Abstract
Mice null for the T-cell protein tyrosine phosphatase (Tcptp−/−) die shortly after birth due to complications arising from the development of a systemic inflammatory disease. It was originally reported that Tcptp−/− mice have increased numbers of macrophages in the spleen; however, the mechanism underlying the aberrant growth and differentiation of macrophages in Tcptp−/− mice is not known. We have identified Tcptp as an important regulator of colony-stimulating factor 1 (CSF-1) signaling and mononuclear phagocyte development. The number of CSF-1-dependent CFU is increased in Tcptp−/− bone marrow. Tcptp−/− mice also have increased numbers of granulocyte-macrophage precursors (GMP), and these Tcptp−/− GMP yield more macrophage colonies in response to CSF-1 relative to wild-type cells. Furthermore, we have identified the CSF-1 receptor (CSF-1R) as a physiological target of Tcptp through substrate-trapping experiments and its hyperphosphorylation in Tcptp−/− macrophages. Tcptp−/− macrophages also have increased tyrosine phosphorylation and recruitment of a Grb2/Gab2/Shp2 complex to the CSF-1R and enhanced activation of Erk after CSF-1 stimulation, which are important molecular events in CSF-1-induced differentiation. These data implicate Tcptp as a critical regulator of CSF-1 signaling and mononuclear phagocyte development in hematopoiesis.
Mice null for the T-cell protein tyrosine phosphatase (Tcptp) develop a progressive inflammatory disease characterized by chronic myocarditis, gastritis, nephritis, and increased production of gamma interferon (IFN-γ) and tumor necrosis factor alpha in vivo (27, 59). Macrophages from Tcptp−/− mice are hypersensitive to IFN-γ due to deregulated Jak/Stat signaling, which leads to increased protein levels of the inducible nitric oxide synthase and nitric oxide production (49). Due to the severity of this phenotype, Tcptp−/− mice die shortly after birth. It has been reported that Tcptp−/− mice have increased numbers of splenic macrophages (59), suggesting that Tcptp also plays a role in regulating macrophage development.
Colony-stimulating factor 1 (CSF-1) is the primary growth factor that regulates the differentiation, proliferation, and survival of mononuclear phagocytes in vivo (42, 50, 51). The CSF-1 receptor (CSF-1R) is expressed at low levels on multipotent hematopoietic progenitor cells, and at higher levels on mononuclear phagocytic cells from the committed macrophage progenitor cell to the fully differentiated macrophage. In fact, mice null for either CSF-1 or CSF-1R have severely reduced numbers of macrophages in vivo.
CSF-1 binding to the receptor results in the formation of noncovalent homodimers of the CSF-1R that undergo autophosphorylation at seven known tyrosine residues. Tyrosine phosphorylation of the CSF-1R results in the recruitment and activation of Src family kinases (14), Plc-γ2 (8), and c-Cbl (57). Binding of the Grb2 and Gads/Mona (10) adaptor proteins to the CSF-1R results in the recruitment of members of the Gab family of docking proteins. When tyrosine phosphorylated, Gab family members bind to the protein tyrosine phosphatase family member Shp2, which plays an important role in extracellular signal-regulated kinase (Erk) activation (24). Although many signaling pathways downstream of the receptor have been identified, their contributions to the biological response to CSF-1 during hematopoiesis remains elusive. Little is also known about the regulation of receptor phosphorylation and its relation to the differentiation, proliferation, and survival signals initiated after CSF-1 binding (9, 26).
In this report, we demonstrate that Tcptp regulates differentiation signals initiated by CSF-1. The number of macrophage CFU (CFU-M) in Tcptp−/− bone marrow is significantly increased due to the increased responsiveness of granulocyte-macrophage precursors (GMP) to CSF-1. We identify CSF-1R, the receptor for CSF-1, as a physiological substrate of Tcptp and demonstrate enhanced Erk activation in Tcptp−/− bone marrow-derived macrophages (BMDMs). These data demonstrate that the loss of Tcptp results in increased production of mononuclear phagocytes through deregulated CSF-1 signaling and provide insight into the mechanism underlying the aberrant macrophage development in Tcptp−/− mice.
MATERIALS AND METHODS
Mice, cell culture, and transfections.
The generation of Tcptp−/− mice has been reported previously (59). All mice were maintained at the McGill University animal facility and treated in accordance with the guidelines approved by this institution. BMDMs were obtained by flushing the femurs of 16-to-20-day-old mice with Dulbecco's modified Eagle's medium (DMEM) and antibiotics (5 mg/ml penicillin, 5 U/ml streptomycin sulfate; Wisent, Saint-Jean-Baptiste de Rouville, Quebec, Canada) supplemented with 10% (vol/vol) endotoxin-free fetal bovine serum (FBS; Invitrogen). Cells were resuspended in the same medium containing 120 ng/ml human CSF-1 (a kind gift of the Chiron Corp.). Nucleated bone marrow cells were plated in CSF-1-supplemented medium, and stromal cells and mature macrophages were removed by adherence to tissue culture plates for 24 h. Suspension cells were plated at 1.5 × 105 cells/ml, and BMDMs were allowed to differentiate for 7 days (49). For stimulations, BMDMs were washed with phosphate-buffered saline (PBS) and depleted of CSF-1 for 16 h. BMDMs were then stimulated for the indicated times with 100 ng/ml mouse CSF-1 (R&D Systems, Minneapolis, MN) in DMEM.
FDC-P1 cells expressing exogenous CSF-1R (FD-Fms) have been previously described (44). FD-Fms cells were cultured in DMEM containing 10% (vol/vol) FBS, antibiotics, and 5% WEHI-conditioned medium as a source of interleukin 3 (IL-3). Once cells reached a density between 5 × 105 and 7.5 × 105 cells/ml, they were washed in serum and antibiotic-free RPMI medium (Wisent) and resuspended at 20 × 106 cells/400 μl in a 0.4-cm-gap electroporation cuvette (Bio-Rad, Mississauga, Ontario). A total of 40 μg of pEF-Tcptp-WT, pEF-Tcptp-DA, or empty pEF vector was added, and cells were incubated for 10 min at room temperature. Cells were pulsed at 960 μF/280V and then chilled on ice for 10 min before being resuspended in 20 ml of FD-Fms culture medium. Twenty hours after transfection, cells were washed with PBS and depleted of IL-3 for 4 h. Transfectants were then stimulated with 100 ng/ml CSF-1 (R&D Systems) in DMEM for the indicated times.
Immunoprecipitations, glutathione S-transferase (GST) mixing experiments, and Western blotting.
Cells were lysed with HNMETG buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1.5 mM MgCl2, 1.0 mM EGTA, 1.0% Triton X-100, 10% glycerol) supplemented with 10 mM NaF, 2 mM Na3VO4, and complete protease inhibitor tablets (Roche). For substrate-trapping experiments, Na3VO4 was excluded from the lysis buffer. Lysates were cleared by centrifugation for 10 min at 10,000 × g and 4°C. For BMDM experiments, 100 μg protein in 500 μl lysis buffer was used for immunoprecipitation, while 1 mg of protein in 1 ml lysis buffer was used in the FD-Fms experiments. Immunoprecipitates were collected on protein A or G Sepharose beads (Sigma, St. Louis, MO), washed three times with HNMETG buffer containing 0.1% Triton X-100, eluted into sodium dodecyl sulfate (SDS) sample buffer, and resolved by SDS-polyacrylamide gel electrophoresis (PAGE).
The GST-Grb2-SH2 fusion protein was produced in DH5α cells by diluting an overnight culture with an equal amount of LB medium containing 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Roche). Bacteria were lysed by sonication in PBS-1% Triton X-100, and GST fusion proteins were purified on glutathione-Sepharose beads (Amersham Pharmacia, Baie D'Urfé, Quebec, Canada) and washed thoroughly with lysis buffer. A total of 4 μg GST-Grb2-SH2 was incubated with 100 μg of BMDM lysates for 90 min and then washed three times with HNMETG lysis buffer. Western blotting was performed as described previously (49).
Antibodies.
The anti-Tcptp antibody 6F3 has been previously described (49). The antiphosphotyrosine antibody 4G10, anti-CSF-1R antibody for immunoprecipitation, and anti-Gab2 antibody were from Upstate Biotechnology (Lake Placid, NY). The anti-CSF-1R (C-20) antibody for Western blotting, the anti-Shp2 (C-18) antibody, the anti-Plc-γ2 (Q-20) antibody, and the anti-Csk (C-20) antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Affinity-purified anti-CSF-1R pY807 (catalog no. 3154), anti-phospho p42/44 mitogen-activated protein kinase (Thr202/Tyr204, catalog no. 4377), anti-phospho-Akt (Ser473, catalog no. 4058), and anti-phospho-PDK1 (Ser241, catalog no. 3061) as well as anti-p44/42 mitogen-activated protein kinase (catalog no. 9012) and anti-Akt (catalog no. 9272) antibodies were from Cell Signaling Technology (Beverly, MA). Mouse monoclonal antiactin antibodies were from Sigma (Oakville, Canada). Affinity-purified anti-Shc antibodies have been described previously (32). The rabbit anti-p62Dok-1 antibody was generated against a GST fusion protein encoding the C terminus of mouse Dok-1.
Tissue staining.
Rat monoclonal antibody to F4/80 was a gift from David Hume (Department of Microbiology, University of Queensland). Tissues were fixed in 10% neutral buffered formalin and paraffin embedded, and 5-μm sections were cut. An anti-rat ABC elite staining kit, an antiavidin blocking kit, and hematoxylin for counterstaining were from Vector laboratories (Burlingame, CA). Staining was performed as outlined by the manufacturer. A minimum of three animals per tissue were analyzed. Pictures were taken at original magnifications of ×20 and ×40 on a light microscope (Leica).
Differentiation and flow cytometry of mononuclear phagocytic precursors.
The culture of committed mononuclear phagocytic precursor cells was performed as described previously (52). Briefly, nucleated bone marrow cells from Tcptp+/+ and Tcptp−/− mice (2 × 105/ml) were cultured in RPMI medium supplemented with 10% FBS, 5 ng/ml recombinant mouse IL-3 (R&D Systems), 12 ng/ml human CSF-1, and 55 μM β-mercaptoethanol for 3 days. Each day, cells were transferred to new flasks to remove adherent macrophages.
For flow cytometry analysis, cells were stained with 5 μg/ml ethidium monoazide (Molecular Probes, Eugene, OR) in 100 μl flow cytometry buffer (PBS plus 2% FBS plus 0.1% sodium azide) for 10 min on ice in the dark before exposure to light for 10 min. Fc receptors were blocked with anti-CD16/32 (eBioscience, San Diego, CA) for 15 min prior to the addition of anti-CD11b-fluorescein isothiocyanate (FITC)-conjugated antibodies (BD Pharmingen, San Diego, CA) for 30 min. Stained cells were washed twice with fluorescence-activated cell sorter (FACS) buffer before fixing the cells in 1% paraformaldehyde in PBS. Analysis was performed on Beckman-Coulter EPICS-Elite (Fullerton, CA). A total of 10,000 events were analyzed for each experiment. Data were analyzed using CellQuest software (BD Biosciences).
Carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling was performed as indicated by the manufacturer (CFSE cell tracer kit no. V12883; Molecular Probes), and committed mononuclear phagocytes were allowed to proliferate for 3 days before analysis. Annexin V-propidium iodide staining for apoptotic cells was performed using an annexin V-PE apoptosis detection kit (Pharmingen; catalog no. 559763) prior to labeling with CFSE. Mac-1-positive cells were gated and analyzed for annexin V-propidium iodide staining.
Clonogenic assays.
All assays were performed in duplicate according to the manufacturer's instructions (Stem Cell Technology, Vancouver, British Columbia). For the mixed clonogenic assays, 10,000 nucleated bone marrow cells were plated in 1 ml of MethoCult GF (M3434) and colonies were scored after 12 days. For CSF-1-dependent CFU (CFU-C) assays, MethoCult (M3231) (containing serum with no additional growth factors) was supplemented with 100 ng/ml mouse CSF-1 (R&D Systems), and cells were incubated for 7 days. The identity of the colony types was scored visually. For GMP assays, 100 GMP were plated in MethoCult (M3231) (containing serum with no additional growth factors) supplemented with 100 ng/ml mouse CSF-1 (R&D Systems), and cells were incubated for 7 days. Macrophage identity was confirmed by four-color flow cytometry using anti-Ly6G-FITC, anti-CD14-phycoerythrin, and CD11b-allophycocyanin-conjugated antibodies and propidium iodide.
Isolation of GMP.
Approximately 10 × 106 bone marrow cells from Tcptp+/+ and Tcptp−/− mice were stained and analyzed by four-color flow cytometry using the following markers: CD16/32 (FcγRII/III)-FITC, lineage markers (CD3, CD4, CD5, CD8, CD11b, CD19, DX5, and TER119), and CD127 (IL-7Rα) as well as Sca-1 (Ly6A/E) conjugated to phycoerythrin, CD117 (c-kit)-PECy7, and CD34-Alexa 647. The acquisition of 1 × 106 live bone marrow cells was performed for the analysis of GMP cells. GMP are defined as Lin−/CD127−/Sca-1−/CD117+/CD34+/CD16/32hi. Antibodies were purchased from BD Biosciences (Mississauga, Ontario, Canada), BioLegend (Vineland, Ontario, Canada), Serotec (Raleigh, NC).
RESULTS
Tissue macrophages are increased in Tcptp−/− mice.
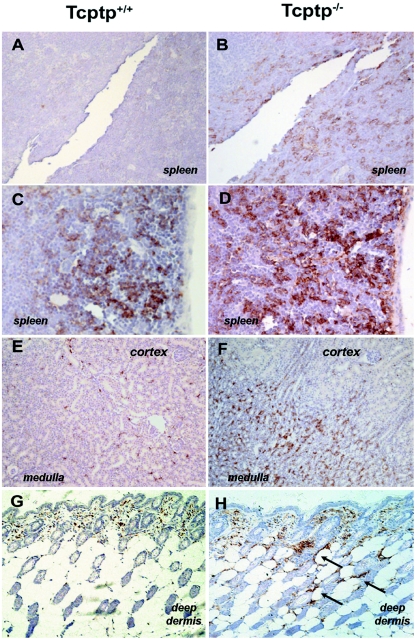
Tcptp−/− mice develop a progressive inflammatory disease characterized by splenomegaly, lymphadenopathy, and nephritis and die prematurely at 3 to 5 weeks of age (27). Using an anti-F4/80 antibody that recognizes macrophages (3), we observed a significant increase in the number of tissue macrophages in sections from kidney and skin as well as spleen. Kidney sections from Tcptp−/− mice had a pronounced increase in the number of macrophages in the medulla (Fig. 1, compare E and F), and in Tcptp−/− skin sections, there was a marked increase in deep-dermal macrophages (Fig. 1, compare G and H). We also observed a substantial increase in the number of splenic macrophages in sections from Tcptp−/− mice as previously reported (59) (Fig. 1, compare panels A and C with panels B and D). In previous studies of osteopetrotic mice, which are null for CSF-1 expression, there is a severe loss of F4/80+ staining in both the kidney and the deep dermis (17, 58). This known requirement for CSF-1 in the development of both kidney and deep-dermal macrophages led us to investigate the role of Tcptp in CSF-1 signaling.
FIG. 1.
Tissues from Tcptp−/− mice are infiltrated with F4/80+ macrophages. Tissue sections from Tcptp+/+ (A, C, E, and G) or Tcptp−/− (B, D, F, and H) mice were stained with an anti-F4/80 antibody to detect tissue macrophages. Sections from spleen (A, B, C, and D), kidney (E and F), and skin (G and H) are shown. Panels A, B, E, and F were photographed at an original magnification of ×20, while panels C, D, G, and H were photographed at an original magnification of ×40. For each panel, a minimum of three mice of each genotype were analyzed. Arrows indicate staining of deep dermal macrophages.
Increased differentiation of mononuclear phagocytic precursors in Tcptp−/− mice.
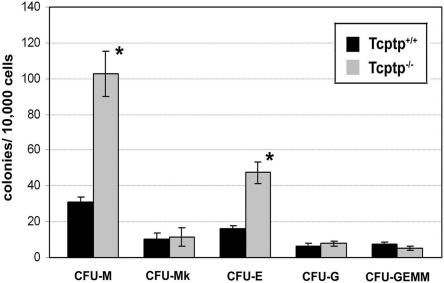
CSF-1 is known to play an important role in myelopoiesis in vivo. To assess any defects in hematopoiesis due to the deletion of Tcptp, we performed a clonogenic assay to determine the frequency of hematopoietic progenitors in Tcptp+/+ and Tcptp−/− bone marrow cells. Using a mix of cytokines (IL-3, stem cell factor [SCF], IL-6, and erythropoietin [Epo]), we observed a fourfold increase in the number of CFU-M-derived macrophage colonies from Tcptp−/− bone marrow cells, while granulocyte CFU (CFU-G)-derived granulocytic colonies remained unchanged relative to wild-type controls (Fig. 2). In addition, no significant change was observed in the number of megakaryocyte-CFU-derived megakaryocytic colonies (CFU-Mk) or mixed multipotent progenitor colonies derived from mixed granulocyte, erythroid, megakaryocyte, and macrophage CFU (CFU-GEMM). Under high magnification, the Tcptp−/− macrophage colonies did not differ significantly in size or density from Tcptp+/+ macrophage colonies. These data indicate a role for Tcptp in hematopoiesis and demonstrate that the loss of Tcptp results in increased generation of macrophage precursors.
FIG. 2.
Increased numbers of macrophage precursors in Tcptp−/− bone marrow. Tcptp+/+ or Tcptp−/− bone marrow cells (1 × 104) were plated in methylcellulose containing IL-3, SCF, IL-6, and Epo. At day 12, colonies were scored under high magnification to determine colony constitution and size (means ± SD [error bars]; n = 7; *, P < 0.01).
Interestingly, we observed a threefold increase in the number of erythroid-CFU (CFU-E)-derived erythroid colonies in Tcptp−/− bone marrow (Fig. 2). The increase in the number of CFU-E most likely arises from an increase in Jak signaling through the Epo receptor. Our work (49) and the work of other groups (35, 54) have demonstrated that Tcptp regulates Jak signaling events, and the role of Tcptp in Epo signaling is currently being investigated.
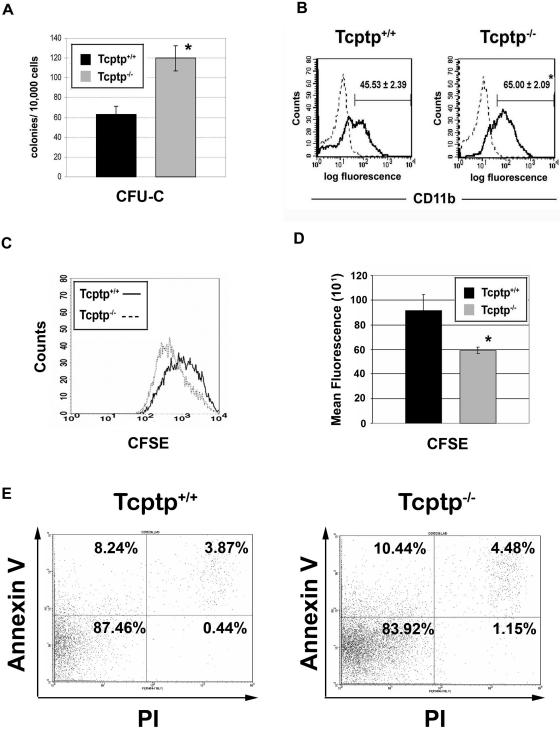
CSF-1 alone cannot promote the differentiation of mononuclear phagocytes from multipotent hematopoietic progenitor cells. To promote commitment to the mononuclear phagocytic lineage, CSF-1 must synergize with other growth factors, such as IL-3, to act upon multipotent progenitor cells (4, 28). This step, involving proliferation and differentiation, generates committed mononuclear phagocytic precursors, which, supported by CSF-1 alone, can proliferate and differentiate further to mononuclear phagocytes (monoblasts, promonocytes, monocytes, and macrophages). To determine the number of committed mononuclear phagocytic precursor cells present in Tcptp+/+ and Tcptp−/− bone marrow (CFU-C), we performed clonogenic assays in the presence of CSF-1 alone. Under these conditions, we observed a twofold increase in the number of CFU-C-derived macrophage colonies in Tcptp−/− bone marrow (Fig. 3A).
FIG. 3.
Increased mononuclear phagocytic precursors in Tcptp−/− bone marrow. (A) Macrophage colonies derived from CFU-C in Tcptp+/+ or Tcptp−/− bone marrow cells (1 × 104) cultured in the presence of 100 ng/ml CSF-1. Colonies were scored at day 7 (n = 6; means ± SD [error bars]; *, P < 0.01). (B) Bone marrow cells from Tcptp+/+ or Tcptp−/− mice were cultured for 3 days in the presence of IL-3 and CSF-1 to allow proliferation and differentiation of multipotent progenitors to nonadherent mononuclear phagocytic precursors. Cells were stained with anti-CD11b-FITC antibodies (solid line) to determine the percentage of mononuclear phagocytic precursors in both cell populations. Staining with an isotype-matched control antibody is shown as a dashed line (means ± SD [error bars]; n = 4, *, P < 0.01). (C) Increased proliferation of Tcptp−/− committed mononuclear phagocyte precursor cells. Bone marrow cells were cultured as described for panel A to generate committed mononuclear phagocytes, and cells were labeled with CFSE and allowed to proliferate for 3 days. CFSE fluorescence was measured by flow cytometry, and cell populations were plotted as histograms. (D) Geometric means from histograms in panel C were plotted to quantitate CFSE dilution. Data were analyzed by an unpaired, two-tailed Student t test (means ± SD [error bars]; n = 3; *, P < 0.05). (E) Annexin V-propidium iodide staining of apoptotic cells in the mononuclear phagocyte cultures used in CFSE proliferation assay.
To determine whether the increased number of CFU-C observed in Tcptp−/− bone marrow reflects an increase in the proliferation and differentiation of multipotent progenitor cells in response to CSF-1, nucleated bone marrow progenitors were cultured to yield committed mononuclear phagocytic precursors. The staining of nonadherent mononuclear phagocytic precursor cells with the monocyte/macrophage lineage marker CD11b indicated a significant increase in CD11b+ cells derived from Tcptp−/− bone marrow progenitors (65.00% ± 2.09%) (Fig. 3B) compared to those derived from Tcptp+/+ bone marrow progenitors (45.53% ± 2.39%) (Fig. 3B).
To determine whether this increased generation of mononuclear phagocyte precursors cells cultured from Tcptp−/− bone marrow cells was due to increased proliferation, Tcptp−/− mononuclear phagocyte precursors were labeled with CFSE and allowed to proliferate for 3 days. Proliferation results in the dilution of the CFSE label, which can be quantitated by flow cytometry. CFSE dilution was 1.5-fold greater in Tcptp−/− mononuclear phagocyte precursors, indicating increased proliferation of the Tcptp−/− cells (Fig. 3C and D). To ensure that this increase in proliferation of Tcptp−/− mononuclear phagocyte precursors was not due to increased apoptosis of Tcptp+/+ cells or decreased apoptosis of Tcptp−/− cells, annexin V-propidium iodide staining was performed. No difference in the level of apoptosis of either Tcptp+/+ or Tcptp−/− mononuclear phagocyte precursors was observed under the same conditions used in the CFSE proliferation assay (Fig. 3E). In fact, upon growth factor withdrawal, a greater percentage of Tcptp−/− precursors underwent apoptosis relative to wild-type precursor cells (data not shown). Therefore, Tcptp−/− mononuclear phagocyte precursors proliferate to a greater extent in the presence of CSF-1 than wild-type cells. Furthermore, the increased frequency of CFU-C in vivo or generation of mononuclear phagocyte precursors from Tcptp−/− bone marrow in vitro was not due to any significant increase in bone marrow cellularity because Tcptp−/− mice in fact have a threefold reduction in the number of nucleated bone marrow cells relative to their wild-type littermates (59). Together, these data indicate that Tcptp regulates the development of committed mononuclear phagocytic precursors from primitive multipotential hematopoietic progenitors.
Increased generation of macrophages from GMP in response to CSF-1.
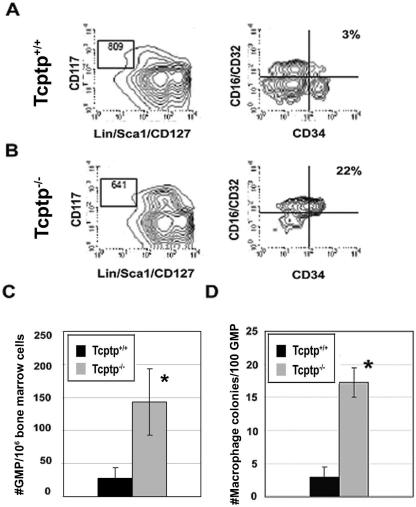
Prior to the generation of committed mononuclear phagocytic precursors, hematopoietic stem cells differentiate to a common myeloid precursor and then to a bipotent GMP (41). To assess the role of Tcptp in early myeloid commitment, we isolated and determined the number of GMP in Tcptp+/+ and Tcptp−/− mice (Fig. 4A and B). In Tcptp−/− mice, the GMP number was increased fourfold (Fig. 4C). Therefore, these data implicate Tcptp in the generation of early myeloid progenitors in addition to the generation of committed mononuclear phagocyte precursors.
FIG. 4.
Increased sensitivity of GMP from Tcptp−/− bone marrow to CSF-1. GMP from Tcptp+/+ (A) and Tcptp−/− (B) bone marrow were purified by FACS. These cells are defined as Lin−/Sca1−/CD127(IL-7Rα)−, CD117(c-KIT)+, FcR+, and CD34+. (C) Graphical representation of the number of GMP in Tcptp+/+ and Tcptp−/− per 106 bone marrow cells. Error bars indicate standard deviations. (n = 4; *, P < 0.01). (D) Colonies derived from 100 GMP in clonogenic assays with 100 ng/ml CSF-1. (means ± SD [error bars]; n = 4, *, P < 0.01).
Although CSF-1 alone cannot effectively support the growth and differentiation of primitive hematopoietic precursors, we hypothesized that Tcptp−/− GMP may be sensitive to the effects of CSF-1, which would provide an explanation for the increased numbers of CFU-C observed in Tcptp−/− bone marrow. To determine whether GMP from Tcptp−/− mice could respond to CSF-1 alone, we performed clonogenic assays with 100 GMP from both Tcptp+/+ and Tcptp−/− mice in the presence of CSF-1. In the presence of CSF-1 alone, Tcptp−/− GMP yielded fivefold more macrophage colonies than GMP from Tcptp+/+ mice (Fig. 4D), which, as expected, produced few macrophage colonies in the presence of CSF-1 alone. Therefore, these data demonstrate that GMP from Tcptp−/− mice are responsive to CSF-1 and implicate Tcptp in the negative regulation of myelopoiesis and CSF-1 signaling in early hematopoietic progenitors.
Tcptp−/− BMDMs have increased CSF-1R tyrosine phosphorylation.
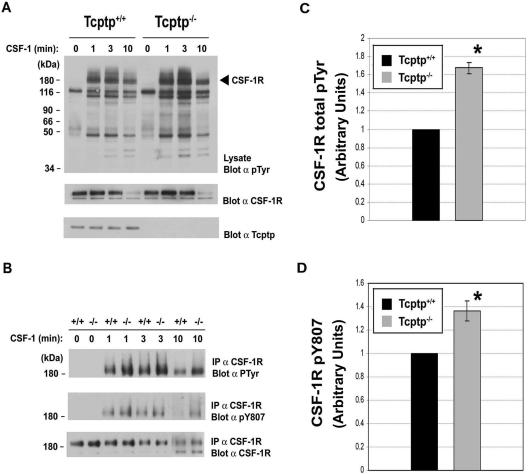
The observation that Tcptp−/− bone marrow contains increased numbers of macrophage precursors is consistent with the known role of CSF-1 in macrophage development. Therefore, we examined the role of Tcptp in CSF-1R signaling in macrophages. To examine the tyrosine phosphorylation status of the CSF-1R following CSF-1 binding, Tcptp+/+ and Tcptp−/− BMDMs were stimulated with CSF-1, whole-cell lysates were separated by SDS-PAGE, and Western blotting was performed using antiphosphotyrosine antibodies. In these experiments, we observed increases in the level of tyrosine phosphorylation of several proteins in extracts from Tcptp−/− BMDMs compared to that in extracts from wild-type BMDMs.
One of the most highly phosphorylated proteins migrated at 170 kDa, which corresponds to the approximate size of the CSF-1R (Fig. 5A, top panel). At all time points, tyrosine phosphorylation of this protein was increased in extracts from Tcptp−/− BMDMs relative to wild-type BMDMs, and the difference became more apparent at later time points. As determined by Western blotting using anti-CSF-1R antibodies, the increase in tyrosine phosphorylation was not due to aberrant expression of the CSF-1R in Tcptp−/− macrophages (Fig. 5A, middle panel).
FIG. 5.
The CSF-1R is hyperphosphorylated in Tcptp−/− macrophages. (A) BMDMs from Tcptp+/+ or Tcptp−/− mice were stimulated with 100 ng/ml CSF-1 for the indicated time points. Equal amounts of lysates were resolved by SDS-PAGE and analyzed by Western blotting with an antiphosphotyrosine antibody (top panel). The blot was reprobed with anti-CSF-1R antibodies (middle panel) and anti-Tcptp antibodies (bottom panel) to demonstrate the correct genotype of the cells. (B) The CSF-1R was immunoprecipitated from Tcptp+/+ or Tcptp−/− BMDMs stimulated with 100 ng/ml CSF-1 as indicated. The immunoprecipitates were resolved by SDS-PAGE and analyzed by Western blotting with antiphosphotyrosine (top panel), anti-CSF-1R phospho-Y807 (middle panel), and anti-CSF-1R (bottom panel) antibodies. (C and D) Graphical representation of total tyrosine phosphorylation of the CSF-1R (C) or phosphorylation of Y807 (D) in Tcptp+/+ or Tcptp−/− BMDMs at 10 min after CSF-1 stimulation. Western blots were quantitated by densitometry. Data were analyzed by an unpaired, two-tailed Student t test with the results of the wild-type set to 1 (means ± SD [error bars]; n = 3; *, P < 0.05).
To further investigate whether the CSF-1R itself was hyperphosphorylated in Tcptp−/− macrophages, we immunoprecipitated the CSF-1R from Tcptp+/+ and Tcptp−/− BMDMs and blotted the immunoprecipitates with antiphosphotyrosine antibodies (Fig. 5B). We observed hyperphosphorylation of the CSF-1R in Tcptp−/− BMDMs at 1, 3, and 10 min of stimulation with CSF-1 compared to the receptor from wild-type macrophages (Fig. 5B, top panel, and C). We also blotted CSF-1R immunoprecipitates with a phosphorylation-specific antibody directed against Y807, the activation loop tyrosine of the kinase. The phosphorylation of Y807 is a critical event required for the activation of the CSF-1R to transmit signals required for macrophage differentiation (16). In immunoprecipitates of the CSF-1R from Tcptp−/− BMDMs, hyperphosphorylation of Y807 was apparent at all time points but became more pronounced at later time points (Fig. 5B, middle panel, and D). Immunoblotting with anti-CSF-1R antibodies confirmed equal immunoprecipitation and loading of the receptor in these experiments (Fig. 5B, bottom panel).
Tcptp forms a substrate-trapping complex with the CSF-1R.
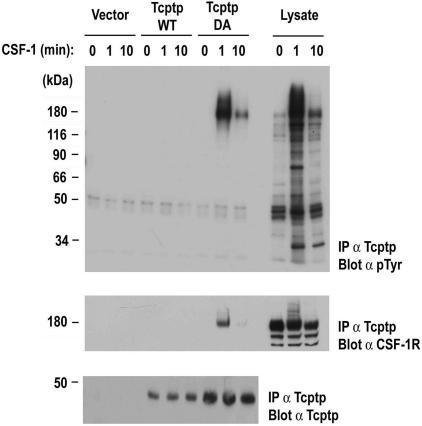
The observed hyperphosphorylation of the CSF-1R in Tcptp−/− BMDMs suggested that the receptor itself could be a direct substrate of Tcptp. To address this, we used a substrate-trapping approach to determine whether the CSF-1R could form an in vivo complex with Tcptp (21). In these experiments, FDC-P1 cells expressing exogenous CSF-1R (FD-Fms) were transfected with vector alone, wild-type Tcptp (Tcptp-WT), or Tcptp-DA, substrate-trapping mutant that can bind to potential substrates in vivo but cannot hydrolyze the tyrosine phosphate ester.
Overexpressed Tcptp-WT or Tcptp-DA was immunoprecipitated with anti-Tcptp antibodies and analyzed by Western blotting with antiphosphotyrosine antibodies to reveal potential coimmunoprecipitating substrates. We observed a single phosphorylated species that coimmunoprecipitated with Tcptp-DA (Fig. 6, top panel). Significantly, no tyrosine phosphorylated proteins coimmunoprecipitated with either the vector alone control or the Tcptp-WT transfectants, indicating the specificity of the interaction with the catalytic domain of Tcptp.
FIG. 6.
The CSF-1R coimmunoprecipitates with Tcptp substrate-trapping mutants in vivo. FD-Fms cells were transfected with pEF-Tcptp-WT, pEF-Tcptp-DA, or empty vector, and then stimulated with 100 ng/ml CSF-1. Tcptp immunoprecipitates were analyzed by Western blotting with antiphosphotyrosine antibodies to reveal coimmunoprecipitating substrates (top panel). Immunoprecipitates were then blotted with anti-CSF-1R antibodies (middle panel) and anti-Tcptp antibodies (bottom panel) to ensure overexpression of the indicated constructs. The three right lanes contain whole-cell lysates of vector transfected cells to demonstrate the specificity of Tcptp for the tyrosine phosphorylated CSF-1R.
Based on the inducible tyrosine phosphorylation of this protein and its molecular weight, we hypothesized that this protein was the CSF-1R. Reblotting of the membrane with anti-CSF-1R antibodies confirmed that the CSF-1R coprecipitated with Tcptp-DA in vivo (Fig. 6, middle panel). Immunoblotting with anti-Tcptp antibodies confirmed the overexpression of the indicated proteins (Fig. 6, bottom panel). Coimmunoprecipitation of the CSF-1R with the Tcptp-DA trapping mutant and hyperphosphorylation of the CSF-1R in Tcptp−/− BMDMs (Fig. 5) indicate that the CSF-1R is a physiological substrate of Tcptp.
Tcptp−/− macrophages have increased Erk activation in response to CSF-1.
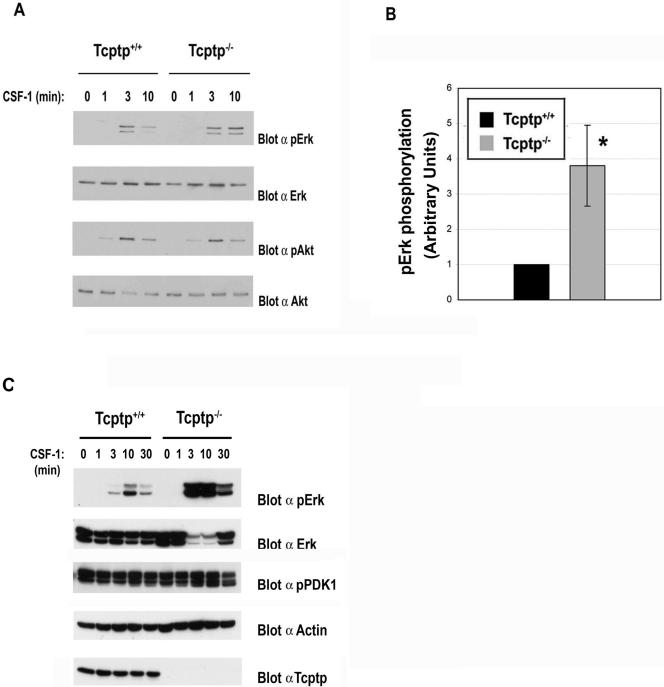
To examine the effect of the loss of Tcptp on signaling pathways downstream of the CSF-1R, extracts from CSF-1-stimulated Tcptp+/+ and Tcptp−/− BMDMs were examined for the activation of Erk and Akt kinases after CSF-1 stimulation. In Tcptp−/− BMDMs, we observed a dramatic increase in the level of Erk activation relative to wild-type samples (Fig. 7A and B) but no difference in Akt activation between CSF-1-stimulated Tcptp+/+ and Tcptp−/− macrophages was observed (Fig. 7A). We also did not observe any change in the activation of PDK1 (2), an upstream activator of Akt (Fig. 7C). Furthermore, the increased Erk activation in Tcptp−/− BMDMs was not drastically prolonged, since at 30 min after CSF-1 stimulation, Erk activation was attenuated in both Tcptp+/+ and Tcptp−/− macrophages (Fig. 7C). These data indicate that Tcptp regulates Erk activation downstream of the CSF-1R. Erk activation is well known to be a requisite event for the differentiation of several cell types (1, 56) and has been recently shown to play an important role in CSF-1-induced macrophage differentiation (22).
FIG. 7.
Tcptp−/− macrophages have increased Erk activation. (A) Lysates from Tcptp+/+ or Tcptp−/− BMDMs stimulated with 100 ng/ml CSF-1 were analyzed by Western blotting with anti-phospho-Erk and anti-phospho-Akt antibodies. (B) Graphical representation of total Erk1/2 TEY phosphorylation in Tcptp+/+ or Tcptp−/− BMDMs at 10 min CSF-1 stimulation. Western blots were quantitated by densitometry. Data were analyzed by an unpaired, two-tailed Student t test with the results of the wild-type set to 1 (means ± SD [error bars]; n = 3, *, P < 0.05). (C) Increased Erk phosphorylation after CSF-1 sitmulation in Tcptp−/− BMDMs is not prolonged. PDK1 activity in lysates from Tcptp+/+ or Tcptp−/− BMDMs stimulated with 100 ng/ml CSF-1 was also analyzed by Western blotting with anti-phospho-PDK1 antibodies.
Tcptp−/− macrophages have increased tyrosine phosphorylation of Gab2 and Shp2 after CSF-1 stimulation.
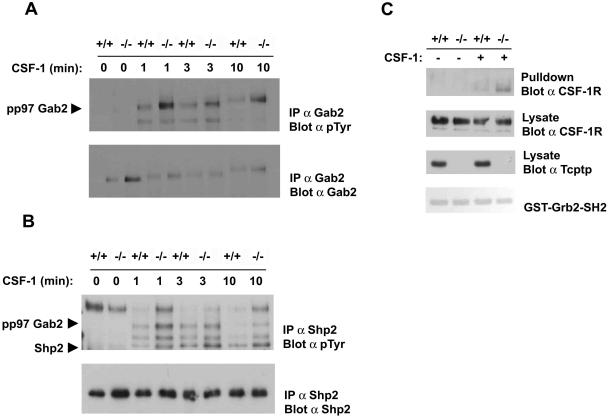
The recruitment and phosphorylation of a Gab2/Shp2 complex to the CSF-1R is a critical signaling event in macrophage differentiation. The expression of a Gab2 mutant that cannot bind Shp2 was shown previously to inhibit CSF-1-induced macrophage differentiation (30). Furthermore, the phosphorylation and activation of Shp2 is required for Erk activation downstream of several other receptor tyrosine kinases (24, 31, 37). Therefore, we examined the tyrosine phosphorylation of both Gab2 and Shp2 in response to CSF-1 in Tcptp+/+ and Tcptp−/− BMDMs. Tyrosine phosphorylation of both Gab2 and Shp2 (Fig. 8A and B) immunoprecipitated from Tcptp−/− BMDMs was increased in response to CSF-1 relative to immunoprecipitates from Tcptp+/+ BMDMs.
FIG. 8.
Increased recruitment and tyrosine phosphorylation of a Grb2/Gab2/Shp2 complex after CSF-1 stimulation in Tcptp−/− macrophages. (A) Gab2 was immunoprecipitated from CSF-1-stimulated Tcptp+/+ or Tcptp−/− BMDMs. Protein complexes were resolved by SDS-PAGE and analyzed by antiphosphotyrosine blotting and then by blotting for total Gab2 protein. (B) Anti-Shp2 immunoprecipitates from CSF-1-stimulated Tcptp+/+ or Tcptp−/− BMDMs were analyzed by antiphosphotyrosine immunoblotting as described for panel A. (C) Lysates from unstimulated and CSF-1-stimulated Tcptp+/+ or Tcptp−/− BMDMs were incubated with a GST-Grb2-SH2 domain fusion protein. The complexes were resolved by SDS-PAGE and analyzed by Western blotting with an anti-CSF-1R antibody. Input lysates from the experiment were blotted with an anti-CSF-1R antibody to ensure equal amounts of the CSF-1R were used and with an anti-Tcptp antibody to ensure the genotype of the cells used in the experiment.
Since the recruitment and activation of the Gab2/Shp2 complex is dependent on a Gab2/Grb2 interaction in other receptor systems, we used a Grb2-SH2 domain fusion protein to determine the extent of Grb2 binding to the CSF-1R in Tcptp+/+ and Tcptp−/− BMDMs after CSF-1 stimulation. In these experiments, we observed increased Grb2 binding to the CSF-1R in extracts from Tcptp−/− BMDMs relative to wild-type cells (Fig. 8C). Therefore, increased Erk activation in Tcptp−/− BMDMs is likely mediated by increased recruitment of a Grb2/Gab2/Shp2 complex to the CSF-1R.
We also looked for changes in the tyrosine phosphorylation of other proteins involved in Erk activation, including the recently reported Shp2 substrate, Pag (61). The Shp2 protein tyrosine phosphatase dephosphorylates the transmembrane protein Pag (also known as Cbp), which recruits the tyrosine kinase Csk that negatively regulates Src family tyrosine kinases. As well, it has been reported recently that Tcptp can target members of the Src family of kinases downstream of the tumor necrosis factor alpha receptor (55). In Tcptp−/− BMDMs, we did not observe a significant change in the tyrosine phosphorylation of Pag bound to Csk, nor did we observe any changes in the tyrosine phosphorylation of Plc-γ2 (8), a downstream target of this Src activation pathway (6, 13) or other Src targets Shc and p62Dok-1 (data not shown).
DISCUSSION
Macrophages and CSF-1 play important roles in the development of inflammatory diseases. Tcptp−/− mice die shortly after birth and suffer from splenomegaly, lymphadenopathy, chronic myocarditis, gastritis, and nephritis, which are all symptoms of an inflammatory disorder (12, 27). Although Tcptp−/− macrophages are sensitive to activating stimuli, such as IFN-γ and lipopolysaccharide, the mechanism by which Tcptp regulates the growth and development of this lineage remained unclear. In this study, we have identified Tcptp as a novel regulator of CSF-1 signaling and mononuclear phagocyte development.
We have demonstrated that several tissues in Tcptp−/− mice have increased numbers of F4/80+ macrophages. Since CSF-1 had been implicated as the primary growth factor regulating the growth and development of macrophages in vivo, we investigated the role of Tcptp in CSF-1 signaling and macrophage differentiation. Analysis of the number and type of CFU present in bone marrow revealed a fourfold increase in CFU-M in Tcptp−/− mice relative to wild-type controls. It has been previously demonstrated that CFU-C are the most primitive cells upon which CSF-1 can act alone to promote differentiation. CSF-1 can also act in concert with other cytokines to promote the proliferation and differentiation of multipotent hematopoietic precursor cells (4, 29). When we assessed the number of CFU-C in Tcptp+/+ and Tcptp−/− mice, we observed a twofold increase in the number of committed mononuclear phagocyte precursors present in Tcptp−/− bone marrow relative to the wild-type and increased proliferation of Tcptp−/− committed mononuclear phagocyte precursors. These data implicate Tcptp in the regulation of the proliferation and differentiation of multipotent cells to committed mononuclear phagocyte precursors during hematopoiesis. Furthermore, when we cultured GMP from Tcptp−/− mice in the presence of CSF-1 alone, these cells generated fourfold more macrophage colonies relative to Tcptp+/+ GMP. Therefore, the loss of Tcptp results in the sensitivity of early myeloid progenitors to the effects of CSF-1.
The phosphorylation of the activation loop tyrosine in protein tyrosine kinases is critical for the complete activation of kinases. Similarly, the phosphorylation of Y807 in the CSF-1R activation loop is required for proper phosphorylation and activation of the receptor. In fact, Erk activation has been shown to be dependent on the phosphorylation of Y807 (16). Furthermore, the phosphorylation of Y807 is also an important event in differentiation since FD-Fms cells expressing the Y807F mutant CSF-1R proliferate in response to CSF-1 but have a reduced capacity to differentiate (7). Our data identify Tcptp as a novel regulator of CSF-1R Y807 phosphorylation and Erk activation in CSF-1 signaling—two known molecular events induced by CSF-1 that are required for effective macrophage differentiation.
Recent studies have also demonstrated that the activation of Erk is important for macrophage differentiation in response to CSF-1 (22). However, the recruitment of Grb2/Sos complexes to the receptor, which is the “classical” mechanism of Ras and Erk activation, does not play a major role in promoting the prolonged activation of Erk that is required for macrophage differentiation. In fact, the disruption of Grb2/Sos complexes in FD-Fms cells with cell-permeable peptides did not affect Erk activation or the ability of these cells to differentiate in response to CSF-1 (22). Therefore, these data implicate the recruitment of Gab2/Shp2 as a critical pathway for Erk signaling and differentiation.
Shp2 recruitment through members of the Gab family of scaffold proteins is an important mechanism for Erk activation in numerous signaling pathways, and the function of Gab family members in the activation of Erk is conserved from Drosophila to mammals (20, 24, 31, 38, 46). The differentiation of Shp2-null embryonic stem cells using in vitro CFU assays was unable to yield any granulocyte and macrophage precursors, thereby implicating the activation of Shp2 as an important event in macrophage development (43). In fact, the overexpression of a Gab2 mutant lacking the Shp2 binding sites in FD-Fms cells inhibited macrophage differentiation in response to CSF-1 (30). In accordance with a role for Gab2 in CSF-1-induced Erk activation, Gab2-null mice have severely reduced Erk activation in mast cells after treatment with both immunoglobulin E and SCF (25) and the differentiation of mast cells was also found to be severely impaired in Gab2−/− animals (39). Shp2 also can also attenuate Akt activation via the dephosphorylation of PI3K binding sites on Gab proteins in a negative feedback loop mechanism (60). However, in Tcptp−/− macrophages we did not observe any significant changes in the level of Akt activation or the activity of PDK1, an upstream activator of Akt, relative to the wild type. This is likely due to the fact that PI3K can bind the CSF-1R directly, which can result in Akt activation independently of Gab2 recruitment. The precise mechanism underlying the increased recruitment of a Grb2/Gab2/Shp2 complex in Tcptp−/− macrophages is unclear. Although our data imply that hyperphosphorylation of one or both of the known Grb2 binding sites (Y697 or Y921) may be involved, we are unable to test this directly since relevant phosphospecific antibodies are not available. However, the hyperphosphorylation of Y807 in Tcptp−/− macrophages likely leads to changes in the overall phosphorylation status and kinetics of CSF-1R activation, which could in turn favor the formation of a specific signaling protein complex.
Another protein tyrosine phosphatase family member, Shp1, also plays an important role in myeloid cell differentiation, proliferation, and activation (48). In fact, motheaten mice, which express either a null or hypomorphic mutant of Shp1, have increased numbers of macrophages in the extremities, resulting in arthritis, and increased levels of macrophages in the lungs, resulting in fatal pneumonitis (23, 47). The CSF-1R is also hyperphosphorylated in Shp1 mutant macrophages (5, 11). Shp1 mutant mice also have increases in the numbers of CSF-1-responsive CFU-C and granulocyte-macrophage CFU in bone marrow (36), indicating that Shp1 plays an important role in myeloid differentiation. However, unlike Tcptp−/− mice, motheaten mice have increased numbers of both granulocytes and macrophages (53). We observed changes in only the number of CFU-M in Tcptp−/− bone marrow, while the number of CFU-G was comparable to the wild-type. Recent studies have also demonstrated that the expression of a dominant-negative Shp1 mutant affects the differentiation of both granulocytes and macrophages in a cell autonomous manner (40). These data indicate a significant difference between the functions of Tcptp and Shp-1 in myeloid cells. Shp1 most likely acts as a general regulator of myelopoiesis and proliferation, while Tcptp specifically acts to balance mononuclear phagocyte differentiation in vivo through its action upon the CSF-1R.
The lineage commitment of hematopoietic stem cells is thought to be determined by stochastic changes in the levels of lineage-specific transcription factors in these cells (19), but the effects of the extracellular environment, in the form of cytokines and growth factors, cannot be completely excluded (34). One model of hematopoiesis proposes that lineage-specific growth factors and cytokines promote the survival and growth of committed cells, while another model implicates these extrinsic signals in commitment decisions. The negative regulation of CSF-1 signaling by Tcptp partly explains the increase in CFU-M that we observed in Tcptp−/− mice; however, we cannot exclude changes in other growth factor and cytokine signaling pathways involved in early myelopoiesis that are directly affected by the loss of Tcptp. However, the increase in GMP in Tcptp−/− mice indicates that CSF-1 also plays a role in early myeloid differentiation.
It is possible that the expression levels of lineage-specific transcription factors involved in myelomonocytic differentiation may be affected in Tcptp−/− hematopoietic progenitor cells, thereby influencing lineage commitment. The level of one such transcription factor, PU.1, has been shown to regulate the development of the myelomonocytic lineage, and its regulation may prove to be an important mechanism underlying the phenotype observed in Tcptp−/− mice (18, 33, 45). However, our results do demonstrate that the loss of a single specific regulator can bias early cell fate decisions during hematopoiesis. Recently, the deletion of Socs3 demonstrated its role as a specific negative regulator of G-CSF signaling and neutrophil proliferation and development (15).
In this report, we have identified Tcptp as a novel regulator of CSF-1 signaling, and we have also demonstrated that the production of committed mononuclear phagocyte precursors from Tcptp−/− bone marrow progenitors is increased. These data implicate Tcptp and CSF-1 in the early regulation of myelopoiesis. The identification of specific negative regulators of hematopoiesis, such as Tcptp, is an important step in understanding how cellular responses to extracellular cues affect the intracellular mechanisms of lineage selection.
Acknowledgments
P. D. Simoncic thanks the National Cancer Institute of Canada for a Terry Fox Studentship. A. Bourdeau thanks the Cancer Research Institute of New York for a cancer immunology fellowship. C. J. McGlade is a research scientist of the National Cancer Institute of Canada supported with funds from the Canadian Cancer Society. This work was supported with funds from the Canadian Institutes of Health Research (to C. J. McGlade), the Leukemia and Lymphoma Society of Canada (to C. J. McGlade), the National Cancer Institute of Canada (to M. L. Tremblay), and the National Institutes of Health (CA26504, CA32551, and P30 CA13330-31 to E. R. Stanley and CA40987 to L. R. Rohrschneider).
We thank Dwayne Barber for critical reading of the manuscript. We also thank Cheryl Smith for help with the FACS analysis.
REFERENCES
- 1.Alberola-Ila, J., and G. Hernandez-Hoyos. 2003. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 191:79-96. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 3.Austyn, J. M., and S. Gordon. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11:805-815. [DOI] [PubMed] [Google Scholar]
- 4.Bartelmez, S. H., T. R. Bradley, I. Bertoncello, D. Y. Mochizuki, R. J. Tushinski, E. R. Stanley, A. J. Hapel, I. G. Young, A. B. Kriegler, and G. S. Hodgson. 1989. Interleukin 1 plus interleukin 3 plus colony-stimulating factor 1 are essential for clonal proliferation of primitive myeloid bone marrow cells. Exp. Hematol. 17:240-245. [PubMed] [Google Scholar]
- 5.Berg, K. L., K. A. Siminovitch, and E. R. Stanley. 1999. SHP-1 regulation of p62(DOK) tyrosine phosphorylation in macrophages. J. Biol. Chem. 274:35855-35865. [DOI] [PubMed] [Google Scholar]
- 6.Bivona, T. G., I. Perez De Castro, I. M. Ahearn, T. M. Grana, V. K. Chiu, P. J. Lockyer, P. J. Cullen, A. Pellicer, A. D. Cox, and M. R. Philips. 2003. Phospholipase Cγ activates Ras on the Golgi apparatus by means of RasGRP1. Nature 424:694-698. [DOI] [PubMed] [Google Scholar]
- 7.Bourette, R. P., G. M. Myles, K. Carlberg, A. R. Chen, and L. R. Rohrschneider. 1995. Uncoupling of the proliferation and differentiation signals mediated by the murine macrophage colony-stimulating factor receptor expressed in myeloid FDC-P1 cells. Cell Growth Differ. 6:631-645. [PubMed] [Google Scholar]
- 8.Bourette, R. P., G. M. Myles, J. L. Choi, and L. R. Rohrschneider. 1997. Sequential activation of phoshatidylinositol 3-kinase and phospholipase C-γ2 by the M-CSF receptor is necessary for differentiation signaling. EMBO J. 16:5880-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourette, R. P., and L. R. Rohrschneider. 2000. Early events in M-CSF receptor signaling. Growth Factors 17:155-166. [DOI] [PubMed] [Google Scholar]
- 10.Bourgin, C., R. P. Bourette, S. Arnaud, Y. Liu, L. R. Rohrschneider, and G. Mouchiroud. 2002. Induced expression and association of the Mona/Gads adapter and Gab3 scaffolding protein during monocyte/macrophage differentiation. Mol. Cell. Biol. 22:3744-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, H. E., S. Chang, T. Trub, and B. G. Neel. 1996. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol. Cell. Biol. 16:3685-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitu, V., and E. R. Stanley. 2006. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18:39-48. [DOI] [PubMed] [Google Scholar]
- 13.Chiu, V. K., T. Bivona, A. Hach, J. B. Sajous, J. Silletti, H. Wiener, R. L. Johnson, Jr., A. D. Cox, and M. R. Philips. 2002. Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell Biol. 4:343-350. [DOI] [PubMed] [Google Scholar]
- 14.Courtneidge, S. A., R. Dhand, D. Pilat, G. M. Twamley, M. D. Waterfield, and M. F. Roussel. 1993. Activation of Src family kinases by colony stimulating factor-1, and their association with its receptor. EMBO J. 12:943-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croker, B. A., D. Metcalf, L. Robb, W. Wei, S. Mifsud, L. DiRago, L. A. Cluse, K. D. Sutherland, L. Hartley, E. Williams, J. G. Zhang, D. J. Hilton, N. A. Nicola, W. S. Alexander, and A. W. Roberts. 2004. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity 20:153-165. [DOI] [PubMed] [Google Scholar]
- 16.Csar, X. F., N. J. Wilson, K. A. McMahon, D. C. Marks, T. L. Beecroft, A. C. Ward, G. A. Whitty, V. Kanangasundarum, and J. A. Hamilton. 2001. Proteomic analysis of macrophage differentiation. p46/52(Shc) tyrosine phosphorylation is required for CSF-1-mediated macrophage differentiation. J. Biol. Chem. 276:26211-26217. [DOI] [PubMed] [Google Scholar]
- 17.Dai, X. M., X. H. Zong, V. Sylvestre, and E. R. Stanley. 2004. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood 103:1114-1123. [DOI] [PubMed] [Google Scholar]
- 18.DeKoter, R. P., J. C. Walsh, and H. Singh. 1998. PU.1 regulates both cytokine-dependent proliferation and differentiation of granulocyte/macrophage progenitors. EMBO J. 17:4456-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enver, T., C. M. Heyworth, and T. M. Dexter. 1998. Do stem cells play dice? Blood 92:348-352. [PubMed] [Google Scholar]
- 20.Feng, G. S. 1999. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res. 253:47-54. [DOI] [PubMed] [Google Scholar]
- 21.Flint, A. J., T. Tiganis, D. Barford, and N. K. Tonks. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobert Gosse, S., C. Bourgin, W. Q. Liu, C. Garbay, and G. Mouchiroud. 2005. M-CSF stimulated differentiation requires persistent MEK activity and MAPK phosphorylation independent of Grb2-Sos association and phosphatidylinositol 3-kinase activity. Cell. Signal. 17:1352-1362. [DOI] [PubMed] [Google Scholar]
- 23.Green, M. C., and L. D. Shultz. 1975. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J. Hered. 66:250-258. [DOI] [PubMed] [Google Scholar]
- 24.Gu, H., and B. G. Neel. 2003. The “Gab” in signal transduction. Trends Cell Biol. 13:122-130. [DOI] [PubMed] [Google Scholar]
- 25.Gu, H., K. Saito, L. D. Klaman, J. Shen, T. Fleming, Y. Wang, J. C. Pratt, G. Lin, B. Lim, J. P. Kinet, and B. G. Neel. 2001. Essential role for Gab2 in the allergic response. Nature 412:186-190. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton, J. A. 1997. CSF-I signal transduction: what is of functional significance? Immunol. Today 18:313-317. [DOI] [PubMed] [Google Scholar]
- 27.Heinonen, K. M., F. P. Nestel, E. W. Newell, G. Charette, T. A. Seemayer, M. L. Tremblay, and W. S. Lapp. 2004. T-cell protein tyrosine phosphatase deletion results in progressive systemic inflammatory disease. Blood 103:3457-3464. [DOI] [PubMed] [Google Scholar]
- 28.Kriegler, A. B., T. R. Bradley, I. Bertoncello, J. A. Hamilton, P. H. Hart, D. S. Piccoli, and G. S. Hodgson. 1990. Progenitor cells in murine bone marrow stimulated by growth factors produced by the AF1-19T rat cell line. Exp. Hematol. 18:372-378. [PubMed] [Google Scholar]
- 29.Kriegler, A. B., S. M. Verschoor, D. Bernardo, and I. Bertoncello. 1994. The relationship between different high proliferative potential colony-forming cells in mouse bone marrow. Exp. Hematol. 22:432-440. [PubMed] [Google Scholar]
- 30.Liu, Y., B. Jenkins, J. L. Shin, and L. R. Rohrschneider. 2001. Scaffolding protein Gab2 mediates differentiation signaling downstream of Fms receptor tyrosine kinase. Mol. Cell. Biol. 21:3047-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, Y., and L. R. Rohrschneider. 2002. The gift of Gab. FEBS Lett. 515:1-7. [DOI] [PubMed] [Google Scholar]
- 32.McGlade, J., A. Cheng, G. Pelicci, P. G. Pelicci, and T. Pawson. 1992. Shc proteins are phosphorylated and regulated by the v-Src and v-Fps protein-tyrosine kinases. Proc. Natl. Acad. Sci. USA 89:8869-8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKercher, S. R., B. E. Torbett, K. L. Anderson, G. W. Henkel, D. J. Vestal, H. Baribault, M. Klemsz, A. J. Feeney, G. E. Wu, C. J. Paige, and R. A. Maki. 1996. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 15:5647-5658. [PMC free article] [PubMed] [Google Scholar]
- 34.Metcalf, D. 1998. Lineage commitment and maturation in hematopoietic cells: the case for extrinsic regulation. Blood 92:345-347, 352. [PubMed] [Google Scholar]
- 35.Myers, M. P., J. N. Andersen, A. Cheng, M. L. Tremblay, C. M. Horvath, J. P. Parisien, A. Salmeen, D. Barford, and N. K. Tonks. 2001. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276:47771-47774. [DOI] [PubMed] [Google Scholar]
- 36.Nakayama, K., K. Takahashi, L. D. Shultz, K. Miyakawa, and K. Tomita. 1997. Abnormal development and differentiation of macrophages and dendritic cells in viable motheaten mutant mice deficient in haematopoietic cell phosphatase. Int. J. Exp. Pathol. 78:245-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neel, B. G., H. Gu, and L. Pao. 2003. The ′Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28:284-293. [DOI] [PubMed] [Google Scholar]
- 38.Nishida, K., and T. Hirano. 2003. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 94:1029-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishida, K., L. Wang, E. Morii, S. J. Park, M. Narimatsu, S. Itoh, S. Yamasaki, M. Fujishima, K. Ishihara, M. Hibi, Y. Kitamura, and T. Hirano. 2002. Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood 99:1866-1869. [DOI] [PubMed] [Google Scholar]
- 40.Paling, N. R., and M. J. Welham. 2005. The tyrosine phosphatase SHP-1 acts at different stages of development to regulate hematopoiesis. Blood 105:4290-4297 [DOI] [PubMed] [Google Scholar]
- 41.Passegue, E., C. H. Jamieson, L. E. Ailles, and I. L. Weissman. 2003. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc. Natl. Acad. Sci. USA 100(Suppl. 1):11842-11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pixley, F. J., and E. R. Stanley. 2004. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 14:628-638. [DOI] [PubMed] [Google Scholar]
- 43.Qu, C. K., Z. Q. Shi, R. Shen, F. Y. Tsai, S. H. Orkin, and G. S. Feng. 1997. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol. Cell. Biol. 17:5499-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohrschneider, L. R., and D. Metcalf. 1989. Induction of macrophage colony-stimulating factor-dependent growth and differentiation after introduction of the murine c-fms gene into FDC-P1 cells. Mol. Cell. Biol. 9:5081-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott, E. W., M. C. Simon, J. Anastasi, and H. Singh. 1994. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265:1573-1577. [DOI] [PubMed] [Google Scholar]
- 46.Shi, Z. Q., D. H. Yu, M. Park, M. Marshall, and G. S. Feng. 2000. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell. Biol. 20:1526-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shultz, L. D., D. R. Coman, C. L. Bailey, W. G. Beamer, and C. L. Sidman. 1984. “Viable motheaten,” a new allele at the motheaten locus. I. Pathology. Am. J. Pathol. 116:179-192. [PMC free article] [PubMed] [Google Scholar]
- 48.Shultz, L. D., T. V. Rajan, and D. L. Greiner. 1997. Severe defects in immunity and hematopoiesis caused by SHP-1 protein-tyrosine-phosphatase deficiency. Trends Biotechnol. 15:302-307. [DOI] [PubMed] [Google Scholar]
- 49.Simoncic, P. D., A. Lee-Loy, D. L. Barber, M. L. Tremblay, and C. J. McGlade. 2002. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr. Biol. 12:446-453. [DOI] [PubMed] [Google Scholar]
- 50.Stanley, E. R., K. L. Berg, D. B. Einstein, P. S. Lee, F. J. Pixley, Y. Wang, and Y. G. Yeung. 1997. Biology and action of colony-stimulating factor-1. Mol. Reprod. Dev. 46:4-10. [DOI] [PubMed] [Google Scholar]
- 51.Stanley, E. R., K. L. Berg, D. B. Einstein, P. S. Lee, and Y. G. Yeung. 1994. The biology and action of colony stimulating factor-1. Stem Cells 12(Suppl. 1):15-25. [PubMed] [Google Scholar]
- 52.Stanley, I. J., N. A. Nicola, and A. W. Burgess. 1989. Growth factor-induced phosphorylation of c-ras p21 in normal hemopoietic progenitor cells. Growth Factors 2:53-59. [DOI] [PubMed] [Google Scholar]
- 53.Tapley, P., N. K. Shevde, P. A. Schweitzer, M. Gallina, S. W. Christianson, I. L. Lin, R. B. Stein, L. D. Shultz, J. Rosen, and P. Lamb. 1997. Increased G-CSF responsiveness of bone marrow cells from hematopoietic cell phosphatase deficient viable motheaten mice. Exp. Hematol. 25:122-131. [PubMed] [Google Scholar]
- 54.ten Hoeve, J., M. de Jesus Ibarra-Sanchez, Y. Fu, W. Zhu, M. Tremblay, M. David, and K. Shuai. 2002. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol. Cell. Biol. 22:5662-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Vliet, C., P. E. Bukczynska, M. A. Puryer, C. M. Sadek, B. J. Shields, M. L. Tremblay, and T. Tiganis. 2005. Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat. Immunol. 6:253-260. [DOI] [PubMed] [Google Scholar]
- 56.Vaudry, D., P. J. Stork, P. Lazarovici, and L. E. Eiden. 2002. Signaling pathways for PC12 cell differentiation: making the right connections. Science 296:1648-1649. [DOI] [PubMed] [Google Scholar]
- 57.Wang, Y., Y. G. Yeung, and E. R. Stanley. 1999. CSF-1 stimulated multiubiquitination of the CSF-1 receptor and of Cbl follows their tyrosine phosphorylation and association with other signaling proteins. J. Cell. Biochem. 72:119-134. [PubMed] [Google Scholar]
- 58.Wiktor-Jedrzejczak, W., A. Bartocci, A. W. Ferrante, Jr., A. Ahmed-Ansari, K. W. Sell, J. W. Pollard, and E. R. Stanley. 1990. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA 87:4828-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.You-Ten, K. E., E. S. Muise, A. Itie, E. Michaliszyn, J. Wagner, S. Jothy, W. S. Lapp, and M. L. Tremblay. 1997. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 186:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, S. Q., W. G. Tsiaras, T. Araki, G. Wen, L. Minichiello, R. Klein, and B. G. Neel. 2002. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 22:4062-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, S. Q., W. Yang, M. I. Kontaridis, T. G. Bivona, G. Wen, T. Araki, J. Luo, J. A. Thompson, B. L. Schraven, M. R. Philips, and B. G. Neel. 2004. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 13:341-355. [DOI] [PubMed] [Google Scholar]