Abstract
To elucidate the mechanism of an irradiance-dependent adjustment in the chlorophyll (Chl) antenna size of Dunaliella salina, we investigated the regulation of expression of the Chl a oxygenase (CAO) and light-harvesting complex b (Lhcb) genes as a function of Chl availability in the photosynthetic apparatus. After a high-light to low-light shift of the cultures, levels of both CAO and Lhcb transcripts were rapidly induced by about 6-fold and reached a high steady-state level within 1.5 h of the shift. This was accompanied by repair of photodamaged photosystem II (PSII) reaction centers, accumulation of Chl a and Chl b (4:1 ratio), photosystem I (PSI), light-harvesting complex, and by enlargement of the Chl antenna size of both photosystems. In gabaculine-treated cells, induction of CAO and Lhcb transcripts was not affected despite substantial inhibition in de novo Chl biosynthesis. However, cells were able to synthesize and accumulate some Chl a and Chl b (1:1 ratio), resulting in a marked lowering of the Chl a to Chl b ratio in the presence of this inhibitor. Assembly incorporation of light-harvesting complex and a corresponding Chl antenna size increase, mostly for the existing photosystems, was noted in the presence of gabaculine. Repair of photodamaged PSII was not affected by gabaculine. However, assembly accumulation of new PSI was limited under such conditions. These results suggest a coordinate regulation of CAO and Lhcb gene transcription by irradiance, independent of Chl availability. The results are discussed in terms of different signal transduction pathways for the regulation of the photosynthetic apparatus organization by irradiance.
Long-term variation in light intensity often brings about pronounced changes in the organization and function of the photosynthetic apparatus in both higher plants and algae (Leong et al., 1985; Anderson, 1986; Eskins et al., 1989; Smith et al., 1990; Falkowski and LaRoche, 1991; Maxwell et al., 1995; Wilson and Huner, 2000). The physiological response of plants to variation in light intensity entails adjustment and optimization in the light-harvesting and energy conversion capacity of the photosynthetic apparatus, including adjustments in photosystem stoichiometry (Melis, 1991, 1996). However, when irradiance is in excess of that required for the saturation of photosynthesis, photoinhibition may occur. This adverse phenomenon is manifested as loss in photosystem II (PSII) activity and oxygen evolution (Powles, 1984) and accumulation of photodamaged PSII reaction centers in the chloroplast thylakoids (Melis, 1999). The primary target of photo-oxidative damage in plants is a functional component within D1, the 32-kD reaction-center protein of PSII (Cleland et al., 1986; Prasil et al., 1992). Photodamage to the D1 protein irreversibly inactivates PSII. Recovery from this photodamage requires removal of the photochemically inert D1 protein from the PSII holocomplex, its degradation, and replacement by a newly synthesized D1. Photo-oxidative damage, the turnover of the D1 protein, and the ensuring repair of PSII are steps in the so-called PSII damage and repair cycle (Melis, 1991; Aro et al., 1993).
Previous work from this laboratory has shown that growth of the unicellular green alga Dunaliella salina Teod. under high irradiance (high light [HL]; 2,200 μmol photons m−2 s−1) causes photoinhibition of photosynthesis and elicits a truncated chlorophyll (Chl) antenna size (Smith et al., 1990). Compared with low-light (LL; 50 μmol photons m−2 s−1)-grown cells, HL-grown D. salina chloroplasts assembled a small amount of photosystem I (PSI). They contained about the same amount of PSII as LL-grown cells; however, up to approximately 80% of PSII were photochemically inactive because of photodamage (Vasilikiotis and Melis, 1994). When HL-acclimated cells were switched to LL conditions, recovery from photoinhibition occurred concomitantly with an increase in the levels of cellular Chl, light-harvesting complex II (LHC-II), and PSI in the chloroplast (Webb and Melis, 1995; Neidhardt et al., 1998). In the course of such adjustments, Chl molecules are distributed to the two photosystems and their respective light-harvesting complex (LHC) proteins. The biosynthesis of pigments is coordinated with that of the LHC apoproteins such that normally no excess pigment is synthesized. Conversely, no excess of LHC protein accumulates without the coordinate synthesis of pigments. The mechanism of coordination of these two distinctly different biosynthetic pathways is unclear (Johanningmeier and Howell, 1984; Hoober et al., 1990).
In higher plants, Chl may be supplied to apoproteins by distribution of newly synthesized Chl (Tanaka and Tsuji, 1985) or by redistribution of existing Chl molecules that have been previously incorporated into Chl-protein complexes (Tanaka and Tsuji, 1982, 1983). Based on experiments with greening seedlings under intermittent illumination (Akoyunoglou and Argyroudi-Akoyunoglou, 1986), it has been suggested that reaction center polypeptides have a higher affinity for Chl than those of the LHC. Under these conditions, reaction center polypeptides accumulated, but LHC apoproteins were unstable in the absence of pigment and were subsequently degraded (Akoyunoglou and Argyroudi-Akoyunoglou, 1986).
In the present study, gabaculine (3-amino-2,3-dihydrobenzoic acid) was used to slow down Chl biosynthesis under conditions when rapid Chl accumulation would normally be elicited in the chloroplast. The experimental protocol entailed shifting an HL-acclimated culture of D. salina to LL conditions. After the HL → LL shift, and as a function of time under LL, we monitored parameters of the photosynthetic apparatus such as recovery from photoinhibition in the presence or absence of substantial Chl biosynthesis. Furthermore, the effect of an HL → LL transition on the LHC composition and cellular PSII and PSI contents was investigated. Our results show that a substantial slowdown of Chl biosynthesis by gabaculine differentially affects the two photosystems. Recovery of PSII from photoinhibition was not affected by inhibition in Chl biosynthesis. Although the transcription of LHC-II (Lhcb) and Chl a oxygenase (CAO) genes was not affected by gabaculine, biosynthesis and assembly of the full complement of the LHC-II was prevented by the limited amount of new Chl. Assembly and accumulation of new PSI complexes was also affected by the lack in Chl biosynthesis. The results suggested a distinct hierarchy in the distribution of newly synthesized Chl with priority given to enlarging the Chl antenna size of existing photosystems over the assembly accumulation of new ones. Furthermore, the Chl antenna size of photosynthesis is posttranscriptionally regulated by Chl availability.
RESULTS
Analysis of Photosynthetic Pigments
When HL-acclimated (2,200 μmol photons m−2 s−1) D. salina cells were transferred to LL conditions (50 μmol photons m−2 s−1), recovery of the photosynthetic apparatus from photoinhibition occurred with a concomitant increase in Chl content and photosystem Chl antenna size (Neidhardt et al., 1998). To delineate Chl biosynthesis and adjustments in the Chl antenna size from the recovery of the photosynthetic apparatus from photoinhibition, Chl biosynthesis was blocked by treatment of the algae with gabaculine. Gabaculine inhibits the transamination of Glu 1-semialdehyde to 5-aminolevulinic acid, which is the first committed precursor of Chl biosynthesis (Avissar and Beale, 1989; Beale, 1999). The effect of gabaculine on cell recovery from photoinhibition, pigment biosynthesis and accumulation, gene expression, and Chl antenna size of the photosystems was monitored upon transferring an HL-grown D. salina culture to LL-growth conditions (HL → LL shift).
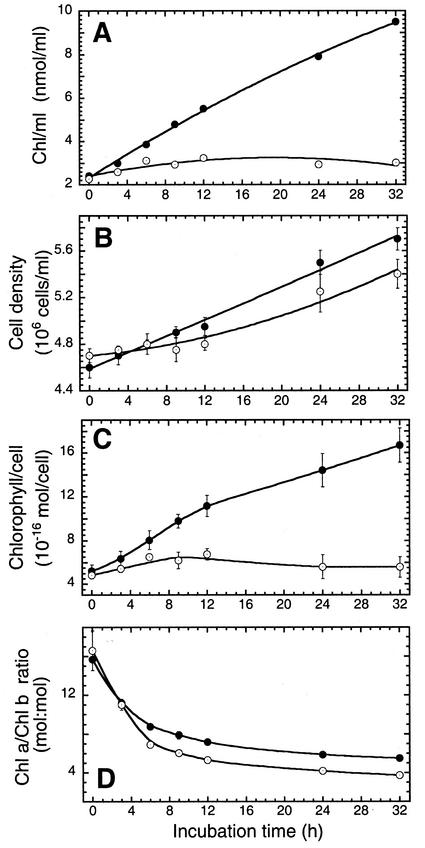
In the control cultures, an HL → LL shift induced a rapid biosynthesis and accumulation of Chl (Fig. 1A). A linear increase in the Chl content of the culture was sustained for at least 24 h, occurring with an initial slope of approximately 0.26 nmol Chl mL−1 culture h−1 under these conditions. In the presence of 1 mm gabaculine, limited Chl biosynthesis took place during the first 12 h after the HL → LL shift, occurring with a rate of 0.075 nmol Chl mL−1 culture h−1 (Fig. 1A). This was only approximately 30% of the control rate. However, this residual Chl biosynthesis activity ceased at times longer than 12 h in the presence of gabaculine.
Figure 1.
Effect of gabaculine on Chl accumulation and cell growth after an HL → LL shift of D. salina cultures. A, Chl accumulation in the culture. B, Cell density increase. C, Cellular Chl content. D, The Chl a to Chl b ratio. Control (●) and gabaculine-treated cells (○) were shifted from HL to LL growth irradiance at zero time. Data are means from two independent experiments with n = 3 to 5. Error bars represent sd.
Cell density also increased as a linear function of time in the control (Fig. 1B). Cell density increase was also noted in the gabaculine-treated samples (Mortain-Bertrand et al., 1990), although the latter was observed after an initial lag period (Fig. 1B). In consequence, within the first 32 h after an HL → LL shift, Chl content increased from about 5 × 10−16 to about 17 × 10−16 mol per cell in the control (Fig. 1C, black circles). Concomitantly, the Chl a to Chl b ratio of the cells decreased from approximately 16:1 to approximately 6:1 over the same time period (Fig. 1D, black circles). On the basis of these quantitative measurements, we estimated that newly synthesized Chl, after the HL → LL shift, was partitioned between Chl a and Chl b in a 4:1 ratio.
In the presence of 1 mm gabaculine, some Chl/cell increase was noted during the first approximately 12 h after an HL → LL shift (Fig. 1C). Thereafter, Chl/cell declined as a cell density increase was not accompanied by increase in the content of Chl under these conditions. Surprisingly, in the presence of gabaculine, cells showed a steep decline in the Chl a to Chl b ratio from approximately 16:1 to approximately 4:1 over the 32-h period after an HL → LL shift. This decline was kinetically similar to that of the control cells, occurring with a half-time of about 3 h (Fig. 1D). We estimated that newly synthesized Chl, after the HL → LL shift in the present of gabaculine, was partitioned between Chl a and Chl b in a nearly 1:1 ratio. This represents a marked difference from the partitioning of Chl into Chl a and Chl b in the control.
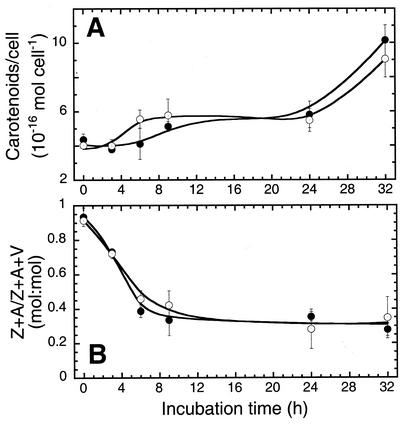
Figure 2 shows the effect of an HL → LL shift on cellular carotenoids in the presence or absence of gabaculine. In the control culture, the level of total carotenoids per cell increased from about 4 × 10−16 to about 10 × 10−16 mol per cell within 32 h after the light shift (Fig. 2A). The de-epoxidation state of the xanthophyll pool (zeaxanthin + antheraxanthin/zeaxanthin + antheraxanthin + violaxanthin) decreased with a half-time of approximately 4 h from about 0.95:1 to about 0.35:1, apparently reflecting changes in xanthophyll cycle activity (Fig. 2B). Gabaculine did not affect these adjustments in carotenoid per cell or changes in the composition of xanthophylls. Furthermore, gabaculine did not affect changes in the composition of other carotenoids, such as lutein, neoxanthin, and β-carotene during this time period (data not shown), indicating that biosynthesis of carotenoids and activity of the xanthophyll cycle were independently regulated from that of Chl availability in D. salina.
Figure 2.
A, Effects of gabaculine on carotenoid content in D. salina after an HL → LL shift. B, Effect of gabaculine on cellular de-epoxidation state of xanthophyll pools after an HL → LL shift. The molar ratio of zeaxanthin (Z) + antheraxanthin (A) per Z + A + violaxanthin (V) was calculated after HPLC analysis. Control (●) and gabaculine-treated cells (○) were shifted from HL to LL growth irradiance at zero time. Data are means from two independent experiments with n = 3. Error bars represent sd.
Recovery from Photoinhibition
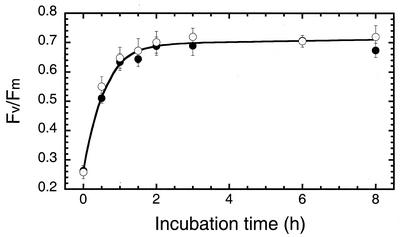
An HL → LL transition in D. salina cultures entails not only Chl accumulation and increase in the light-harvesting Chl antenna size of the photosystems but, independently, repair of the sizable pool of photodamaged PSII centers and de novo biosynthesis/assembly of PSI centers to match the increasing electron-transport capacity of PSII in the thylakoid membrane (Neidhardt et al., 1998). The effect of gabaculine on the repair of photodamaged PSII was measured after an HL → LL shift. Repair was measured in vivo by the Chl fluorescence Fv to Fm ratio, which is a measure of the photochemical charge separation efficiency of PSII in the chloroplast thylakoids (Butler and Kitajima, 1975).
In the control culture, the Fv to Fm ratio increased upon an HL → LL shift, with a half-time of about 40 min, from 0.26 to about 0.7 (Fig. 3). The presence of gabaculine did not have any effect on this process, suggesting that recovery of PSII from photoinhibition is largely independent of a Chl increase. Thus, photodamaged PSII reaction centers were completely repaired after an HL → LL shift, irrespective of the presence or absence of gabaculine.
Figure 3.
Effects of gabaculine on recovery of the in vivo Fv/Fm Chl fluorescence ratio after an HL → LL shift. Control (●) and gabaculine-treated cells (○) were shifted from HL to LL growth irradiance at zero time. Data are representative of three independent measurements.
Levels of Lhcb and CAO Transcripts
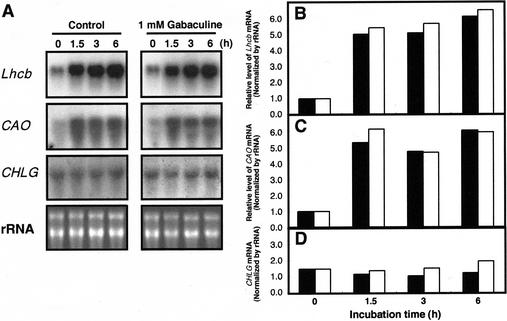
To examine the effect of Chl biosynthesis on the expression of genes related to the assembly of the Chl antenna, we measured the effect of gabaculine on the mRNA levels of the Lhcb genes and genes encoding Chl biosynthetic enzymes. Lhcb genes encode the apoproteins of the LHC-II. Quantitation of mRNA levels after northern-blot analysis and densitometric scanning showed that the Lhcb transcripts increased approximately 6-fold within 1.5 h after the HL → LL shift (Fig. 4, A and B). The presence of gabaculine did not prevent this light-dependent up-regulation of the Lhcb gene expression. Among six different genes encoding Chl biosynthetic enzymes, i.e. Glu 1-semialdehyde aminotransferase (GSA), subunits of magnesium chelatase (CHLI, CHLD, CHLH), Chl a synthetase (CHLG), CAO, and actin, only the CAO gene showed an irradiance-dependent mRNA profile similar to that of the Lhcb gene. As an example, Figure 4A shows the prompt and substantial induction of Lhcb and CAO mRNA in comparison with the lack of induction for the CHLG mRNA after an HL → LL shift. These findings provide important evidence that not all Chl biosynthesis-related genes are subject to regulation by irradiance. Conversely, the prompt response of CAO gene expression to a change in irradiance suggests an important role for CAO in the regulation of the Chl antenna size. The CAO gene encodes the Chl a oxygenase, which catalyzes the conversion of Chl a to Chl b (Tanaka et al., 1998). CAO transcripts increased approximately 5-fold and reached a high steady state within 1.5 h after the HL → LL shift (Fig. 4A and C). The presence of gabaculine did not block this light-dependent up-regulation in the CAO gene expression. In contrast, levels of the CHLG transcript remained constant after the HL → LL shift in the presence or absence of 1 mm gabaculine (Fig. 4, A and D). Moreover, run-on nuclear transcription experiments demonstrated that the induction of CAO and Lhcb genes is caused by transcriptional activation, rather than by enhancement of mRNA stability (data not shown).
Figure 4.
Effect of gabaculine on the induction of Lhcb, CAO and CHLG mRNA after an HL → LL shift. Treatment with 1 mm gabaculine and the shift in growth irradiance occurred at zero time. Samples were harvested at the indicated times during incubation. A, Autoradiogram of northern blots and ethidium bromide staining of rRNA. Histogram of the quantitative analysis of Lhcb (B), CAO (C), and CHLG (D) mRNA normalized to the concentration of rRNA in control (black bars) and gabaculine-treated cells (white bars).
Accumulation of LHC-II, PSI, and PSII Reaction Center Apoproteins
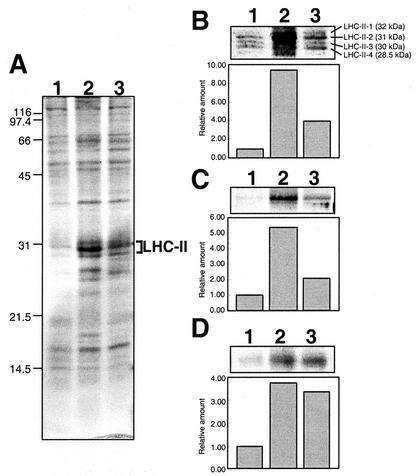
The profile of thylakoid membrane proteins from control and gabaculine-treated cells was examined by SDS-PAGE. Cells were harvested immediately before an HL → LL shift and after a 24-h incubation in LL. Thylakoid membranes were isolated and solubilized, and proteins were resolved based on equal cell basis. Figure 5A shows a Coomassie-stained gel from such an experiment. It is evident that LHC-II proteins were elicited upon a 24-h incubation in LL. However, the increase in LHC-II was considerably greater for the control (Fig. 5A, lane 2) than for the gabaculine-treated sample (Fig. 5A, lane 3).
Figure 5.
Effects of gabaculine on the accumulation of key thylakoid membrane proteins after an HL → LL shift. A, Coomassie Brilliant Blue-stained SDS-PAGE gel. Molecular mass markers (kD) are indicated on the left. Western blot analysis of the LHC-II apoproteins (B), the PsaA/PsaB PSI reaction center proteins (C), and the D1/32-kD reaction center protein (D). The nitrocellulose filters were probed with specific polyclonal antibodies. Cross-reactions were quantitated by densitometric scan (block diagrams in B, C, and D). Lanes for the western blotting of LHC-II proteins were loaded with solubilized thylakoid membranes corresponding to 1.3 × 106 cells. All other runs were loaded with thylakoid membranes equivalent to 5 × 106 cells per lane. Lane 1, HL-grown cells. Lane 2, HL-grown cells after a 24-h incubation under LL conditions in the absence of gabaculine (control). Lane 3, HL-grown cells after a 24-h incubation under LL conditions in the presence of gabaculine.
The effect of gabaculine on key thylakoid membrane proteins was assessed in greater detail by western blot analysis. Figure 5B shows the accumulation of LHC-II apoprotein. Four distinct protein bands, termed LHC-II-1 through LHC-II-4, with apparent molecular masses of 32, 31, 30, and 28.5 kD were identified by the polyclonal antibodies (Tanaka and Melis, 1997). In thylakoid membranes from HL-grown cells, LHC-II-1 was absent and LHC-II-2 was greatly depleted. In the control culture, the total amount of LHC-II apoprotein increased after an HL → LL shift (Fig. 5B, lane 2). This primarily reflected the appearance of LHC-II-1 and enhancement in the amount of LHC-II-2, which greatly increased relative to the LHC-II-3 and LHC-II-4 apoproteins. In gabaculine-treated cells (Fig. 5B, lane 3), all four LHC-II bands were detected. However, increase of the LHC-II-2 was prevented relative to the other LHC-II proteins. Quantitation of western blots by densitometric scanning showed that, in the control samples, the total amount of LHC-II apoprotein per cell increased approximately 10-fold after an HL → LL shift, whereas in the presence of gabaculine increase in the LHC-II apoprotein was limited to only approximately 4-fold.
Figure 5C shows the effect of gabaculine on the accumulation of the PSI reaction center proteins PsaA/PsaB. After an HL → LL shift, substantial de novo biosynthesis of PSI apoprotein was detected in the control (lane 2). This is consistent with the measured increase in total cellular P700 after an HL → LL shift (Neidhardt et al., 1998; see also Table I). In the presence of gabaculine, de novo biosynthesis of PSI apoprotein (Fig. 5C, lane 3) was limited to only about 20% to 25% of that seen in the control. The limited PsaA/PsaB protein accumulation in the presence of gabaculine is attributed directly to the lack of Chl a supply under these conditions.
Table I.
Photosynthetic apparatus characteristics of D. salina after a 24-h incubation under LL conditions, either in the absence (control) or in the presence of 1 mm gabaculine
| Parameter | HL | Control | 1 mm Gabaculine |
|---|---|---|---|
| Fv/Fm | 0.26 ± 0.02 | 0.67 ± 0.02 | 0.72 ± 0.01 |
| QA/total Chl (mmol:mol) | 2.63 ± 0.19 | 2.09 ± 0.02 | 4.90 ± 0.17 |
| P700/total Chl (mmol:mol) | 1.10 ± 0.05 | 1.61 ± 0.06 | 1.61 ± 0.02 |
| QA/cell (10−18 mol cell−1) | 0.77 ± 0.23 | 2.45 ± 0.02 | 1.91 ± 0.06 |
| P700/cell (10−18 mol cell−1) | 0.27 ± 0.03 | 1.69 ± 0.07 | 0.63 ± 0.01 |
Thylakoid membranes were isolated from D. salina as described in “Materials and Methods.” Data are representative of at least three independent membrane preparations. Values shown are means ± se.
Figure 5D shows the amounts of the 32-kD form of D1, representing functional PSII reaction centers (Kim et al., 1993; Baroli and Melis, 1996), in thylakoid membranes isolated before and after 24 h in the presence or absence of 1 mm gabaculine. In the control culture, the amount of functional D1 protein per cell increased approximately 3.5-fold within 24 h after an HL → LL shift (Fig. 5D, lane 2). Qualitatively similar results were obtained with the gabaculine-treated cells (Fig. 5D, lane 3), again suggesting that repair of the D1 protein from photodamage was not prevented by the gabaculine treatment.
Functional Analysis of the Photosynthetic Apparatus
The above results show that inhibition of Chl biosynthesis by gabaculine differentially affects the recovery of the two photosystems from photoinhibition. To gain further insight into the concentration of photochemically competent PSII and PSI centers in the thylakoid membranes of control and gabaculine-treated D. salina cells, we measured the functional QA and P700 content spectrophotometrically from the light-induced amplitude of the absorbance change at 320 and 700 nm, respectively (Melis, 1989). Table I shows the result of such quantitations in cells grown under HL conditions and after 24-h incubation following an HL → LL shift in the presence or absence of gabaculine. It is shown that the QA/cell increased within 24 h from 0.77 × 10−18 mol cell−1 in the HL-grown cells to 2.45 × 10−18 in the control and to 1.91 × 10−18 mol cell−1 in the gabaculine-treated sample, consistent with the repair of photodamaged PSII. P700 content increased within 24 h after the HL → LL shift from 0.27 × 10−18 mol cell−1 in the HL-grown to 1.69 × 10−18 mol cell−1 in the control. In the gabaculine-treated sample, P700 content increased only slightly to 0.63 × 10−18 mol cell−1. These results are qualitatively consistent with the western blot analyses (Fig. 5, C and D) and corroborate the notion that gabaculine affects the accumulation of new PSI but has no effect on the repair of PSII.
Photosystem Chl Antenna Size
To examine whether different levels of LHC-II protein in control and gabaculine-treated cells (Fig. 5, A and B) are reflected in the functional Chl antenna size of the photosystems in D. salina, estimates of the number of Chl molecules associated with PSI and PSII were obtained (Melis and Anderson, 1983). According to this spectrophotometric method, Chl molecules are functionally assigned to PSI and PSII in direct proportion to the rate of light absorption/utilization by the two photosystems, measured from the kinetics of P700 photooxidation and QA photoreduction in isolated and 3-(3,4-dichlorophenyl)- 1,1-dimethylurea–poisoned thylakoid membranes (Melis, 1989).
As reported previously (Melis et al., 1999), HL-grown cells had a substantially truncated Chl antenna size for both PSI and PSII in their chloroplasts. There was no antenna heterogeneity in PSII, and the number of Chl (a and b) molecules specifically associated with PSII and PSI were 55 and 112, respectively (Table II). After a 24-h incubation under LL conditions, enlargement in the Chl antenna size was observed in the control samples, concomitant with the appearance of two populations of PSII noted for their dissimilar Chl antenna size (Lavergne and Briantais, 1996). In the control, about 36% of the functional PSII centers became PSIIα with an antenna size of approximately 570 Chl (a and b) molecules (Melis, 1996). The remaining 64% of the functional PSII were of the PSIIβ-type with an antenna size of approximately 150 Chl (a and b) molecules. This translated into an average PSII antenna size of about 300 Chl (a+b) molecules (Table II). In contrast to the control, cells treated with 1 mm gabaculine contained PSII with a uniform Chl antenna size of about 132 Chl molecules. Thus, the PSII Chl antenna size of 132 in the gabaculine-treated cells was substantially smaller than that of the control cells (300). Interestingly, the PSI Chl antenna size of 220 in the gabaculine-treated cells was essentially the same as that of the control cells (228), suggesting enlargement of the PSI Chl antenna size in the presence of gabaculine.
Table II.
Chl antenna sizes of PSII and PSI in D. salina after a 24-h incubation under LL conditions, either in the absence (control) or in the presence of 1 mm gabaculine
| Parameter | HL | Control | 1 mm Gabaculine |
|---|---|---|---|
| PSIIα | – | 566 ± 40 | – |
| PSIIβ | – | 151 ± 13 | – |
| PSII | 55 ± 10 | 300 ± 23 | 132 ± 2 |
| PSI | 112 ± 11 | 228 ± 6 | 220 ± 15 |
Thylakoid membranes were isolated from D. salina as described in “Materials and Methods.” Data are representative of at least three independent membrane preparations. Values shown are means ± se.
Measurements of Photosynthetic Capacity
Information about the capacity of photosynthesis can be obtained from the light-saturation curve (the so-called photosynthesis-versus-irradiance curve), in which the rate of O2 evolution is measured and plotted as a function of the actinic light intensity. In these measurements, the rate of O2 evolution first increases linearly with irradiance and then levels off as the saturating irradiance (Is) is approached. The light-saturated rate (Pmax) provides a measure of the capacity of photosynthesis for the particular sample (Powles and Critchley, 1980).
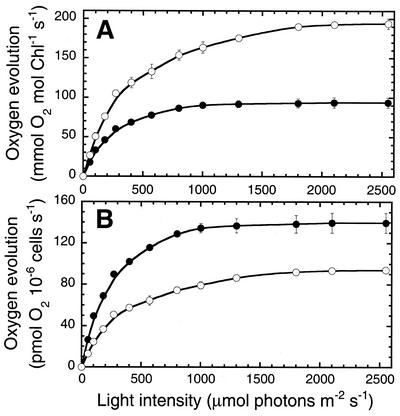
Figure 6A shows light-saturation curves of photosynthesis in D. salina for the control and gabaculine-treated cells, measured 24 h after an HL → LL shift. The control culture showed a Pmax of approximately 90 mmol O2 mol−1 Chl s−1, whereas that of the gabaculine-treated cells was about 2-fold greater (Pmax= approximately 200 mmol O2 mol−1 Chl s−1). The gabaculine-treated cells showed a saturating irradiance significantly greater than that of the control, consistent with a smaller Chl antenna size for PSII. Figure 6B shows the light-saturation curves of photosynthesis plotted on a per cell basis. The control culture had a Pmax of approximately 140 pmol O2 10−16 cells s−1, whereas that of the gabaculine-treated cells was about 35% lower (Pmax = approximately 95 pmol O2 10−16 cells s−1).
Figure 6.
The light-saturation curve of photosynthesis in control and gabaculine-treated D. salina. A, Rates of oxygen evolution on a per Chl basis were measured as a function of incident light intensity. B, Rates of oxygen evolution on a per cell basis were measured as a function of incident intensity. HL-grown cells were incubated under LL growth conditions for 24 h, either in the absence (control; ●) or in the presence (○) of 1 mm gabaculine. Data are means from two independent experiments with n = 3. Error bars represent sd.
An interesting observation derived from these measurements is that the half-saturation intensity of photosynthesis and the Pmax level appear to depend on the Chl antenna size and relative concentration of PSII, respectively, and not on that of PSI. For example, the Pmax amplitude in Figure 6A closely matches the relative QA content (either on a per Chl or cell basis) but not that of P700 in the cells (Table II). Similarly, the half-saturation intensity of photosynthesis is inversely proportional to the Chl antenna size of PSII but not to that of PSI (Table II). These results show that functional PSII singularly determines the properties of the light-saturation curve of photosynthesis in D. salina.
DISCUSSION
Results in this work showed that inhibition of Chl biosynthesis by gabaculine exerts a differential effect on the recovery of the various photosynthetic apparatus parameters from irradiance stress. In the presence of gabaculine, and after an HL → LL shift, the initial rate of Chl biosynthesis was inhibited by about 70% compared with that of the control. Despite the substantially smaller amounts of newly synthesized Chl, the Chl a to Chl b ratio of gabaculine-treated cells declined with kinetics similar to those of the control (Fig. 1D). The ratio of newly synthesized Chl a to Chl b was estimated to be approximately 4:1 in the control and approximately 1:1 in the gabaculine-treated cells, respectively. Assuming that newly synthesized Chl a molecules are the sole substrate for Chl b biosynthesis, it would appear that a greater fraction of the newly synthesized Chl was shunted toward Chl b biosynthesis in gabaculine-treated D. salina. In this case, a slower rate of new Chl biosynthesis in combination with an unimpeded expression of CAO and Lhcb genes may have shifted the Chl a ⇌ Chl b equilibrium toward Chl b, leading to a lower Chl a:Chl b = 1:1 ratio. In HL-grown D. salina, the Chl b to LHC-II ratio was less than 30% of that in LL-grown cells (Tanaka and Melis, 1997; Nishigaki et al., 2000), suggesting vacant Chl b sites. Thus, it is possible that a slanted Chl b biosynthesis in gabaculine-treated samples may serve to fill vacant Chl b positions in pre-existing LHC-II (Polle et al., 2000). Newly synthesized LHC also requires Chl molecules to become properly assembled. In the gabaculine-treated samples, we estimated that newly synthesized LHC-II and LHC-I would require Chl a and Chl b molecules at a Chl a to Chl b ratio of about 2:1. Thus, newly synthesized Chl b molecules in the gabaculine-treated samples may be divided about evenly between filling vacant Chl b positions in existing LHC and serving in the assembly of de novo synthesized LHC-II and LHC-I.
An alternative consideration by which to explain the low newly synthesized Chl a:Chl b = 1:1 ratio in gabaculine-treated samples in to invoke conversion of pre-existing Chl a molecules to Chl b. These Chl a molecules probably had become incorporated into light-harvesting proteins in the HL-acclimated D. salina. In higher plants, it has been shown that redistribution of pre-existing Chl molecules occurs from LHC-II proteins to the photosystem reaction center proteins, because the latter have a higher affinity for Chl a and priority of assembly over that of the LHC-II (Tanaka et al., 1990). It is possible that something analogous occurs after an HL → LL shift in the presence of gabaculine, whereby pre-existing Chl a are converted into Chl b.
It is important to note that gabaculine did not affect adjustments in cellular carotenoid content and xanthophyll de-epoxidation state, which were similar to that of the control in D. salina (Fig. 2). In contrast, it was reported that gabaculine in wheat (Triticum aestivum) decreased neoxanthin and β-carotene levels as well as Chl a and Chl b in primary and secondary leaves of etiolated and greened seedlings (Duysen et al., 1993). This discrepancy can be explained by the longer incubation of gabaculine in wheat (7 d) than in our experiment (24 h), and by the relatively greater stability of LHC-II proteins in D. salina than in higher plants (see below). It is apparent from this work that biosynthesis of carotenoids and activity of the xanthophyll cycle in D. salina were not significantly affected by the lack of Chl availability.
Inhibition of Chl biosynthesis by gabaculine did not affect the repair of PSII from photoinhibition (Fig. 5D, Table I). In contrast to the rapid repair of D1 (half-time of 40 min, Fig. 3), it has been reported that, after an HL → LL shift, increases in PSII, PSI and LHC-II per cell occurred with half-times of 3 to 4 h, approximately 12 h and approximately 16 h, respectively (Webb and Melis, 1995; Neidhardt et al., 1998), consistent with the notion of a distinct hierarchy in the temporal order of D1 repair > PSII > PSI > LHC-II assembly. Under limited Chl biosynthesis in the presence of gabaculine, however, this hierarchy appears to have been altered with de novo LHC-II biosynthesis and assembly advancing relative to that of PSII and PSI in the order of preference.
It was further shown that levels of Lhcb and CAO mRNA were rapidly induced and reached a high steady state within 1.5 h after an HL → LL shift (Fig. 4), which coincided with a period of rapid LHC-II apoprotein accumulation in D. salina (LaRoche et al., 1990b; Webb and Melis, 1995). We found that induction of CAO and Lhcb genes is caused by transcriptional activation, rather than by enhancement of mRNA stability. Thus, a larger Chl antenna size occurs by coordinate induction of Chl biosynthesis and Lhcb and CAO gene expression. Gabaculine did not inhibit the Lhcb and CAO mRNA accumulation, consistent with the observation that this inhibitor did not affect rates of Chl b biosynthesis. This result suggests that in D. salina, the conversion of Chl a to Chl b and the regulation of the Chl antenna size strongly depend on CAO gene expression, and that such regulation occurs independently from the regulation of Chl biosynthesis. Regulation of CAO and Lhcb gene expression may occur mechanistically via the redox state of the plastoquinone pool (Escoubas et al., 1995; Wilson and Huner, 2000; Huner et al., 1998). Regulation of Chl biosynthesis may occur, conversely, via accumulated Chl biosynthesis intermediates (Johanningmeier and Howell, 1984; Johanningmeier, 1988; Kropat et al., 1997; Kropat et al., 2000).
Measurement of the functional Chl antenna size for PSII and PSI provided further insight into the assembly of the photochemical apparatus in gabaculine-treated cells. After an HL → LL shift, the Chl antenna size of PSII increased from 55 to 300 Chl molecules in the control, whereas in the gabaculine-treated cells, increase in the PSII Chl antenna size was limited, i.e. from 55 to 130 Chl molecules. This result demonstrates that, in gabaculine-treated cells, only a limited complement of the LHC-II was assembled. In higher plants, LHC-II has been considered to bind a fixed number of Chl a and Chl b molecules. As a consequence, lack of sufficient Chl b in the chloroplast prevented proper folding of the LHC-II, thereby leading to LHC-II protein degradation (Bennett, 1981; Bellamare et al., 1982; Ghirardi et al., 1986). However, in D. tertiolecta, it was reported that the Chl a to Chl b ratio of isolated LHC-II was variable and that this ratio changed in response to growth irradiance (Sukenik et al., 1987). Moreover, recent results have suggested the assembly of inner complements of the LHC-II in the absence of Chl b in green algae (Nishigaki et al., 2000; Polle et al., 2000). These studies indicated a relative stability of LHC-II proteins in green algae without the full complement of Chl molecules, resulting in a variable Chl to LHC-II ratio in the thylakoid membrane.
In summary, our results show that in D. salina, after an HL → LL shift, cell recovery from photoinhibition and Chl antenna size increase were differentially affected upon inhibition of Chl biosynthesis by gabaculine. The repair of PSII was minimally affected by a limited de novo biosynthesis of Chl. However, de novo assembly/accumulation of PSI was suppressed because of the lack of Chl. Moreover, although the transcription of Lhcb and CAO genes was not affected by gabaculine, biosynthesis and assembly of the full complement of the LHC-II was prevented by the limited amount of Chl, suggesting that the Chl antenna size of PSII is posttranscriptionally regulated by Chl availability.
MATERIALS AND METHODS
Cell Growth Conditions
The unicellular green alga Dunaliella salina Teod. (UTEX collection; Starr, 1978) was grown photoautotrophically in an artificial hypersaline medium (Pick et al., 1986) in the presence of 25 mm NaHCO3 as a supplemental inorganic carbon source. Cells were grown in flat bottles (3-cm optical path length) at 30°C under continuous illumination at 2,200 μmol photons m−2 s−1 (HL). Care was exercised, by means of shaking and by the use of reflectors, to ensure as uniform illumination to the culture as possible. Cells were grown until the late-exponential growth phase and then transferred to LL conditions (50 μmol photons m−2 s−1) with or without the addition of gabaculine (1 mm). The number of cells per milliliter of suspension was counted using the improved Neubauer ultraplane (Reichert Co., Buffalo, NY) and an Olympus (Tokyo) BH-2 light microscope at an amplification of 200×.
Photosynthetic Pigment Determination
For Chl measurements, cells or isolated thylakoid membranes were extracted in 80% acetone, and debris were removed by centrifugation at 10,000g for 5 min. The absorbance of the supernatant at 710, 663, and 645 nm was measured with a Shimadzu (Kyoto) UV-160U spectrophotometer. The Chl (a and b) concentration of the samples was determined according to Arnon (1949), with equations corrected as in Melis et al. (1987).
For HPLC analysis, a Hewlett-Packard Series 1100 (Hewlett-Packard, Palo Alto, CA) equipped with a Waters (Waters, Milford, MA) Spherisorb S5 ODS1 4.6- × 250-mm cartridge column was used. Five mL of cell culture was harvested by centrifugation, and pigments were extracted from the cells by adding 200 μL of 100% (v/v) acetone to the pellet and vortexing at maximum speed for 1 min. The extract was centrifuged in a microfuge and 15 μL of the filtered supernatant (0.2-μm nylon filter) was subjected to HPLC analysis. The latter was performed using a modification method of Garcia-Plazaola and Becerril (1999). Pigments were eluted with a linear gradient of solvents, beginning with 100% (v/v) of solvent A (acetonitrile:methanol:0.1 m Tris-HCl [pH 8.0]; 84:2:14) and ending with 100% (v/v) of solvent B (methanol:ethyl acetate; 68:32). The solvent flow rate was 1.2 mL min−1 and the elution lasted for 15 min, followed by 3 min of elution by solvent B. Pigments were detected by A445, with a reference at 550 nm. Concentrations of individual pigments were determined using standard curves of purified pigments (VKI, Hørsholm, Denmark) at known concentrations.
RNA Isolation and Northern Hybridization
Thirty to 50 mL of cell culture (approximately 2 × 106 cells mL−1) was harvested by centrifugation and the total RNA was isolated with S.N.A.P. total RNA isolation kit (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Ten to 20 μg of RNA/lane was fractionated by electrophoresis through 1% (w/v) agarose/formaldehyde gels and then transferred to nylon membrane. RNA blots were probed with a 1-kb EcoRI fragment containing a cDNA of Lhcb gene (pDTcab1) cloned from D. tertiolecta (accession no. M35860; LaRoche et al., 1990a), with a 1.9-kb XhoI-EcoRI fragment containing a CAO gene cDNA cloned from D. salina (accession no. AB021312), or with a 0.4-kb fragment containing a Chl a synthetase gene (CHLG) from Chlamydomonas reinhardtii (accession no. AV623758). For the Lhcb and CAO probes, hybridizations were carried out at 65°C for 16 h, and the membranes were washed twice with 2 × SSC/0.1% SDS at 65°C for 15 min, and twice with 0.2× SSC/0.1% SDS at 65°C for 15 min. For the CHLG probe, hybridization and washing was carried out at 55°C. The relative amounts of mRNA were estimated by densitometric scanning of the autoradiograms. Peak areas from the densitometric scans were used to calculate the relative abundance for each sample.
Thylakoid Membrane Isolation
Cells were harvested by centrifugation at 3,000g for 3 min at 4°C. Pellets were resuspended in 1 to 2 mL of growth medium and stored frozen at −80°C until all samples were ready for processing. Samples were thawed on ice and diluted with 20 mL of a hypotonic buffer containing 50 mm Tris-HCl (pH 7.8), 10 mm NaCl, 5 mm MgCl2, 0.2% polyvinylpyrrolidone 40, 0.2% sodium ascorbate, 1 mm aminocaproic acid, 1 mm aminobenzamidine, and 0.1 mm phenylmethylsulfonylfluoride. Cells were broken by sonication in a Branson (Danbury, CT) 200 Cell Disruptor operated at 4°C for 30 s at a power output of 5 and a 50% duty cycle. Unbroken cells and starch grains were removed by centrifugation at 3,000g for 3 min at 4°C. The thylakoid membranes were collected by centrifugation of the supernatant at 75,000g for 30 min at 4°C. The thylakoid membrane pellet was resuspended in a buffer containing 250 mm Tris-HCl (pH 6.8), 20% (w/v) glycerol, 7% (w/v) SDS. Solubilization of thylakoid proteins was carried out upon incubation for 30 min at room temperature, a procedure designed to prevent the formation of protein aggregates during denaturation. Samples were centrifuged in a microfuge for 5 min to remove unsolubilized material.
Protein Analysis by SDS-PAGE and Western Blotting
Samples were brought to room temperature before loading for electrophoresis. Gel lanes were loaded with an equal amount of extract, equivalent to 5 × 106 cells per lane, for staining and western blotting of the PsaA/PsaB and D1 proteins, or the extract of 1.3 × 106 cells per lane for western blotting of LHCII proteins. The proteins were separated electrophoretically in a gel containing 12.5% (w/v) acrylamide without urea (Laemmli, 1970) at a constant current of 9 mA for 16 h. Gels were stained with 1% Coomassie Brilliant Blue R for protein visualization.
Electrophoretic transfer of the SDS-PAGE resolved proteins onto nitrocellulose was carried out for 3 to 5 h at a constant current of 800 mA, in transfer buffer containing 50 mm Tris, 380 mm Gly (pH 8.5), 20% (v/v) methanol, and 1% (w/v) SDS. Identification of thylakoid membrane proteins was accomplished with specific antibodies raised in rabbit against the reaction center D1 protein, the LHC-II apoproteins (Harrison and Melis, 1992; Kim et al., 1993), and PsaA/PsaB proteins (PSI; Kashino et al., 1990). Cross-reaction with the antibodies was visualized by a chromogenic reaction with anti-IgG secondary antibodies conjugated with alkaline phophatase (Bio-Rad Laboratories, Hercules, CA) or by enhanced chemiluminescence western-blotting detection reagents with IgG secondary antibodies conjugated with horseradish peroxidase (Amersham Biosciences, Piscataway, NJ). The amounts of proteins were estimated by densitometric scanning of western blots.
Photosynthesis Measurements
The initial (F0), variable (Fv), and maximum (Fm) Chl fluorescence yields of intact cells were measured at 690 nm. Actinic excitation of the cultures was provided by green light at an incident intensity of 35 μmol photons m−2 s−1 (Melis, 1989). An aliquot from the culture was incubated in the dark for 10 min before the measurement and the Chl fluorescence was recorded in the absence or presence of 3-(3, 4-dichlorophenyl)-1,1-dimethylurea (2.5 μm final concentration).
Oxygen evolution activity of the cells was measured at 22°C with a Clark-type oxygen electrode illuminated with a slide projector lamp. An aliquot of 5-mL cell suspension (2 μm Chl) was transferred to the oxygen electrode chamber. To ensure that oxygen evolution was not limited by the carbon source available to the cells, 100 μL of 0.5 m sodium bicarbonate solution (pH 7.4) was added to the suspension before the oxygen evolution measurements. Measurements of the light-saturation curve of photosynthesis were obtained with the oxygen electrode, beginning with the registration of dark respiration in the cell suspension, and followed by measurement of the rate of oxygen evolution in steps at 55, 100, 180, 270, 400, 570, 800, 1,000, 1,300, 1,800, 2,100, and 2,550 μmol photons m−2 s−1. The rate of oxygen evolution at each light intensity step was recorded for about 2.5 min.
For photochemical reaction center charge separation measurements, thylakoid membranes were isolated as described above with a buffer containing 50 mm Tricine-KOH (pH 8.0), 10 mm NaCl, 5 mm MgCl2, 1 mm aminocaproic acid, 1 mm aminobenzamidine, and 100 μm phenylmethylsulfonylfluoride. The thylakoid membranes were resuspended in a buffer containing 50 mm Tricine-KOH (pH 8.0), 10 mm NaCl, and 5 mm MgCl2. The concentration of the photosystems in thylakoid membranes was estimated spectrophotometrically from the amplitude of the light-minus-dark absorbance difference signal at 700 nm (P700) for PSI, and 320 nm (QA) for PSII (Melis and Brown, 1980). The functional light-harvesting Chl antenna size of PSI and PSII was measured from the kinetics of P700 photooxidation and QA photoreduction, respectively (Melis, 1989).
ACKNOWLEDGMENTS
We thank Dr. I. Enami for the generous gift of the PSI antibodies, Dr. A. Tanaka for the D. salina CAO gene probe, and Dr. K.K. Niyogi for use of the HPLC apparatus.
Footnotes
This work was produced under a U.S. Department of Energy-University of California, Berkeley Cooperative Agreement (no. DE–FC36–00GO10536).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010595.
LITERATURE CITED
- Akoyunoglou G, Argyroudi-Akoyunoglou J. Post-translation regulation of chloroplast differentiation. In: Akoyunoglou G, Senger H, editors. Regulation of chloroplast differentiation. A.R. New York: Liss; 1986. pp. 571–582. [Google Scholar]
- Anderson JM. Photoregulation of the composition, function and structure of thylakoid membranes. Annu Rev Plant Physiol. 1986;37:93–136. [Google Scholar]
- Arnon D. Cooper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Avissar YJ, Beale SI. Biosynthesis of tetrapyrrole pigment precursors: pyridoxal requirement of the aminotransferase step in the formation of δ-aminolevulinate from glutamate in extracts of Chlorella vulgaris. Plant Physiol. 1989;89:852–859. doi: 10.1104/pp.89.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroli I, Melis A. Photoinhibition and repair in Dunaliella salinaacclimated to different growth irradiances. Planta. 1996;198:640–646. doi: 10.1007/BF00262653. [DOI] [PubMed] [Google Scholar]
- Beale SI. Enzymes of chlorophyll biosynthesis. Photosynth Res. 1999;60:43–73. [Google Scholar]
- Bellamare G, Bartlett SG, Chua N-H. Biosynthesis of chlorophyll a/b binding polypeptides in wild type and the chlorina f2mutant of barley. J Biol Chem. 1982;257:7762–7767. [PubMed] [Google Scholar]
- Bennett J. Biosynthesis of the light-harvesting chlorophyll a/bprotein. Polypeptide turnover in darkness. Eur J Biochem. 1981;118:61–70. doi: 10.1111/j.1432-1033.1981.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Butler WL, Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta. 1975;376:116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Cleland RE, Melis A, Neale PJ. Mechanism of photoinhibition: photochemical reaction center inactivation in system II of chloroplasts. Photosynth Res. 1986;9:79–88. doi: 10.1007/BF00029734. [DOI] [PubMed] [Google Scholar]
- Duysen M, Freeman T, Eskins K, Guikema JA. Gabaculine impaired accumulation of pigments and apoproteins in light-harvesting complexes and reaction centers of wheat thylakoids. Photosynthetica. 1993;29:329–339. [Google Scholar]
- Escoubas JM, Lomas M, LaRoche J, Falkowski PG. Light intensity regulation of cabgene transcription is signaled by redox state of the plastoquinone pool. Proc Natl Acad Sci USA. 1995;92:10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskins K, Westhoff P, Beremand PD. Light quality and irradiance level interaction in the control of expression of light-harvesting complex of photosystem II. Plant Physiol. 1989;91:163–169. doi: 10.1104/pp.91.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski PG, LaRoche J. Acclimation to spectral irradiance in algae. J Phycol. 1991;26:8–14. [Google Scholar]
- Garcia-Plazaola JI, Becerril JM. A rapid high-performance liquid chromatography method to measure lipophilic antioxidants in stressed plants: simultaneous determination for carotenoids and tocopherols. Phytochem Anal. 1999;10:307–313. [Google Scholar]
- Ghirardi ML, McCauley SW, Melis A. Photochemical apparatus organization in the thylakoid membrane of Hordeum vulgare wild type and chlorophyll b-less chlorina-f2mutant. Biochim Biophys Acta. 1986;851:331–339. [Google Scholar]
- Harrison MA, Melis A. Organization and stability of polypeptides associated with the chlorophyll a-blight-harvesting complex of photosystem-II. Plant Cell Physiol. 1992;33:627–637. [Google Scholar]
- Hoober JK, Maloney MA, Asbury LR, Marks DB. Accumulation of chlorophyll a/b-binding polypeptides in Chlamydomonas reinhardtiiy-1 in the light or dark at 38°C. Plant Physiol. 1990;92:419–426. doi: 10.1104/pp.92.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner NPA, Oquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998;3:224–230. [Google Scholar]
- Johanningmeier U. Possible control of transcript levels by chlorophyll precursors in Chlamydomonas. Eur J Biochem. 1988;177:417–424. doi: 10.1111/j.1432-1033.1988.tb14391.x. [DOI] [PubMed] [Google Scholar]
- Johanningmeier U, Howell SH. Regulation of light-harvesting chlorophyll-binding protein mRNA accumulation in Chlamydomonas reinhardtii. J Biol Chem. 1984;259:13541–13549. [PubMed] [Google Scholar]
- Kashino Y, Enami I, Satoh K, Katoh S. Immunological cross-reactivity among corresponding proteins of photosystem I and II from widely divergent photosynthetic organisms. Plant Cell Physiol. 1990;31:479–488. [Google Scholar]
- Kim JH, Nemson JA, Melis A. Photosystem II reaction center damage and repair in D. salinagreen alga. Analysis under physiological and irradiance-stress conditions. Plant Physiol. 1993;103:181–189. doi: 10.1104/pp.103.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J, Oster U, Rüdiger W, Beck CF. Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc Natl Acad Sci USA. 1997;94:14168–14172. doi: 10.1073/pnas.94.25.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J, Oster U, Rüdiger W, Beck CF. Chloroplast signalling in the light induction of nuclear HSP70 genes requires the accumulation of chlorophyll precursors and their accessibility to cytoplasm/nucleus. Plant J. 2000;24:523–531. doi: 10.1046/j.1365-313x.2000.00898.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LaRoche J, Bennett J, Falkowski PG. Characterization of a cDNA encoding for the 28.5-kDa LHCII apoprotein from the unicellular marine chlorophyte, Dunaliella tertiolecta. Gene. 1990a;95:165–171. doi: 10.1016/0378-1119(90)90358-x. [DOI] [PubMed] [Google Scholar]
- LaRoche J, Mortain-Bertrand A, Falkowski PG. Light intensity-induced changes in cab mRNA and light harvesting complex II apoprotein levels in the unicellular chlorophyte Dunaliella tertiolecta. Plant Physiol. 1990b;97:147–153. doi: 10.1104/pp.97.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne J, Briantais J-M. Ort DR,Yocum CF, eds, Oxygenic Photosynthesis: The Light Reactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. Photosystem-II heterogeneity; pp. 265–287. [Google Scholar]
- Leong T-Y, Goodchild DJ, Anderson JM. Effects of light quality on the composition, function, and structure of photosynthetic thylakoid membranes of Asplenium australasicum(Sm.) Hook. Plant Physiol. 1985;78:561–567. doi: 10.1104/pp.78.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Laudenbach DE, Huner NPA. Redox regulation of light-harvesting complex II and cab mRNA abundance in Dunaliella salina. Plant Physiol. 1995;109:787–795. doi: 10.1104/pp.109.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A. Spectroscopic methods in photosynthesis: photosystem stoichiometry and chlorophyll antenna size. Philos Trans R Soc Lond B. 1989;323:397–409. [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. [Google Scholar]
- Melis A. Excitation energy transfer: functional and dynamic aspects of Lhc (cab) proteins. In: Ort DR, Yocum CF, editors. Advance in Photosynthesis. 4. Oxygenic Photosynthesis: The Light Reactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 523–538. [Google Scholar]
- Melis A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage? Trends Plant Sci. 1999;4:130–135. doi: 10.1016/s1360-1385(99)01387-4. [DOI] [PubMed] [Google Scholar]
- Melis A, Anderson JM. Structural and functional organization of the photosystems in spinach chloroplasts: antenna size, relative electron transport capacity, and chlorophyll composition. Biochim Biophys Acta. 1983;724:473–484. [Google Scholar]
- Melis A, Brown JS. Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci USA. 1980;77:4712–4716. doi: 10.1073/pnas.77.8.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A, Neidhardt J, Benemann JR. Dunaliella salina(Chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J Appl Phycol. 1999;10:515–525. [Google Scholar]
- Melis A, Spangfort M, Andersson B. Light-absorption and electron transport balance between photosystem-II and photosystem-I in spinach chloroplasts. Photochem Photobiol. 1987;45:129–136. [Google Scholar]
- Mortain-Bertrand A, Bennett J, Falkowski PG. Photoregulation of the light-harvesting chlorophyll protein complex associated with photosystem II in Dunaliella tertiolecta. Plant Physiol. 1990;94:304–311. doi: 10.1104/pp.94.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt J, Benemann JR, Zhang L, Melis A. Photosystem II repair and chloroplast recovery from irradiance stress: relationship between chronic photoinhibition, light-harvesting chlorophyll antenna size and photosynthetic productivity in Dunaliella salina(green algae) Photosynth Res. 1998;56:175–184. [Google Scholar]
- Nishigaki A, Ohshima S, Nakayama K. Characterization of three forms of light-harvesting chlorophyll a/b-protein complexes of photosystem II isolated from the green alga Dunaliella salina. Plant Cell Physiol. 2000;41:591–599. doi: 10.1093/pcp/41.5.591. [DOI] [PubMed] [Google Scholar]
- Pick U, Karni L, Avron M. Determination of ion content and ion fluxes in the halotolerant alga Dunaliella salina. Plant Physiol. 1986;81:92–96. doi: 10.1104/pp.81.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle JEW, Benemann JR, Tanaka A, Melis A. Photosynthetic apparatus organization and function in the wild type and a chlorophyll b-less mutant of Chlamydomonas reinhardtii. Dependence on carbon source. Planta. 2000;211:335–344. doi: 10.1007/s004250000279. [DOI] [PubMed] [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol. 1984;35:15–44. [Google Scholar]
- Powles SB, Critchley C. Effect of light intensity during growth on photoinhibition of intact attached bean leaflets. Plant Physiol. 1980;65:1181–1187. doi: 10.1104/pp.65.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasil O, Adir N, Ohad I. Dynamics of photosystem II: mechanism of photoinhibition and recovery processes. In: Barbar J, editor. The Photosystems: Structure, Function and Molecular Biology. Amsterdam: Elsevier Scientific Publications; 1992. pp. 295–342. [Google Scholar]
- Smith BM, Morrissey PF, Guenther JE, Nemson JA, Harrison MA, Melis A. Response of the photosynthetic apparatus in Dunaliella salina(green alga) to irradiance stress. Plant Physiol. 1990;93:1433–1440. doi: 10.1104/pp.93.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr RC. The culture collection of algae at the University of Texas at Austin. J Phycol. 1978;14:47–100. [Google Scholar]
- Sukenik A, Wyman KD, Bennett J, Falkowski PG. A novel mechanism for regulating the excitation of photosystem II in a green alga. Nature. 1987;327:704–707. [Google Scholar]
- Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci USA. 1998;95:12719–12723. doi: 10.1073/pnas.95.21.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Melis A. Irradiance-dependent changes in the size and composition of the chlorophyll a-b light-harvesting pigment-protein complex in the green alga Dunaliella salina. Plant Cell Physiol. 1997;38:17–24. [Google Scholar]
- Tanaka A, Tsuji H. Calcium-induced formation of chlorophyll b and light-harvesting chlorophyll a/b-protein complex in cucumber cotyledons in the dark. Biochim Biophys Acta. 1982;680:265–270. [Google Scholar]
- Tanaka A, Tsuji H. Formation of chlorophyll-protein complexes in greening cucumber cotyledons in light and then in darkness. Plant Cell Physiol. 1983;24:101–108. [Google Scholar]
- Tanaka A, Tsuji H. Appearance of chlorophyll-protein complexes in greening barley seedlings. Plant Cell Physiol. 1985;26:893–902. [Google Scholar]
- Tanaka A, Yamamoto Y, Tsuji H. Formation of chlorophyll-protein complexes during greening. 2. Redistribution of chlorophyll among apoproteins. Plant Cell Physiol. 1990;32:195–204. [Google Scholar]
- Vasilikiotis C, Melis A. Photosystem II reaction center damage and repair cycle: chloroplast acclimation strategy to irradiance stress. Proc Natl Acad Sci USA. 1994;91:7222–7226. doi: 10.1073/pnas.91.15.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb MR, Melis A. Chloroplast response in Dunaliella salinato irradiance stress: effect on thylakoid membrane protein assembly and function. Plant Physiol. 1995;107:885–893. doi: 10.1104/pp.107.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KE, Huner NPA. The role of growth rate, redox-state of the plastoquinone pool and the trans-thylakoid ΔpH in photoacclimation of Chlorella vulgaristo growth irradiance and temperature. Planta. 2000;212:93–102. doi: 10.1007/s004250000368. [DOI] [PubMed] [Google Scholar]