Abstract
In cells capable of entering the cell cycle, including cancer cells, β-catenin has been termed a master switch, driving proliferation over differentiation. However, its role as a transcriptional activator in terminally differentiated cells is relatively unknown. Herein we utilize conditional, cardiac-specific deletion of the β-catenin gene and cardiac-specific expression of a dominant inhibitory mutant of Lef-1 (Lef-1Δ20), one of the members of the T-cell factor/lymphocyte enhancer factor (Tcf/Lef) family of transcription factors that functions as a coactivator with β-catenin, to demonstrate that β-catenin/Tcf/Lef-dependent gene expression regulates both physiologic and pathological growth (hypertrophy) of the heart. Indeed, the profound nature of the growth impairment of the heart in the Lef-1Δ20 mouse, which leads to very early development of heart failure and premature death, suggests β-catenin/Tcf/Lef targets are dominant regulators of cardiomyocyte growth. Thus, our studies, employing complementary models in vivo, implicate β-catenin/Tcf/Lef signaling as an essential growth-regulatory pathway in terminally differentiated cells.
Growth in the postnatal mammalian heart is principally via hypertrophy, as opposed to hyperplasia, since the vast majority of cardiac myocytes are terminally differentiated. Hypertrophy can be either physiologic (i.e., normal growth or exercise-induced growth) or pathological, induced by stresses, such as pressure overload secondary to hypertension or valvular disease, or occurring as a result of mutations in a number of proteins making up the contractile apparatus of the myocyte (14, 17, 44). Growth of cardiac myocytes is regulated by a number of parallel but intersecting signaling pathways that transduce signals to the nucleus, leading to the reprogramming of gene expression (reviewed in references 14 and 17). One central pathway for which abundant evidence exists, implicating it in the regulation of both normal and pathological stress-induced growth, is the phosphatidylinositol 3-kinase pathway and its downstream targets (38, 47, 50). One such target is the protein kinase glycogen synthase kinase 3β (GSK-3β) (2, 20, 43). This kinase appears to be a negative regulator of both normal and pathological cardiac growth in vivo, although this conclusion is based entirely on studies employing transgenic mice overexpressing GSK-3β (1, 40).
GSK-3β has a number of substrates that are known or putative regulators of growth, including transcription factors (e.g., GATA-4 and members of the NF-AT family) and a regulator of protein translation (eIF2B) (1, 20, 22, 42, 63, 64). However, the roles of GATA-4 and eIF2B in postnatal heart growth in vivo are not clear. Furthermore, while NF-ATc3 regulates, in part, the hypertrophic response to pressure overload, it appears to play little role in normal cardiac growth (64). Therefore, the profound growth retardation of the heart we observed in the transgenic mouse expressing GSK-3β (40) suggested additional factors downstream of GSK-3β might be relevant to growth control. Herein we explore the role of one candidate, the transcriptional regulatory pathway controlled by β-catenin/T-cell factor/lymphocyte enhancer factor (β-catenin/Tcf/Lef).
β-Catenin functions as both a component of the adherens junction, where it links cadherins to the cytoskeleton, and as a transcriptional activator (4, 46). Cytosolic β-catenin levels are regulated by a multimolecular complex assembled on the scaffolding proteins Axin and Presenilin 1 (reviewed in references 16, 46, and 52). Within the Axin complex is the adenomatous polyposis coli gene product, APC, which binds both β-catenin and Axin, tethering the former to the latter. GSK-3β is also in this complex, and when β-catenin is phosphorylated by GSK-3β, it is targeted for ubiquitination and degradation by the proteasome (35). Inhibition of GSK-3β is thus essential to the stabilization of β-catenin.
When cytosolic levels of β-catenin increase, such as that which occurs following stimulation with Wnts in the canonical pathway, β-catenin translocates to the nucleus, where it partners with members of the Tcf/Lef family to induce transactivation of genes containing Tcf/Lef promoter elements (25). (β-Catenin contains a transactivation domain but does not bind DNA in the absence of a cofactor, whereas Tcf/Lefs bind DNA but do not contain a transactivation domain.) This induces the expression of a large number of genes that regulate a host of developmental processes, including embryonic axis formation (for a list of Wnt target genes, please see the Wnt homepage at www.stanford.edu/∼rnusse/wntwindow.html.).
During cardiac development, the canonical Wnt/β-catenin pathway plays various roles. Since mice deleted for β-catenin have multiple ectopic hearts (33), the pathway appears to antagonize cardiomyocyte differentiation and/or to restrict the size of the cardiogenic field (15). In addition, later in development, the pathway, acting in part via Pitx2, a member of the bicoid family of transcription factors, is critical for cushion morphogenesis, outflow tract development, and valve formation (26, 27).
Postnatally, in cells that are capable of entering the cell cycle, β-catenin drives cells toward proliferation and away from differentiation (58). However, it is not clear what role, if any, the transactivating activity of β-catenin plays in the growth of cardiomyocytes or, for that matter, the growth of any terminally differentiated cell.
Previously, we demonstrated that β-catenin was stabilized and a Tcf/Lef reporter was activated by hypertrophic stimuli in terminally differentiated neonatal cardiomyocytes in culture, and that this occurred via a Wnt-independent mechanism (21). In addition, overexpression of a stabilized mutant of β-catenin via adenovirus-mediated gene transfer was sufficient to drive hypertrophic growth in these cells (21). However, it is unclear whether this pathway plays any role in vivo in physiologic growth or in pathological stress-induced growth of the heart. Herein, utilizing Cre/loxP technology (7) and a tamoxifen-inducible, cardiac-specific Cre transgenic mouse (54), we demonstrate that targeted deletion of β-catenin in the heart leads to a blunted hypertrophic response to pathological stress-induced growth. We further demonstrate that expression of a dominant inhibitory mutant of Lef-1, which blocks expression of β-catenin/Tcf/Lef-dependent genes, leads to dramatic reductions in cardiomyocyte growth, suggesting that the transcriptional activating activity of β-catenin, as opposed to its role at the adherens junction, is critical to heart growth. These data are the first of which we are aware to demonstrate an essential role in vivo for the β-catenin/Tcf/Lef pathway in the biology of terminally differentiated cells.
MATERIALS AND METHODS
Protocol for conditional deletion of β-catenin.
The breeding scheme for conditional deletion of β-catenin involved crossing the β-cateninfl/fl mouse obtained from Jackson Laboratories (7, 33) with the heterozygous Mer-Cre-Mer mouse. The latter expresses Cre flanked by two modified estrogen receptors that are responsive to tamoxifen (54). The Mer-Cre-Mer transgene is driven by the α-myosin heavy chain (MHC) promoter and therefore is only expressed in cardiomyocytes. This led to the generation of two genotypes: β-cateninfl/fl/wild type (WT) and β-cateninfl/fl/Cre. An additional control was WT/Cre. Genotyping was performed with PCR using specific primers as described previously (7, 33, 54). Mer-Cre-Mer expression levels, determined by immunoblotting with an anti-Cre antibody, were equal in the β-cateninfl/fl/Cre versus WT/Cre mice (data not shown). At 5 weeks of age, mice from the three genotypes underwent intraperitoneal injection with tamoxifen (20 mg/kg of body weight [Sigma] dissolved at a final concentration of 4 mg/ml in 30% ethanol-sterile phosphate-buffered saline) or vehicle daily for 5 days. Success of recombination in the β-cateninfl/fl/Cre mice was determined based on immunocytochemistry and immunoblotting for β-catenin (Fig. 1). This system has no detectable recombination in the absence of tamoxifen treatment (30, 51, 54).
FIG. 1.
Deletion of β-catenin in tamoxifen-treated β-cateninfl/fl/Cre mice. Cardiomyocytes were isolated from hearts of β-cateninfl/fl/Cre mice that had been treated with tamoxifen or vehicle (−Tamoxifen) for 5 days and then were sacrificed 10 days (top panels) or 6 weeks (A, bottom panel, and B) later. Myocytes were immunostained (A) or immunoblotted (B) for expression of β-catenin. (A, top left panel) The arrow identifies residual β-catenin remaining in the adherens junction. (A, bottom panel) The arrows identify cardiomyocytes in which β-catenin has been deleted by Cre-mediated recombination. (B) GAPDH serves as a loading control.
Creation of the Lef-1Δ20 transgenic mouse.
The cDNA encoding Lef-1 deleted for the first 20 amino acids and containing an amino-terminal hemagglutinin (HA) epitope tag was subcloned into the 5.5-kb murine α-myosin heavy chain promoter expression vector (a gift from Jeffrey Robbins, Children's Hospital, Cincinnati, OH). The injection fragment was gel purified, reconstituted in 2 mM Tris-HCl (pH 7.5)-20 μM EDTA, and injected into fertilized eggs from FVB/N mice. The eggs were then transferred to the oviducts of pseudopregnant females. Founders were then crossed into C57Bl6/J. Line 1 was studied as F1 but then was lost due to early postnatal lethality (see Results). Line 2 was studied at F5.
Immunoblotting and quantification.
Cell lysates and lysates from hearts for Western blotting were prepared as previously described (21). After sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transfer to nitrocellulose, membranes were incubated with one of the following antibodies: hemagglutinin (HA) and c-Myc from Santa Cruz Biotechnology, Inc.; β-catenin from BD Transduction Labs, Inc.; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Research Diagnostics, Inc.; vinculin from Sigma; and Cre from Novagen. The Lef-1 antibody was a kind gift of Elaine Fuchs, Rockefeller University. Blots were incubated with primary antibodies overnight and with the appropriate secondary antibody for 1 h at room temperature. Antibody binding was detected with a peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G (DAKO) and chemiluminescence (Amersham). Quantification of expression levels of proteins was done by determining band density on immunoblots using a ChemiImager 4400 imaging system with the AlphaEase software package. For quantification, band density for the protein of interest was normalized to either GAPDH or vinculin, which served as a loading control.
RT-PCR.
RNA from hearts was isolated with Tri-Reagent (Sigma) following the manufacturer's suggested protocol. A ThermoScript reverse transcriptase PCR (RT-PCR) kit (Invitrogen) was used for cDNA synthesis. Semiquantitative RT-PCR was carried out for β-myosin heavy chain (β-MHC), α-skeletal actin, atrial natriuretic factor (ANF), and brain natriuretic peptide (BNP) using primers described previously (45). Amplification was for 26 cycles. For normalization control, primers specific for GAPDH were the following: 5′-ATGGTGAAGGTCGGTGTGAAC-3′ and 5′-CGTTCACACCGACCTTCACCAC-3′. PCR products were separated on an agarose gel and visualized and quantified with the Quantity One system from Bio-Rad.
Thoracic aortic constriction (TAC).
The method of thoracic aortic constriction was that developed by Rockman and coworkers (53). In brief, a 27-gauge needle is placed adjacent to the thoracic aorta, and then a nylon suture ligature is tied around the vessel and the needle. The needle is then withdrawn, leaving a stenosis of approximately 65 to 70% in the aorta between the innominate artery and the left carotid artery.
Morphometric measures.
For determination of myocardial mass, animals were anesthetized and then were sacrificed with an injection of cardioplegia solution (at 4°C) (21). The hearts were carefully excised, and heart weight was determined. The left ventricle (LV) was then carefully trimmed away from the right ventricle and atria, and it was weighed. The length of the right tibia was also determined. Heart weight and LV weight were expressed relative to body weight and to tibial length.
Cardiomyocyte isolation and immunocytochemistry.
Adult mouse cardiomyocytes were isolated as previously described (40). Briefly, mice were administered heparin intraperitoneally (200 U) and were then anesthetized with intraperitoneal doses of ketamine (150 mg/kg) and xylazine (15 mg/kg). The hearts were quickly excised and perfused with 1.8 mM Ca2+ Tyrode buffer (137 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.5 mM MgCl2, 10 mM HEPES, 10 mM glucose, pH 7.4, at 37°C) followed by Ca2+-free Tyrode (135 mM NaCl, 4 mM KCl, 1 mM MgCl2, 10 mM HEPES, 0.33 mM NaH2PO4, 10 mM glucose, 10 mM 2,3-butanedione monoxime, pH 7.2, at 37°C). Hearts were then digested with Ca2+-free Tyrode containing 0.014% collagenase B and 0.05% collagenase D (Boehringer Mannheim) as well as 0.005% protease XIV (Sigma Chemical Co.). After the hearts were palpably flaccid, the LV was separated, minced, and gently agitated in Ca2+-free Tyrode buffer, allowing the myocytes to be dispersed. The cells were subsequently resuspended in Tyrode buffers with gradually increasing Ca2+ concentrations (0.06, 0.24, 0.6, and 1.2 mM Ca2+). Cells were then plated on glass slides precoated with laminin, fixed in formalin, washed in phosphate-buffered saline, and stored in phosphate-buffered saline until used.
For determination of cardiomyocyte width, slides were incubated with the anti-β-catenin antibody at 1:500 dilution for 1 h at room temperature and then incubated with Cy3-conjugated rabbit anti-mouse secondary antibody (1:5,000) for 1 h at room temperature, followed by incubation in 4′,6′-diamidino-2-phenylindole (DAPI; 1:10,000) for 5 min. Cells were imaged with a Nikon Eclipse E800 microscope and photographed with a Hammamatsu Orca digital camera. Cardiomyocyte width was determined with OpenLab software (Improvision).
Determination of cardiomyocyte cross-sectional area in heart sections.
Paraffin-imbedded sections were deparaffinized, rehydrated, and incubated for 1 h with fluorescein isothiocyanate (FITC)-conjugated wheat germ agglutinin per the manufacturer's recommendations (Biomeda). Slides were rinsed in phosphate-buffered saline and then mounted in Vectashield mounting solution.
Random fields from both the septum and LV free wall were photographed by an observer blinded to the genotypes. Cardiomyocyte cross-sectional area was then determined by the same observer, who remained blinded. Only cardiomyocytes cut in short axes were measured. Data analysis was with OpenLab software.
Echocardiography.
Transthoracic echocardiography was performed under light sedation with 1.5% isoflurane administered via nose cone while the core body temperature was maintained at 37.0 ± 0.2°C using a closed loop system (Barnant Company, Barrington, IL). Cardiac dimensions and wall thicknesses were obtained from two-dimensional-guided M-mode echocardiograms as described previously (21, 59). LV mass calculated with the formula LV mass = [(2 × PWT) + EDD]3 − EDD3, where PWT is posterior wall thickness and EDD is end-diastolic dimension. Although absolute values for LV mass by echocardiography are consistently greater than direct LV mass measurements, the two are highly correlated (r = 0.97 for these studies).
Invasive hemodynamic monitoring.
Hemodynamic evaluation was performed as described previously (21, 59) using 2.0% isoflurane anesthesia administered via nose cone. Pressures were acquired from the right internal carotid artery, ascending aorta, and the left ventricle. A 1.4 French microtip pressure transducer (Millar Instruments, Inc., Houston, TX), connected to a computerized data acquisition system (Powerlab; ADInstruments, Grand Junction, CO), was used to record hemodynamic measurements. Chart 4 data analysis software (ADInstruments, Grand Junction, CO) was used to calculate the first derivative of the pressure curves (dP/dt) and the heart rates. LV end diastolic pressures were obtained by averaging five measurements taken at the end of expiration.
Statistical analyses.
Data were analyzed by one-way analysis of variance followed by Fisher's test, except for the determination of differences between data sets in Fig. 3B. For this figure, a single linear regression model of the two sets of data was completed, using an indicator variable to distinguish experimental sets. The initial model allowed both intercept and slope to differ by experiment. If the intercepts were not significantly different, this term was dropped from the model, and a test of slope differences was then considered. Computations were completed in SAS version 9.1. Significance was set at P < 0.05. All data are presented as means ± standard errors of the means unless otherwise noted.
FIG. 3.
Characterization of the hypertrophic response in hearts of mice deleted for β-catenin. A. Five-week-old female β-cateninfl/fl/Cre (β-catfl/fl/Cre) mice were injected with tamoxifen or vehicle (placebo), and β-cateninfl/fl/WT mice were injected with tamoxifen. Six weeks later, mice underwent echocardiographic examination (top panel, baseline) and then were subjected to TAC or sham TAC. Two weeks later, echocardiography was performed (top panel, TAC), and then animals were sacrificed for determination of morphometric measures (bottom panel). LV mass is normalized to tibial length. B. Relationship between LVSP and LV mass/tibial length (LVM/TL) in β-cateninfl/fl/Cre mice, treated with tamoxifen or placebo, and subjected to TAC or sham TAC. Mice underwent the protocol described for panel A. Prior to sacrifice, they underwent invasive hemodynamic assessment with a micromanometer-tipped catheter followed by determination of the ratio of LV mass to tibial length (LVM/TL). Correlation coefficients for the two groups (r = 0.76 and 0.92) were significant at P < 0.001. The slope of the regression line for β-cateninfl/fl/Cre mice treated with tamoxifen, identified with an arrow, is significantly less than that for the β-cateninfl/fl/Cre mice treated with placebo. n = 8 for each TAC group, and n = 5 for each sham group. C. Direct determination of cardiomyocyte width. Cardiomyocytes were isolated from β-cateninfl/fl/Cre mice treated with tamoxifen or placebo (Tamoxifen−) or from WT/Cre mice (Cre) treated with tamoxifen that had been subjected to TAC or sham TAC. Cardiomyocyte width was determined after staining for β-catenin. *, P < 0.05; #, P < 0.01. n = 3 to 4 in TAC groups, and n = 2 in sham groups. A minimum of 100 cardiomyocytes was measured per animal. Statistical significance was determined based on comparing the mean value of myocyte width for each animal, not total myocytes (i.e., n = 3 to 4). D. Analysis of TAC-induced gene expression by RT-PCR. Expression of β-MHC, α-skeletal actin, and ANF in hearts of β-cateninfl/fl/Cre mice treated with tamoxifen or placebo and subjected to TAC or sham TAC. n = 3 for TAC groups, and n = 1 to 2 for sham groups.
RESULTS
Deletion of the β-catenin gene is uniformly embryonic lethal at about embryonic day 8.5 (19). Therefore, we turned to the β-cateninfl/fl mouse (7, 33), crossing it with a cardiac-specific transgenic mouse expressing a fusion protein of a modified estrogen receptor flanking the Cre protein under the control of the α-MHC promoter (54). The activity of the Cre protein can be induced following administration of tamoxifen, which binds to the modified estrogen receptors, displacing heat shock proteins that constitutively bind to and inhibit Cre activity (54).
We first determined the success of Cre-mediated recombination in tamoxifen-treated β-cateninfl/fl/Cre mice. We isolated myocytes from the hearts of β-cateninfl/fl/Cre mice treated with tamoxifen and quantified the percentage of cardiomyocytes in which β-catenin was successfully deleted. At 10 days after completion of the tamoxifen injections, some β-catenin remained in the adherens junctions, consistent with this pool not being freely interchangeable with the cytosolic pool (Fig. 1A, top left panel). Six weeks after treatment with tamoxifen, however, β-catenin was virtually undetectable in 54% ± 8% of cardiomyocytes (n = 7; Fig. 1A, bottom panel). However, immunoblotting whole-heart lysates for β-catenin showed a more marked (87%) decrease in expression levels (Fig. 1B). This suggests that many of the cardiomyocytes that did stain positive for β-catenin after tamoxifen treatment may have had one allele deleted by Cre-mediated recombination (note the intermediate level of staining in two of the myocytes in bottom panel of Fig. 1A).
β-Catenin regulates pressure overload-induced hypertrophy.
We next examined what role, if any, β-catenin plays in pathological stress-induced hypertrophy following pressure overload. We first asked whether deletion of β-catenin had the expected effect on expression levels of β-catenin/Tcf/Lef target genes. This is particularly relevant, since upregulation of either γ-catenin/plakoglobin or p120-catenin could compensate for the loss of β-catenin (48), although we did not detect this in our model (data not shown). We found that expression of two bona fide targets, c-Myc and c-Fos (23, 48), was reduced in tamoxifen-treated β-cateninfl/fl/Cre mice compared to that for vehicle-treated mice (Fig. 2). For example, expression of c-Myc was 2.5-fold greater in vehicle-treated β-cateninfl/fl/Cre mice post-TAC compared to that of mice treated with tamoxifen (Fig. 2). We also found a 33% reduction in expression of Connexin 43, a gap junction structural protein that is also a target of β-catenin (P = 0.028; data not shown). We examined other targets of β-catenin, including cyclin D1 and Pitx2, but consistent with prior reports in the literature, cyclin D1 (as with D2 and D3) was virtually undetectable in our adult hearts (49). Similarly, we did not detect Pitx2, consistent with it not having been found to be expressed in adult heart based on the National Center for Biotechnology Information analysis of expression patterns of expressed sequence tags. In summary, although the regulation of c-Myc and c-Fos expression, like many β-catenin targets, is complex (3), these data confirm that expression of c-Myc and c-Fos in the heart post-TAC is, in large part, dependent on β-catenin, and that β-catenin/Tcf/Lef-dependent gene expression is indeed reduced in the β-cateninfl/fl/Cre mouse treated with tamoxifen.
FIG. 2.
Expression of β-catenin targets in the hearts of β-cateninfl/fl/Cre (β-catfl/fl/Cre) mice. Immunoblot for c-Myc (top panel) and c-Fos (middle panel) in lysates from hearts of β-cateninfl/fl/Cre mice treated with tamoxifen or vehicle (−Tamoxifen) and subjected to TAC versus sham TAC (−TAC). (Bottom panel) Quantification of c-Myc expression by densitometry, normalized to vinculin as a loading control. Data are from groups of 3 to 5 mice. *, P < 0.05.
We then tested whether deletion of β-catenin impaired the hypertrophic response to pressure overload induced by TAC. Five-week-old female β-cateninfl/fl/Cre mice were intraperitoneally injected for 5 days with tamoxifen or placebo. At 11 weeks of age, animals underwent echocardiographic examination followed by TAC or sham TAC. Two weeks later animals again underwent echocardiography, as well as hemodynamic assessment for determination of the pressure load placed on the ventricle by the TAC, and then were sacrificed for determination of morphometric measures of hypertrophy.
The echocardiographic evaluation prior to TAC (to screen for any differences in baseline LV size or function in the β-cateninfl/fl/Cre mice treated with tamoxifen versus placebo) showed no differences in heart rate, chamber size (EDD), contractile function (FS), LV mass, or LV mass indexed to body weight and tibial length (Table 1). Similarly, there were no differences in any of the morphometric measures between β-cateninfl/fl/Cre mice treated with tamoxifen versus those treated with placebo and subjected to sham surgery (Table 2). However, following TAC, there was significantly less hypertrophy, despite equivalent pressure loads (see Table 3), in the β-cateninfl/fl/Cre mice treated with tamoxifen compared to those treated with placebo, whether assessed by echocardiography or by morphometric measures (Tables 1 and 2; Fig. 3A). Not surprisingly, the reduced hypertrophy in the β-cateninfl/fl/Cre mice treated with tamoxifen and subjected to TAC was not caused by the 5 days of tamoxifen injections given 6 weeks prior to banding, since the β-cateninfl/fl/WT mice treated with tamoxifen had normal baseline parameters and TAC-induced hypertrophy was normal (i.e., equivalent to that of β-cateninfl/fl/Cre mice treated with placebo and significantly greater than that of β-cateninfl/fl/Cre mice treated with tamoxifen [Fig. 3A]). Of note, the inability of the β-cateninfl/fl/Cre mice treated with tamoxifen to hypertrophy normally in response to hemodynamic stress did not result in impaired LV systolic or diastolic function or in LV dilatation as assessed by both echocardiography (Table 1) and invasive hemodynamic evaluation (Table 3).
TABLE 1.
Baseline and post-TAC echocardiographic measurements in the β-cateninfl/fl/Cre mice, treated with tamoxifen (T) or placebo (P)a
| Mouse type and treatment status | HR (bpm) | EDD (mm) | FS (%) | LVM (mg) | LVM/BW (mg/g) | LVM/TL (mg/mm) |
|---|---|---|---|---|---|---|
| β-Cateninfl/fl/Cre, P (n = 17), baseline | 534 ± 13 | 3.32 ± 0.09 | 41.6 ± 1.4 | 89.0 ± 5.3 | 4.71 ± 0.25 | 5.10 ± 0.32 |
| β-Cateninfl/fl/Cre, T (n = 14), baseline | 513 ± 10 | 3.51 ± 0.08 | 39.2 ± 0.6 | 96.3 ± 6.3 | 4.93 ± 0.32 | 5.35 ± 0.31 |
| β-Cateninfl/fl/Cre, P (n = 9), TAC | 540 ± 12 | 3.56 ± 0.14 | 36.2 ± 2.7 | 138 ± 11 | 7.32 ± 0.64 | 7.99 ± 0.61 |
| β-Cateninfl/fl/Cre, T (n = 11), TAC | 525 ± 15 | 3.50 ± 0.09 | 39.1 ± 2.4 | 111 ± 4b | 5.74 ± 0.21b | 6.36 ± 0.23b |
HR, heart rate; EDD, end-diastolic dimension; FS, fractional shortening; LVM, left ventricular mass; TL, tibial length; BW, body weight; TAC, transverse aortic constriction; P, placebo-treated; and T, tamoxifen treated; bpm, beats per minute.
P < 0.05 versus β-cateninfl/fl/Cre mice treated with placebo and subjected to TAC.
TABLE 2.
Morphometric measurements in the β-cateninfl/fl/Cre mice, treated with tamoxifen or placebo and subjected to TAC or sham TACa
| Mouse type and treatment status | BW (g) | HW (mg) | LVM (mg) | TL (mm) | HW/BW (mg/g) | HW/TL (mg/mm) | LVM/BW (mg/g) | LVM/TL (mg/mm) |
|---|---|---|---|---|---|---|---|---|
| β-Cateninfl/fl/Cre, P (n = 5), sham | 19.2 ± 0.3 | 78.6 ± 3.0 | 63.8 ± 2.7 | 17.3 ± 0.1 | 4.10 ± 0.16 | 4.56 ± 0.18 | 3.33 ± 0.15 | 3.70 ± 0.16 |
| β-Cateninfl/fl/Cre, T (n = 5), sham | 19.4 ± 0.6 | 80.7 ± 1.6 | 62.7 ± 0.8 | 17.5 ± 0.3 | 4.17 ± 0.19 | 4.61 ± 0.12 | 3.24 ± 0.13 | 3.59 ± 0.08 |
| β-Cateninfl/fl/Cre, P (n = 8), TAC | 19.6 ± 0.1 | 104.9 ± 3.5 | 88.0 ± 2.6 | 17.4 ± 0.1 | 5.37 ± 0.18 | 6.02 ± 0.21 | 4.50 ± 0.11 | 5.05 ± 0.16 |
| β-Cateninfl/fl/Cre, T (n = 8), TAC | 19.4 ± 0.2 | 92.4 ± 3.3# | 76.3 ± 3.1# | 17.3 ± 0.2 | 4.76 ± 0.17* | 5.35 ± 0.19* | 3.94 ± 0.17* | 4.42 ± 0.17* |
BW, body weight; HW, heart weight; LVM, left ventricular mass; TL, tibial length; P, placebo treated; T, tamoxifen treated; TAC, transverse aortic constriction. *, P < 0.05 versus P and TAC; #, P < 0.01 versus P and TAC. All morphometric measures (HW, LVM, HW/BW, HW/TL, LVM/BW, and LVM/TL) in the TAC groups were significantly different from those of the respective sham groups at P < 0.05.
TABLE 3.
Hemodynamic measurements post-TAC in the β-cateninfl/fl/Cre mice and β-cateninfl/fl/WT mice, treated with tamoxifen (T) or placebo (P)a
| Mouse type and treatment | HR (bpm) | LVSP (mm Hg) | LVEDP (mm Hg) | +dP/dt | −dP/dt |
|---|---|---|---|---|---|
| β-Cateninfl/fl/Cre, P (n = 8) | 585 ± 11 | 142 ± 8 | 10.7 ± 2.0 | 8,494 ± 305 | 8,256 ± 364 |
| β-Cateninfl/fl/Cre, T (n = 7) | 550 ± 21 | 143 ± 5 | 12.8 ± 1.6 | 8,389 ± 398 | 8,691 ± 453 |
| β-Cateninfl/fl/WT, T (n = 8) | 568 ± 13 | 146 ± 5 | 12.8 ± 2.0 | 7,959 ± 355 | 8,326 ± 341 |
HR, heart rate; LVSP, LV systolic pressure; LVEDP, LV end-diastolic pressure; +dP/dt, first derivative of the rate of rise of LV pressure in systole; −dP/dt, first derivative of the rate of fall of LV pressure in diastole; bpm, beats per minute. The P value was not significant for all parameters.
Since the increase in LV mass is critically dependent on the pressure load faced by the left ventricle, we plotted LV mass/tibial length (TL) determined at sacrifice as a function of LV systolic pressure determined by a micromanometer-tipped catheter immediately prior to sacrifice. The correlation coefficients for the regression lines relating LV systolic pressure (LVSP) to LV mass/TL were highly significant in both groups (P < 0.001). There was a statistically significant reduction in the slope of the regression line for the β-cateninfl/fl/Cre mice treated with tamoxifen (P = 0.047; Fig. 3B). Across the range of LVSPs in the groups, the β-cateninfl/fl/Cre mice treated with tamoxifen required ∼20 to 25 mm Hg more LVSP to develop an amount of hypertrophy equivalent to that of the β-cateninfl/fl/Cre mice treated with placebo (Fig. 3B).
To get a more direct assessment of cardiomyocyte hypertrophy, we examined width (concentric hypertrophy) and length (eccentric hypertrophy) of cardiomyocytes isolated from sham and banded β-cateninfl/fl/Cre mice treated with tamoxifen, comparing width and length in cells in which β-catenin had been deleted versus those in which it had not been deleted. Cardiomyocyte length was not affected by deletion of β-catenin, either in the sham or TAC group (data not shown). Of note, width of cardiomyocytes isolated from sham-operated animals in which β-catenin had been deleted was very slightly smaller than the width of the cells in which β-catenin had not been deleted (Fig. 3C, bars 1 and 2). This was somewhat surprising, since over 85% of heart growth is achieved by 5 weeks of age (36), the time at which the tamoxifen injections were started. Thus, with only 15% of growth remaining to occur after the injections, even the modest reduction in cardiomyocyte growth, as determined by cardiomyocyte width, suggests that β-catenin may also play a role in normal growth of the heart, a contention that is supported by the complementary studies with the Lef-1Δ20 transgenic line described below.
When we examined the effects of deletion of β-catenin on cardiomyocyte hypertrophy post-TAC, we found that cardiomyocyte width increased significantly less in the myocytes in which β-catenin was deleted (Fig. 3C, bar 4) compared to those in which β-catenin was not deleted (bar 3). Of note, the comparison of width of cardiomyocytes in bar 4 versus bar 3 is comparing cardiomyocytes from the same hearts. Thus, differences in width cannot be due to differences in loading conditions, since the myocytes were exposed to identical pressure loads.
The reduction in growth was not due to tamoxifen treatment per se or tamoxifen treatment in the setting of Cre expression, since hypertrophic growth in the WT/Cre transgenic cells treated with tamoxifen (bar 6) was comparable to growth in the cells of the β-cateninfl/fl/Cre mice treated with tamoxifen in which β-cateninfl/fl was not deleted (bar 3) and was significantly greater than growth in the cells in which β-catenin was deleted (bar 4). Growth in β-cateninfl/fl/Cre mice treated with placebo (in which β-catenin is not deleted; bar 5) was also greater than that in cells deleted for β-catenin (bar 4). The reduction in hypertrophy was also not due to enhanced apoptosis or reduced fibrosis in the β-cateninfl/fl/Cre mice treated with tamoxifen, since we saw very low rates of apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling and minimal fibrosis by trichrome staining in these hearts and no differences between tamoxifen- and placebo-treated groups (data not shown).
We also asked if deletion of β-catenin led to alterations in the expression of the hypertrophic gene program, a collection of genes, the expression of which is reinitiated by the stress of pressure overload. We found, consistent with the reduction in growth in the β-cateninfl/fl/Cre mice treated with tamoxifen, that there was less of an increase in expression of β-MHC and α-skeletal actin (the so-called fetal isoforms of these contractile elements) by semiquantitative RT-PCR (Fig. 3D). Interestingly, deletion of β-catenin had no effect on upregulation of expression of the neurohormonal mediators ANF (Fig. 3D) and BNP (data not shown), consistent with a divergence in mechanisms underlying induction of various components of the hypertrophic gene program.
Tcf/Lef signaling regulates cardiac growth.
These data demonstrate that deletion of β-catenin in cardiomyocytes reduces the hypertrophic response to pressure overload. In addition, the cardiomyocyte width data from the sham-operated animals (Fig. 3C) suggest that β-catenin/Tcf/Lef signaling may also regulate normal growth of the heart. To address this issue and to confirm that β-catenin/Tcf/Lef transactivating activity, rather than effects of loss of β-catenin at the adherens junction, was responsible for growth regulation, we chose to manipulate β-catenin/Tcf/Lef signaling by creating a cardiac-specific transgenic mouse expressing an inhibitory mutant of one of the four Tcf/Lef family members. This strategy has been employed by numerous investigators in multiple model systems, including our work in cardiomyocytes in culture (21), as a means to study the effects of blocking β-catenin/Tcf/Lef-dependent gene expression (9, 24, 29, 32, 41, 57, 58, 61). Based on RT-PCR, Tcf3 and Tcf4 are expressed in the heart and in cardiomyocytes isolated from the heart (Fig. 4D and data not shown). We chose to express an inhibitory mutant of Lef-1 based both on the concept that the four family members are largely interchangeable and redundant (61) and the fact that Lef-1, in contrast to Tcf3 and Tcf4, does not interact with the corepressor C-terminal binding protein (5, 56, 60). Thus, we reasoned that using Lef-1 would help assure that the effects we observed were more likely due to loss of β-catenin/Tcf/Lef transactivating activity than to transcriptional repression by the C-terminal binding protein.
FIG.4.
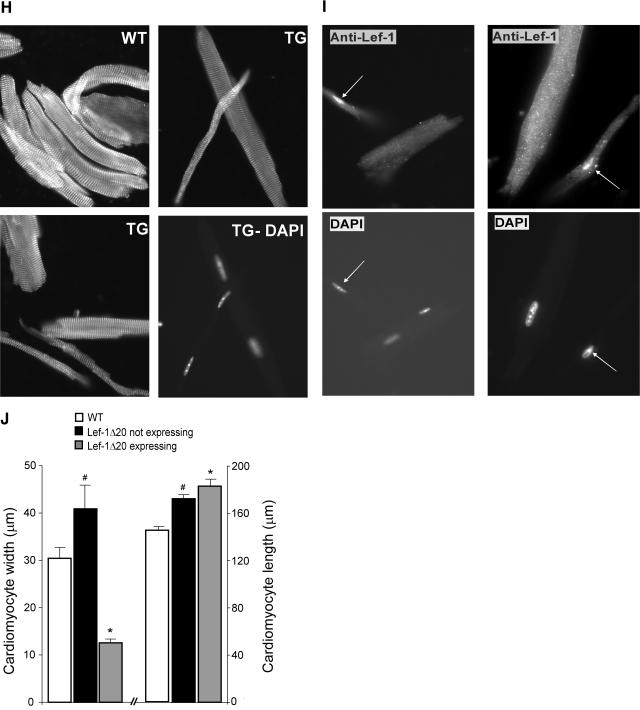
Characterization of the Lef-1Δ20 transgenic line. A. Expression of HA-Lef-1Δ20. Anti-HA immunoblot of lysates from transgenic and wild-type hearts demonstrating expression of the Lef-1Δ20 transgene. B. Neonatal hearts expressing Lef-1Δ20 have reduced cardiac growth. Shown are hearts from a 5-day-old Lef-1Δ20 transgenic mouse (TG) from line 1 and a wild-type (WT) littermate. Note the foreshortened long axis of the ventricle in the TG. C. Cardiomyocyte size is reduced in the Lef-1Δ20 transgenic neonate. Sections of hearts from 5-day-old Lef-1Δ20 transgenic and wild-type mice were stained with FITC-conjugated wheat germ agglutinin to delineate the cardiomyocyte sarcolemma. The transgenic sample is notable for significantly decreased cross-sectional areas of the cardiomyocytes. Cardiomyocyte cross-sectional area is quantified in the lower panel. D. Expression of Lef-1Δ20 and Tcf4 by RT-PCR in TG and WT hearts. E. M-mode echocardiograms of 5-week-old wild-type and Lef-1Δ20 transgenic hearts, in which the transgene has inserted into the X chromosone, showing markedly decreased posterior wall (PW) and anterior wall (AW) thicknesses as well as a dilated ventricle (as evidenced by an increased end-diastolic dimension [EDD]) and reduced contractile function (as evidenced by an increased end-systolic dimension [ESD]). F. Hematoxylin- and eosin (H and E)-stained sagittal section of an X chromosome-inserted Lef-1Δ20 transgenic heart. Note the markedly reduced wall thicknesses and the dilated ventricle. G. Cardiomyocyte size in the Lef-1Δ20 transgenic. H- and E-stained sections of the heart of a mouse from the X chromosome-inserted Lef-1Δ20 transgenic line. Notable are two vastly different populations of cardiomyocytes, one characterized by extremely small myocytes and the other by normal-sized myocytes. Also note the mosaic pattern with areas of hypotrophic myocytes adjacent to areas of normal-sized myocytes. H. Cardiomyocyte size in Lef-1Δ20 transgenic mice. Myocytes isolated from the X-inserted Lef-1Δ20 transgenic mouse versus wild-type littermates were stained with anti-α-actinin antibody. Again, two populations are readily apparent in the transgenic. The lower right panel is a DAPI stain of the cells shown in the upper right panel, identifying nuclei. This demonstrates that the smaller myocyte is not a fragment of a larger normal myocyte. These grossly hypotrophic myocytes were never seen in the wild-type mice. I. Hypotrophic cardiomyocytes express Lef-1Δ20. Cardiomyocytes were stained with anti-Lef-1 antibody (upper panels) or DAPI (lower panels). Arrows identify nuclei of cells expressing Lef-1Δ20. All cells staining positive for Lef-1Δ20 expression were markedly hypotrophic. J. Quantification of cardiomyocyte width and length in the Lef-1Δ20-expressing cardiomyocytes versus those not expressing Lef-1Δ20. Also shown are values for myocytes isolated from wild-type mice. *, P < 0.01 versus cells not expressing Lef-1Δ20 and versus wild type. #, P < 0.05 versus the wild type.
The mutant (Lef-1Δ20) is deleted for the amino-terminal 20 amino acids, a region which contains the primary β-catenin binding domain (13). Thus, Lef-1Δ20 can occupy Tcf/Lef promoter elements but cannot efficiently interact with β-catenin and, thus, is transcriptionally inert (13, 61). When expressed in cardiomyocytes in culture, the construct does function as a dominant negative based on its ability to block activation of a Tcf/Lef reporter in response to α-adrenergic agonists and expression of a stabilized mutant of β-catenin (21; unpublished data).
We generated two lines of Lef-1Δ20 mice (Fig. 4A). In one of the lines (line 1), all transgenics died within a few days of birth. Characterization of 5-day-old neonates from this line revealed that the left ventricles of the transgenics were smaller (Fig. 4B). In addition, when we characterized cardiomyocyte size of the transgenic neonates, this was significantly reduced (by ∼30%) compared to that of wild-type littermates (Fig. 4C).
In the second line, very few transgenic males survived to birth, and none survived for more than 1 to 2 days. However, transgenic females were born in approximately normal numbers (generally 4 to 6 per litter), consistent with the transgene having integrated into the X chromosome. We confirmed expression of the transgene in the heart both by immunoblot (Fig. 4A) and by RT-PCR (Fig. 4D). Echocardiography at 5 weeks of age confirmed that LV posterior wall thickness was markedly reduced (by an average of 22%), compatible with a limited ability of cardiomyocytes from these mice to undergo concentric hypertrophy (Fig. 4E; Table 4). In addition, the ventricles were significantly dilated with depressed contractile function (Fig. 4E; Table 4), a phenotype consistent with a severe dilated cardiomyopathy. This was confirmed with hematoxylin and eosin staining of sagittal sections of hearts from these mice (Fig. 4F).
TABLE 4.
Echocardiographic measurements in the Lef-1Δ20 transgenic micea
| Mouse type | HR (bpm) | EDD (mm) | ESD (mm) | FS (%) | PWT (mm) | LV mass (mg) |
|---|---|---|---|---|---|---|
| Wild type (n = 7) | 505 ± 9 | 3.1 ± 0.1 | 1.8 ± 0.1 | 41.8 ± 1.7 | 0.79 ± .03 | 78.9 ± 6.6 |
| Lef-1Δ20 (n = 5) | 580 ± 22* | 4.1 ± 0.1* | 3.3 ± 0.2* | 20.7 ± 4.4* | 0.62 ± .03* | 88.7 ± 2.3 |
HR, heart rate; EDD, left ventricular end-diastolic dimension; ESD, left ventricular end-systolic dimension; FS, fractional shortening; PWT, posterior wall thickness; bpm, beats per minute. Animals were imaged at 5 weeks of age. *, P < 0.05 versus wild type.
We then examined cardiomyocyte size in hematoxylin- and eosin-stained sections of the hearts. Not surprisingly, given that the transgene had integrated into the X chromosome and given the phenomenon of X chromosome inactivation (8), we found two very distinct populations of cells. One population was dramatically reduced in size, and the other population appeared normal (Fig. 4G). The hypotrophic cells were clustered in a mosaic pattern, consistent with the origins of these clusters from single cells that have undergone X inactivation of the chromosome not containing the transgene (8). We then examined cardiomyocytes isolated from the hearts of Lef-1Δ20 transgenics. Again we found two populations of cells, one that appeared normal and one demonstrating a remarkable inability to concentrically hypertrophy (Fig. 4H and I). Critically, immunocytochemistry confirmed that it was specifically the hypotrophic cardiomyocytes that expressed Lef-1Δ20 (Fig. 4I). Quantification confirmed the marked inability to undergo concentric hypertrophy (Fig. 4J). Of note, the cardiomyocytes that did not express Lef-1Δ20 were significantly larger (by ∼25%) compared to the wild type, likely consistent with reactive hypertrophy in response to increased wall stress in the transgenics (Fig. 4J). Interestingly, cardiomyocyte length was increased in the transgenics, irrespective of whether cells did or did not express Lef-1Δ20, suggesting eccentric hypertrophy was not impaired. In summary, in the same heart with the two populations of cells exposed to identical conditions, the cells expressing Lef-1Δ20 exhibited a profound inability to concentrically hypertrophy. These data confirm that Lef-1Δ20 acts in a cell-autonomous manner to markedly restrict cardiomyocyte growth.
DISCUSSION
In this paper, we demonstrate that β-catenin/Tcf/Lef signaling regulates both physiologic hypertrophy (normal growth) and pathological hypertrophy of the heart. Canonical Wnt signaling has been shown to play a role in axon remodeling in neurons, which, like cardiomyocytes, are terminally differentiated. However, the pathway regulating this diverges from the canonical pathway downstream of GSK-3β and does not require β-catenin or Tcf/Lef (12). Thus, we believe that regulation of growth may be the first function identified in vivo for β-catenin/Tcf/Lef-dependent genes in terminally differentiated cells of the postnatal mammal.
The GSK-3β/β-catenin/Tcf/Lef signaling pathway and cardiomyocyte growth.
GSK-3β is a potent inhibitor of both physiologic and pathological hypertrophic growth in vivo, and inhibition of GSK-3β appears to be necessary for hypertrophic growth to occur (1, 40). The best studied route to inhibition of GSK-3β and subsequent activation of β-catenin signaling is the canonical Wnt pathway. Following Wnt stimulation, GSK-3β is inhibited by the concerted actions of Dvl and Frat1/GBP, though the precise mechanism of inhibition of GSK-3β is not clear (62). This inhibition of GSK-3β allows β-catenin to escape ubiquitination and degradation via the proteasome pathway (35). As a result, cytosolic levels of β-catenin rise, resulting in nuclear translocation and transcriptional activation. We have found that in cardiomyocytes, hypertrophic stimuli (α-adrenergic or endothelin-1 stimulation in vitro or pressure overload in vivo) can also lead to the stabilization of β-catenin, but this occurs via a Wnt-independent mechanism that involves Akt-mediated phosphorylation and inhibition of GSK-3β occurring within the Axin complex (21). Others have more recently confirmed the ability of heterotrimeric G protein-coupled receptors to co-opt Wnt pathway components, including Axin, to signal downstream to β-catenin (10).
β-Catenin is known to play a critical role in cardiac development. It is essential for endothelial-mesenchymal transformation, a process that is required for formation of the endocardial cushions which give rise to the cardiac outflow tract and valves (26, 27, 34). Since this process does not involve β-catenin signaling in cardiomyocytes, not surprisingly the outflow tract and valves were normal in our mice, including the Lef-1Δ20 mouse, based on echocardiogrpahic, Doppler, and postmortem examination (data not shown).
We first studied the APC−/+ mouse, which is heterozygous for the multiple intestinal neoplasia mutation (55). This mutation is in the gene coding for APC. However, in concordance with the findings in colonic carcinoma cells heterozygous for the minus mutation, in which deletion or mutation of the remaining wild-type allele must occur before the cells are transformed (31), the APC−/+ mouse demonstrated only very minimal increases in cytosolic β-catenin in the heart (data not shown). Despite this minimal increase, heart weight/body weight ratios in the APC−/+ mouse were statistically, though very minimally (4%), larger (P < 0.05; data not shown).
Therefore, we turned to more robust models. Studies with the first model, conditional cardiomyocyte-specific deletion of β-catenin, confirmed a role for the pathway in stress-induced hypertrophic growth and in stress-induced expression of at least part of the hypertrophic gene program. Since only 50 to 60% of the cardiomyocytes were completely deleted for β-catenin, it is likely that our data significantly underestimate the contribution of the β-catenin/Tcf/Lef pathway to stress-induced growth.
Determination of cardiomyocyte size in this model also suggested a role for β-catenin/Tcf/Lef signaling in normal growth, despite the fact that the tamoxifen injections were started at 5 weeks of age, a time before which we did not think it was reasonable to begin injections but at which point the majority of heart growth has already occurred. Thus, evaluating the role of the pathway in regulating normal growth required a second model. Given the vast experience of numerous investigators employing inhibitory mutants of Tcf/Lef factors to modulate activity of the pathway in model systems from Drosophila melanogaster to mammalian cells (9, 24, 29, 32, 41, 57, 58, 61), we chose cardiac-specific transgenic expression of a dominant inhibitory mutant of Lef-1. The alternative of employing Tcf3/Tcf4 gene-targeted mice was limited by the early lethality of these models, with Tcf3−/− embryos dying at approximately embryonic day 9.5 (expanded and duplicated axial mesoderm structures [39]) and Tcf4−/− mice dying shortly after birth with a phenotype of complete absence of the stem cell compartment in the crypts of the small intestine (28). Other models were considered, including transgenic expression of inhibitors of Wnt signaling (e.g., secreted frizzled-related proteins or Dickkopf proteins), but these seemed illogical since, as noted, our data suggest that hypertrophic stress-induced stabilization of β-catenin is Wnt-independent (21). Thus, although we realized the caveats that go along with expression of any dominant inhibitory mutant, we thought it was the best option, especially since it would be used to complement data from the conditional knockout.
In the Lef-1Δ20 transgenic mice, there was a dramatic reduction in cardiomyocyte growth. Indeed, we were unable to find examples in the literature of more profound growth retardation than that seen in the Lef-1Δ20 transgenic cardiomyocytes, and we asked why the phenotype was more extreme than that of the β-catenin conditional knockout. When β-catenin is not bound to Tcf/Lef family members, the Groucho/Grg/Transducin-like Enhancer of Split family of repressors interacts with the Tcf/Lef family members ands recruit histone deacetylases, changing Tcf/Lefs from transcriptional activators to repressors (6, 11, 18, 25). The Groucho binding domain is not deleted in the Lef-1Δ20 construct we employed (13). Thus, Lef-1Δ20 not only prevents activation of β-catenin/Tcf/Lef-dependent genes but also allows Groucho-mediated repression of these genes. This could be expected to produce a more complete inhibition of β-catenin/Tcf/Lef-dependent gene expression and thus a more profound phenotype than simple loss of β-catenin. The second reason is that deletion of β-catenin in the conditional knockout did not occur until 5 to 6 weeks of age, after the phase of rapid growth of the heart is completed. In contrast, the Lef-1Δ20 transgene is expressed at birth, and thus β-catenin/Tcf/Lef-dependent gene expression is suppressed during the first few weeks of life, the period of most rapid growth of the heart. This was likely the major contributor to the more profound cardiomyocyte phenotype of the transgenic mouse. Of note, low levels of expression do occur during development as well, as evidenced by the marked growth retardation and early postnatal mortality in the first transgenic line, in which the Lef-1Δ20 transgene was expressed in all cardiomyocytes.
Although the cardiomyocyte growth phenotypes of the β-catenin conditional knockout and the Lef-1Δ20 transgenic line were concordant, there were important differences between the two models. First, the Lef-1Δ20 transgenic line in which the transgene inserted into the X chromosome had markedly depressed contractile function. This is likely due to the very limited ability of the islands of severely hypotrophic cardiomyocytes (Fig. 4G) to generate much force during contraction. In addition, wall stress, a measure of the load placed on the heart during contraction, is inversely proportional to the square of the wall thickness. Since wall thickness was ∼20% less in the Lef-1Δ20 transgenic line, increased wall stress can be expected to have contributed to the impaired contractile function. The early postnatal lethality in the first transgenic line, in which all cardiomyocytes are markedly reduced in size, probably arose from contractile failure.
Another finding of note was that total LV mass was maintained in the X-integrated transgenic line, despite the markedly reduced size of roughly half of the cardiomyocytes. This was not explained by increased fibrosis in the transgenic line (data not shown). Rather, it appears that the myocytes not expressing Lef-1Δ20 hypertrophied, presumably in an attempt to compensate for the depressed contractile function, since they were on average ∼30% larger in width than cardiomyocytes isolated from wild-type mice.
Finally, we were surprised that complete loss of β-catenin within the adherens junction did not have any apparent adverse consequences for the heart. We have now monitored the mice out to 7 months of age, and by echocardiography there is no obvious alteration in LV structure or function. It is possible that increases in γ-catenin may have substituted for the loss of β-catenin (37), although in the short term we saw no clear evidence of this.
In summary, β-catenin/Tcf/Lef signaling is central to both the physiologic and pathological hypertrophic growth of cardiomyocytes. Thus, it appears that β-catenin/Tcf/Lef signaling is not only a regulator of proliferation versus differentiation in cycling cells but is also capable of regulating growth of noncycling, terminally differentiated cells.
Acknowledgments
We thank Elaine Fuchs for providing anti-Lef-1 antibody, Paul Hamel for providing the Lef-1Δ20 construct and helpful advice, and Terry Hyslop for assistance with statistical analyses.
This work was supported by NIH grants HL61688 and HL67371 to T.F.
REFERENCES
- 1.Antos, C. L., T. A. McKinsey, N. Frey, W. Kutschke, J. McAnally, J. M. Shelton, J. A. Richardson, J. A. Hill, and E. N. Olson. 2002. Activated glycogen synthase kinase-3β suppresses cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 99:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badorff, C., H. Ruetten, S. Mueller, M. Stahmer, D. Gehring, F. Jung, C. Ihling, A. M. Zeiher, and S. Dimmeler. 2002. Fas receptor signaling inhibits glycogen synthase kinase-3β and induces cardiac hypertrophy following pressure overload. J. Clin. Investig. 109:373-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baek, S. H., C. Kioussi, P. Briata, D. Wang, H. D. Nguyen, K. A. Ohgi, C. K. Glass, A. Wynshaw-Boris, D. W. Rose, and M. G. Rosenfeld. 2003. Regulated subset of G1 growth control genes in response to derepression by the Wnt pathway. Proc. Natl. Acad. Sci. USA 100:3245-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bienz, M. 2005. Beta-catenin: a pivot between cell adhesion and Wnt signaling. Curr. Biol. 15:R64-R67. [DOI] [PubMed] [Google Scholar]
- 5.Brannon, M., J. D. Brown, R. Bates, D. Kimelman, and R. T. Moon. 1999. XCtBP is a XTcf-3 co-repressor with roles throughout Xenopus development. Development 126:3159-3170. [DOI] [PubMed] [Google Scholar]
- 6.Brantjes, H., J. Roose, M. van de Wetering, and H. Clevers. 2001. All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res. 29:1410-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brault, V., R. Moore, S. Kutsch, M. Ishibashi, D. H. Rowitch, A. P. McMahon, L. Sommer, O. Boussadia, and R. Kemler. 2001. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128:1253-1264. [DOI] [PubMed] [Google Scholar]
- 8.Brown, C. J., and W. P. Robinson. 2000. The causes and consequences of random and non-random X chromosome inactivation in humans. Clin. Genet. 58:353-363. [DOI] [PubMed] [Google Scholar]
- 9.Cadigan, K. M., A. D. Jou, and R. Nusse. 2002. Wingless blocks bristle formation and morphogenetic furrow progression in the eye through repression of Daughterless. Development 129:3393-3402. [DOI] [PubMed] [Google Scholar]
- 10.Castellone, M. D., H. Teramoto, B. O. Williams, K. M. Druey, and J. S. Gutkind. 2005. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science 310:1504-1510. [DOI] [PubMed] [Google Scholar]
- 11.Chen, G., and A. J. Courey. 2000. Groucho/TLE family proteins and transcriptional repression. Gene 249:1-16. [DOI] [PubMed] [Google Scholar]
- 12.Ciani, L., and P. C. Salinas. 2005. Wnts in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 6:351-362. [DOI] [PubMed] [Google Scholar]
- 13.Daniels, D. L., and W. I. Weis. 2005. β-Catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol. 12:364-371. [DOI] [PubMed] [Google Scholar]
- 14.Dorn, G. W., and T. Force. 2005. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Investig. 115:527-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley, A. C., and M. Mercola. 2004. Heart induction: embryology to cardiomyocyte regeneration. Trends Cardiovasc. Med. 14:121-125. [DOI] [PubMed] [Google Scholar]
- 16.Frame, S., and P. Cohen. 2001. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frey, N., H. A. Katus, E. N. Olson, and J. A. Hill. 2004. Hypertrophy of the heart: a new therapeutic target. Circulation 109:1580-1589. [DOI] [PubMed] [Google Scholar]
- 18.Gasperowicz, M., and F. Otto. 2005. Mammalian Groucho homologs: redundancy or specificity? J. Cell Biochem. 95:670-687. [DOI] [PubMed] [Google Scholar]
- 19.Haegel, H., L. Larue, M. Ohsugi, L. Federov, K. Herrenknecht, and R. Kemler. 1995. Lack of β-catenin affects mouse development at gastrulation. Development 121:3529-3537. [DOI] [PubMed] [Google Scholar]
- 20.Haq, S., G. Choukroun, Z. B. Kang, K.-H. Lee, H. Ranu, T. Matsui, A. Rosenzweig, A. Alessandrini, J. D. Molkentin, J. Woodgett, R. Hajjar, and T. Force. 2000. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J. Cell Biol. 151:117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haq, S., A. Michael, M. Andreucci, K. Bhattacharya, P. Dotto, B. Walters, J. R. Woodgett, H. Kilter, and T. Force. 2003. Stabilization of β-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc. Natl. Acad. Sci. USA 100:4610-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardt, S. E., H. Tomita, H. A. Katus, and J. Sadoshima. 2004. Phosphorylation of eukaryotic translation initiation factor 2Bɛ by glycogen synthase kinase-3β regulates β-adrenergic cardiac myocytes hypertrophy. Circ. Res. 94:926-935. [DOI] [PubMed] [Google Scholar]
- 23.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-Myc as a target of the APC pathway. Science 281:1509-1512. [DOI] [PubMed] [Google Scholar]
- 24.Hovanes, K., T. W. Li, J. E. Munguia, T. Truong, T. Milovanovic, J. Lawrence-Marsh, R. F. Holcombe, and M. L. Waterman. 2001. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat. Genet. 28:53-57. [DOI] [PubMed] [Google Scholar]
- 25.Hurlstone, A., and H. Clevers. 2002. T-cell factors: turn-ons and turn-offs. EMBO J. 21:2303-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurlstone, A., A. P. Haramis, E. Wienholds, H. Begthel, J. Korving, F. Van Eeden, E. Cuppen, D. Zivkovic, R. H. Plasterk, and H. Clevers. 2003. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature 425:633-637. [DOI] [PubMed] [Google Scholar]
- 27.Kioussi, C., P. Briata, S. H. Baek, D. W. Rose, N. S. Hamblet, T. Herman, K. A. Ohgi, C. Lin, A. Gleiberman, J. Wang, V. Brault, P. Ruiz-Lozano, H. D. Nguyen, R. Kemler, C. K. Glass, A. Wynshaw-Boris, and M. G. Rosenfeld. 2002. Identification of a Wnt/Dvl/β-catenin-Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111:673-685. [DOI] [PubMed] [Google Scholar]
- 28.Korinek, V., N. Barker, K. Willert, M. Molenaar, J. Roose, G. Wagenaar, M. Markman, W. Lamers, O. Destree, and H. Clevers. 1998. Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol. Cell. Biol. 18:1248-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korswagen, H. C., M. A. Herman, and H. Clevers. 2000. Distinct beta-catenins mediate adhesion and signalling functions in C. elegans. Nature 406:527-532. [DOI] [PubMed] [Google Scholar]
- 30.Kostetskii, I., J. Li, Y. Xiong, R. Zhou, V. A. Ferrari, V. V. Patel, J. D. Molkentin, and G. L. Radice. 2005. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ. Res. 96:346-354. [DOI] [PubMed] [Google Scholar]
- 31.Lengauer, C., K. W. Kinzler, and B. Vogelstein. 1998. Genetic instabilities in human cancers. Nature 396:643-649. [DOI] [PubMed] [Google Scholar]
- 32.Li, B., D. R. Mackay, Q. Dai, T. W. Li, M. Nair, M. Fallahi, C. P. Schonbaum, J. Fantes, A. P. Mahowald, M. L. Waterman, E. Fuchs, and X. Dai. 2002. The LEF1/beta-catenin complex activates movo1, a mouse homolog of Drosophila ovo required for epidermal appendage differentiation. Proc. Natl. Acad. Sci. USA 99:6064-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lickert, H., S. Kutsch, B. Kanzler, K. Tamai, M. M. Taketo, and R. Kemler. 2002. Formation of multiple hearts in mice following deletion of β-catenin in the embryonic endoderm. Dev. Cell 3:171-181. [DOI] [PubMed] [Google Scholar]
- 34.Liebner, S., A. Cattelino, R. Gallini, N. Rudini, M. Iurlaro, S. Piccolo, and E. Dejana. 2004. β-Catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol. 166:359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, C., Y. Li, M. V. Semenov, C. Han, G.-H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 36.Lupu, F., J. D. Terwilliger, K. Lee, G. V. Segre, and A. Efstratiadis. 2001. Roles of growth hormone and IGF-1 in mouse postnatal growth. Dev. Biol. 229:141-162. [DOI] [PubMed] [Google Scholar]
- 37.Maeda, O., N. Usami, M. Kondo, M. Takahashi, H. Goto, K. Shimokata, K. Kusugami, and Y. Sekido. 2004. Plakoglobin (γ-catenin) has TCF/LEF family-dependent transcriptional activity in β-catenin-deficient cell line. Oncogene 23:964-972. [DOI] [PubMed] [Google Scholar]
- 38.McMullen, J. R., T. Shioi, L. Zhang, O. Tarnavski, M. C. Sherwood, P. M. Kang, and S. Izumo. 2003. Phosphoinositide 3-kinase (p110α) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 100:12355-12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merrill, B. J., H. A. Pasolli, L. Polak, M. Rendl, M. J. Garcia-Garcia, K. V. Anderson, and E. Fuchs. 2004. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development 131:263-274. [DOI] [PubMed] [Google Scholar]
- 40.Michael, A., S. Haq, X. Chen, E. Hsich, L. Cui, B. Walters, Z. Shao, K. Bhattacharya, H. Kilter, G. S. Huggins, M. Andreucci, M. Periasamy, R. N. Solomon, R. Liao, R. D. Patten, J. D. Molkentin, and T. Force. 2004. Glycogen synthase kinase-3β regulates growth, calcium homeostasis, and diastolic function in the heart. J. Biol. Chem. 279:21383-21393. [DOI] [PubMed] [Google Scholar]
- 41.Molenaar, M., M. van de Wetering, M. Oosterwegel, J. Peterson-Maduro, S. Godsave, V. Korinek, J. Roose, O. Destree, and H. Clevers. 1996. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86:391-399. [DOI] [PubMed] [Google Scholar]
- 42.Morisco, C., K. Seta, S. E. Hardt, Y. Lee, S. F. Vatner, and J. Sadoshima. 2001. Glycogen synthase kinase 3β regulates GATA4 in cardiac myocytes. J. Biol. Chem. 276:28586-28597. [DOI] [PubMed] [Google Scholar]
- 43.Morisco, C., D. Zebrowski, G. Condorelli, P. Tsichlis, S. F. Vatner, and J. Sadoshima. 2000. The Akt-glycogen synthase kinase 3β pathway regulates transcription of atrial natriuretic factor induced by β-adrenergic receptor stimulation in cardiac myocytes. J. Biol. Chem. 275:14466-14475. [DOI] [PubMed] [Google Scholar]
- 44.Morita, H., J. Seidman, and C. E. Seidman. 2005. Genetic causes of human heart failure. J. Clin. Investig. 115:518-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Most, P., S. T. Pleger, M. Volkers, B. Heidt, M. Boerries, D. Weichenhan, E. Loffler, P. M. Janssen, A. D. Eckhart, J. Martini, M. L. Williams, H. A. Katus, A. Remppis, and W. J. Koch. 2004. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J. Clin. Investig. 114:1550-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson, W. J., and R. Nusse. 2004. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303:483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oudit, G. Y., H. Sun, B. Kerfant, M. Crackower, J. M. Penninger, and P. H. Backx. 2004. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J. Mol. Cell Cardiol. 37:449-471. [DOI] [PubMed] [Google Scholar]
- 48.Park, J., S. W. Kim, J. P. Lyons, H. Ji, T. T. Nguyen, K. W. Cho, M. C. Barton, T. Deroo, K. Vleminckx, and P. D. McCrea. 2005. Kaiso/p120-catenin and Tcf/β-catenin complexes coordinately regulate canonical Wnt gene targets. Dev. Cell 8:843-854. [DOI] [PubMed] [Google Scholar]
- 49.Pasumarthi, K. B. S., H. Nakajima, H. O. Nakajima, M. H. Soonpaa, and L. J. Field. 2005. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ. Res. 96:110-118. [DOI] [PubMed] [Google Scholar]
- 50.Patrucco, E., A. Notte, L. Barberis, G. Selvetella, A. Maffei, M. Brancaccio, S. Marengo, G. Russo, O. Azzolino, S. D. Rybalkin, L. Silengo, F. Altruda, R. Wetzker, M. P. Wyman, G. Lembo, and E. Hirsch. 2004. PI3Kγ modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118:375-387. [DOI] [PubMed] [Google Scholar]
- 51.Petrich, B. G., J. D. Molkentin, and Y. Wang. 2003. Temporal activation of c-Jun N-terminal kinase in adult transgenic heart via cre-loxP-mediated DNA recombination. FASEB J. 17:749-751. [DOI] [PubMed] [Google Scholar]
- 52.Polakis, P. 2001. More than one way to skin a catenin. Cell 105:563-566. [DOI] [PubMed] [Google Scholar]
- 53.Rockman, H. A., R. S. Ross, A. N. Harris, K. U. Knowlton, M. E. Steinhelper, L. J. Field, J. Ross, and K. R. Chien. 1991. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo model of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 88:8277-8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohal, D. S., M. Nghiem, M. A. Crackower, S. A. Witt, T. F. Kimball, K. M. Tymitz, J. M. Penninger, and J. D. Molkentin. 2001. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ. Res. 89:20-25. [DOI] [PubMed] [Google Scholar]
- 55.Su, L. K., K. W. Kinzler, B. Vogelstein, A. C. Preisinger, A. R. Moser, C. Luongo, K. A. Gould, and W. F. Dove. 1992. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256:668-670. [DOI] [PubMed] [Google Scholar]
- 56.Valenta, T., J. Lukas, and V. Korinek. 2003. HMG box transcription factor TCF4's interaction with CtBP1 controls the expression of the Wnt target Axin/Conductin in human embryonic kidney cells. Nucleic Acids Res. 31:2369-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van de Wetering, M., R. Cavallo, D. Dooijes, M. van Beest, J. van Es, J. Loureiro, A. Ypma, D. Hursh, T. Jones, A. Bejsovec, M. Peifer, M. Mortin, and H. Clevers. 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88:789-799. [DOI] [PubMed] [Google Scholar]
- 58.van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A. P. Haramis, M. Tjon-Pon-Fong, P. Moerer, M. van den Born, G. Soete, S. Pals, M. Eilers, R. Medema, and H. Clevers. 2002. The beta-catenin/Tcf4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241-250. [DOI] [PubMed] [Google Scholar]
- 59.van Eickels, M., R. D. Patten, M. J. Aronovitz, A. Alsheikh-Ali, K. Gostyla, F. Celestin, C. Grohe, M. E. Mendelsohn, and R. H. Karas. 2003. 17-Beta-estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J. Am. Coll. Cardiol. 41:2084-2092. [DOI] [PubMed] [Google Scholar]
- 60.van Noort, M., and H. Clevers. 2002. TCF transcription factors, mediators of Wnt-signaling in development and cancer. Dev. Biol. 244:1-8. [DOI] [PubMed] [Google Scholar]
- 61.Waterman, M. L. 2004. Lymphoid enhancer factor/T cell factor expression in colorectal cancer. Cancer Metastasis Rev. 23:41-52. [DOI] [PubMed] [Google Scholar]
- 62.Wharton, K. A. 2003. Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253:1-17. [DOI] [PubMed] [Google Scholar]
- 63.Wilkins, B. J., Y. S. Dai, O. F. Bueno, S. A. Parsons, J. Xu, D. M. Plank, F. Jones, T. R. Kimball, and J. D. Molkentin. 2004. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 94:110-118. [DOI] [PubMed] [Google Scholar]
- 64.Wilkins, B. J., L. J. De Windt, O. F. Bueno, J. C. Braz, B. J. Glascock, T. F. Kimble, and J. D. Molkentin. 2002. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol. Cell. Biol. 22:7603-7613. [DOI] [PMC free article] [PubMed] [Google Scholar]