Abstract
Chlamydomonas reinhardtii activates Cpx1, Cyc6, and Crd1, encoding, respectively, coproporphyrinogen oxidase, cytochrome c6, and a novel di-iron enzyme when transferred to oxygen-deficient growth conditions. This response is physiologically relevant because C. reinhardtii experiences these growth conditions routinely, and furthermore, one of the target genes, Crd1, is functionally required for normal growth under oxygen-depleted conditions. The same genes are activated also in response to copper-deficiency through copper-response elements that function as target sites for a transcriptional activator. The core of the copper-response element, GTAC, is required also for the hypoxic response, as is a trans-acting locus, CRR1. Mercuric ions, which antagonize the copper-deficiency response, also antagonize the oxygen-deficiency response of these target genes. Taken together, these observations suggest that the oxygen- and copper-deficiency responses share signal transduction components. Nevertheless, whereas the copper-response element is sufficient for the nutritional copper response, the oxygen-deficiency response requires, in addition, a second cis-element, indicating that the response to oxygen depletion is not identical to the nutritional copper response. The distinction between the two responses is also supported by comparative analysis of the response of the target genes, Cyc6, Cpx1, and Crd1, to copper versus oxygen deficiency. A Crr1-independent pathway for Hyd1 expression in oxygen-depleted C. reinhardtii demonstrates the existence of multiple oxygen/redox-responsive circuits in this model organism.
In plants, hypoxia is relevant in several physiological contexts, but it has been studied most extensively in the context of root biology where several situations resulting in an oxygen-depleted environment have been described. At the cellular level, the responses to oxygen deprivation involve changes in energy and oxidative metabolism, which are effected through regulatory processes at all levels, including transcriptional activation of genes, post-transcriptional processes, translational control, and enzyme activation (for review, see Bailey-Serres and Dawe, 1996; Sachs et al., 1996; Germain et al., 1997; Rivoal et al., 1997). The alcohol dehydrogenase gene has been studied as a prototypical target for the root hypoxic response. Its activation in anaerobiosis requires cis-regulatory sequences that were identified through reporter gene analysis (Olive et al., 1991; Walker et al., 1997) and transcription factors of which one has been identified recently (Hoeren et al., 1998). Experiments involving differential screening have revealed additional genes that are induced in oxygen deficiency and, not surprisingly, most encode enzymes involved in sugar metabolism, including glycolysis and fermentation. Although these metabolic pathways have been substantially unraveled, the signal transduction mechanisms resulting in differential gene expression remain an open question.
Target genes are activated with different kinetics in response to oxygen deprivation and to different extents (e.g. Peschke and Sachs, 1993; Huq and Hodges, 1999), indicating the presence of multiple sensing and response pathways that may allow adaptation to be tuned to organ function and different ranges of oxygen tension. An important example of this is the response of photosynthetic organs to oxygen deprivation, which is distinct from the root response (Okimoto et al., 1980; Ellis et al., 1999), and is not well described. In particular, chloroplast metabolism is largely unexplored, except for adaptive processes in anaerobic algal cells, including Chlamydomonas reinhardtii. Metabolic, physiological, and molecular responses within the chloroplast to oxygen-deficient or anaerobic growth conditions have long been known for C. reinhardtii (Wood, 1978; Happe et al., 1994; Melis et al., 2000). One of these is the accumulation of chloroplast cytochrome c6 in poorly aerated medium (Wood, 1978). Cytochrome c6 functions in copper-deficient cells as a heme-containing substitute for a copper-containing protein, plastocyanin, in the photosynthetic apparatus (Merchant and Bogorad, 1986b; Merchant, 1998). Cytochrome c6 is induced in copper-deficiency by transcriptional activation of the Cyc6 gene through copper-response elements (CuREs). C. reinhardtii also up-regulates Cpx1 (encoding coprogen oxidase, an enzyme of tetrapyrrole biosynthesis) and Crd1 (encoding a putative di-iron enzyme) during adaptation to copper deficiency (Hill and Merchant, 1995; Moseley et al., 2000; Quinn et al., 2000a). Because each copper-responsive gene is also expressed in hypoxic cells, a mechanistic and physiological connection between copper- and oxygen-deficiency-induced gene expression was proposed (Moseley et al., 2000; Quinn et al., 2000a). The availability of well-characterized target genes and the amenability of C. reinhardtii for molecular genetic analysis of signaling pathways indicated that oxygen deficiency-activated expression of Cyc6, Cpx1, and Crd1 might serve as another important model for hypoxic responses in photosynthetic eukaryotes, particularly in the context of chloroplast metabolism.
Here, we show that the target gene Crd1 is physiologically essential for normal chloroplast biogenesis under oxygen-deficient conditions, that the core of the CuRE associated with the Cpx1 gene is necessary but not sufficient for transcriptional activation under oxygen deficiency, which requires also a hypoxia-response element (HyRE), and that Crr1, a trans-acting master regulator of the copper-deficiency response, is also required for the oxygen deficiency response. Anoxic induction of a well-known anaerobically induced gene, Hyd1 (encoding an Fe-hydrogenase that is induced under anaerobic conditions), in the crr1 mutant speaks to multiple hypoxia/anoxia-sensing mechanisms in C. reinhardtii.
RESULTS
Hypoxic Induction of Copper Deficiency Responsive Genes
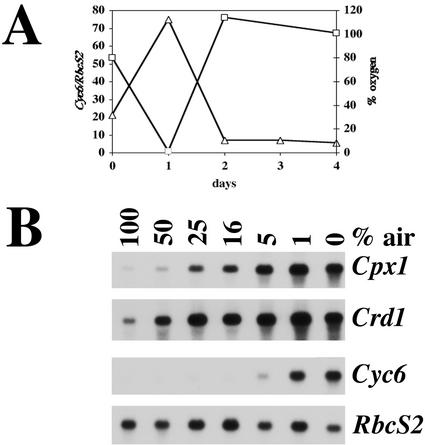
Cyc6, Cpx1, and Crd1 are three target genes of a copper deficiency signal transduction pathway. Each is activated during adaptation to copper deficiency. We noted that these three genes are also induced in copper-replete cells under oxygen-deficient or near anoxic conditions as might be experienced in their natural environment or during normal laboratory growth conditions (Moseley et al., 2000; Quinn et al., 2000a). For instance, when heterotrophic C. reinhardtii cultures are suspended by slow basal stirring (versus vigorous agitation on a shaker at 250 rpm) under normal room lighting (approximately 10–15 μmol m−2 s−1), they can become oxygen depleted within a day as a consequence of respiratory activity (Fig. 1A). Under these conditions, Cyc6 mRNA accumulates (Fig. 1A; Wood, 1978). As photosynthetic activity predominates after depletion of acetate in the culture, the oxygen content is restored and the mRNA falls in response (Fig. 1A). We established that the critical variable was oxygen (rather than CO2 or pH; Quinn et al., 2000a), and that Cpx1 and Crd1 mRNAs also increased when the oxygen content of the culture was decreased. In all experiments, oxygen content was varied by mixing air with nitrogen. The CO2 content was kept constant at 2%.
Figure 1.
A, Cyc6 expression in copper-supplemented laboratory cultures of wild-type C. reinhardtii as a consequence of oxygen depletion. Cells were grown in Erlenmeyer flasks fitted with cotton plugs and were suspended by low basal stirring using a magnetic stir bar and stir plate under normal room lighting (approximately 1–15 μmol m−2 s−1 illumination). The oxygen content of the culture was measured with a standardized oxygen electrode each day (□). The culture was sampled at the same time for preparation of RNA and was analyzed by hybridization (▵). B, Hypoxia-induced gene expression in C. reinhardtii. Wild-type strain CC125 was grown in copper-supplemented (6 μm) Tris-acetate-phosphate medium under normal aeration to a concentration of 1 × 106 cells mL−1, and it was then bubbled with a gas mixture containing the indicated amount of air plus 2% CO2 with the balance as N2. Total RNA was prepared after 24 h and was analyzed by hybridization.
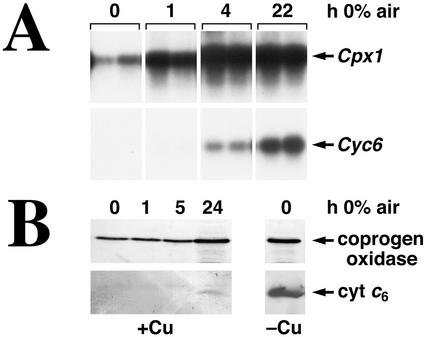
For the Crd1 and Cpx1 genes, even a small change in oxygen concentration, corresponding to 24-h growth at 50% air (2% CO2 and balance N2), results in increased transcript accumulation (Fig. 1B). As the amount of oxygen in the culture is decreased, each of the genes is induced more strongly. In contrast to Crd1 and Cpx1, Cyc6 expression is not as sensitive to oxygen depletion. Activation of Cyc6 is not detected until the concentration of oxygen is decreased to the amount in 5% air (about 1% O2). The time course of response of Cpx1 and Crd1 versus Cyc6 is also different. The hypoxic response of the Cpx1 and Crd1 genes is noticeable within 30 min, but the hypoxic response of the Cyc6 gene is barely detectable even when the cells have been oxygen deficient for at least 1 h (Quinn et al., 2000a). In accordance with this, the Cpx1 response is saturated quickly (within 4 h of transfer to 0% air, 2% CO2, and balance N2), whereas the Cyc6 response is still increasing for up to 24 h (Fig. 2A). The response time course and threshold of sensitivity of the Crd1 gene to oxygen deprivation parallels Cpx1 exactly (not shown).
Figure 2.
Time course of the Cyc6 and Cpx1 responses. A, Cultures were bubbled with 98% N2/2% CO2, sampled in duplicate at the indicated times, and analyzed for RNA abundance by blot hybridization. B, In a comparable time course experiment, cultures were sampled and analyzed for protein accumulation by immunoblotting. The lanes marked −Cu represent the accumulation of coprogen oxidase and cytochrome c6 in copper-deficient, fully aerated cultures.
The production of cytochrome c6 and coprogen oxidase in copper-deficient cells follows the abundance of the Cyc6 and Cpx1 transcripts, except that the proteins are more stable than the corresponding mRNAs and hence persist even when the mRNAs are completely degraded following copper supplementation (Hill et al., 1991). The same is true in oxygen-deficient cells. Protein abundance parallels mRNA abundance with a slight lag (compare Fig. 2, A with B). For example, the increase in coprogen oxidase is not evident until 24 h after oxygen deprivation, whereas the corresponding Cpx1 mRNA increase peaks by 4 h. Upon re-aeration of cells, the proteins persist long after the mRNA has decayed to undetectable levels as a result of cessation of transcription (not shown).
Why are three targets of the copper deficiency signal transduction pathway also turned on by oxygen deficiency? A simple explanation for the complete overlap of the set of target genes is that low oxygen results in copper deficiency, which activates the copper deficiency response. For instance, oxygen deprivation could inhibit copper uptake, resulting in intracellular copper deficiency even in copper-replete medium. Therefore, intracellular copper availability in oxygen-depleted versus aerated cells was assessed. One measure of intracellular copper availability is the abundance of the copper protein plastocyanin in the thylakoid lumen. Plastocyanin does not accumulate unless copper is present in the lumen for holoprotein formation (Merchant and Bogorad, 1986a; Li and Merchant, 1995; Tottey et al., 2001). Other metals cannot substitute for copper, which makes this assay highly selective for intracellular copper (Hill et al., 1991).
Wild-type cells were depleted of holoplastocyanin simply by growth under copper-deficiency (Merchant and Bogorad, 1986a), and were maintained in air or shifted to oxygen-depleted conditions (2% air) prior to initiation of holoplastocyanin formation by copper addition (Fig. 3). Holoplastocyanin is synthesized and accumulates to similar levels within hours in aerated and oxygen-deprived cultures (Fig. 3, lanes 5, 6, 9, and 10), indicating that copper is available inside the cell for de novo holoplastocyanin formation regardless of oxygen supply. RNA was isolated from the same cultures to confirm that oxygen removal was effective in activating the target genes, and hybridization analysis confirmed that the abundance of the Cyc6 and Cpx1 transcripts displayed the expected pattern of expression. That is, the transcripts decreased transiently in response to copper supplementation and then increased again in response to activation by oxygen deficiency (data not shown). From these results, which show that copper can access intracellular compartments, we conclude that the oxygen deficiency response is not manifested merely through an effect on intracellular copper metabolism/mobilization. We conclude that the oxygen deficiency response of Cyc6, Cpx1, and Crd1 is a separate physiological response from the nutritional copper response.
Figure 3.
Intracellular copper availability in hypoxic-treated cells. Duplicate copper-deficient cultures of wild-type cells were kept in air or transferred to 2% air (CO2 was kept constant at 2%, balance N2) for 1 h prior to addition of copper to 6 μm final concentration (t = 0). Soluble protein was prepared from an aliquot of each culture at the indicated times after addition of copper and was analyzed by native gel electrophoresis and immunoblotting using anti-plastocyanin antiserum at 1:1,000 dilution. The antiserum, generated against plastocyanin, cross-reacts with cytochrome c6 and, therefore, both proteins are detected (lanes 1 and 2).
The Oxygen Deficiency Response Is Physiologically Relevant
That an oxygen deficiency response might be biologically relevant for species of C. reinhardtii is evident upon consideration of their natural habitats, which include damp soil, bogs, and sewage lagoons. Even laboratory cultures can become oxygen depleted quite rapidly as a consequence of respiration (Fig. 1A). The availability of strains carrying loss-of-function mutations in one of the hypoxic target genes, namely Crd1, gave us the opportunity to assess whether the oxygen deficiency response under study in this project was physiologically relevant and significant for acclimation to low oxygen.
Crd1 encodes a candidate redox enzyme required for normal accumulation of photosystem I and its associated light-harvesting complexes in copper-deficient cells. Therefore, copper-deficient crd1 strains are chlorotic (Moseley et al., 2000). If expression of Crd1 in hypoxic cells represents a requirement for its gene product in hypoxic cells, crd1 mutants should display chlorosis under hypoxic growth conditions. When strain crd1-1 is grown in 2% air, it becomes chlorotic, accumulating approximately 5-fold less chlorophyll per cell compared with wild-type hypoxic cells or to crd1-1 or wild-type cells grown with normal aeration (Fig. 4). We conclude that Crd1 function is critical in oxygen-deficient cells for normal development of the photosynthetic apparatus. Hence, the activation of Crd1 expression under oxygen-deficient growth conditions is biologically meaningful.
Figure 4.
Effect of oxygen-deficient growth on a crd1 mutant strain. Strain crd1 and wild-type (WT) cells were grown under normal aeration to 1 × 106 cells mL−1. Each culture was then bubbled with a mixture of 2% air (constant 2% CO2, balance N2) or with 100% air, and was grown continuously under these conditions for about two generations.
Oxygen-Responsive Expression Requires CuREs and a HyRE
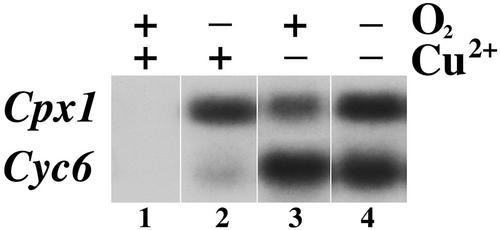
To test whether the two signals, i.e. copper and oxygen deficiency, might feed into the same pathway, we compared the expression of each target gene in copper or oxygen deficiency versus copper and oxygen deficiency (Fig. 5). The Cyc6 gene is induced maximally by copper deficiency, and oxygen deprivation does not further activate it (Fig. 5, compare lanes 3 and 4). Likewise, Cpx1 is maximally induced in oxygen-deficient cells, and copper deficiency does not further activate it (Fig. 5, compare lanes 2 and 4). This is consistent with a model in which the oxygen and copper deficiency responses share signal transduction components.
Figure 5.
Effect of copper and oxygen deficiency on Cpx1 and Cyc6 expression. Total RNA isolated from cultures grown under the indicated conditions was analyzed. −O2, 1% air (constant 2% CO2, balance N2).
In previous work, we have shown that the copper deficiency response requires CuREs associated with the target genes (Quinn et al., 2000a). A functionally essential GTAC sequence forms the core of the CuREs. One CuRE was identified in the Cpx1 promoter. Constructs a and b (Fig. 6) consist of the indicated portions of Cpx1 fused to a reporter gene (Ars2 encoding arylsulfatase). These constructs are expressed in Cu-deficient cells coordinately with the endogenous Cpx1 gene. The same is true when these constructs are tested for oxygen deficiency expression, which indicates that all the information required for induction of Cpx1 in oxygen deficiency is within the region from −197 to +207. When a GTAC sequence corresponding to the core of the previously identified CuRE is mutated (Fig. 6, compare constructs c and b), the oxygen deficiency response of the reporter gene is lost (Quinn et al., 2000a). Construct c does not induce Ars2 at any concentration of air. Construct d, in which a downstream GTAC sequence that is not part of a CuRE is mutated, also fails to respond to oxygen deficiency. This suggests that induction of Cpx1 in oxygen deficiency requires a HyRE beyond the CuRE. The sequence GTAC is also a critical component of the HyRE. The GTAC cores of both elements must be intact to confer oxygen deficiency responsive expression to a reporter gene, whereas only the GTAC core of the CuRE is required for the nutritional copper response (Quinn et al., 2000a).
Figure 6.
Oxygen deficiency expression of Cpx1-Ars2 reporter gene constructs. Strains containing the indicated Cpx1 5′-upstream sequences fused to the Ars2 reporter gene were grown to 2 × 106 cells mL−1 and were bubbled with the indicated concentrations of air for 24 h prior to preparation and analysis of total RNA. CO2 was kept constant at 2%. Endogenous Cpx1 was probed as a positive control for the efficacy of the oxygen deprivation, and RbcS was probed as a control for loading (not shown). Copper-responsive expression of the same constructs was analyzed by Quinn et al. (2000a). Multiple, independent transformants were generated for each construct. The data shown are from a single representative transformant.
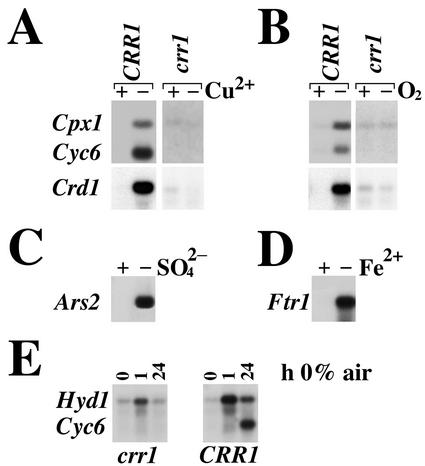
The CRR1 Locus, Which Encodes a trans-Acting Component Required for Adaptation to Copper Deficiency, Is Also Required for Oxygen Deficiency Responsive Expression
Genetic analysis of the copper deficiency response led to the identification of the CRR1 locus, which is required for adaptation to copper deficiency (M Eriksson and S Merchant, unpublished data). A crr1 mutant grows at a reduced rate in copper-deficient medium and cannot induce any of the target genes under Cu-deficient conditions (Crd1, Cyc6, or Cpx1 in Fig. 7A). We tested whether the CRR1 locus might also represent a shared signal transduction component in the copper- versus oxygen-sensing pathways. The crr1 strain was blocked in the oxygen-deficiency response for all target genes tested (Fig. 7B). To rule out the possibility that CRR1 is a “general” factor required for multiple nutritional stresses, we analyzed the expression of target genes of other nutrient-responsive pathways. The crr1 mutant was capable of responding to other nutritional deficiencies: Under sulfate deficiency, Ars2, encoding a sulfatase, is induced (de Hostos et al., 1989; Fig. 7C), and under iron deficiency, Ftr1, encoding a ferric transporter, is induced (Quinn et al., 2000b; Fig. 7D). The sulfur and iron deficiency responses occur even in copper-deficient crr1 cells that are growth compromised, and the extent of activation is comparable with that of sulfate- or iron-deficient wild-type cells (not shown). Thus, the specific absence of a response to oxygen deficiency in the crr1 strain indicates that there is a mechanistic connection between the copper and oxygen deficiency responses. We conclude that the oxygen deficiency response of the Cyc6, Cpx1, and Crd1 genes requires a cis- and trans-component of the nutritional copper signaling pathway, i.e. a CuRE and Crr1. This oxygen deficiency response is distinct from mechanisms operating to control hydrogenase production in anaerobic C. reinhardtii cells because crr1 can activate Hyd1 expression under anaerobic conditions (Fig. 7E).
Figure 7.
The crr1 mutant is blocked in the oxygen deficiency response. Total RNA isolated from the crr1 mutant and a wild-type strain (CRR1) grown under the indicated copper- (A) or oxygen-deficient (B) conditions was analyzed for Crd1, Cpx1, and Cyc6 expression. Total RNA from copper-deficient crr1 was analyzed for Ars2 expression in response to sulfur deficiency (C) or Ftr1 (encoding a ferric transporter) in response to iron deficiency (D). Comparable expression of these genes in wild-type cells is not shown. Total RNA was analyzed for Hyd1 expression (E) in wild-type versus crr1 cells. A time course of response to oxygen deprivation (achieved by transfer to 98% N2/2% CO2) is shown with Cyc6 expression as an internal reference.
An Antagonist of the Copper Deficiency Response Blocks the Oxygen Deficiency Response
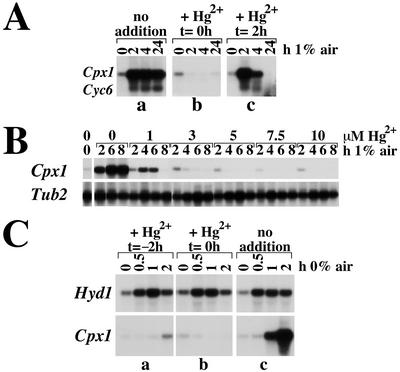
Genes that are activated in copper-deficient conditions are deactivated upon provision of mercuric salts at subtoxic concentrations (Hill et al., 1991; Quinn et al., 2000a). If oxygen deficiency signaling shares factors involved in copper deficiency signaling, HgII salts might deactivate the oxygen deficiency response just as they do the copper deficiency response. When HgCl2 is added to C. reinhardtii cells simultaneously with the shift to oxygen-deprived conditions, activation of the Cyc6 and Cpx1 genes is blocked (Fig. 8A, compare b and a). If the target genes are already induced by oxygen deficiency, the response is turned off when HgCl2 is added (Fig. 8A, compare 2 and 4 h 1% air in c and a).
Figure 8.
Mercuric ions specifically block the oxygen deficiency response of Cpx1 and Cyc6. A, Wild-type cells were grown to 2 × 106 cells mL−1 and were divided into three subcultures. Each subculture was bubbled with 1% air (2% CO2, balance N2) for the indicated times and HgCl2 was added to 10 μm final concentration at the indicated times. A, No added HgCl2; B, HgCl2 added at t = 0 h; C, HgCl2 added 2 h after initiation of hypoxic treatment. B, Response of Tub2 (encoding β-tubulin). A culture of CC125 was grown to 2 × 106 cells mL−1 and was divided into six subcultures. Each subculture was bubbled with 1% air for the indicated times prior to sampling for RNA preparation. HgCl2 was added to the final indicated concentrations after 2 h of treatment with 1% air. C, Response of Hyd1 (encoding Fe-hydrogenase). Mercuric chloride was added to a final concentration of 1 μm 2 h before or immediately prior to transfer to 0% air (2% CO2/98% N2; A and B). As a positive control, no addition was made before or after transfer to 0% air (2% CO2/98% N2; C).
The deactivation of the oxygen deficiency response by mercuric ions cannot be attributed to toxicity because the accumulation of other transcripts such as Pcy1 (encoding plastocyanin) and RbcS2 is not affected under these conditions (Hill et al., 1991; Quinn et al., 2000a). Nevertheless, we tested the effect of mercuric chloride on short-lived transcripts whose accumulation might be more sensitive to cytotoxic agents. HgCl2, at concentrations from 1 to 10 μm, had no effect on the abundance of Tub2 transcripts [which has a t1/2 similar to that of Cpx1 (Baker et al., 1984)]. At these concentrations, HgCl2 effectively inhibited hypoxic induction of Cpx1 (Fig. 8B). Also, expression of the Hyd1 gene, which is induced in oxygen-depleted cells by a different pathway (see above), is unaffected by HgCl2 addition (Fig. 8C), verifying the selectivity of the mercuric response.
DISCUSSION
Function of the Oxygen Deficiency Response in C. reinhardtii
C. reinhardtii, like other organisms, responds to changes in oxygen supply with alteration of metabolism (Harris, 1989). Some species in this genus are found in naturally oxygen-deficient habitats like peat bogs and sewage lagoons (Harris, 1989) where a hypoxic response is probably critical for survival. Three oxygen deficiency-induced genes are described in this work: Cyc6, Cpx1, and Crd1. Increased Cpx1 expression in oxygen-deficient conditions has already been observed in other organisms. In Saccharomyces cerevisiae, coproporphyrinogen oxidase, an oxygen-dependent enzyme, becomes rate limiting for heme synthesis in oxygen-depleted growth conditions, and the organism responds by synthesis of more enzyme (Zagorec et al., 1988). Many bacteria have two versions of this enzyme, an aerobic form that uses oxygen as a substrate and an anaerobic form that is oxygen independent (Keithly and Nadler, 1983; Xu et al., 1992). Therefore, the dramatic regulation of Cpx1 in C. reinhardtii is well precedented. Increased expression of Crd1 might be explained on the same basis. Crd1 is proposed to catalyze an oxygen-dependent oxidation in an Fe deficiency-sensitive pathway for cofactor biosynthesis in the plastid (Moseley et al., 2000; Pinta et al., 2002). Perhaps Crd1 becomes rate limiting for this pathway in oxygen-deficient cells. Crd1 function is known to be required in oxygen-deficient cells because the crd1 mutant exhibits iron deficiency-type chlorosis when it is grown at low oxygen conditions (Fig. 4). The modulation of cofactor biosynthetic pathways through changes in Cpx1 and Crd1 expression might also serve to signal oxygen status to the nucleus. This is precedented by the heme-dependent O2-sensing system in S. cerevisiae (Zhang and Hach, 1999) and by the Mg-protoporphyrin IX-dependent plastid-to-nucleus signaling system in C. reinhardtii and plants (Kropat et al., 1997; Mochizuki et al., 2001).
At present, an explanation for why Cyc6 expression is activated in oxygen-deficient cells eludes us. The gene product, cytochrome c6, which accumulates in oxygen-depleted cells following the increase in mRNA (Fig. 2), is an electron transfer catalyst whose function is apparent and necessary only when plastocyanin is absent (Wood, 1978; Merchant and Bogorad, 1987a, 1987b). Because anaerobic cells accumulate holoplastocyanin, cytochrome c6 is not necessary for its replacement (Fig. 3). One possibility is that in anaerobic cells, cytochrome c6 is used for a metabolic pathway other than photosynthesis. Another possibility is that Cyc6 expression in anoxic cells is simply fortuitous, being a consequence of the gene containing two CuREs that have the same core as the candidate HyRE (see below).
Mechanism of the Oxygen Deficiency Response
The commonality of the copper deficiency and oxygen deficiency responses might be trivially explained if oxygen-deficient cells are internally copper deficient despite plentiful supply in the medium. Nevertheless, this is not the case because oxygen-deprived cells are capable of de novo synthesis of the copper protein holoplastocyanin, indicating that copper is available intracellularly (Fig. 3). Also, even 100-fold excess copper provided in the medium cannot repress the oxygen deficiency response (data not shown). Furthermore, comparison of the oxygen and copper deficiency responses of each target gene indicates that the two responses are not identical (Fig. 5). If the oxygen deficiency response worked by creating internal copper deficiency, we would expect that the oxygen deficiency response would recapitulate the copper deficiency response. In this context, we note the distinct pattern of the oxygen deficiency response of Cyc6 versus Cpx1 and Crd1 (Fig. 1B). The Cpx1 and Crd1 genes are much more sensitive to hypoxia than is the Cyc6 gene, whereas the opposite is true of the copper deficiency response (Moseley et al., 2000; Quinn et al., 2000a) The oxygen deficiency responses of Cyc6, Cpx1, and Crd1 are a separate physiological response, but with a mechanistic and perhaps evolutionary connection to the copper deficiency response.
Because the same three genes are regulated by both copper and oxygen deficiency, we tested whether components of the nutritional copper-sensing pathway are involved in oxygen deficiency signaling. One component is the CuRE, which was defined previously by mutational analysis (Quinn et al., 2000a). The core of the CuRE is the sequence GTAC, which is absolutely essential for CuRE activity. This sequence is also essential for the oxygen deficiency response (Fig. 6), indicating that the two signaling pathways have some common components. Yet, the oxygen deficiency signaling pathway is not exactly the same, and this is demonstrated by the observation that although the copper deficiency response of Cpx1 is dependent on a single CuRE (defined by the upstream GTAC core), a second element is required for the hypoxic response of Cpx1. Mutation of the core GTAC of the second element abolishes hypoxic expression of the Cpx1-Ars2 reporter gene (Fig. 6), but does not affect its response to copper deficiency (Quinn et al., 2000a). Perhaps the much greater degree of activation of Cpx1 in oxygen deficiency relative to its activation in copper deficiency can be explained by the presence of more than one cis-regulatory element for the former response. We refer to the second element as a HyRE. The two elements are regulated by virtue of the common GTAC core, but are distinct because they are not functionally identical. The distinction resides probably in flanking nucleotides that are part of the element beyond the GTAC core.
Another regulatory component of the copper deficiency response is Crr1, defined by a mutation at the CRR1 locus (M. Eriksson and S. Merchant, unpublished data). The crr1 strains are not able to activate any of the copper deficiency responses and hence are modeled to be defective in a “master” regulator of the nutritional copper response. In this work, we show that a functional CRR1 locus is required for the oxygen deficiency response, but not for other nutrition starvation responses (Fig. 7), indicating that Crr1 is a specific rather than a general regulatory factor.
A heme- or iron-based sensor is the favored model for direct oxygen or indirect redox sensing in various hypoxia-responsive pathways in eukaryotes (for review, see Semenza, 1999; Wenger, 2000; Zhu and Bunn, 2001). There is considerable precedence for the use of iron in biological reactions where oxygen is a substrate and iron is also a common cofactor in enzymes that catalyze redox reactions, which influences our thinking about mechanisms for oxygen and redox sensing. Yet bacterial sensors are designed with a variety of redox-sensitive cofactors besides iron (for review, see Bauer et al., 1999), so other non-iron based redox or oxygen sensors are likely to occur in eukaryotic cells as well. The parallels between copper and iron biochemistry (Kaim and Schwederski, 1984) raised the possibility of a mechanistic connection between the copper deficiency and the oxygen deficiency responses.
Hyd1 Expression Is Independent of Copper and of CRR1
The genetic handle on a putative hypoxia-signaling pathway in C. reinhardtii presented us with the opportunity to test whether there might be a second, Crr1-independent mechanism. Hydrogenase production in anaerobic C. reinhardtii cells occurs, in part, by increased accumulation of Hyd1 mRNA (J.M. Quinn, unpublished data). The anaerobic response of the Hyd1 gene is the same in a crr1 mutant compared with wild-type cells (Fig. 7E) Also, Hg2+, which blocks the hypoxic response of Cyc6, Cpx1, and Crd1, has no effect on the hypoxic accumulation of Hyd1 transcripts (Fig. 8C, compare b and c). This indicates that a second signaling pathway activates Hyd1 through a different (copper-independent) signal transduction mechanism. The existence of at least one other oxygen deficiency signaling pathway may allow the organism to perceive and respond to a broad range of oxygen supply (for discussion, see Poyton, 1999).
MATERIALS AND METHODS
Chlamydomonas reinhardtii Strains and Culture Conditions
C. reinhardtii wild-type strain CC125, mutant strains crr1-1, crd1, and transformants of strain CC425 were typically cultured under 100 to 125 μmol m−2 s−1 illumination in copper-supplemented or copper-deficient Tris-acetate-phosphate media (Quinn and Merchant, 1998). For growth under specified oxygen concentrations, cultures were bubbled with mixtures of indicated concentrations of air plus 2% CO2 with the balance as N2 (Quinn et al., 2000a). For experiments involving assessment of gene expression (e.g. to assay reporter gene constructs), cultures were bubbled with 98% N2/2% CO2 to maximize differences, but when the experimental design required cell growth and division (e.g. to monitor de novo protein synthesis), the cultures were bubbled with 2% air/2% CO2 in N2. Where indicated, cultures were supplemented with HgCl2 or AgCl from stock solutions. All experiments were performed at least twice, and were often repeated multiple times.
Amplification of Ftr1 and Hyd1 cDNAs
Expressed sequence tags corresponding to C. reinhardtii hydrogenase (Hyd1) were identified by BLAST search using the N terminus of the protein as the input sequence. An expressed sequence tag corresponding to a putative ferric transporter (Ftr1) was identified by a BLAST search with the Saccharomyces cerevisiae Ftr1p sequence as input. Primers for amplification of these sequences were designed with BamHI sites to facilitate cloning of the reverse transcriptase-PCR products (Ftr1, 348 bp, and Hyd1, 372 bp) into the BamHI site of pBluescriptIIKS+ (Stratagene, La Jolla, CA).
Nucleic Acid Analysis
Total RNA was prepared and analyzed by hybridization as described by Quinn and Merchant (1995) for Ars2 or as described by Hill et al. (1991) for all other transcripts. Five micrograms of total RNA was loaded per lane. Probes for Cpx1, Cyc6, Ars2, and RbcS2 (encoding the small subunit of Rubisco) were prepared as described (Quinn et al., 1999). The RbcS2 hybridization signal is used for normalization between samples (not shown in every figure). For detection of Crd1 transcripts, the 55 × 102-bp insert from pCrd1-5 (Moseley et al., 2000) was used. The 358-bp insert of pFtr358 and the 372-bp insert of pHyd372 (described above) were used for detection of Ftr1 and Hyd1 transcripts, respectively. The 1,227-bp insert from pGPXβ9 (Leisinger et al., 1999) was used for detection of Gpxh RNA. Specific activities of probes ranged from 3 to 6 × 108 cpm μg−1 DNA. Blots were exposed at −80°C to film (XRP-1; Eastman-Kodak, Rochester, NY) with two intensifying screens, and were typically developed after overnight exposure.
Immunoblot Analysis
Total soluble protein was prepared (Li et al., 1996) and separated on 12% (w/v) SDS-polyacrylamide gels (Li and Merchant, 1992) or 15% (w/v) anionic gels for immunoblot analysis (Hill et al., 1991; Merchant et al., 1991). Blots were incubated overnight with a 1:1,000 dilution of anti-plastocyanin as the primary antibody and a 1:2,000 dilution of alkaline phosphatase-conjugated goat anti-rabbit IgG (Southern Biotechnology Associates, Birmingham, AL) as the secondary antibody. Bound antibody was detected using the alkaline phosphatase color reaction (Sambrook et al., 1989).
ACKNOWLEDGMENTS
We thank Professor Marie-Alda Gilles-Gonzalez (Ohio State University), Prof. Albert Courey (UCLA), Ms. Gloria Turner (UCLA), and the members of our group for helpful comments on the manuscript.
Footnotes
This work was supported by the National Institutes of Health (grant no. GM42143). M.E. was supported, in part, by a European Molecular Biology Organization Long-Term Fellowship, and J.L.M., was supported, in part, by the Molecular Biology Ph.D. program and a Dissertation Year Fellowship from the Graduate Division of the University of California (Los Angeles).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010694.
LITERATURE CITED
- Bailey-Serres J, Dawe R. Both 5′ and 3′ sequences of maize adh1 mRNA are required for enhanced translation under low-oxygen conditions. Plant Physiol. 1996;116:685–695. doi: 10.1104/pp.112.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EJ, Schloss JA, Rosenbaum JL. Rapid changes in tubulin RNA synthesis and stability induced by deflagellation in Chlamydomonas. J Cell Biol. 1984;99:2074–2081. doi: 10.1083/jcb.99.6.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CE, Elsen S, Bird TH. Mechanisms for redox control of gene expression. Annu Rev Microbiol. 1999;53:495–523. doi: 10.1146/annurev.micro.53.1.495. [DOI] [PubMed] [Google Scholar]
- de Hostos EL, Schilling J, Grossman AR. Structure and expression of the gene encoding the periplasmic arylsulfatase of Chlamydomonas reinhardtii. Mol Gen Genet. 1989;218:229–239. doi: 10.1007/BF00331273. [DOI] [PubMed] [Google Scholar]
- Ellis MH, Dennis ES, Peacock WJ. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiol. 1999;119:57–64. doi: 10.1104/pp.119.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain V, Raymond P, Ricard B. Differential expression of two tomato lactate dehydrogenase genes in response to oxygen deficit. Plant Mol Biol. 1997;35:711–721. doi: 10.1023/a:1005854002969. [DOI] [PubMed] [Google Scholar]
- Happe T, Mosler B, Naber JD. Induction, localization and metal content of hydrogenase in the green alga Chlamydomonas reinhardtii. Eur J Biochem. 1994;222:769–774. doi: 10.1111/j.1432-1033.1994.tb18923.x. [DOI] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic Press; 1989. [DOI] [PubMed] [Google Scholar]
- Hill KL, Li HH, Singer J, Merchant S. Isolation and structural characterization of the Chlamydomonas reinhardtii gene for cytochrome c6analysis of the kinetics and metal specificity of its copper-responsive expression. J Biol Chem. 1991;266:15060–15067. [PubMed] [Google Scholar]
- Hill KL, Merchant S. Coordinate expression of coproporphyrinogen oxidase and cytochrome c6 in the green alga Chlamydomonas reinhardtiiin response to changes in copper availability. EMBO J. 1995;14:857–865. doi: 10.1002/j.1460-2075.1995.tb07067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennis ES. Evidence for a role for AtMYB2 in the induction of the Arabidopsisalcohol dehydrogenase gene (ADH1) by low oxygen. Genetics. 1998;149:479–490. doi: 10.1093/genetics/149.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E, Hodges TK. An anaerobically inducible early (aie) gene family from rice. Plant Mol Biol. 1999;40:591–601. doi: 10.1023/a:1006284014613. [DOI] [PubMed] [Google Scholar]
- Kaim W, Schwederski B. Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life: An Introduction and Guide. Chichester, UK: Wiley; 1984. [Google Scholar]
- Keithly JH, Nadler KD. Protoporphyrin formation in Rhizobium japonicum. J Bacteriol. 1983;154:838–845. doi: 10.1128/jb.154.2.838-845.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J, Oster U, Rüdiger W, Beck CF. Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc Natl Acad Sci USA. 1997;94:14168–14172. doi: 10.1073/pnas.94.25.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisinger U, Ruefenacht K, Zehnder AJB, Eggen RIL. Structure of a glutathione peroxidase homologous gene involved in the oxidative stress response in Chlamydomonas reinhardtii. Plant Sci. 1999;149:139–149. [Google Scholar]
- Li HH, Merchant S. Two metal-dependent steps in the biosynthesis of Scenedesmus obliquesplastocyanin: differential mRNA accumulation and holoprotein formation. J Biol Chem. 1992;267:9368–9375. [PubMed] [Google Scholar]
- Li HH, Merchant S. Degradation of plastocyanin in copper-deficient Chlamydomonas reinhardtii. J Biol Chem. 1995;270:23504–23510. doi: 10.1074/jbc.270.40.23504. [DOI] [PubMed] [Google Scholar]
- Li HH, Quinn J, Culler D, Girard-Bascou J, Merchant S. Molecular genetic analysis of plastocyanin biosynthesis in Chlamydomonas reinhardtii. J Biol Chem. 1996;271:31283–31289. doi: 10.1074/jbc.271.49.31283. [DOI] [PubMed] [Google Scholar]
- Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000;122:127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S. Reciprocal, copper-responsive accumulation of plastocyanin and cytochrome c6 in algae and cyanobacteria: a model for metalloregulation of metalloprotein synthesis. In: Silver S, Walden W, editors. Metal Ions in Gene Regulation. New York: Chapman and Hall; 1998. pp. 450–467. [Google Scholar]
- Merchant S, Bogorad L. Rapid degradation of apoplastocyanin in Cu(II)-deficient cells of Chlamydomonas reinhardtii. J Biol Chem. 1986a;261:15850–15853. [PubMed] [Google Scholar]
- Merchant S, Bogorad L. Regulation by copper of the expression of plastocyanin and cytochrome c552 in Chlamydomonas reinhardtii. Mol Cell Biol. 1986b;6:462–469. doi: 10.1128/mcb.6.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S, Bogorad L. The Cu(II)-repressible plastidic cytochrome c: cloning and sequence of a complementary DNA for the pre-apoprotein. J Biol Chem. 1987a;262:9062–9067. [PubMed] [Google Scholar]
- Merchant S, Bogorad L. Metal ion regulated gene expression: use of a plastocyanin-less mutant of Chlamydomonas reinhardtii to study the Cu(II)-dependent expression of cytochrome c-552. EMBO J. 1987b;6:2531–2535. doi: 10.1002/j.1460-2075.1987.tb02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S, Hill K, Howe G. Dynamic interplay between two copper-titrating components in the transcriptional regulation of cyt c6. EMBO J. 1991;10:1383–1389. doi: 10.1002/j.1460-2075.1991.tb07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA. 2001;98:2053–2058. doi: 10.1073/pnas.98.4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J, Quinn J, Eriksson M, Merchant S. The Crd1 gene encodes a putative di-iron enzyme required for photosystem I accumulation in copper deficiency and hypoxia in Chlamydomonas reinhardtii. EMBO J. 2000;19:2139–2151. doi: 10.1093/emboj/19.10.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto R, Sachs MM, Porter E, Freeling M. Patterns of polypeptide synthesis in various maize organs under hypoxia. Planta. 1980;150:89–94. doi: 10.1007/BF00385619. [DOI] [PubMed] [Google Scholar]
- Olive MR, Peacock WJ, Dennis ES. The anaerobic responsive element contains two GC-rich sequences essential for binding a nuclear protein and hypoxic activation of the maize ADH1 promoter. Nucleic Acids Res. 1991;19:7053–7060. doi: 10.1093/nar/19.25.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschke VM, Sachs MM. Multiple pyruvate decarboxylase genes in maize are induced by hypoxia. Mol Gen Genet. 1993;240:206–212. doi: 10.1007/BF00277058. [DOI] [PubMed] [Google Scholar]
- Pinta V, Picaud M, Reiss-Husson F, Astier C (2002) Rubrivivax gelatinosus acsF (previously orf358) codes for a conserved, putative binuclear-iron-cluster-containing oxygenase subunit involved in aerobic oxidative cyclization of Mg-protoporphyrin IX monomethylester. J Bacteriol (in press) [DOI] [PMC free article] [PubMed]
- Poyton RO. Models for oxygen sensing in yeast: implications for oxygen-regulated gene expression in higher eukaryotes. Resp Physiol. 1999;115:119–133. doi: 10.1016/s0034-5687(99)00028-6. [DOI] [PubMed] [Google Scholar]
- Quinn JM, Barraco P, Eriksson M, Merchant S. Coordinate copper- and oxygen-responsive Cyc6 and Cpx1 expression in Chlamydomonasis mediated by the same element. J Biol Chem. 2000a;275:6080–6089. doi: 10.1074/jbc.275.9.6080. [DOI] [PubMed] [Google Scholar]
- Quinn JM, LaFontaine S, Gohre V, Merchant S. Copper-dependent and copper-independent iron metabolism in Chlamydomonas reinhardtii. Plant Biology 2000, July 15–19, San Diego, American Society of Plant Physiologists. 2000b. p. S961. [Google Scholar]
- Quinn JM, Merchant S. Two copper-responsive elements associated with the ChlamydomonasCyc6 gene function as targets for transcriptional activators. Plant Cell. 1995;7:623–638. doi: 10.1105/tpc.7.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JM, Merchant S. Copper-responsive gene expression during adaptation to copper deficiency. Methods Enzymol. 1998;297:263–279. doi: 10.1016/s0076-6879(98)97020-3. [DOI] [PubMed] [Google Scholar]
- Quinn JM, Nakamoto SS, Merchant S. Induction of coproporphyrinogen oxidase in Chlamydomonas chloroplasts occurs via transcriptional regulation of Cpx1mediated by copper-response elements and increased translation from a copper-deficiency-specific form of the transcript. J Biol Chem. 1999;274:14444–14454. doi: 10.1074/jbc.274.20.14444. [DOI] [PubMed] [Google Scholar]
- Rivoal J, Thind S, Pradet A, Ricard B. Differential induction of pyruvate decarboxylase subunits and transcripts in anoxic rice seedlings. Plant Physiol. 1997;114:1021–1029. doi: 10.1104/pp.114.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MM, Subbaiah CC, Saab IM. Anaerobic gene expression and flooding tolerance in maize. J Exp Bot. 1996;47:1–15. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Semenza GL. Perspectives on oxygen sensing. Cell. 1999;98:281–284. doi: 10.1016/s0092-8674(00)81957-1. [DOI] [PubMed] [Google Scholar]
- Tottey S, Rich PR, Rondet SA, Robinson NJ. Two Menkes-type ATPases supply copper for photosynthesis in SynechocystisPCC 6803. J Biol Chem. 2001;276:19999–20004. doi: 10.1074/jbc.M011243200. [DOI] [PubMed] [Google Scholar]
- Walker JC, Howard EA, Dennis ES, Peacock WJ. DNA sequences required for anaerobic expression of the maize ADH1 gene. Proc Natl Acad Sci USA. 1997;84:6624–6629. doi: 10.1073/pnas.84.19.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203:1253–1263. doi: 10.1242/jeb.203.8.1253. [DOI] [PubMed] [Google Scholar]
- Wood PM. Interchangeable copper and iron proteins in algal photosynthesis: studies on plastocyanin and cytochrome c-552 in Chlamydomonas. Eur J Biochem. 1978;87:8–19. doi: 10.1111/j.1432-1033.1978.tb12346.x. [DOI] [PubMed] [Google Scholar]
- Xu K, Delling J, Elliot T. The genes required for heme synthesis in Salmonella typhimuriuminclude those encoding alternative functions for aerobic and anaerobic coproporphyrinogen oxidation. J Bacteriol. 1992;174:3953–3963. doi: 10.1128/jb.174.12.3953-3963.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorec M, Buhler JM, Treich I, Keng T, Guarente L, Labbe-Bois R. Isolation, sequence, and regulation by oxygen of the yeast HEM13gene coding for coproporphyrinogen oxidase. J Biol Chem. 1988;263:9718–9724. [PubMed] [Google Scholar]
- Zhang L, Hach A. Molecular mechanism of heme signaling in yeast: The transcriptional activator Hap1 serves as the key mediator. Cell Mol Life Sci. 1999;56:415–426. doi: 10.1007/s000180050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Bunn HF. Signal transduction: How do cells sense oxygen? Science. 2001;292:449–451. doi: 10.1126/science.1060849. [DOI] [PMC free article] [PubMed] [Google Scholar]