Abstract
Src kinase is a crucial mediator of adhesion-related signaling and motility. Src binds to focal adhesion kinase (FAK) through its SH2 domain and subsequently activates it for phosphorylation of downstream substrates. In addition to this binding function, data suggested that the SH2 domain might also perform an important role in targeting Src to focal adhesions (FAs) to enable further substrate phosphorylations. To examine this, we engineered an R175L mutation in cSrc to prevent the interaction with FAK pY397. This constitutively open Src kinase mediated up-regulated substrate phosphorylation in SYF cells but was unable to promote malignant transformation. Significantly, SrcR175L cells also had a profound motility defect and an impaired FA generation capacity. Importantly, we were able to recapitulate wild-type motile behavior and FA formation by directing the kinase to FAs, clearly implicating the SH2 domain in recruitment to FAK and indicating that this targeting capacity, and not simply Src-FAK scaffolding, was critical for normal Src function.
Src family kinases (SFKs) are a group of ubiquitously expressed nonreceptor tyrosine kinases that are essential for integrin-mediated cell motility and adhesion signaling (17). SFKs contain at least two distinct protein-protein interaction sequences, the eponymously named Src homology 2 and 3 (SH2 and SH3) domains. First identified in vSrc, SH2 domains have since been found in an array of proteins having catalytic, adapter, or regulatory roles.
The SH2 domain is an ∼100-amino-acid module that binds to particular phosphorylated tyrosine residues. SH2 binding specificity is determined by flanking sequences C terminal to the target phosphotyrosine, for Src usually the YEEI motif (16). In SFKs, the SH2 domain functions in both intra- and intermolecular binding. The intramolecular association is with its C-terminal phosphotyrosine, and this interaction maintains the enzyme in a closed, catalytically inactive conformation while also blocking intermolecular SH2 interactions and sequestering the SH3 domain (3, 39). Dephosphorylation of Src's C-terminal tail by tyrosine phosphatases (30, 32, 40) opens and activates the kinase while also allowing the SH2 domain to bind to its downstream substrates.
The determination of unambiguous biological functions for SFKs was impeded for a number of years by redundancy among family members. The development of fibroblasts with the three ubiquitously expressed enzymes, Src, Yes, and Fyn, deleted (SYF cells), identified SFKs as crucial mediators of adhesion signaling (17). In adhesion, an important Src substrate is another nonreceptor tyrosine kinase, focal adhesion kinase (FAK) (22). FAK localizes to structures called focal adhesions (FAs) that form the link between the extracellular matrix (ECM) and the actin cytoskeleton. FAs are plaques containing accumulations of integrins, the cell's ECM receptor, and cytoplasmic proteins that mediate the connection between integrins and actin and regulate important biological functions such as apoptosis and hemostasis (13). Significantly, integrins and FAs also modulate cell migration and fibroblasts null for either SFKs or FAK have a profound defect in motility (17, 28).
The critical phosphotyrosine in FAK that binds to Src's SH2 domain is pY397, and dephosphorylation or mutation of this residue to phenylalanine dramatically reduces Src-FAK interaction (7). Experiments in SYF cells reexpressing Src mutants with defective kinase domains, but intact SH2 domains, indicated that although FAK Y397 was phosphorylated normally, phosphorylation at FAK's catalytic site, the activation loop residues Y576/577, was deficient, as was motility (4, 17). This result implicated wild-type Src in a two-step FAK activation process: first, the Src SH2/FAK pY397 interaction occurred, and second, Src performed its catalytic function to fully activate FAK, enabling cell migration.
However, this model of Src activity, where its SH2 domain performs primarily a binding function for activation of FAK, did not account for the cellular localization of different Src constructs. In adherent fibroblasts, wild-type Src does not localize to FAs and is probably recruited only transiently to FAK at the leading edge during FA assembly (4, 37). However, Src deletion mutants with an accessible SH2 domain do localize stably to FAs (9, 15), suggesting that the SH2 domain may play a role in the temporary recruitment of wild-type Src during FA formation.
In addition to FA localization, there were a number of other lines of evidence to suggest that the substrate binding role was not the sole function of Src's SH2 domain. First, Src-FAK interactions mediated primarily by the SH3 domain supported normal FAK phosphotyrosine levels, indicating that SH2 domain binding was not essential for Src's scaffold function (4). Second, small sequence differences among SH2 family members confer distinct binding specificities, suggesting that SH2 modules have a targeting capacity (16). Third, when FAK was mutated at the specific tyrosine (Y397F) that binds to Src's SH2 domain, Src was unable to phosphorylate FAK at its kinase site (Y576/577) and migration was suppressed (28). This result indicated that despite evidence that Src could perform its substrate binding function in SH2 mutants, probably through SH3-FAK binding, the Src SH2-FAK pY397 interaction could not be entirely replaced by the SH3 domain.
Finally, it had been reported that alteration of the conserved arginine 175 in the SH2 domain to an acidic (E) or uncharged (L) residue attenuated the transforming capability of permanently active kinases (vSrc or SrcY527F) (11, 31). This result suggested that, in transformation, inappropriate targeting of the kinase was as important as up-regulation of catalytic activity. Given the critical role of SFKs in migration, we wondered whether this inhibition of transformation with SH2 mutants may have a correlation with cell motility.
To examine the targeting function of Src's SH2 domain in migration, we wanted to limit the ability of the kinase to associate with FAK. Within the group of SH2 domain-containing proteins, the arginine residue at position 175 (corresponding to vSrc numbering) when replaced with leucine results in a loss of target peptide binding (1, 21). Given the importance of this residue in SH2 substrate association and in transformation, we generated an R175L mutation in full-length cSrc and examined its effects in SFK-null fibroblasts. Unexpectedly, SYF cells expressing this construct were profoundly defective in migration despite high kinase activity. Interestingly, we were subsequently able to restore wild-type motility by targeting the kinase to focal adhesions. This result suggested that an active kinase alone was insufficient to promote normal cell migration and that in wild-type Src the SH2 domain performed a specific targeting function, which is important in motility and transformation.
MATERIALS AND METHODS
Generation of Src mutants and infection of SYF cells.
Standard recombinant DNA techniques were used to make all Src and FAK constructs in the retroviral expression vector pLHCX (Clontech). Restriction enzymes were from Promega. Taq DNA polymerase, used for generating DNA fragments, was from Sigma, and PfuTurbo DNA polymerase and DpnI restriction enzyme, used for degenerate oligonucleotide-mediated site-directed mutagenesis, were from Stratagene. Constructs were sequenced to confirm their fidelity.
Wild-type chicken cSrc and myc-tagged Src251 in the retroviral vector were a gift from Pam Schwarzberg (National Institutes of Health). Point mutations were introduced into cSrc and Src251 to generate R175L and into cSrc to generate Y527F. For SrcR175L-FAT, the terminal stop codon of the SrcR175L was ablated and the human FAK focal adhesion targeting (FAT) domain (residues 840 to 1051) was amplified and ligated to the 3′ end of the coding region. The resulting chimera had a 3-amino-acid linker (IDM) between the Src C terminus and the FAT sequence. Green fluorescent protein (GFP)-FAK was generated as described previously (8) and subcloned into pLHCX. The Y397F point mutation was introduced into GFP-FAK by site-directed mutagenesis.
Retroviral infections were performed as described previously (25). Briefly, viral packaging cells were transfected with the Src or FAK constructs by calcium phosphate precipitation and incubated at 37°C. Twenty-four hours later the supernatant was filtered (0.45 μm), and an 0.25 volume was added to subconfluent 3T3 cells treated with 5 μg/ml Polybrene and incubated for 24 to 48 h. Cells were cultured in selection medium (300 μg/ml hygromycin), and clones were screened by Western blotting and immunofluorescence with anti-avian Src monoclonal antibody (MAb) (EC10; Upstate Biotechnology) or anti-myc MAb (9E10; Santa Cruz Biotechnology).
Adhesion-dependent tyrosine phosphorylation of FA proteins.
Cells were trypsinized and placed in suspension in Dulbecco modified Eagle medium containing 2% bovine serum albumin for 1 h and then plated on coverslips coated with 1 μg fibronectin for 1 h. Cells were lysed in buffer (50 mM HEPES, 150 mM NaCl, 1% Triton X-100, 1 mM CaCl2, 1 mM MgCl2, 2% glycerol, 10 mM NaF, 1 mM pyrophosphate, and 2 mM Na3VO4, pH 7.4) supplemented with mammalian extract protease inhibitor cocktail (Sigma), and lysates were used directly (∼25 μg) for FAK pY397 determination or immunoprecipitated with Gammabind G (Amersham) and either Cas (polyclonal antibody [PAb]; Santa Cruz Biotechnology), FAK (MAb 2A7; Upstate Biotechnology), or paxillin (MAb; Transduction Laboratories) antibody for 2 h at 4°C. Equal amounts of protein were electrophoresed in 7.5% gels and transferred to nitrocellulose membranes (GE Osmonics, Minnetonka, MN). For FAK phosphotyrosine blot assays, membranes were probed with a primary PAb specific for pY397 or pY576 of FAK (Biosource) followed by protein A-peroxidase (Transduction Laboratories) and subsequently reprobed with FAK-specific antibody (Transduction Laboratories) followed by anti-mouse antibody-peroxidase (Chemicon). For Cas and paxillin phosphotyrosine blots, membranes were probed with horseradish peroxidase-conjugated anti-pY antibody (RC20; Transduction Laboratories) and reprobed with Cas (Transduction Laboratories) or paxillin MAbs. Membranes were developed using enhanced chemiluminescence (ECL; Pierce).
Immunofluorescence microscopy.
For immunofluorescence microscopy, cells were plated on fibronectin-coated coverslips in Dulbecco modified Eagle medium-2% bovine serum albumin and incubated at 37°C for various times before fixation (phosphate-buffered saline, 4% paraformaldehyde for 10 min) and permeabilization (phosphate-buffered saline, 0.5% Triton X-100 for 5 min). Cells were stained with rhodamine phalloidin or antibodies to either vinculin (Sigma), FAK pY397, α5 integrin (2), or chicken Src. Secondary antibodies were from Molecular Probes. Epifluorescence microscopy was performed with a Nikon Optiphot microscope using a 60× plan apo objective and filter cubes optimized for fluorescein/Alexa 488 or rhodamine fluorescence (Nikon). Images were obtained with a MicroMax cooled charge-coupled device camera (Kodak KAF 1400 chip; Princeton Instruments, Trenton, NJ) using Metamorph software (Universal Imaging Corp., West Chester, PA). Total internal reflection fluorescence (TIRF) microscopy was performed as described elsewhere (8). Briefly, samples were observed with a 60× apo objective (numerical aperture, 1.45) on a Nikon TE2000-U microscope equipped with a TIRF illuminator and fiber optic-coupled laser illumination with two separate laser lines (Ar ion, 488 nm, and HeNe, 543 nm) and filter cubes optimized for fluorescein/Alexa 488/GFP and rhodamine/red fluorescent protein (Chroma Technology, Rockingham, VT).
Cell migration analysis.
For wound healing assays, cells were plated onto fibronectin-coated coverslips and grown to confluence and the monolayer was injured using an acrylic comb with 0.5-mm-wide teeth. Cells were allowed to migrate at 37°C in mitomycin C-containing medium, fixed in 4% paraformaldehyde at the indicated times, and imaged by phase-contrast microscopy. For random migration, cells were sparsely plated (1.0 × 103 to 1.5 × 103 cells/cm2) onto fibronectin-coated glass-bottom culture dishes (MatTek, Ashland, MA) and allowed to spread for 1 h. Dishes were then placed on a heated stage (35°C), and nuclear positions were tracked from phase images captured every 10 to 20 min over 4 to 6 h.
Analysis of focal adhesion regeneration.
Methods for disassembly and regeneration of FAs after nocodazole treatment have been described previously (8). Briefly, cells were plated onto fibronectin-coated glass coverslips and treated with 10 μM nocodazole (Sigma-Aldrich) for 4 h to depolymerize microtubules. Nocodazole was replaced with serum-free medium, and at the indicated times, cells were either fixed and vinculin stained for immunofluorescence or lysed and processed for FAK pY397 determination by Western blotting. Intensity analysis of Western blots was performed with ImageJ software (National Institutes of Health).
Determination of anchorage-independent growth.
Cells expressing cSrc or Src mutants were plated in triplicate at 5 × 104, 1 × 105, and 2 × 105 cells per ml as described previously (14). After incubation for 2 weeks at 37°C, colonies larger than 0.25 mm were scored by counting 10 low-power fields.
RESULTS
SH2 Arg175 is necessary for recruitment to FAs and FAK Y397 phosphorylation.
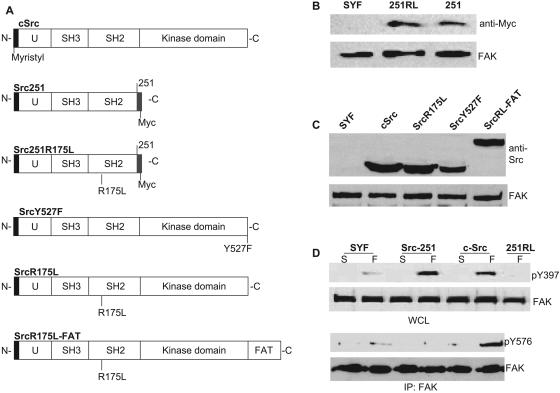
Previous work had described the localization to FAs of a mutant of cSrc having its C terminus from residue 251 deleted (15). The truncated Src (Src251, Fig. 1A) possessed the short unique sequence important for membrane targeting along with its membrane-anchoring myristyl group, as well as two protein interaction modules, the SH2 and SH3 domains (Fig. 1A) (24, 26). This construct was missing the kinase domain, along with the autoinhibitory tyrosine 527, which, when phosphorylated, forms an intramolecular binding partner with the SH2 domain to keep the kinase in an inactive conformation (Fig. 1A) (39). We initially verified that when expressed in SYF cells, Src251, with its SH2 domain constitutively accessible for intermolecular interactions, localized to FAs (Fig. 1B and 2A). This FA localization likely occurred through a stable interaction with FAK pY397, thus protecting the residue from dephosphorylation when adherent (Fig. 1D). However, Src251-expressing cells were nonmotile (data not shown) and FAK activity, measured by Y576 phosphorylation (Fig. 1D), was reduced. These results were consistent with findings that full-length kinase-dead Src (K295R) mediated normal FAK Y397 phosphorylation but had defective pY576 levels and motility (4, 15). The data from these mutated kinases indicated that Src's protein interaction modules, the SH2 and SH3 domains, performed at least two distinct roles: first, to direct Src transiently to FAs—a targeting function—and subsequently, to mediate the association with FAK required for Src to activate it—a scaffolding function.
FIG. 1.
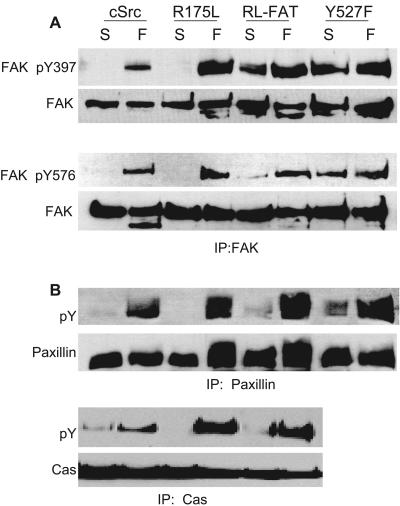
Expression of Src constructs in SFK-null fibroblasts (SYF cells). (A) Representations of cSrc and mutants stably expressed in SYF cells. (B and C) Western blots indicating expression levels of exogenous proteins in SYF cells infected with myc-tagged Src251 (251) and Src251R175L (251RL) (B), as well as cSrc, SrcR175L, SrcY527F, and SrcR175L-FAT (SrcRL-FAT) (C). (D) Western blots of FAK phosphotyrosine levels in SFK-null fibroblasts (SYF) reconstituted with full-length (cSrc) and truncated constructs Src251 and Src251R175L (251RL). Cells were kept in suspension for 1 h (S), and levels of FAK pY397, in whole-cell lysates (WCL), and pY576, in FAK immunoprecipitates (IP), were compared to those for cells spread on fibronectin (F). The membrane was reprobed with anti-FAK MAb.
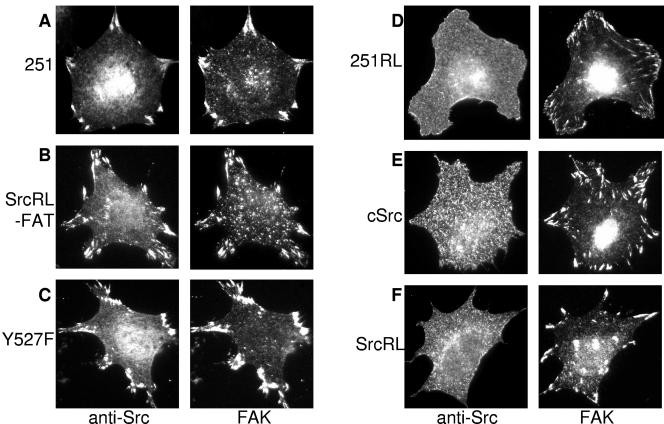
FIG. 2.
Localization of Src constructs reexpressed in SYF cells. The figure shows the cellular localization of chicken Src constructs stably expressed in SYF cells recognized by chicken-specific anti-Src antibody in fully spread cells on fibronectin. FAs were revealed by counterstaining with a FAK pY397-specific PAb. Src251 (A) (251), SrcR175L-FAT (B) (SrcRL-FAT), and SrcY527F (C) (Y527F) localized to FAs upon adhesion, whereas Src251R175L (D) (251RL), cSrc (E), and SrcR175L (F) (SrcRL) did not localize to FAs and were stained diffusely at the cell membrane.
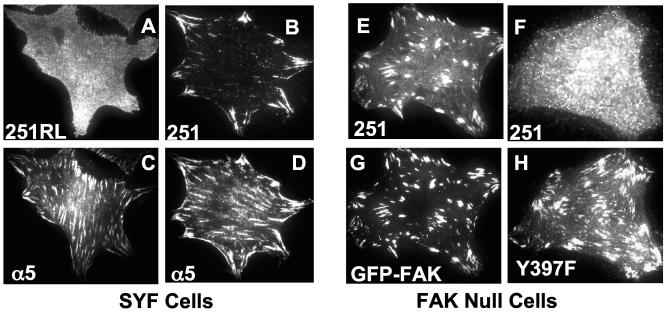
While the scaffolding function had been extensively examined, the ability of cSrc to localize to FAs was less well understood, particularly given that in adherent cells, cSrc was not stably recruited to adhesion plaques (4) (Fig. 2E). SH2-mediated protein-protein interactions are relatively high-affinity associations. Among FA proteins, the Src SH2-FAK connection is certainly more robust than the SrcSH3-FAK connection (23). Consequently, we wanted to analyze the role that the SH2 domain played in Src targeting to FAs. Therefore, we engineered an R-L mutation at residue 175 in the SH2 domain of Src251 and expressed the construct in SYF cells (Fig. 1A and 1B). Others had determined that in vitro this mutation prevented full-length Src from interacting with the pY397 residue of FAK (1, 21). In vivo, using cells expressing other SFKs, it had been reported that Src251R175L localized to FAs, although substantially less well than the wild-type Src251 (15). Furthermore, this localization occurred upon significant overexpression of the construct (15), suggesting that while SH3 domain interactions contributed to FA recruitment, their role was minor compared to SH2 domain associations. Interestingly, in SYF cells, the R175L mutation in Src251 resulted in a reduction of adhesion-dependent FAK Y397 phosphorylation (Fig. 1D). Additionally, in the absence of other SFKs, Src251R175L was unable to localize to FAs when examined by epifluorescent microscopy (Fig. 2D). Because this result contradicted previous findings in other cell types, we used a more sensitive technique to confirm our data. TIRF microscopy permits observation of only the bottom ∼100 nm of the cell (33). We used this technique because it enabled examination of the specific region where integrins interact with the ECM and FAs form and, due to its inherent high signal-to-noise ratio, it is very sensitive for FA localization. When we analyzed FA localization using TIRF microscopy with pools of Src251R175L-infected cells displaying mixed levels of expression, we were unable to identify any FA localization of the construct even in the most strongly positive cells (Fig. 3A). In contrast, Src251 was readily detectable at FAs by TIRF microscopy (Fig. 3B). This result reinforced the idea that the mutant truncated kinase did not localize to FAs in the absence of SFKs. This evidence, along with the lack of adhesion-dependent FAK pY397 in Src251R175L-expressing cells, strongly suggested that the Arg175 residue in Src's SH2 domain, and the interaction with FAK pY397, was the critical factor in directing Src to FAs.
FIG. 3.
TIRF microscopy of truncated Src constructs (Src251 and Src251R175L) in SYF and FAK-null cells. Src251 (B and D)- and Src251R175L (A and C)-expressing SYF cells were plated on fibronectin for 90 min, fixed, and stained with antibodies to chicken Src and the integrin subunit α5. The Src251 construct localizes to α5 integrin-containing focal adhesions when analyzed by TIRF microscopy (B), whereas Src251 with an R-L mutation at residue 175 does not (A). FAK-null cells stably expressing GFP-FAK (E and G) or GFP-FAK-Y397F (F and H) were transiently transfected with Src251 before being plated on fibronectin for 90 min. Cells were fixed and stained for chicken Src and examined for FA localization by TIRF microscopy. Src251 vigorously localized to FAs in wild-type GFP-FAK-expressing FAK-null cells (E) but not in cells expressing GFP-FAK-Y397F (F).
In addition to FAK, at least two other FA proteins, the adapter proteins paxillin and Cas, contain phosphotyrosines with flanking recognition sites compatible with Src's SH2 domain (5, 34). It is possible that Src251 was directed to FAs via these two adapter proteins in addition to, or instead of, FAK. To test this hypothesis, we first examined whether expression of Src251 in SYF cells, which rescued FAK Y397 phosphorylation (Fig. 1D), also restored paxillin or Cas phosphotyrosine levels upon adhesion. However, despite the fact that Src251 localized stably to FAs, paxillin and Cas signaling remained defective (see Fig. S1 in the supplemental material). In addition to the biochemical data, we investigated the localization of Src251 in FAK-null fibroblasts expressing either GFP-FAK or a construct with a mutation at the tyrosine that binds to Src's SH2 domain, GFP-FAK-Y397F. Interestingly, the truncated Src construct vigorously localized to wild-type GFP-FAK-containing FAs (Fig. 3E) but localized very poorly to GFP-FAK-Y397F-containing FAs (Fig. 3F). Furthermore, unlike wild-type GFP-FAK-expressing cells, GFP-FAK-Y397F-expressing FAK-null fibroblasts were defective in migration (see Fig. S2 in the supplemental material) (28). Taken together, these data strongly suggested that the specific Src SH2-FAK pY397 interaction, and not associations with other FA proteins that bind to Src's SH2 domain, was the mediator of Src recruitment to FAs.
SrcR175L cells were nonmotile and not morphologically transformed despite increased kinase activity in spreading.
Given the stark differences in FA recruitment observed after mutation of the R175 residue in Src251, we wanted to examine the effect that this mutation would have on the wild-type kinase, so we engineered the R175L mutation in the SH2 domain of full-length cSrc (SrcR175L, Fig. 1A). By mutating arginine 175 to leucine in full-length cSrc, we concomitantly abolished the intramolecular association with pY527, rendering the kinase permanently open (39). Therefore, it was possible that the kinase would be constitutively active and, consequently, transforming. However, as with the truncated kinase possessing an Arg-Leu mutation, when full-length SrcR175L was expressed in SYF cells, the kinase was not localized to FAs (Fig. 1C and 2F). In contrast, constitutively active SrcY527F localized stably to FAs and induced aberrant cellular morphology, up-regulated signaling, and permitted colony formation in soft agar (Fig. 1A, 1C, and 2C; see also Fig. 7A and C) (4, 15). Interestingly, SrcR175L-expressing cells did not exhibit a notable morphological change compared to the parental SYF cells, nor did the construct induce anchorage-independent growth (Fig. 2F; see also Fig. 7A). This result indicated that the constitutively open SrcR175L mutant was not transforming, perhaps due to a loss of SH2 substrate binding.
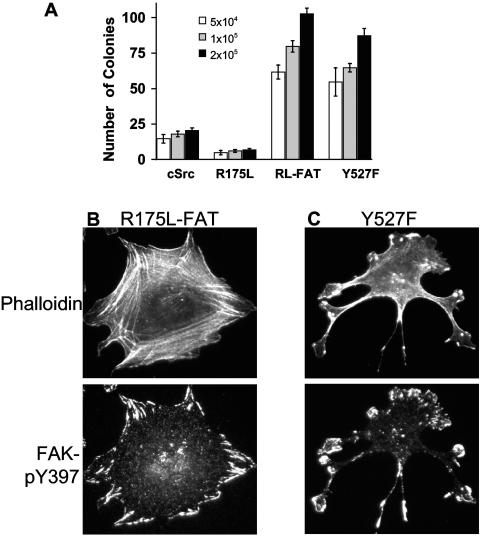
FIG. 7.
Anchorage-independent growth and morphology of SYF cells expressing Src mutants. (A) Colony formation in soft agar of SYF cells expressing SrcR175L (R175L), SrcR175L-FAT (RL-FAT), and SrcY527F (Y527F) relative to cSrc. (B and C) Morphology of SYF cells expressing SrcR175L-FAT (B) and SrcY527F (C) when plated on fibronectin and stained with rhodamine-phalloidin to reveal actin organization and an antibody to FAK pY397 to visualize FAs. Note that while both SrcY527F and SrcR175L-FAT can grow in soft agar, only SrcY527F shows poor actin organization characteristic of morphological transformation.
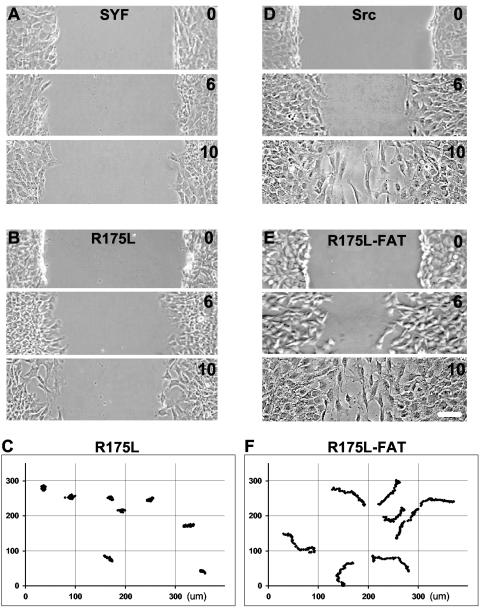
Given that SrcR175L cells were normal morphologically, we wanted to analyze additional aspects of their behavior. One important area of cell biology, and of particular relevance to cell adhesion, is the study of cell motility. In SYF cells transfected with full-length kinase-dead Src, motility improved but was not fully restored to wild-type levels (17). We wanted to determine whether a constitutively open Src kinase, but one that may be defective in FAK recruitment or scaffolding, mediated a similar partial rescue of migration. To examine migration, we employed two different techniques, a directed migration assay in which cells migrate to close a wound made through a confluent monolayer and a random migration assay in which the movements of sparsely plated cells were traced. The two different motility assays were used to confirm that we were assaying primarily integrin-based migration. Interestingly, in directed migration experiments, SrcR175L cells had a defect in mobility similar to that of SYF cells (Fig. 4A and 4B). Furthermore, in random migration, motility was also retarded (Fig. 4C). Indeed, the velocity (μm/h ± standard error of the mean) of SrcR175L cells (5.4 ± 0.6, n = 8) in random migration was not significantly different from that of SYF cells (6.4 ± 0.5, n = 6).
FIG. 4.
Motility of SFK-null fibroblasts expressing variants of cSrc. (A, B, D, and E) Directed cell migration of SYF cells (A) and cells reexpressing full-length Src (D), as well as mutants with an Arg→Leu mutation at residue 175 in the SH2 domain (R175L) (B), and the R175L construct targeted to FAs (R175L-FAT) (D). A confluent monolayer was wounded, and cells were fixed at 0, 6, and 10 h after injury and imaged by phase-contrast microscopy. (C and F) Random motility of R175L cells (C) and R175L-FAT cells (F) on fibronectin over 4.5 h. Cells were sparsely plated onto fibronectin-coated dishes and allowed to spread for 1 h, and nuclear positions were tracked from images captured every 10 min. Both the x and y axes show distances in micrometers. The motility of SYF cells remained defective after reexpression of a Src construct with an R175L mutation in the SH2 domain, but migration was restored after targeting the construct to FAs.
To determine whether the impaired motility of SrcR175L cells was due to an inability to effectively bind to normal Src targets, we measured FAK pY levels and the phosphorylation states of two Src/FAK substrates, Cas and paxillin, upon adhesion (5, 34). Interestingly in respreading experiments, FAK pY397 was hyperphosphorylated in SrcR175L cells compared to cells expressing wild-type Src when adherent (Fig. 5A). Similarly, the phosphorylation status of the kinase domain tyrosine (Y576) of FAK was increased, and the total pY levels of paxillin and Cas were also greater in cells expressing SrcR175L than wild-type cells (Fig. 5A and 5B). This result clearly indicated that in respreading experiments, Src/FAK binding and substrate phosphorylation could occur, possibly through SH3 domain interactions. The hyperphosphorylation status of Src substrates was consistent with SrcR175L being a constitutively active kinase, and it has been reported that Src catalytic activity is sufficient for almost all Src functions (4). Given these results, it was surprising that cells expressing this active kinase had a profound motility defect.
FIG. 5.
Adhesion signaling of SYF cells reconstituted with cSrc, SrcR175L, SrcY527F, and SrcR175L-FAT. The figure shows Western blots indicating phosphorylation levels of FAK at tyrosine residues 397 and 576 (A), as well as total phosphotyrosine of paxillin and Cas (B) in suspension (S) and after 1 h of spreading on fibronectin (F). (A) Phosphorylation of FAK at residue Y397 in whole-cell lysates and Y576 in FAK immunoprecipitates of SYF cells and cells reexpressing cSrc and Src mutants. Membranes were reprobed with anti-FAK MAb for normalization. (B) Reaction of antiphosphotyrosine antibody (RC20) to immunoprecipitates of Cas and paxillin from SFK-null fibroblasts reconstituted with wild-type Src and Src mutants.
FA targeting of SrcR175L restores motility and FA regeneration.
In adherent SYF cells, FAK Y397 is phosphorylated at basal levels: greater than for cells in suspension but dramatically lower than levels in cells reconstituted with wild-type Src (Fig. 1D) (4). We wondered whether this basal level of FAK pY397 in attached SYF cells was analogous to the leading edge of a wild-type cell during migration, where mature FAs had not formed and pY levels may have been locally suppressed. In this situation, the robust Src SH2-FAK pY397 interaction may be required for the rapid recruitment of Src and subsequent activation of FAK, and mutation of the critical R175L in Src may have hindered this localization and consequently impaired migration. To address this, we incorporated the FAT domain of human FAK, which binds to paxillin (20), into the SrcR175L construct (SrcR175L-FAT) to direct the kinase to FAs (Fig. 1A and 2B). Interestingly, when SrcR175L-FAT was expressed in SYF cells, directed cell migration was restored to wild-type levels and random migration improved dramatically (Fig. 1C, 4D, 4E, and 4F). In fact, the velocity of SrcR175L-FAT (19.6 ± 1.0, n = 10) was almost four times that of SrcR175L. The magnitude of the defect in velocity of SrcR175L cells in random migration was surprising given that their kinase activity, measured by substrate pY levels, was at least equal to that of cSrc. These results clearly implicated kinase targeting and not simply activity in the observed motility defect.
During cell migration, stationary adhesions provide traction, and at the leading edge, new FAs are formed, facilitating forward movement of the cell (27, 29). As mentioned, our results suggested that SrcR175L might have been unable to effectively localize to, and consequently activate, FAK at the leading edge of a motile cell. We postulated that this inability to activate FAK might have resulted in an inability to generate new FAs at the front of the cell, thus inhibiting migration.
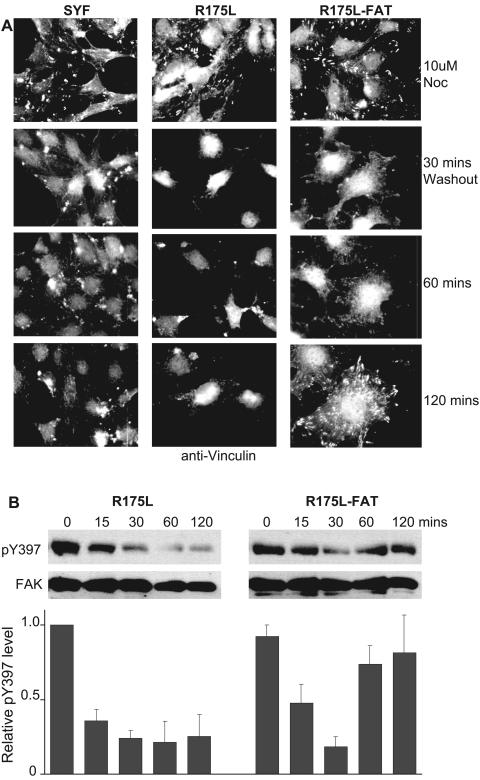
To further examine the generation of adhesions in SrcR175L cells, we employed an assay that measured FA assembly. Recently it has been established that cells treated with the microtubule-depolymerizing agent nocodazole disassembled their FAs after the drug was removed, a process that was FAK dependent and resulted in a reduction in pY397 levels (8). Intriguingly, FAs then reassembled and pY397 levels recovered 2 hours after nocodazole washout. Consequently, the assay provided a convenient method to analyze the synchronous reassembly of FAs by all of the cells on the coverslip. Hence, we decided to use this technique to test the role of the SH2 domain during this synchronous FA reassembly. In SYF cells, although FA disassembly occurred normally (8), FA regeneration at this time point was defective (Fig. 6A). This defect in FA reassembly was clearly consistent with full-length Src being integral to the activation of FAK as a precursor to FA generation. Interestingly, SrcR175L cells were also deficient in FA reassembly and did not recover normal pY397 levels after removal of nocodazole (Fig. 6A and 6B). However, when the R175L mutant was targeted to focal adhesions, FA regeneration occurred normally and FAK Y397 phosphorylation levels recovered after 2 hours (Fig. 6A and B). Consequently, in accordance with the rescue of motility of SrcR175L cells, restoration of FA regeneration after nocodazole washout required targeting the kinase to FAs.
FIG. 6.
FA formation and FAK pY397 levels in SFK-null fibroblasts and cells reexpressing Src SH2 domain mutants after removal of the microtubule-depolymerizing agent nocodazole. (A) Vinculin staining of SYF cells and cells expressing SrcR175L and SrcR175L-FAT at various times after nocodazole washout. (B) Phosphorylation of FAK Y397 in SrcR175L and SrcR175L-FAT cells at 0, 15, 30, 60, and 120 min after nocodazole washout. The chart represents the average relative pY397 levels (± standard errors of the means) at the same time points from two independent experiments. FA regeneration and recovery of FAK phosphorylation after nocodazole washout are restored in SrcR175L cells by targeting the kinase to FAs.
Transforming capability of SrcR175L and SrcR175L-FAT.
Until the findings that Src was essential for adhesion signaling (17), the most commonly studied aspect of Src function was its role in cell transformation. Our data showing that SrcR175L, despite having up-regulated kinase activity, did not transform SYF cells (Fig. 2F and 7A) were consistent with previous data in other cell types. Studies have established that deletions and mutations in the SH2 domain of vSrc attenuated its ability to induce transformation, defined primarily by aberrant morphology in culture and anchorage-independent growth (6, 12, 18, 35, 36). We have shown that even though highly catalytically active after respreading, SrcR175L does not transform cells. Since the levels of expression of all the exogenous Src constructs were similar (Fig. 1C), the differences observed in transforming capability were not likely to be due to differences in the levels of protein expression.
It has been reported that FA targeting of vSrc is sufficient to transform cells (19). In accordance with this finding, SrcR175L-FAT induced anchorage-independent growth at a level approximately equivalent to that of the transforming SrcY527F construct (Fig. 7A). However, in culture the cells were flat and did not show any tendency to form foci when confluent, as SrcY527F did (data not shown). Importantly, there was a striking difference in the morphology and actin organization of the two cell types when plated on fibronectin. SrcR175L-FAT cells displayed typical fibroblast-like morphology: the cells were well polarized and had extensive formation of actin stress fibers anchored by prominent FAs formed at the cell's periphery (Fig. 7B). In contrast, SrcY527F cells, although well spread, had little or no actin stress fiber formation, were not polarized, and had aberrant FA formation and cell shape (Fig. 7C), indicators of a morphologically transformed cell.
DISCUSSION
Our results show that although SrcR175L-FAT supported anchorage-independent growth, the construct was not frankly morphologically transforming. It is likely that FA targeting directed the mutant kinase to substrates associated with the adhesion apparatus required for migration (i.e., FAK). However, the construct did not “restore” morphological transformation observed with activated Src (4, 15), suggesting that the substrates necessary to induce morphological transformation were not localized to FAs. A reexamination of previous data suggests that directing vSrc to FAs may actually attenuate its transforming capacity (the FA-targeted vSrc induced a less severe fusiform morphology in culture and did not generate the large foci characteristic of vSrc) (19). The ability of FA-targeted vSrc to transform cells was likely due to its intact SH2 domain allowing recruitment of transformation-specific substrates, which the SrcR175L-FAT construct used in the current study was unable to do. However, in the soft agar assay, SrcR175L-FAT was able to support anchorage-independent growth, perhaps because the construct could mediate phosphorylation of FAK tyrosine 397 in suspension (Fig. 5A).
One of our initial findings was that Src251R175L did not localize to FAs in SYF cells. However, the construct had been reported to be recruited to adhesion plaques in other cell types, albeit poorly (15). It is possible that the dramatically higher adhesion signaling levels in cell lines expressing other SFKs permit a more vigorous recruitment to FAs of adapter proteins, such as paxillin and Cas. Importantly, Cas also contains a proline-rich domain (5) which may interact with the constitutively accessible SH3 domain in Src251R175L, thus providing an alternate mechanism for recruitment of the construct to FAs. However, it is clear that the primary mechanism by which Src251 localizes to FAs in mouse embryonic fibroblasts is via an SH2-mediated interaction, because the R175L mutation within this domain prevents the truncated kinase from localizing to FAs in SYF cells.
Since the full-length SrcR175L was defective in FA recruitment, it was surprising that FAK was hyperphosphorylated at its kinase domain in spreading experiments. Importantly, it has been shown that SH3 interactions, although less robust than SH2 associations, can facilitate Src-FAK binding to mediate phosphorylation of downstream substrates in fully spread cells (4). In our experiments, the fact that even the lower-affinity SH3 domain binding provided sufficient scaffolding capacity for greater-than-wild-type substrate phosphotyrosine levels was likely due to SrcR175L's kinase domain being permanently open. Importantly, the high degree of FAK phosphorylation shown for SrcR175L cells represents the cell's total phosphotyrosine levels measured in a respreading assay, which are likely to be different from phosphorylation levels in migration. More significantly, levels of phosphorylated proteins in distinct regions of the cell, such as the tail and the leading edge, may differ substantially during migration. Thus, the total phosphotyrosine levels determined in a respreading assay probably do not reflect this more complicated and dynamic situation.
Interestingly, the investigation of the biochemistry and localization of truncated Src constructs in SFK- or FAK-null cells has provided important insights into Src targeting to FA proteins. First, FAK pY397 is phosphorylated at low levels in SYF cells while paxillin and Cas are not (4). Second, Src251 localizes to FAs, which rescues FAK pY397 to wild-type levels but does not restore Cas or paxillin phosphotyrosine levels. Third, mutation of the R175 residue in Src251 prevents the construct from localizing to FAs and also ablates the rescue of FAK pY397. Finally, in the reciprocal localization experiment, Src251 is recruited vigorously to FAs in FAK-null cells expressing GFP-FAK but very poorly when these cells express GFP-FAK-Y397F. Thus, the localization of Src to FAs is both SH2 dependent and FAK pY397 dependent. We believe that these data strongly suggest that while Src SH2-paxillin/Cas interactions may occur, in adhesion, the initial interaction of Src SH2 is with FAK.
Despite the phosphorylation of downstream substrates in spreading SrcR175L cells, normal motility did not occur in the absence of SH2 domain associations. Importantly, the fact that the motility defect was rescued upon FA targeting of the SrcR175L construct adds further evidence that in wild-type Src, the specific Src SH2-FAK interaction was necessary for appropriate recruitment to FAs in migration.
In recent years there has been keen interest in understanding the turnover or disassembly of FAs at the tail of a migrating cell and the role that this plays in the motile behavior of cells (8, 38). However, we reasoned that as the generation of new adhesions at the leading edge was the initiating step in migration, it was, therefore, likely a critically important aspect of cell motility.
For our experiments, we decided to use a novel assay of focal adhesion reassembly to determine the effect of the loss of targeting to FAK on this process. Performing assays of FAK phosphorylation on migrating cells is difficult, since all directed cell migration assays have only a small, nonsynchronized fraction of the cells that are moving, and the vast majority of stationary cells would dominate the biochemistry. Furthermore, in our experience, the immunofluorescent staining of focal adhesions with FAK Y397P antibody is not as quantitatively reliable as staining of Western blots. Other assays such as observing assembly of focal adhesions in spreading cells are not as vigorous as the synchronized, massive reassembly that we observed with our nocodazole washout assay.
Interestingly, using this assay we find that in addition to the rescue of migration, FA targeting of SrcR175L also corrected the defect in FA reassembly upon nocodazole washout. Taken together, these results suggest that under the dynamic conditions of migration or reassembly after nocodazole washout, the targeting of Src to FAs is critical in the rapid phosphorylation of FAK and in FA assembly.
We have attempted to understand the interactions occurring among molecules comprising the adhesion apparatus in a rapidly changing system, a migrating cell. Src's involvement in FA signaling under these dynamic conditions is somewhat perplexing as Src does not localize stably to FAs. However, there is substantial evidence that there is an important, albeit transient, interaction with proteins found in FAs. It is probable that at the leading edge of a migrating cell, prior to the formation of bona fide FAs, Src interacts transiently with FAK, phosphorylating the protein at its kinase domain. This event initiates a cascade leading to the generation of mature FAs, which facilitates the forward motion of the cell. Thus, if the localization of Src to FAs is disrupted either by mutation of the SH2 domain (SrcR175L) or by mutation of the docking site on FAK (Y397F), then motility is lost. This motility is restored with rescue of the FA localization of SrcR175L-FAT.
It has been established that cell lines null for individual cytoplasmic adhesion proteins have motility defects (10, 17, 28). However, in wild-type cells, the biological mechanisms that regulate interactions between the different FA proteins to modulate migration are poorly understood. We have been able to identify a specific behavioral defect when Src's SH2 domain was mutated and corrected it by targeting the kinase to FAs. In doing so we have characterized a substrate targeting function for the SH2 domain that is critical for normal Src function in cell migration. Future work may enable us to use these differences in Src constructs to identify targets specifically involved in morphological transformation versus motility.
Supplementary Material
Acknowledgments
We thank L. Hughes for expert technical assistance and Pam Schwarzberg for the generous gift of Src constructs.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bibbins, K. B., H. Boeuf, and H. E. Varmus. 1993. Binding of the Src SH2 domain to phosphopeptides is determined by residues in both the SH2 domain and the phosphopeptides. Mol. Cell. Biol. 13:7278-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briesewitz, R., A. Kern, and E. E. Marcantonio. 1993. Ligand-dependent and -independent integrin focal contact localization: the role of the alpha chain cytoplasmic domain. Mol. Biol. Cell 4:593-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, M. T., and J. A. Cooper. 1996. Regulation, substrates and functions of src. Biochim. Biophys. Acta 1287:121-149. [DOI] [PubMed] [Google Scholar]
- 4.Cary, L. A., R. A. Klinghoffer, C. Sachsenmaier, and J. A. Cooper. 2002. SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22:2427-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chodniewicz, D., and R. L. Klemke. 2004. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim. Biophys. Acta 1692:63-76. [DOI] [PubMed] [Google Scholar]
- 6.DeClue, J. E., and G. S. Martin. 1989. Linker insertion-deletion mutagenesis of the v-src gene: isolation of host- and temperature-dependent mutants. J. Virol. 63:542-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eide, B. L., C. W. Turck, and J. A. Escobedo. 1995. Identification of Tyr-397 as the primary site of tyrosine phosphorylation and pp60src association in the focal adhesion kinase, pp125FAK. Mol. Cell. Biol. 15:2819-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezratty, E. J., M. A. Partridge, and G. G. Gundersen. 2005. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7:581-590. [DOI] [PubMed] [Google Scholar]
- 9.Felsenfeld, D. P., P. L. Schwartzberg, A. Venegas, R. Tse, and M. P. Sheetz. 1999. Selective regulation of integrin-cytoskeleton interactions by the tyrosine kinase Src. Nat. Cell Biol. 1:200-206. [DOI] [PubMed] [Google Scholar]
- 10.Hagel, M., E. L. George, A. Kim, R. Tamimi, S. L. Opitz, C. E. Turner, A. Imamoto, and S. M. Thomas. 2002. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 22:901-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirai, H., and H. E. Varmus. 1990. Mutations in src homology regions 2 and 3 of activated chicken c-src that result in preferential transformation of mouse or chicken cells. Proc. Natl. Acad. Sci. USA 87:8592-8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirai, H., and H. E. Varmus. 1990. Site-directed mutagenesis of the SH2- and SH3-coding domains of c-src produces varied phenotypes, including oncogenic activation of p60c-src. Mol. Cell. Biol. 10:1307-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hynes, R. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 14.Iida, S., P. H. Rao, M. Butler, P. Corradini, M. Boccadoro, B. Klein, R. S. Chaganti, and R. Dalla-Favera. 1997. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat. Genet. 17:226-230. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan, K. B., K. B. Bibbins, J. R. Swedlow, M. Arnaud, D. O. Morgan, and H. E. Varmus. 1994. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine 527. EMBO J. 13:4745-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimber, M. S., J. Nachman, A. M. Cunningham, G. D. Gish, T. Pawson, and E. F. Pai. 2000. Structural basis for specificity switching of the Src SH2 domain. Mol. Cell 5:1043-1049. [DOI] [PubMed] [Google Scholar]
- 17.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebl, E. C., L. J. England, J. E. DeClue, and G. S. Martin. 1992. Host range mutants of v-src: alterations in kinase activity and substrate interactions. J. Virol. 66:4315-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liebl, E. C., and G. S. Martin. 1992. Intracellular targeting of pp60src expression: localization of v-src to adhesion plaques is sufficient to transform chicken embryo fibroblasts. Oncogene 7:2417-2428. [PubMed] [Google Scholar]
- 20.Liu, G., C. D. Guibao, and J. Zheng. 2002. Structural insight into the mechanisms of targeting and signaling of focal adhesion kinase. Mol. Cell. Biol. 22:2751-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer, B. J., P. K. Jackson, R. A. Van Etten, and D. Baltimore. 1992. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol. Cell. Biol. 12:609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitra, S. K., D. A. Hanson, and D. D. Schlaepfer. 2005. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 6:56-68. [DOI] [PubMed] [Google Scholar]
- 23.Pawson, T. 1995. Protein modules and signalling networks. Nature 373:573-580. [DOI] [PubMed] [Google Scholar]
- 24.Pawson, T., and G. D. Gish. 1992. SH2 and SH3 domains: from structure to function. Cell 71:359-362. [DOI] [PubMed] [Google Scholar]
- 25.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Resh, M. D. 1994. Myristylation and palmitylation of Src family members: the fats of the matter. Cell 76:411-413. [DOI] [PubMed] [Google Scholar]
- 27.Rottner, K., A. Hall, and J. V. Small. 1999. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9:640-648. [DOI] [PubMed] [Google Scholar]
- 28.Sieg, D. J., C. R. Hauck, and D. D. Schlaepfer. 1999. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112:2677-2691. [DOI] [PubMed] [Google Scholar]
- 29.Smilenov, L. B., A. Mikhailov, R. J. Pelham, E. E. Marcantonio, and G. G. Gundersen. 1999. Focal adhesion motility revealed in stationary fibroblasts. Science 286:1172-1174. [DOI] [PubMed] [Google Scholar]
- 30.Su, J., M. Muranjan, and J. Sap. 1999. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 9:505-511. [DOI] [PubMed] [Google Scholar]
- 31.Tian, M., and G. S. Martin. 1996. Reduced phosphotyrosine binding by the v-Src SH2 domain is compatible with wild-type transformation. Oncogene 12:727-734. [PubMed] [Google Scholar]
- 32.Tonks, N. K., and B. G. Neel. 2001. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13:182-195. [DOI] [PubMed] [Google Scholar]
- 33.Toomre, D., and D. J. Manstein. 2001. Lighting up the cell surface with evanescent wave microscopy. Trends Cell Biol. 11:298-303. [DOI] [PubMed] [Google Scholar]
- 34.Turner, C. E. 2000. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2:E231-E236. [DOI] [PubMed] [Google Scholar]
- 35.Verderame, M. F., J. M. Kaplan, and H. E. Varmus. 1989. A mutation in v-src that removes a single conserved residue in the SH-2 domain of pp60v-src restricts transformation in a host-dependent manner. J. Virol. 63:338-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verderame, M. F., and H. E. Varmus. 1994. Highly conserved amino acids in the SH2 and catalytic domains of v-src are altered in naturally occurring, transformation-defective alleles. Oncogene 9:175-182. [PubMed] [Google Scholar]
- 37.von Wichert, G., G. Jiang, A. Kostic, K. De Vos, J. Sap, and M. P. Sheetz. 2003. RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J. Cell Biol. 161:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb, D. J., K. Donais, L. A. Whitmore, S. M. Thomas, C. E. Turner, J. T. Parsons, and A. F. Horwitz. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6:154-161. [DOI] [PubMed] [Google Scholar]
- 39.Xu, W., S. C. Harrison, and M. J. Eck. 1997. Three-dimensional structure of the tyrosine kinase c-Src. Nature 385:595-602. [DOI] [PubMed] [Google Scholar]
- 40.Zheng, X. M., R. J. Resnick, and D. Shalloway. 2000. A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO J. 19:964-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.