Abstract
Selenocyanate (SeCN−) is a major contaminant in the effluents from some oil refineries, power plants, and in mine drainage water. In this study, we determined the potential of Indian mustard (Brassica juncea) and muskgrass (a macroalga, Chara canescens) for SeCN− phytoremediation in upland and wetland situations, respectively. The tolerance of Indian mustard to toxic levels of SeCN− was similar to or higher than other toxic forms of Se. Indian mustard treated with 20 μm SeCN− removed 30% (w/v) of the Se supplied in 5 d, accumulating 554 and 86 μg of Se g−1 dry weight in roots and shoots, respectively. Under similar conditions, muskgrass removed approximately 9% (w/v) of the Se supplied as SeCN− and accumulated 27 μg of Se g−1 dry weight. A biochemical pathway for SeCN− degradation was proposed for Indian mustard. Indian mustard and muskgrass efficiently degraded SeCN− as none of the Se accumulated by either organism remained in this form. Indian mustard accumulated predominantly organic Se, whereas muskgrass contained Se mainly as selenite and organic Se forms. Indian mustard produced volatile Se from SeCN− in the form of less toxic dimethylselenide. Se volatilization by Indian mustard accounted for only 0.7% (w/v) of the SeCN− removed, likely because the biochemical steps in the production of dimethylselenide from organic Se were rate limiting. Indian mustard is promising for the phytoremediation of SeCN−-contaminated soil and water because of its remarkable abilities to phytoextract SeCN− and degrade all the accumulated SeCN− to other Se forms.
Selenocyanate (SeCN−) is a major pollutant in effluents from some oil refineries and power plants, and especially in mining wastewater when cyanide leaches selenide (Se2−) ores. Physicochemical methods for the removal of SeCN− have been investigated; these methods include ion exchange and precipitation with Cu, Ag, Au, Cd, Hg, Th, and Pb (Manceau and Gallup, 1997). These methods are, unfortunately, very expensive, often requiring large and toxic amounts of heavy metals to precipitate SeCN−.
A more cost-effective method for the clean up of large volumes of SeCN− may be the use of constructed wetlands, as was shown for oil refinery effluent contaminated with selenite (SeO32−; Hansen et al., 1998). Vegetated flow-through wetland microcosms successfully cleaned up SeCN−-contaminated wastewater from a power plant, removing 79% and 54% (w/v) of the mass of Se and CN−, respectively, from the inflow (S.N. Whiting and N. Terry, unpublished data). However, before vegetated wetlands are used for the remediation of SeCN−-contaminated water, it is essential to determine the fate of the two toxic components of SeCN−, i.e. Se and cyanate (OCN−), in plant tissues and sediments. X-ray absorption spectroscopy (XAS) is an excellent analytical tool for identifying different forms of Se in vivo. In the current study, we have used XAS to determine the fate of SeCN− in Indian mustard (Brassica juncea) and muskgrass (a macroalga, Chara canescens). These two species were selected because they are excellent candidates for the phytoremediation of many different trace elements, including Se, and are potentially important for phytoremediation in two different situations, uplands and wetlands.
Indian mustard is useful for the phytoremediation of contaminated upland soil via phytoextraction, the accumulation of contaminants in biomass (Kumar et al., 1995). Experiments with Se-contaminated agricultural soil have shown that Indian mustard is one of the best plants tested so far for Se phytoremediation, with almost 50% (w/v) of the soil Se removed by the plants in three crops (Bañuelos and Meek, 1990; Bañuelos et al., 1995). Indian mustard was also effective in removing contaminants from water in a process known as rhizofiltration (Dushkenov et al., 1995). In addition to being an excellent candidate for the phytoextraction of Se from soil and water, Indian mustard is also promising for phytovolatilization, i.e. the production of volatile Se from inorganic or organic Se present in contaminated soil and water (Terry et al., 2000). Phytovolatilization has the advantage of removing Se from the site in a relatively nontoxic form (Lin et al., 2000). Dimethylselenide (DMSe), the major volatile form of Se produced by most nonhyperaccumulator plants like Indian mustard, is 500 to 700 times less toxic than selenate or selenite (Wilber, 1980).
Chara sp. are macroalgae that make excellent candidates for metal(loid) phytoremediation because they produce a large biomass under field conditions (Carneiro et al., 1994; Herrera-Silveira, 1994) and bioconcentrate large amounts of trace elements (Ye et al., 2001). Muskgrass in the Allegheny Power Service wetland at Springdale, Pennsylvania, accumulated concentrations of iron and manganese from coal ash leachate that were orders of magnitude higher than vascular plants such as cattail growing in the same wetland (Ye et al., 2001). Muskgrass has also been suggested as a candidate for the remediation of selenate at the Kesterson Reservoir in the San Joaquin Valley, California (Horne, 2000).
The two major objectives of the research described here are to determine whether Indian mustard and muskgrass may be used for the phytoremediation of SeCN− and to determine the metabolic fate of SeCN− in plant tissues. To achieve the first objective, the tolerance of Indian mustard and muskgrass to toxic levels of SeCN− was determined, along with their abilities to phytoextract and phytovolatilize Se when supplied with SeCN−. In this regard, Indian mustard and muskgrass were treated with SeCN− and their biomass production and rates of Se accumulation and volatilization were measured under controlled conditions. With regard to the second objective, XAS was used to determine the fate of SeCN− accumulated in tissues. These data were used to propose a model for SeCN− assimilation in Indian mustard as a model plant.
RESULTS
For plants to be used for SeCN− phytoremediation, they must be able to tolerate high levels (200 μm or 16 mg L−1) of Se similar to or higher than the levels found in SeCN−-contaminated sites. The wastewater from the sour water stripper of a coal gasification plant in Indiana contained SeCN− at 1.4 mg L−1 (S.N. Whiting and N. Terry, unpublished data). When the different Se forms were supplied at 200 μm, Indian mustard seedlings were more tolerant of SeCN− and selenate than selenite, as they had significantly higher fresh weights as compared with those grown with 200 μm selenite (Fig. 1; P < 0.05). Although the root lengths of plants grown with 200 μm SeCN− were not significantly different from those grown in the presence of 200 μm selenate or selenite (P > 0.05), inferences cannot be made regarding Se tolerance because seedlings grown with 200 or 20 μm SeCN− had multiple short roots. Seedlings grown under control conditions or those grown with 20 or 200 μm selenate or selenite had single roots. Seedlings in the 200 μm selenate treatment had significantly longer roots than those in the 200 μm selenite treatment (P < 0.05), suggesting that Indian mustard is more tolerant of toxic levels of selenate than selenite. At 20 μm Se, the fresh weights of Indian mustard grown in the presence of selenate, selenite, or SeCN− were similar to each other, but the root lengths of the SeCN− treatment were significantly shorter than those in the selenate or selenite treatments because they had multiple roots.
Figure 1.
Tolerance of Indian mustard seedlings to selenate, selenite, and SeCN−. Seedlings were germinated in one-half-strength Murashige and Skoog agar containing the different chemical forms of Se at concentrations of 0, 20, or 200 μm. Vertical bars indicate 1 se from the mean, n = 24. Bars showing the same letter code within each graph are not significantly different (P > 0.05).
Indian mustard seedlings rapidly accumulated Se and cyanide in their tissues when supplied with 100 μm SeCN− (197 μg of Se and 32.5 μg of cyanide). In 0.5 h, the seedlings accumulated 15 ± 3 μg Se and 9.9 ± 1.7 μg cyanide. Since plants were shown to take up SeCN−, the next experiments were designed to determine the capacity of Indian mustard and muskgrass for the phytoextraction and phytovolatilization of Se when they were supplied with SeCN−.
Indian mustard showed a remarkable ability to accumulate Se in its tissues when the plants were supplied with 20 μm SeCN− in hydroponic solution. The root Se concentrations reached 554 μg g−1 dry weight after treatment for 5 d with SeCN− in hydroponic solution (Fig. 2). The shoots (harvestable biomass) of the SeCN−-supplied plants accumulated lower concentrations of Se (86 μg g−1 dry weight) as compared with the shoots or roots of the selenate-supplied plants and the roots of the selenite-supplied plants (124–177 μg of Se g−1 dry weight). The accumulation of Se from SeCN− did not involve a bacterial role as was shown earlier for selenate accumulation in Indian mustard (de Souza et al., 1999). Axenic Indian mustard seedlings accumulated 190 ± 22 μg g−1 dry weight of tissue (root + shoot) when they were grown for a 10 d period on agar containing 20 μm SeCN−. When axenic seeds were coated with a mixture of rhizosphere bacteria and germinated on agar containing SeCN−, the seedlings contained 182 ± 17 μg Se g−1 dry weight, a concentration that was not significantly different (P > 0.05) from the similarly treated axenic seedlings.
Figure 2.
Se concentrations in tissues of Indian mustard and muskgrass supplied with selenate (A), selenite (B), or SeCN− (C). All Se species were supplied at 20 μm in hydroponic solution. Vertical bars indicate 1 se from the mean, n = 4. Bars showing the same letter code in all parts of the figure are not significantly different from each other (P > 0.05). The biomass (fresh weight) of the plants did not change over the 5-d exposure to Se. The dry weights at the end of the 5-d period for selenate, selenite, and SeCN−-supplied Indian mustard were 0.58 ± 0.21, 0.62 ± 0.14, and 0.60 ± 0.10, respectively, and for muskgrass were 0.40 ± 0.028, 0.37 ± 0.028, 0.40 ± 0.046, respectively.
Unlike Indian mustard where only the shoots can be easily harvested, all the tissue of muskgrass is harvestable biomass because this macroalga is free living or loosely attached to the substrate. Unfortunately, muskgrass accumulated relatively lower Se concentrations in its tissues when supplied with SeCN−, selenate, or selenite (11, 7, and 30 μg g−1 dry weight), compared with Indian mustard (Fig. 2).
Indian mustard and muskgrass treated with 20 μm SeCN− in hydroponic solution were able to produce volatile Se from SeCN− (Fig. 3). The volatile Se species produced by Indian mustard treated with SeCN−, selenate, or selenite was identified by gas chromatography/mass spectrometry (GC/MS) as DMSe. No dimethyldiselenide (DMDSe) or hydrogen selenide (H2Se) was detected. The production of CH3SeCN cannot be ruled out because its mass is similar to DMSe and it may have had a similar retention time to DMSe on the GC column. Therefore, the rates of Se volatilization by Indian mustard and muskgrass were measured by trapping all volatile Se species produced in an alkaline peroxide solution using the chamber and trap method described earlier (Zayed and Terry, 1992). Volatile Se production by Indian mustard and muskgrass supplied with SeCN−, selenate, or selenite increased linearly with time (Fig. 3). The rate of Se volatilization by SeCN−-supplied muskgrass was not significantly different from that measured from SeCN−-treated Indian mustard or from selenate-supplied macroalgae (Table I). The rate of Se volatilization from SeCN−-supplied Indian mustard was 2-fold higher than the rate obtained when the plant was supplied with selenate (Table I). Selenite-supplied Indian mustard had a significantly higher rate of Se volatilization than SeCN− or selenate-supplied plants (1.3- and 2.8-fold, respectively). Selenite-supplied muskgrass had 10- to 27-fold higher rates than those measured from the Indian mustard treatments and 6- and 13-fold higher than SeCN− or selenate supplied macroalgae, respectively.
Figure 3.
Se volatilization by Indian mustard (A) and muskgrass (B) supplied with selenate, selenite, or SeCN−. All Se species were supplied at 20 μm in hydroponic solution. Vertical bars indicate 1 se from the mean, n = 4. The rates of Se volatilization and the statistical differences between the different lines are presented in Table I. The dry weights of the plants are shown in the legend to Figure 2.
Table I.
Rates of Se volatilization by Indian mustard and muskgrass
| Treatment | Muskgrass | Indian Mustard | ||
|---|---|---|---|---|
| μg Se g−1 dry wt d−1 | R2 | μg Se g−1 dry wt d−1 | R2 | |
SeO
|
0.53 a | 0.9931 | 0.26 c | 0.9847 |
SeO
|
7.11 b | 0.9922 | 0.74 d | 0.9924 |
| SeCN− | 1.16 ad | 0.9769 | 0.54 a | 0.9978 |
These rates are the slopes of the best-fit lines for the data shown in Figure 3. Also shown are the R2 values for the curve fit and the statistical comparison of the different Se volatilization curves shown in Figure 3. Values with the same letter code are not significantly different from each other (P > 0.05).
The fate of the accumulated Se in SeCN−-supplied plants was studied by XAS. The spectrum of a reference solution of SeCN− was very different from that of solutions of selenate or selenite (Fig. 4). The SeCN− edge was similar to that of Se-Met, but showed very different post-edge characteristics. When the XAS spectra of the plant samples were fitted to the references, it was clear that none of the Se accumulated in the tissues of Indian mustard or muskgrass remained in the form of SeCN− (Table II). In Indian mustard, most of the Se in SeCN−-supplied mature plants showed a spectrum very similar to organic Se forms such as Se-Met and selenocystine. Similar results were seen in earlier work where selenite-supplied Indian mustard accumulated organic forms of Se (de Souza et al., 1998). In contrast, selenate-supplied Indian mustard plants transformed very little of the selenate to reduced forms because ATP sulfurylase, which activates selenate for reduction, is a major rate-limiting step in selenate assimilation (Pilon-Smits et al., 1999). The XAS spectra of Se in SeCN−-supplied Indian mustard seedlings that were grown axenically or in the presence of bacteria were similar to each other and similar to the spectra obtained for mature plants (data not shown). These XAS data for seedlings indicate that bacteria do not play a role in SeCN− assimilation by Indian mustard.
Figure 4.
Se K near-edge x-ray absorption spectra of selenium accumulated by Indian mustard and muskgrass supplied with 20 μm SeCN− (top) compared with the spectra for aqueous solutions of selenate, selenite, l-selenocystine (seleno-Cys dimer), and Se-Met, which were used as Se standards.
Table II.
Results of fitting Se K near-edge x-ray absorption spectra of Indian mustard and muskgrass
| Treatment | SeCN− | SeO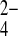
|
SeO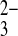
|
SeMet | Selenocystine | R × 10 | |
|---|---|---|---|---|---|---|---|
| Indian mustard | |||||||
| SeCN− | Shoots | 0 | 0 | 0.07 (2) | 0.75 (6) | 0.18 (5) | 0.315 |
| Roots | 0 | 0 | 0.09 (2) | 0.32 (6) | 0.59 (5) | 0.329 | |
| Muskgrass | |||||||
SeO
|
0 | 0.40 (1) | 0.13 (2) | 0.23 (7) | 0.24 (6) | 0.441 | |
SeO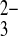
|
0 | 0 | 0.34 (1) | 0.39 (5) | 0.27 (4) | 0.199 | |
| SeCN− | 0 | 0 | 0.45 (2) | 0.31 (6) | 0.24 (6) | 0.351 |
The fractional contribution of standard spectra to the spectrum of the unknown is shown. The values in parentheses are three times the estimated sd of the last figure(s). R is the least-squares residual, Σ(Iobs − Icalc)/N, where Iobs and Icalc are the observed and calculated data, and N is the number of data points. Elemental Se was also included in the fits, but did not contribute. The selenocystine standard is a stable dimer of selenocysteine.
Muskgrass transformed SeCN− in a manner very different from Indian mustard. The Se that had accumulated in the tissues of SeCN−-supplied muskgrass had a spectrum that was fit by equal contributions of selenite and organic Se. This suggests that muskgrass does not produce organic Se as efficiently as Indian mustard. The XAS data from selenite-treated muskgrass support this hypothesis. When selenite was supplied, the XAS spectrum of Se accumulated by muskgrass could be fit with approximately 66% organic Se and 34% selenite. As mentioned above, selenite-supplied Indian mustard accumulated mainly organic Se. Thus, Indian mustard appears to be more effective at converting SeCN− and selenite to organic Se than muskgrass. However, muskgrass was more efficient than Indian mustard at reducing selenate because a significant proportion (approximately 47%) of the Se accumulated in the tissues of selenate-supplied muskgrass could be attributed to organic Se, with approximately 40% selenate and 13% selenite. Thus, selenate reduction does not appear to be rate limiting for Se assimilation in muskgrass, as it appears to be in Indian mustard.
DISCUSSION
Use of Indian Mustard and Muskgrass for the Phytoremediation of SeCN
The results clearly show that Indian mustard and muskgrass are suitable for the phytoremediation of SeCN− in upland and wetland environments, respectively, because they were able to tolerate, take up, and assimilate SeCN− to organic Se forms and less toxic DMSe. SeCN− supplied at a relatively high concentration of 200 μm (16 mg L−1) was not much more toxic to Indian mustard than selenate or selenite supplied at the same concentration, suggesting that plants should be able to tolerate the relatively lower concentrations of SeCN− encountered in contaminated sites; e.g. the wastewater from the sour water stripper of a coal gasification plant in Indiana contained SeCN− at 1.4 mg L−1 (S.N. Whiting and N. Terry, unpublished data).
Indian mustard was much more efficient than muskgrass at removing Se from the supplied SeCN− in the hydroponic solution. SeCN− was supplied at a concentration of 20 μm (1.6 μg of Se mL−1). Because each plant was maintained in 200 mL of hydroponic solution, 320 μg of Se was supplied to each plant. The mass of Se removed from the hydroponic solution by Indian mustard and muskgrass was calculated from the biomass values shown in the legend to Figure 2 and from the tissue Se concentrations and Se volatilization values shown in Figures 2 and 3. Indian mustard roots removed 44 μg, shoots removed 50 μg, and Se volatilization removed 0.7 μg of the Se supplied as SeCN−. Thus, in 5 d, Indian mustard removed approximately 98 μg of Se, i.e. 30% (w/v) of the Se supplied as SeCN−. Muskgrass, however, removed only 9% (w/v) of the 320 μg of SeCN− supplied, with 27 μg of Se phytoextracted into its tissue and 2.3 μg of Se volatilized. Muskgrass is better suited for the phytoremediation of selenite-contaminated water because it removed approximately 13% (w/v) of the Se supplied, approximately 4% and 9% (w/v) by volatilization and phytoextraction, respectively.
Indian mustard was very efficient at accumulating Se from SeCN−-contaminated water because one-third of the Se supplied as SeCN− was accumulated in its tissue. One-half of the mass of Se removed from SeCN−-contaminated solution was removed by phytoextraction into the shoots. The shoots of Indian mustard plants used for the phytoremediation of SeCN−-contaminated soil may be harvested to physically remove Se from the site. For Indian mustard to be used for the phytoremediation of SeCN−-contaminated water, plants may be grown in a rhizofiltration setup where shoots and roots may be harvested.
Muskgrass could be used in constructed wetlands treating SeCN−-contaminated water, but its ability to phytoextract Se over a short period of time was not as efficient as the upland plant, Indian mustard. There may be other wetland species more suited for the phytoremediation of SeCN−-contaminated water. Indian mustard and muskgrass were efficient in degrading SeCN− because XAS revealed that none of the Se accumulated in the tissues of plants treated with SeCN− remained in the form of SeCN−. Thus, plants have good potential for the detoxification of SeCN−, regardless of whether they are an upland or wetland species.
A Possible Mechanism for the Assimilation and Phytodegradation of SeCN
Indian mustard and muskgrass were able to remove the cyanide moiety of SeCN− and convert the Se to organic Se and less toxic DMSe. The biochemical processes that are responsible for the accumulation and assimilation of SeCN− to volatile Se have not yet been elucidated.
Although cyanogenesis is well studied because of the toxicological role of hydrogen cyanide production from cyanogenic glycosides in some food plants (Jones, 1998; Vetter, 2000), OCN− degradation has received little attention. The Brassicaceae are not cyanogenic, but they produce thiocyanate, the chemical analog of SeCN−, as a toxic byproduct of glucosinolate hydrolysis (Fenwick et al., 1983; Angus et al., 1994; Brown and Morra, 1996). The thiocyanate is believed to act as a plant defense mechanism during attack by fungal pathogens or insect pests (Fenwick et al., 1983; Angus et al., 1994). Sulfur from thiocyanate may enter the sulfur assimilation pathway to produce other volatile sulfur gases, e.g. dimethylsulfide (Forney and Jordan, 1998). Thus, it is possible that plants use the biochemical pathways for the conversion of thiocyanate to dimethylsulfide for the assimilation of SeCN− to DMSe. Indian mustard has been shown to assimilate selenate via the sulfate assimilation pathway (Pilon-Smits et al., 1999; Terry et al., 2000). Therefore, a pathway of SeCN− metabolism was proposed in analogy to thiocyanate metabolism in plants (Fig. 5).
Figure 5.
Proposed pathway of SeCN− assimilation by Indian mustard to the volatile Se forms DMSe and H2Se. Also shown is a simplified version of the selenate assimilation pathway for Indian mustard (Terry et al., 2000). The numbers by the arrows represent the enzymes involved. The two major rate-limiting enzymes for selenate assimilation to DMSe are ATP sulfurylase (1) and Met methyltransferase (MMT, 2). Enzyme 3 is a novel thiol methyltransferase (Attieh et al., 2000) that methylates SeCN− to CH3SeCN. Enzyme 4 is unknown or a plant homolog for the bacterial thiocyanate hydrolase. This enzyme degrades SeCN− to volatile H2Se or the Se2− that enters the Se assimilation pathway for DMSe production. OCN− is detoxified by the enzyme cyanase (5), which has been cloned from Arabidopsis (Aichi et al., 1998).
The first step in the proposed SeCN− assimilation pathway is the uptake of SeCN−. Given the rapid accumulation of Se and cyanide into Indian mustard seedlings and the high Se concentrations accumulated in tissue of mature plants after 5 d of treatment, it is possible the SeCN− is accumulated actively in a manner similar to selenate (Leggett and Epstein, 1956) and Se-Met (Abrams et al., 1990).
In the second step, SeCN− may be degraded to Se2− and OCN− by an unknown enzyme, as suggested for bacteria (Youatt, 1954; Happold et al., 1958). OCN− is then degraded to ammonia and CO2 by the enzyme cyanase. The gene encoding cyanase was recently cloned in Arabidopsis (Aichi et al., 1998), which is a member of the Brassicaceae. In an alternate manner, plants could produce Se2− via an alternative pathway of thiocyanate degradation, where the enzyme thiocyanate hydrolase mediates the degradation of thiocyanate to ammonia and carbonylsulfide (Katayama et al., 1992, 1993, 1998). The carbonylsulfide is then degraded to S2− and CO2 by an unknown enzyme.
In the third step, Se2− enters the pathway described for the assimilation of inorganic Se to DMSe (Terry et al., 2000). The incorporation of Se2− into an amino acid backbone provided by O-acetyl-Ser results in the production of seleno-Cys (Ng and Anderson, 1978), which is likely to serve as a precursor of Se-Met (Terry et al., 2000). The XAS data is consistent with this proposed pathway because the Se accumulated by SeCN−-supplied Indian mustard plants was mainly in the form of organic Se forms with no Se remaining in the form of SeCN− (Fig. 4). Se-Met is thought to be methylated by MMT to methylSe-Met, which is cleaved to form DMSe. It was recently shown that MMT is a key enzyme for DMSe production by Arabidopsis plants treated with different forms of Se (A. Tagmount, A. Berken, and N. Terry, unpublished data).
The GC/MS analysis identified DMSe as the major volatile Se form produced by SeCN−-treated Indian mustard. However, the production of volatile CH3SeCN could not be ruled out. Therefore, the pathway of SeCN− metabolism in Figure 5 includes its methylation to CH3SeCN in a manner similar to the detoxification of thiocyanate by a novel thiol methyltransferase produced by cabbage (Brassica oleracea capitata L.; Attieh et al., 2000).
Indian mustard plants accumulated large amounts of organic Se in their tissues when supplied with SeCN− (29% [w/v] of the Se supplied), but they did not produce large amounts of volatile Se (0.7% [w/v] of the SeCN− removed was in the form of volatile Se). It is very likely that one or more biochemical steps in the conversion of organic Se to DMSe is rate limiting, e.g. the methylation of Se-Met by MMT. Transgenic Indian mustard plants that overexpress MMT may overcome the rate limitation and produce more DMSe when treated with SeCN−.
In conclusion, plants such as Indian mustard are promising for the phytoremediation of SeCN−-contaminated soil and water because of their remarkable abilities to phytoextract SeCN− and degrade all the accumulated SeCN− to other Se forms. Furthermore, the tolerance of plants to toxic levels of SeCN− was similar to or higher than other forms of Se and they were able to convert some of the SeCN− to less toxic DMSe, which may be removed from the contaminated site into the atmosphere.
MATERIALS AND METHODS
Seeds of Indian mustard (Brassica juncea; accession no. 173874) were obtained from the North Central Regional Plant Introduction Station (Ames, IA). The ability of Indian mustard to take up SeCN− was determined. Indian mustard seeds were surface sterilized by treatment with 70% (w/v) ethanol for 30 s and 20% (w/v) bleach for 30 min, followed by five washes with sterile water. During these treatments, the seeds were kept in sterile plastic tubes that were closed tightly and kept on a rocking platform to ensure equal access of the bleach or alcohol to all the seeds. After blotting the seeds on sterile filter paper in a laminar flow hood, they were transferred into sterile Magenta boxes containing 50 mL of sterile one-half-strength Hoagland solution (Hoagland and Arnon, 1938) solidified with 0.4% (w/v) Phytagar (Invitrogen, Carlsbad, CA). The boxes were placed under constant light in a controlled environment room that was maintained at 25°C and 40% humidity. After 1 week of growth, a solution of 100 μm SeCN− was added to the boxes, overlaying the agar. After 30 min, the seedlings were gently removed with forceps, washed in running deionized water, and dried at 55°C overnight. One-half of the samples were ground, weighed, and digested for Se analysis as described below. The rest of the samples were analyzed for their total cyanide content by Ana-Lab (Kilgore, TX) using EPA method 9012.
In another experiment, the role of bacteria in SeCN− accumulation by Indian mustard was determined. Seeds were surface sterilized as described above. One batch of axenic seeds was inoculated with three randomly selected strains of bacteria that we had isolated previously from the rhizosphere of Indian mustard (de Souza et al., 1999). The three strains (BJ1, BJ5, and BJ10) were identified as close relatives of Microbacterium saperdae, Pseudomonas monteilii, and Enterobacter cancerogenes, based on >99% similarity in their 16S rDNA sequences (M.P. de Souza and N. Terry, unpublished data). A mixed inoculum of these three strains was prepared to add to the seeds: one loopful of each bacterial strain was added to 5 mL of sterile 0.85% (w/v) saline (NaCl) solution in a sterile plastic tube and vortexed to suspend the bacteria. One milliliter of this suspension was added to 4 mL of sterile 0.5% (w/v) methylcellulose solution (Sigma, St. Louis) prepared with 0.85% (w/v) sterile saline solution, and one batch of axenic seeds were soaked in this methylcellulose solution containing bacteria. An additional tube containing a second batch of seeds and 2.5 mL of sterile methylcellulose provided an axenic control. After 20 min of soaking time, all seeds were removed from the methylcellulose solutions and dried on sterile filter papers in a laminar flow cabinet. The seeds were sown in Magenta boxes containing 50 mL of one-half-strength Hoagland solution containing 0.4% (w/v) Phytagar (Invitrogen) and 0.22-μm filter-sterilized KSeCN (20 μm), which was added just before the media solidified. The boxes were placed under constant light in a controlled environment growth room, and were harvested after 10 d. At harvest, the seedlings were removed from the boxes, rinsed thoroughly in deionized water to remove adhering agar, dried at 80°C, and weighed. The concentrations of Se in the seedlings were determined after acid digestion and analysis by hydride-generation atomic absorption spectroscopy, as described below. To determine the role of microbes in SeCN− assimilation, a separate batch of Indian mustard seedlings was grown similarly, and after harvest, the fresh tissues from axenic and bacteria-treated seedlings were ground in liquid nitrogen. These tissues were then frozen at −80°C for XAS analysis as described below.
To determine the tolerance of Indian mustard seedlings to SeCN−, seeds were surface sterilized as described above and sowed in Magenta boxes containing 50 mL of one-half-strength Murashige and Skoog agar medium (Sigma), 1% (w/v) Suc, and 0.4% (w/v) Phytagar (pH 5.8). After the media had been autoclaved and cooled, sodium selenate, sodium selenite, or KSeCN were added from 0.22-μm filter-sterilized stock solutions to make triplicate Magenta boxes containing 20 and 200 μm Se for each Se species. Eight seeds were sowed in each Magenta box. The boxes were incubated in a controlled environment growth room under constant light. After 7 d, the seedlings were gently removed from the agar, washed to remove any agar sticking to the roots, and the root lengths and fresh weights of individual seedlings were measured.
For the mature plant experiments, Indian mustard seeds were germinated on moistened filter paper and transferred after 2 d into 3.5-inch pots containing coarse sand. The pots were placed in flats that were filled halfway to the top with one-half-strength Hoagland solution and they were maintained in a greenhouse with controlled temperature (25°C–30°C) and a long-day (16-h) photoperiod. The plants were watered twice a day, once with tap water and once with one-half-strength Hoagland solution. After 2 weeks, the plants were carefully removed from the sand to avoid damage to the roots, washed in deionized water, and placed in containers containing 18 L of one-half-strength Hoagland solution for 10 d. The hydroponic solutions were aerated.
Muskgrass (Chara canescens) was collected from the Allegheny Power Services constructed wetland (Springdale, PA; Ye et al., 2001), washed with deionized water, and cultured with aerated one-half-strength Hoagland solution in the greenhouse for 1 week. After 1 week in hydroponic solution, five replicates each of Indian mustard and muskgrass were transferred into one-half-strength Hoagland solutions containing 20 μm Se supplied as sodium selenate, sodium selenite, or KSeCN (Sigma). Conditions of 1 week pretreatment with 20 μm Se were chosen because the kinetics of Se accumulation and Se volatilization by Indian mustard was linear at concentrations between 0.2 and 200 μm Se and up to 14 d when treated with 20 μm Se (de Souza et al., 1998).
After 1 week on Se, one replicate plant from each treatment was washed thoroughly in running deionized water. The muskgrass tissue and the roots and shoots of Indian mustard were ground separately in liquid nitrogen. These tissues were then frozen at −80°C for XAS analysis as described below.
Se volatilization was measured from the other four replicates of each treatment by placing the Indian mustard plants or the muskgrass in Magenta boxes (Sigma) containing 200 mL of one-half-strength Hoagland solution and 20 μm selenate, selenite, or SeCN−. The Magenta boxes were placed in gastight acrylic volatilization chambers (3-L volume) through which a continuous air flow (1.5 L min−1) was passed by applying suction at the outlet and by bubbling incoming air into the hydroponic solution. Volatile Se was quantitatively trapped in alkaline peroxide liquid traps as described previously (Zayed and Terry, 1992). The Se volatilization chambers were placed in the greenhouse. Aliquots (10 mL) of trap solution were collected every 24 h, after which the solutions were replaced. The trap solution samples were heated at 95°C to remove the peroxide. The selenate-Se was reduced to selenite by adding an equal volume of concentrated HCl and heating at 95°C for 30 min. The Se concentration was measured by vapor-generation atomic absorption spectroscopy as described below.
In a separate experiment, GC/MS was used to determine the form of volatile Se given off by Indian mustard. Briefly, axenic seedlings of Indian mustard were grown in 15-mL glass serum vials (Fisher Scientific, Pittsburgh) containing 20 μm SeCN−, selenate, or selenite. The vials were closed with Teflon-faced butyl rubber stoppers (Wheaton, Millville, NJ), crimp sealed, incubated in a growth chamber for 7 d, and frozen at −20°C. After thawing, the gas phase was sampled (100 μL) with a 1710SL gas tight syringe (Hamilton, Reno, NV) and injected into the GC with the injection port split flow off for 10 s then on at 20 mL min−1 thereafter. GC/MS analyses were run on a 4500 mass spectrometer (Thermo Finnigan, San Jose, CA). A 30-m Rtx-5 column (Restek, Bellefonte, PA) was used to separate volatile compounds in the headspace. The column oven was maintained at room temperature for 3 min and was then ramped at 10°C min−1 to 125°C. The mass spectrometer was scanned in selected ion mode looking at two ions for each analyte, e.g. 80 and 82 for H2Se, 94 and 96 for methane selenol (CH3SeH), 107 and 109 for methylselenocyanate (CH3SeCN), 95 and 110 for DMSe, and 188 and 190 for DMDSe. Known standards of DMDSe (Aldrich Chemical, Milwaukee, WI) and DMSe (Alfar Aesar, Ward Hill, MA) were also run along with the samples. There are no commercially available standards for H2Se, CH3SeH, and CH3SeCN, which are three other potential volatile Se forms that may be produced from SeCN−.
After measuring Se volatilization for 5 d, the Indian mustard or muskgrass was washed thoroughly in running water to remove any Se adhering to the tissue, and was dried at 55°C for 3 d. Roots and shoots of Indian mustard and whole tissue of muskgrass were weighed and ground separately using a Wiley mill. The tissues were digested by stepwise additions of 70% (w/v) nitric acid, 30% (w/v) hydrogen peroxide, and concentrated HCl at 95°C in a modification of EPA protocols 3052 B and 7742 (Bañuelos and Pflaum 1990). A wheat flour standard (National Institute of Science and Technology, 1.1 mg of Se kg−1) and a blank were used with all digestions. The Se content in the acid digests of plant tissues or in the acid-treated trap solution was measured by hydride-generation atomic absorption spectroscopy (Martin, 1975). The background Se concentrations were measured in untreated plants and were subtracted from the values obtained for plants treated with Se. The detection limit of this analytical method was 1 μg of Se L−1. Selenium dioxide reference solution (Fisher Scientific) was diluted in 6 m HCL and used as a standard. All samples were diluted in 6 m HCl to give absorbances in the linear portion of the standard curve.
The forms of Se accumulated in the frozen samples of seedlings and mature plants were determined by XAS at the Stanford Synchrotron Radiation Laboratory on Beam Line 4–3. Frozen samples were positioned in a liquid He cryostat at a 45° angle to the x-ray beam. A Si(220) double-crystal monochromator was used with an upstream vertical aperture of 1 mm, and harmonic rejection was achieved by detuning one crystal by 50%. The electron energy was 3.0 GeV with a current of ≈50 to 100 mA. Selenium K-edge x-ray absorption spectra were collected by monitoring the Se Kα fluorescence using a Canberra 13-element Ge detector in a series of replicate scans dependent on trace element concentration. Spectra were also collected for reference solutions, i.e. 10 mm solutions of sodium selenate, sodium selenite, potassium SeCN−, Se-Met, and selenocystine (Sigma). All samples were calibrated against a reference of hexagonal Se(0) collected simultaneously with the data in transmission; the first energy-inflection of the reference was assumed to be 12658.0 eV. Data were collected using the program XAS-Collect (George, 2000) and were analyzed using the EXAFSPAK suite of programs (http://ssrl.slac.stanford.edu/exafspak.html). Quantitative analysis using an edge-fitting method was carried out according to the method of Pickering et al. (1995).
Statistical analyses were performed using the JMP IN statistical package (SAS Institute, Cary, NC) using analysis of variance procedures.
ACKNOWLEDGMENTS
We thank Danika LeDuc for helpful comments on the manuscript, Patricia Fox, Marina Ma, May Zhao, and Morgan Bauer for technical assistance, Zhiqing Lin for collecting the Chara, and the staff of the University of California (Berkeley) herbarium for help with algal identification.
Footnotes
This work was supported by the Cinergy Corporation and by the Electric Power Research Institute (grant nos. W08021–30 and W04163). The XAS analysis was performed at the Stanford Synchrotron Radiation Laboratory, which is funded by the U.S. Department of Energy, Offices of Basic Energy Sciences and Biological and Environmental Research, by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and by the National Institute of General Medical Sciences.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010686.
LITERATURE CITED
- Abrams MM, Shennan C, Zasoski RJ, Burau RG. Selenomethionine uptake by wheat seedlings. Agron J. 1990;82:1127–1130. [Google Scholar]
- Aichi M, Nishida I, Omata T. Molecular cloning and characterization of a cDNA encoding cyanase from Arabidopsis thaliana. Plant Cell Physiol Suppl. 1998;39:S135. [Google Scholar]
- Angus JF, Gardner PA, Kirkegaard JA, Desmarchelier JM. Biofumigation: isothiocyanates released from Brassicaroots inhibit growth of the take-all fungus. Plant Soil. 1994;162:107–112. [Google Scholar]
- Attieh J, Kleppinger-Sparace KF, Nunes C, Sparace SA, Saini HS. Evidence implicating a novel thiol methyltransferase in the detoxification of glucosinolate hydrolysis products in Brassica oleraceaL. Plant Cell Environ. 2000;23:165–174. [Google Scholar]
- Bañuelos G, Pflaum T. Determination of selenium in plant tissue with optimal digestion conditions. Commun Soil Sci Plant Anal. 1990;21:1717–1726. [Google Scholar]
- Bañuelos GS, Meek DW. Accumulation of selenium in plants grown on selenium-treated soil. J Environ Qual. 1990;19:772–777. [Google Scholar]
- Bañuelos GS, Terry N, Zayed A, Wu L. Managing high soil Se with phytoremediation. In: Schuman GE, Vance GF, editors. Selenium, Mining, Reclamation, and Environmental Impact. Gillete, WY: American Society of Surface Mining and Reclamation; 1995. pp. 394–405. [Google Scholar]
- Brown PD, Morra MJ. Hydrolysis products of glucosinolates in Brassica napustissues as inhibitors of seed germination. Plant Soil. 1996;181:307–316. [Google Scholar]
- Carneiro MER, Azevedo C, Ramalho NM, Knoppers B. The biomass of Chara hornemanniiin relation to the physical and chemical conditions of the Piratininga lagoon. An Acad Bras Cienc. 1994;66:213–222. [Google Scholar]
- de Souza MP, Chu D, Zhao M, Zayed A, Ruzin SE, Schichnes D, Terry N. Rhizosphere bacteria enhance Se accumulation and volatilization by Indian mustard (Brassica junceaL.) Plant Physiol. 1999;119:565–573. doi: 10.1104/pp.119.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza MP, Pilon-Smits EAH, Lytle CM, Hwang S, Tai J, Honma TSU, Yeh L, Terry N. Rate limiting steps in Se assimilation and volatilization by Brassica juncea. Plant Physiol. 1998;117:1487–1494. doi: 10.1104/pp.117.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushkenov V, Kumar PBAN, Motto H, Raskin I. Rhizofiltration: the use of plants to remove heavy metals from aqueous streams. Environ Sci Technol. 1995;29:1239–1245. doi: 10.1021/es00005a015. [DOI] [PubMed] [Google Scholar]
- Fenwick GR, Heaney RK, Mullin WT. Glucosinolates and their breakdown products in food and food plants. CRC Crit Rev Food Sci Nutr. 1983;18:123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- Forney CF, Jordan MA. Induction of volatile compounds in broccoli by postharvest hot-water dips. J Agric Food Chem. 1998;46:5295–5301. [Google Scholar]
- George MJ. XAS-Collect: a computer program for X-ray absorption spectroscopic data acquisition. J Synchrotron Rad. 2000;7:283–286. doi: 10.1107/S090904950000683X. [DOI] [PubMed] [Google Scholar]
- Hansen D, Duda P, Zayed AM, Terry N. Selenium removal by constructed wetlands: role of biological volatilization. Environ Sci Technol. 1998;32:591–597. doi: 10.1021/es0260216. [DOI] [PubMed] [Google Scholar]
- Happold FC, Jones GL, Pratt DB. Utilization of thiocyanate by Thiobacillus thioparus and T. thiooxidans. Nature. 1958;182:266–267. doi: 10.1038/182266a0. [DOI] [PubMed] [Google Scholar]
- Herrera-Silveira JA. Phytoplankton productivity and submerged macrophyte biomass variation in a tropical coastal lagoon with ground water discharge. Vie et Mileu. 1994;44:257–266. [Google Scholar]
- Hoagland D, Arnon DI (1938) The water culture method for growing plants without soil. Bull Calif Agric Stat 346
- Horne AJ. Phytoremediation by constructed wetlands. In: Terry N, Bañuelos G, editors. Phytoremediation of Contaminated Soil and Water. New York: Lewis Publishers; 2000. pp. 13–39. [Google Scholar]
- Jones DA. Why are so many food plants cyanogenic? Phytochemistry. 1998;47:155–162. doi: 10.1016/s0031-9422(97)00425-1. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Kanagawa T, Kuraishi H. Emission of carbonyl sulfide by Thiobacillus thioparusgrown with thiocyanate in pure and mixed cultures. FEMS Microbiol Lett. 1993;114:223–228. [Google Scholar]
- Katayama Y, Narahara Y, Inoue Y, Amano F, Kanagawa T, Kuraishi H. A thiocyanate hydrolase of Thiobacillus thioparus: a novel enzyme catalyzing the formation of carbonyl sulfide from thiocyanate. J Biol Chem. 1992;267:9170–9175. [PubMed] [Google Scholar]
- Katayama Y, Narahara Y, Inoue Y, Amano F, Kanagawa T, Kuraishi H. Cloning of genes coding for the subunits of thiocyanate hydrolase of Thiobacillus thioparusTHI 115 and their evolutionary relationships to nitrile hydratase. J Bacteriol. 1998;180:2583–2589. doi: 10.1128/jb.180.10.2583-2589.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PBAN, Dushkenov V, Motto H, Raskin I. Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol. 1995;29:1232–1238. doi: 10.1021/es00005a014. [DOI] [PubMed] [Google Scholar]
- Leggett JE, Epstein E. Kinetics of sulfate adsorption by barley roots. Plant Physiol. 1956;31:222–226. doi: 10.1104/pp.31.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZQ, Schemenauer RS, Cervinka V, Zayed A, Lee A, Terry N. Selenium volatilization from a soil-plant system for the remediation of contaminated water and soil in the San Joaquin Valley. J Environ Qual. 2000;29:1048–1056. [Google Scholar]
- Manceau A, Gallup DL. Removal of selenocyanate in water by precipitation: characterization of copper-selenium precipitate by x-ray diffraction, infrared, and x-ray absorption spectroscopy. Environ Sci Technol. 1997;31:968–976. [Google Scholar]
- Martin TD. Determining selenium in wastewater sediment and sludge by flameless atomic absorption. Atomic Abs Newslett. 1975;14:109–116. [Google Scholar]
- Ng BH, Anderson JW. Synthesis of selenocysteine by cysteine synthases from selenium accumulator and non-accumulator plants. Phytochemistry. 1978;18:573–580. [Google Scholar]
- Pickering IJ, Brown GE, Jr, Tokunaga TK. Quantitative speciation of selenium in soils using X-ray absorption spectroscopy. Environ Sci Technol. 1995;29:2456–2459. doi: 10.1021/es00009a043. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Hwang S, Lytle CM, Zhu Y, Tai JC, Bravo RC, Chen Y, Leustek T, Terry N. Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999;119:123–132. doi: 10.1104/pp.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N, Zayed AM, de Souza MP, Tarun AS. Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:401–432. doi: 10.1146/annurev.arplant.51.1.401. [DOI] [PubMed] [Google Scholar]
- Vetter J. Plant cyanogenic glycosides. Toxicon. 2000;38:11–36. doi: 10.1016/s0041-0101(99)00128-2. [DOI] [PubMed] [Google Scholar]
- Wilber CG. Toxicology of selenium: a review. Clin Toxicol. 1980;17:171–230. doi: 10.3109/15563658008985076. [DOI] [PubMed] [Google Scholar]
- Ye ZH, Whiting SN, Lin ZQ, Lytle CM, Qian JH, Terry N. Removal and distribution of Fe, Mn, Co, and Ni within a constructed wetland treating coal combustion by-product leachate. J Environ Qual. 2001;30:1464–1473. doi: 10.2134/jeq2001.3041464x. [DOI] [PubMed] [Google Scholar]
- Youatt JB. Studies on the metabolism of Thiobacillus thiocyanooxidans. J Gen Microbiol. 1954;11:139–149. doi: 10.1099/00221287-11-2-139. [DOI] [PubMed] [Google Scholar]
- Zayed A, Terry N. Selenium volatilization in broccoli as influenced by sulfate supply. J Plant Physiol. 1992;140:646–652. [Google Scholar]







