google Scholar]
- 22.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 23.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libermann, T. A., and D. Baltimore. 1990. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol. Cell. Biol. 10:2327-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, L., M. T. Eby, N. Rathore, S. K. Sinha, A. Kumar, and P. M. Chaudhary. 2002. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the IκB kinase complex. J. Biol. Chem. 277:13745-13751. [DOI] [PubMed] [Google Scholar]
- 26.Matta, H., and P. M. Chaudhary. 2004. Activation of alternative NF-κB pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1β-converting enzyme inhibitory protein (vFLIP). Proc. Natl. Acad. Sci. USA 101:9399-9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriuchi, H., M. Moriuchi, and A. S. Fauci. 1997. Nuclear factor-κB potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J. Immunol. 158:3483-3491. [PubMed] [Google Scholar]
- 28.Nakamura, S., S. Z. Salahuddin, P. Biberfeld, B. Ensoli, P. D. Markham, F. Wong-Staal, and R. C. Gallo. 1988. Kaposi's sarcoma cells: long-term culture with growth factor from retrovirus-infected CD4+ T cells. Science 242:426-430. [DOI] [PubMed] [Google Scholar]
- 29.Osborn, L., C. Hession, R. Tizard, C. Vassallo, S. Luhowskyj, G. Chi-Rosso, and R. Lobb. 1989. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 59:1203-1211. [DOI] [PubMed] [Google Scholar]
- 30.Pan, H., F. Zhou, and S. J. Gao. 2004. Kaposi's sarcoma-associated herpesvirus induction of chromosome instability in primary human endothelial cells. Cancer Res. 64:4064-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce, J. W., R. Schoenleber, G. Jesmok, J. Best, S. A. Moore, T. Collins, and M. E. Gerritsen. 1997. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272:21096-21103. [DOI] [PubMed] [Google Scholar]
- 32.Podgrabinska, S., P. Braun, P. Velasco, B. Kloos, M. S. Pepper, and M. Skobe. 2002. Molecular characterization of lymphatic endothelial cells. Proc. Natl. Acad. Sci. USA 99:16069-16074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salahuddin, S. Z., S. Nakamura, P. Biberfeld, M. H. Kaplan, P. D. Markham, L. Larsson, and R. C. Gallo. 1988. Angiogenic properties of Kaposi's sarcoma-derived cells after long-term culture in vitro. Science 242:430-433. [DOI] [PubMed] [Google Scholar]
- 35.Schreck, R., and P. A. Baeuerle. 1990. NF-κB as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol. Cell. Biol. 10:1281-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skobe, M., L. F. Brown, K. Tognazzi, R. K. Ganju, B. J. Dezube, K. Alitalo, and M. Detmar. 1999. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi's sarcoma. J. Investig. Dermatol. 113:1047-1053. [DOI] [PubMed] [Google Scholar]
- 37.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, Q., H. Matta, and P. M. Chaudhary. 2003. The human herpes virus 8-encoded viral FLICE inhibitory protein protects against growth factor withdrawal-induced apoptosis via NF-κB activation. Blood 101:1956-1961. [DOI] [PubMed] [Google Scholar]
- 39.Sun, Q., H. Matta, and P. M. Chaudhary. 2005. Kaposi's sarcoma associated herpes virus-encoded viral FLICE inhibitory protein activates transcription from HIV-1 long terminal repeat via the classical NF-κB pathway and functionally cooperates with Tat. Retrovirology 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun, Q., S. Zachariah, and P. M. Chaudhary. 2003. The human herpes virus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NF-κB activation. J. Biol. Chem. 278:52437-52445. [DOI] [PubMed] [Google Scholar]
- 41.Sun, S. C., and G. Xiao. 2003. Deregulation of NF-κB and its upstream kinases in cancer. Tat. Retrovirology 22:405-422. [DOI] [PubMed] [Google Scholar]
- 42.Tang, J., G. M. Gordon, M. G. Muller, M. Dahiya, and K. E. Foreman. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen induces expression of the helix-loop-helix protein Id-1 in human endothelial cells. J. Virol. 77:5975-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meinl, F. Neipel, C. Mattmann, K. Burns, J. L. Bodmer, M. Schroter, C. Scaffidi, P. H. Krammer, M. E. Peter, and J. Tschopp. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517-521. [DOI] [PubMed] [Google Scholar]
- 44.Tzima, E., M. A. Del Pozo, W. B. Kiosses, S. A. Mohamed, S. Li, S. Chien, and M. A. Schwartz. 2002. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 21:6791-6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36:687-693. [DOI] [PubMed] [Google Scholar]
- 46.Weninger, W., T. A. Partanen, S. Breiteneder-Geleff, C. Mayer, H. Kowalski, M. Mildner, J. Pammer, M. Sturzl, D. Kerjaschki, K. Alitalo, and E. Tschachler. 1999. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi's sarcoma tumor cells. Lab. Investig. 79:243-251. [PubMed] [Google Scholar]
- 47.Witte, M. H., C. L. Witte, D. L. Way, and M. Fiala. 1990. AIDS, Kaposi sarcoma, and the lymphatic system: update and reflections. Lymphology 23:73-80. [PubMed] [Google Scholar]