Abstract
Shigellosis is a diarrheal disease caused by the gram-negative bacterium Shigella flexneri. Following ingestion of the bacterium, S. flexneri interferes with innate immunity, establishes an infection within the human colon, and initiates an inflammatory response that results in destruction of the tissue lining the gut. Examination of host cell factors required for S. flexneri pathogenesis in vivo has proven difficult due to limited host susceptibility. Here we report the development of a pathogenesis system that involves the use of Caenorhabditis elegans as a model organism to study S. flexneri virulence determinants and host molecules required for pathogenesis. We show that S. flexneri-mediated killing of C. elegans correlates with bacterial accumulation in the intestinal tract of the animal. The S. flexneri virulence plasmid, which encodes a type III secretory system as well as various virulence determinants crucial for pathogenesis in mammalian systems, was found to be required for maximal C. elegans killing. Additionally, we demonstrate that ABL-1, the C. elegans homolog of the mammalian c-Abl nonreceptor tyrosine kinase ABL1, is required for S. flexneri pathogenesis in nematodes. These data demonstrate the feasibility of using C. elegans to study S. flexneri pathogenesis in vivo and provide insight into host factors that contribute to S. flexneri pathogenesis.
The gram-negative bacterium Shigella flexneri is the causative agent of shigellosis, a diarrheal disease which affects up to 150 million people annually (22). The study of shigellosis in vivo has been hampered by the lack of a suitable model system. Unlike humans, mice do not develop intestinal disease upon ingestion of S. flexneri. While recent developments of mouse models using newborn mice (17) and infection paired with intraluminal injection of interleukin-8 (34) are promising, their utility in the study of host cell factors involved in shigellosis remains to be determined.
Although Shigella is believed to specifically infect primates, several reports indicate that invertebrates, such as flies and nematodes, may serve as vectors of the bacterium (5, 12, 16, 25, 28, 41, 42). These results indicate that the host range of the pathogen may be broader than is suspected and open the possibility of using invertebrates to study conserved host-pathogen interactions that can be translated to mammalian systems. Caenorhabditis elegans has become a well-established model invertebrate for the study of bacterial pathogenesis and innate immunity. As in mammals, peristalsis, low pH, lytic enzymes, and antimicrobial substances prevent microbial colonization of the C. elegans intestine. However, pathogenic bacteria are capable of proliferating and killing C. elegans using different mechanisms. The lists of bacterial pathogens that induce nematode killing include both gram-negative pathogens such as Salmonella enterica, Pseudomonas aeruginosa, and Serratia marcescens and gram-positive bacteria such as Streptococcus pneumoniae, Staphylococcus aureus, and Enterococcus faecalis (reviewed in references 1, 4, and 33). The relatively simple innate immune system of C. elegans and the number of traits that facilitate genetic and genomic analysis using this organism, including a hermaphroditic lifestyle and short 2- to 3-week life span, have nurtured rapid advances into the understanding of C. elegans innate immunity during the last few years (26, 30, 31). In addition, the nematode has also been used to study the mechanisms by which specific virulence factors interact with host molecules to promote bacterial pathogenesis (14, 39).
Here we demonstrate that S. flexneri kills C. elegans by an infection-like process that requires live bacteria, correlates with the bacterial accumulation in the intestine of the animals, and requires the S. flexneri virulence plasmid which is crucial for pathogenesis in mammalian systems. In addition, we have shown that C. elegans ABL-1 is required for S. flexneri-induced killing, as loss of ABL-1 through mutation, RNA interference (RNAi) ablation of expression, or pharmacological inhibition results in extension of nematode life span on S. flexneri. These observations suggest that C. elegans can be used to study host-pathogen interactions between S. flexneri and invertebrates, some of which may also take place in mammals.
MATERIALS AND METHODS
Bacterial strains and reagents.
The wild-type strain S. flexneri 2457T was a generous gift from Marcia Goldberg (Harvard University). The virulence plasmid-cured S. flexneri Δinv strain has been described previously (10). S. flexneri was grown on tryptic soy broth (Difco) agar plates containing 0.5% Congo Red. Escherichia coli OP50 (7) and Salmonella. enterica serovar Typhimurium strains SL1344 and SL1344-GFP (3) have been described. All bacterial strains were grown overnight at 37°C in Luria broth (LB). STI571, a generous gift of Brian Druker, was added to the modified nematode growth (NG) agar media, at noted concentrations. Bacterial lawns grown on plates containing STI571 were not visually different from lawns grown on control plates, and growth curves of bacteria grown on LB or LB containing STI571 were not different, indicating that the drug does not have any effect on bacterial growth at the concentrations used in this study.
Nematode strains and maintenance of nematodes.
C. elegans strain Bristol N2 was maintained as hermaphrodites at 20°C, grown on modified NG agar plates (0.35% instead of 0.25% peptone), and fed with E. coli strain OP50 as described previously (36). The abl-1(ok171) mutant strain XR1 was obtained from the Caenorhabditis Genetics Center (University of Minnesota). Worms were observed under a Leica MZ7.5 dissecting microscope. RNAi abl-1 nematodes were prepared by feeding, as described previously (23).
C. elegans killing assays.
Bacterial lawns used for killing assays were prepared by plating 10 μl of the overnight culture on modified NG agar medium in 35-mm dishes. Plates were grown overnight at 37°C and cooled to room temperature before use. Young adult hermaphrodite nematodes were transferred to bacterial lawns and incubated at 25°C. The worms were transferred to fresh lawns each day for 3 days to separate adults from progeny. Worm mortality was scored over time, and a worm was considered dead when it no longer responded to touch. Worms that died as a result of getting stuck to the wall of the plate were excluded from the analysis. The time required for 50% of the nematodes to die (TD50) was calculated using GraphPad Prism software (version 4.01) using the following equation: Y = Bottom + [Top − Bottom]/[1 + 10(log EC50 − X)(Hill slope)], where X is the logarithm of days, Y is the average of dead worms (3), and EC50 is the midpoint of the curve. The TD50 data were analyzed using the paired t test, with a P value of <0.05 denoting a significant result. The relative mortality was calculated using the following equation: (TD50 wild-type nematode on pathogen/TD50 test nematode on pathogen)/(TD50 wild-type nematode on E. coli/TD50 test nematode on E. coli) (2).
Bacterial accumulation assay.
To examine bacterial accumulation, young adult hermaphrodites were transferred to E. coli DH5α-GFP or S. flexneri 2457T-GFP bacterial lawns prepared on modified NG agar plates containing ampicillin (50 μg/ml) and were incubated at 25°C for 48 h. The ampicillin resistance of both E. coli DH5α-GFP or S. flexneri 2457T-GFP is given by the pSMC21 plasmid (6) they carry. Nematodes were then serially transferred to plates containing E. coli OP50, in order to remove any external green fluorescent protein (GFP)-expressing bacteria. The nematodes were washed, lysed in phosphate-buffered saline (PBS) containing 0.1% Triton X-100, and mechanically disrupted using a pestle. The worm lysates were diluted and plated on LB plates containing ampicillin (50 μg/ml). The plates were incubated overnight at 37°C. Colonies were quantified and used to calculate the number of bacteria per nematode. Alternatively, nematodes were anesthetized with sodium azide and analyzed by confocal microscopy, as described previously (3). To examine persistent colonization, young adult hermaphrodites were transferred to E. coli DH5α-GFP, S. enterica 1344-GFP, or S. flexneri 2457T-GFP bacterial lawns prepared on modified NG agar plates containing ampicillin (50 μg/ml) and were incubated at 25°C for 24 h. Nematodes were transferred to lawns of E. coli OP50 for 1 h and then transferred to new plates containing OP50. The worms were transferred to new plates every 24 h and lysed at various time points. The lysates were diluted, plated on LB plates containing ampicillin (50 μg/ml), and examined as described above.
UV treatment of bacteria.
Bacteria on lawns were killed by irradiation using the UV Stratalinker autosetting (1,200 μJ × 100) for 1 min. As a control, a sample of killed bacteria was transferred to LB and grown overnight at 37°C to check for viable bacteria. Experiments were discarded if growth was detected.
RESULTS
S. flexneri killing of C. elegans correlates with bacterial accumulation in the intestine and requires the virulence plasmid.
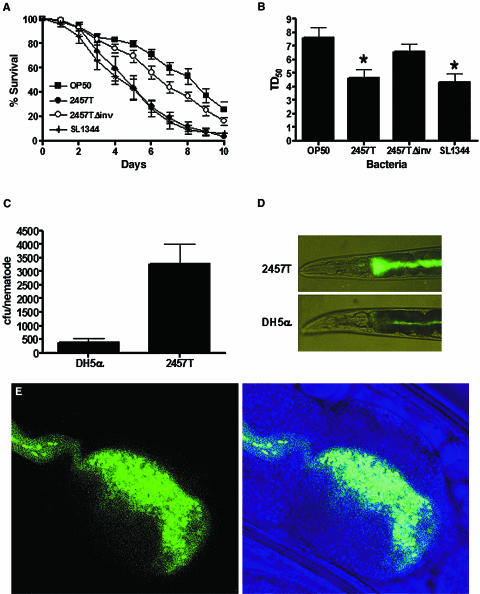
To study S. flexneri interactions with invertebrates, we examined whether S. flexneri was capable of killing C. elegans. As shown in Fig. 1A, wild-type N2 nematodes died more quickly when fed on the S. flexneri strain 2457T than when fed on E. coli OP50, the usual food source for propagating C. elegans in the laboratory. Nematodes grown on S. flexneri die with kinetics similar to those observed for animals infected with S. enterica, which has been previously shown to be pathogenic to C. elegans (3, 27). The TD50 when feeding on S. flexneri lawns (TD50 = 4.64 ± 0.62) was calculated in five independent experiments and determined to be significantly shorter than that for nematodes feeding on E. coli (TD50 = 7.61 ± 0.76) (Fig. 1B).
FIG. 1.
S. flexneri kills C. elegans and accumulates in the nematode intestine. (A) Young adult hermaphrodite N2 nematodes were fed on lawns of either E. coli OP50, S. flexneri 2457T, S. flexneri 2457TΔinv, or S. enterica serovar Typhimurium SL1344 at 25°C, and nematodes were scored daily for survival. The survival curve represents data from 5 independent experiments, each using 20 nematodes. (B) The time required for nematodes to die (TD50) was calculated using the survival data in panel A, representing data from five independent experiments. Asterisks indicate significant differences compared to data for OP50 (P < 0.05). (C) Young adult nematodes were fed on lawns of either E. coli DH5α or S. flexneri 2457T expressing GFP for 48 h and mechanically disrupted. Diluted lysates were plated on LB-ampicillin plates, and colonies were quantified in order to calculate S. flexneri cells associated with individual nematodes. These data represent 3 independent experiments, each using 20 nematodes. (D) Nematodes fed either E. coli DH5α or S. flexneri 2457T expressing GFP for 48 h were analyzed using a fluorescence stereomicroscope. (E) Confocal images show the anterior intestine of an animal fed S. flexneri 2457T expressing GFP for 48 h.
Most of the S. flexneri virulence determinants are located in a large 220-kb virulence plasmid (8, 40) required for full pathogenicity in mammals (32). The S. flexneri virulence plasmid encodes a type III secretory system (TTSS) critical for the translocation of a variety of virulence factors that facilitate bacterial pathogenesis by modulating actin cytoskeleton dynamics and innate immunity. To examine whether the virulence plasmid was required for C. elegans killing, we examined the life span of nematodes fed plasmid-cured S. flexneri (10). Indeed, the nematodes fed the plasmid-cured 2457TΔinv strain exhibited a longer life span (TD50 = 6.56 ± 0.59) than nematodes infected with wild-type S. flexneri, suggesting that the virulence plasmid of S. flexneri is required for full pathogenicity in C. elegans. These observations provide evidence that S. flexneri kills C. elegans using virulence mechanisms important for pathogenesis in mammalians systems and that nematodes may be used to analyze host factors involved in early stages of the pathogenic process.
C. elegans is susceptible to a number of bacterial pathogens, which kill the nematodes using a variety of mechanisms. Under high-osmolarity conditions, Pseudomonas aeruginosa PA14 produces phenazines, which are toxic to the nematode (29). Other bacteria, like S. enterica, establish a persistent infection within the gut of the nematode (3, 27). First we examined whether S. flexneri killing of C. elegans correlates with bacteria accumulation in the intestine. The profile of bacterial accumulation in the gut was examined by scoring the number of live bacteria in the gut and following the accumulation of bacteria expressing GFP in the gut of the animals by direct observation under the fluorescence microscope (3). As shown in Fig. 1C, S. flexneri 2457T accumulated in the nematodes to a much greater extent than E. coli DH5α. Consistent with these results, Fig. 1D shows that after 48 h of continuous feeding on S. flexneri, the intestinal lumen of the nematodes is distended and full of intact bacteria (upper panel). In contrast, reduced numbers of intact bacteria were observed when the worms were fed E. coli DH5α-GFP for 48 h, and the lumen was not as highly distended (Fig. 1D, lower panel). Figure 1E shows a confocal image that indicates that S. flexneri does not invade the intestinal cells of the nematode and remains in the intestinal lumen, which is filled with GFP from disrupted bacteria.
Characterization of the S. flexneri-mediated killing of C. elegans.
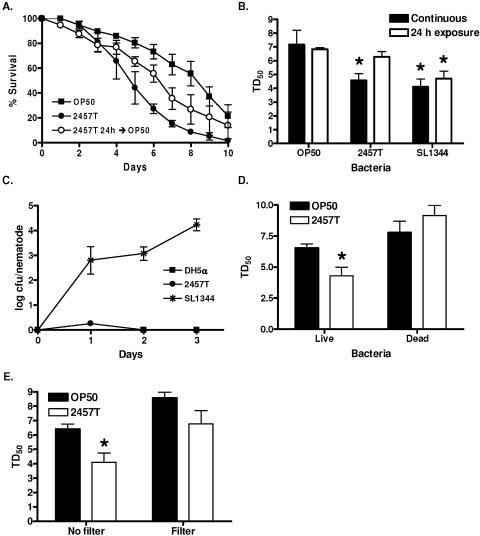
Since we observed N2 nematodes to have a similarly shortened life span when fed S. flexneri or S. enterica, we examined whether S. flexneri-mediated killing resembled the C. elegans killing induced by S. enterica. Short exposures to S. enterica, as little as 5 h, result in a persistent and lethal infection of C. elegans that correlates with bacterial replication in the intestinal lumen (3). To determine whether S. flexneri was capable of persistently colonizing the C. elegans intestine, we exposed C. elegans to S. flexneri for 24 h and then transferred the nematodes back to E. coli OP50 and compared their survival rate to that of nematodes continuously exposed to either E. coli OP50 or S. flexneri 2457T. Nematodes exposed to S. flexneri for 24 h exhibited an extended life span compared to those fed S. flexneri for the duration of the assay (Fig. 2A and B), suggesting that, in contrast to the case with S. enterica, long-term exposure to S. flexneri is required for maximal killing. We also exposed nematodes to either E. coli DH5α, S. flexneri 2457T, or S. enterica SL1344 for 24 h and then transferred back to E. coli OP50. Nematodes were removed every 24 h and mechanically disrupted to release internalized bacteria, which were quantified in a plating assay. While the numbers of internalized S. enterica increased over time, we did not observe a similar increase in either DH5α or S. flexneri (Fig. 2C). Similarly, S. flexneri expressing GFP was not observed in the intestine when nematodes were analyzed by fluorescence microscopy (data not shown), indicating that S. flexneri cannot persistently colonize the intestine of the nematode.
FIG. 2.
The mechanism of S. flexneri-mediated C. elegans killing is distinct from that of S. enterica and P. aeruginosa. (A) Young adult hermaphrodite nematodes were fed on lawns of either E. coli OP50 (squares) or S. flexneri 2457T (closed circles) continuously or fed on S. flexneri 2457T for 24 h and then transferred to OP50 for the duration of the assay (open circles). Nematodes were scored daily for survival. The survival curve represents data from three independent experiments, each using 20 nematodes. (B) The time required for nematodes to die (TD50) was calculated from the survival data in panel A, representing data from three independent experiments. Asterisks indicate significant differences, compared to results for OP50 (P < 0.05). (C) Young adult hermaphrodite nematodes were fed on E. coli DH5α-GFP (squares), S. flexneri 2457T-GFP (circles), or S. enterica serovar Typhimurium SL1344-GFP (asterisks) for 24 h and then transferred to lawns of E. coli OP50. Nematodes were removed every 24 h and mechanically disrupted to release internalized bacteria. Diluted lysates were plated on LB-ampicillin plates, and colonies were scored in order to quantify S. flexneri cells associated with individual nematodes. (D) Young adult hermaphrodites were fed on either live or UV-killed E. coli OP50 or S. flexneri 2457T and scored daily for survival. The time for nematodes to die (TD50) was calculated from the survival data. Asterisks indicate significant differences, compared to results for OP50 (P < 0.05). (E) Young adult nematodes were fed on either E. coli OP50 or S. flexneri 2457T or on E. coli OP50 plated on agar plates incubated with E. coli OP50 or S. flexneri 2457T separated by a 0.45-μm filter. The nematodes were scored daily for survival, and the time required for nematodes to die (TD50) was calculated from the survival data. Asterisks indicate significant differences, compared to results for OP50 (P < 0.05).
We next examined whether S. flexneri might kill C. elegans using a mechanism that involves diffusible toxins. Under conditions of high osmolarity, P. aeruginosa PA14 produces diffusible toxins and remains pathogenic to the nematodes following either antibiotic or heat-mediated killing of the bacteria (29, 38). First we examined whether live S. flexneri was required for C. elegans killing by feeding nematodes on lawns of either live or UV-killed S. flexneri. While nematodes were susceptible to live S. flexneri, there was no corresponding decrease in life span induced by the UV-killed S. flexneri (Fig. 2D). Next we analyzed whether S. flexneri might produce a lethal diffusible toxin. Lawns of E. coli OP50 and S. flexneri 2457T were plated on 0.45-μm filters atop modified NG agar plates, so that any potential toxins produced by the bacteria will diffuse through the filters into the agar. The filters were removed, and E. coli OP50 was added to the plates to prevent starvation. Young adult nematodes were added to the plates and analyzed for shortened life span. As shown in Fig. 2E, we did not observe a decreased life span of the nematodes fed on the S. flexneri 2457T filter plates, suggesting that S. flexneri does not kill C. elegans by producing a potent, diffusible toxin.
Use of the S. flexneri-C. elegans pathogenesis system to study the role of Abl kinases in defense response against S. flexneri infection.
Using a cell culture system, it has been previously demonstrated that loss of Abl kinase activity affects the virulence phenotype of S. flexneri (9, 10). Here we use C. elegans as a whole-animal model host to determine whether the Abl kinases are required for S. flexneri infection in vivo. While there are two mammalian Abl tyrosine kinases, C. elegans has only one homologue, ABL-1 (20). The full-length ABL-1 protein exhibits 32% sequence identity and 62% sequence similarity to human c-Abl. The catalytic domain retains the highest degree of homology, at 67% identity, and retains sensitivity to pharmacological inhibitors, such as STI571. The ABL-1 protein retains the SH2 and SH3 domains to a high degree of similarity but is less conserved at the C terminus (15).
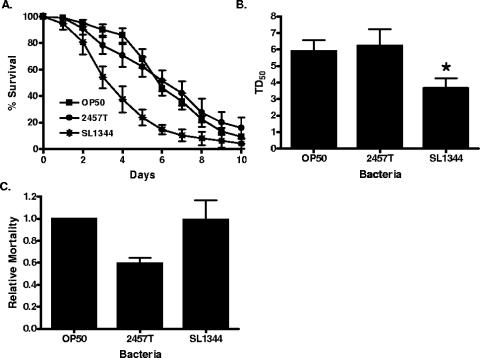
To test the hypothesis that loss of ABL-1 would reduce nematode susceptibility to S. flexneri, we first compared S. flexneri killing of wild-type N2 nematodes to worms carrying the abl-1(ok171) deletion allele. This mutant strain was created by UV irradiation and trimethylpsoralen treatment, resulting in a deletion of exons 8 to 12 and loss of expression of full-length ABL-1 (15). In contrast to N2 nematodes examined within the same experiments (data shown in Fig. 1A and B), the abl-1(ok171) nematodes exhibited no significant difference in life span when fed S. flexneri (TD50 = 6.23 ± 1.00), compared to results with E. coli (TD50 = 5.90 ± 0.68) (Fig. 3A and B). Indeed, the relative mortality of abl-1(ok171) nematodes infected with S. flexneri is 41% lower than the relative mortality of N2 nematodes (Fig. 3C). The relative mortality has the advantage of normalizing any observed change in longevity of the abl-1(ok171) animals feeding on S. flexneri to any change in longevity when feeding on E. coli and, therefore, takes into account changes in life span due to a general modification in fitness rather than a specific defect in innate immunity against S. flexneri. The enhanced resistance to S. flexneri exhibited by abl-1(ok171) animals is specific, since the nematodes remained susceptible to S. enterica (TD50 = 3.67 ± 0.60), and the relative mortality of abl-1(ok171) animals is comparable to that of wild-type animals when infected with S. enterica (Fig. 3A).
FIG. 3.
ABL-1 mutant nematodes are resistant to S. flexneri-mediated C. elegans killing. (A) Young adult hermaphrodite abl-1(ok171) nematodes were fed on lawns of either E. coli OP50, S. flexneri 2457T, or S. enterica serovar Typhimurium SL1344 at 25°C, and nematodes were scored daily for survival. The survival curve represents data from 5 independent experiments, each using 20 nematodes. These experiments also included analysis of N2 nematodes, shown in Fig. 1. (B) The time required for nematodes to die (TD50) was calculated from the survival data in panel A, representing data from three independent experiments. The asterisk indicates significant difference compared to results for OP50 (P < 0.05). (C) The relative mortality was calculated using the TD50 values for N2 and abl-1(ok171) nematodes in order to normalize the survival rates of the nematodes grown on pathogens compared to that of nematodes fed on E. coli OP50 (2).
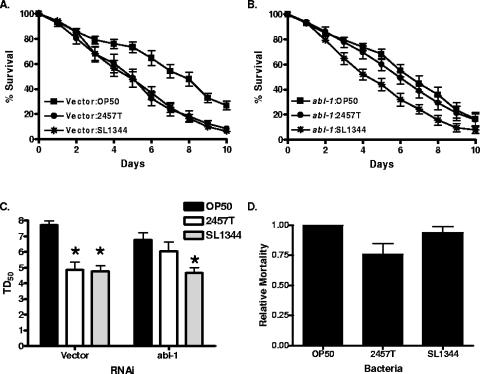
We also examined nematodes in which ABL-1 levels were diminished by RNAi (23) for their susceptibility to S. flexneri. Nematodes fed RNAi vector exhibited a significantly decreased life span when fed S. flexneri (TD50 = 4.84 ± 0.2) than when fed E. coli (TD50 = 7.82 ± 0.15) (Fig. 4A and C). However, nematodes with ablated ABL-1 levels did not show a significant decrease in life span on S. flexneri (TD50 = 5.97 ± 0.7) compared to results on E. coli (TD50 = 6.8 ± 0.2) (Fig. 4B and C). The ABL-1 RNAi nematodes exhibited a 33% decrease in relative mortality on S. flexneri, while there was no significant decrease when fed S. enterica (Fig. 4D), indicating that ABL-1 is specifically required for S. flexneri pathogenesis in C. elegans.
FIG. 4.
ABL-1 knockdown nematodes are resistant to S. flexneri-mediated C. elegans killing. (A and B) Vector (panel A) or abl-1 RNAi (panel B) young adult hermaphrodite nematodes were fed on lawns of either E. coli OP50, S. flexneri 2457T, or S. enterica serovar Typhimurium SL1344 at 25°C, and nematodes were scored daily for survival. The survival curve represents data from 9 independent experiments, each using 20 nematodes. (C) The time for nematodes to die (TD50) was calculated from the survival data in panels A and B. Asterisks indicate significant differences, compared to results for OP50 (P < 0.05), representing data from nine independent experiments. (D) The relative mortality was calculated using the TD50 values for the vector and abl-1 RNAi nematodes in order to normalize the survival rates of the nematodes grown on pathogens compared to that of nematodes fed on E. coli OP50 (2).
ABL-1 kinase activity is required for S. flexneri-mediated killing of C. elegans.
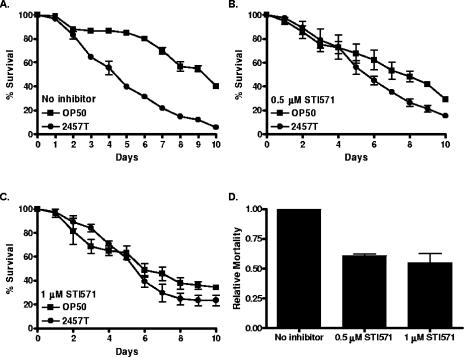
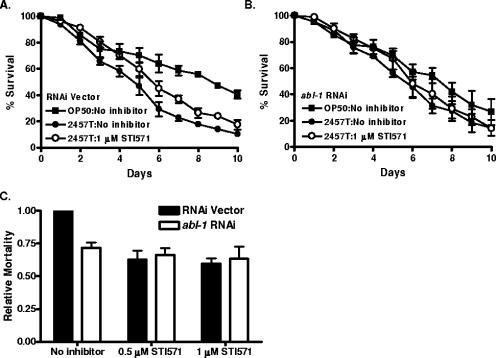
To examine whether ABL-1 kinase activity might be required for S. flexneri-induced killing of C. elegans, we used STI571, a pharmacological inhibitor of the mammalian Abl kinases. STI571 has been demonstrated to inhibit C. elegans ABL-1 (15). STI571 was incorporated into the bacterial media upon which lawns of E. coli and S. flexneri were grown. When N2 nematodes were exposed to S. flexneri, there was an increase in life span of those grown in the presence (TD50 = 5.62 ± 0.23) of 1 μM STI571 compared to results for those grown in the absence of STI571 (TD50 = 4.37 ± 0.14) (Fig. 5A to C). Indeed, S. flexneri feeding in the presence of STI571 reduced the relative mortality of the nematodes by 39% and 45% at the 0.5 μM and 1 μM concentrations (Fig. 5D). To verify that ABL-1 was being specifically targeted by STI571, we analyzed the effect of the inhibitor on nematodes in which abl-1 expression had been inhibited by RNAi. Nematodes fed the RNAi vector had a similar response to untreated N2 nematodes, in that STI571 treatment reduced S. flexneri-induced killing (Fig. 6A and C). However, treatment of abl-1 knockdown nematodes did not result in a significant enhancement of resistance to S. flexneri above that already observed following RNAi ablation of ABL-1 expression (Fig. 6B and C). This observation demonstrates that STI571 prevents S. flexneri-induced killing of the nematodes through the specific inhibition of ABL-1 activity, rather than through a nonspecific mechanism.
FIG. 5.
Nematodes treated with STI571 are resistant to S. flexneri-mediated C. elegans killing. (A to C) Young adult hermaphrodite nematodes were fed on lawns of either E. coli OP50 or S. flexneri 2457T at 25°C in the presence of PBS (A) or 0.5 μM (B) or 1 μM (C) STI571, and nematodes were scored daily for survival. The survival curve represents data from 3 independent experiments, each using 20 nematodes. (D) The relative mortality was calculated using the TD50 values (representing data from three independent experiments) for the PBS and STI571-treated nematodes, in order to normalize the survival rates of the nematodes grown on S. flexneri 2457T compared to that of nematodes fed on E. coli OP50 (2).
FIG. 6.
STI571 is targeting ABL-1 during S. flexneri infection. (A and B) Vector (A) or abl-1 RNAi (B) young adult hermaphrodite nematodes were fed on lawns of either E. coli OP50 or S. flexneri 2457T at 25°C in the presence of PBS (filled symbols) or 1 μM STI571 (open symbols), and nematodes were scored daily for survival. The survival curve represents data from 4 independent experiments, each using 20 nematodes. (C) The relative mortality was calculated using the TD50 values (representing data from four independent experiments) for the PBS and STI571-treated nematodes, in order to normalize the survival rates of the nematodes grown on S. flexneri 2457T compared to that of nematodes fed on E. coli OP50 (2).
DISCUSSION
Our results indicate that the invertebrate C. elegans can be efficiently killed by S. flexneri with kinetics comparable to that of other human pathogens such as S. enterica. Both S. enterica and S. flexneri accumulate within the gut of the nematode; however, S. flexneri does not establish a persistent infection, suggesting that S. enterica and S. flexneri exhibit distinct mechanisms of nematode killing. We have also demonstrated that the S. flexneri virulence plasmid, which encodes a type III secretory system as well as various virulence determinants crucial for pathogenesis in mammalian systems, is required for maximal C. elegans killing (Fig. 1). Since S. flexneri TTSS-related genes are expressed at 37°C but not at 25°C, which is the temperature used to infect C. elegans, it is likely that other S. flexneri virulence-related genes play a role in C. elegans killing. However, since the nematodes are transferred every day to fresh S. flexneri lawns grown at 37°C until day 4 of the assay, we cannot completely rule out the possibility that TTSS-related genes play a role in C. elegans killing.
Moreover, we have demonstrated that the tyrosine kinase ABL-1 is required for maximal S. flexneri pathogenesis in the nematode, as disruption of ABL-1 by mutagenesis, RNAi, or pharmacological inhibition reduces S. flexneri-mediated killing of C. elegans (Fig. 3 to 6). The kinase may act as an inhibitor of innate immune responses against S. flexneri. Although we have shown that germ line apoptosis protects C. elegans from S. enterica infection (2) and abl-1(ok171) animals exhibit higher levels of germ line apoptosis (15), it is unlikely that this is the reason for the increased resistance to S. flexneri of ABL-1-deficient animals. S. flexneri does not induce germ line apoptosis to the extent to which S. enterica does (data not shown), and higher levels of germ line apoptosis do not appear to confer resistance to bacteria (2). In addition, ABL-1-deficient animals are not more susceptible to S. enterica-mediated killing. Another possibility is that ABL-1 is a component of the host machinery required for the activation of S. flexneri virulence factors that enter the C. elegans cells. It has been proposed that IpaC interacts with and activates Src, suggesting that IpaC might be a target of host cell tyrosine kinases (13), and that the TTSS protein Tir from enteropathogenic E. coli is phosphorylated by host cell tyrosine kinases (37). At this point in our investigation, we cannot distinguish between the possibilities that ABL-1 negatively regulates an S. flexneri-specific defense response or that it is required to activate S. flexneri virulence factors.
In addition to the differences in growth temperature between nematodes and mammals, a second limitation of the system to model S. flexneri pathogenesis is the lack of bacterial internalization in the intestinal cells of C. elegans. Despite this limitation, our results indicate that C. elegans can be used to study some aspects of host-pathogen interactions that can be translated to mammalian systems, in addition to interactions that may occur in nature between enteric pathogens and invertebrates. Human enteric pathogens have been found to be associated with free-living nematodes (41, 42), and C. elegans has been suggested to transport Salmonella to fruits and vegetables (11, 24). It is known that Shigella gets in contact with invertebrates such as flies (5, 16, 25, 28) and that it is ingested and defecated by free-living saprozoic nematodes (12). Thus, although the interaction between C. elegans and S. flexneri in the petri dish is artificially caused, the interaction between the two organisms may take place in nature.
In conclusion, we have demonstrated that Shigella kills C. elegans by an active process that requires the virulence plasmid and correlates with the bacterial accumulation in the intestine. Based on the results reported here and various studies that indicate that some of the key features underlying host-pathogen interactions have ancient origins (1, 18, 19, 21, 35), we believe that the C. elegans-S. flexneri pathogenesis system may be useful for studying putative natural interactions between Shigella and invertebrates. The extent to which those interactions can be translated to mammalian systems remains to be determined.
Acknowledgments
This work was supported by NIH training grant CA009111-27 (E.A.B.), NIH grants CA70940 and GM62375 (A.M.P.), and NIH grants GM70977 and AI065641 (A.A.).
REFERENCES
- 1.Aballay, A., and F. M. Ausubel. 2002. Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr. Opin. Microbiol. 5:97-101. [DOI] [PubMed] [Google Scholar]
- 2.Aballay, A., and F. M. Ausubel. 2001. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc. Natl. Acad. Sci. USA 98:2735-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aballay, A., P. Yorgey, and F. M. Ausubel. 2000. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10:1539-1542. [DOI] [PubMed] [Google Scholar]
- 4.Alegado, R. A., M. C. Campbell, W. C. Chen, S. S. Slutz, and M. W. Tan. 2003. Characterization of mediators of microbial virulence and innate immunity using the Caenorhabditis elegans host-pathogen model. Cell. Microbiol. 5:435-444. [DOI] [PubMed] [Google Scholar]
- 5.Bidawid, S. P., J. F. Edeson, J. Ibrahim, and R. M. Matossian. 1978. The role of non-biting flies in the transmission of enteric pathogens (Salmonella species and Shigella species) in Beirut, Lebanon. Ann. Trop. Med. Parasitol. 72:117-121. [DOI] [PubMed] [Google Scholar]
- 6.Bloemberg, G. V., G. A. O'Toole, B. J. Lugtenberg, and R. Kolter. 1997. Green fluorescent protein as a marker for Pseudomonas spp. Appl. Environ. Microbiol. 63:4543-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchrieser, C., P. Glaser, C. Rusniok, H. Nedjari, H. D'Hauteville, F. Kunst, P. Sansonetti, and C. Parsot. 2000. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 38:760-771. [DOI] [PubMed] [Google Scholar]
- 9.Burton, E. A., T. N. Oliver, and A. M. Pendergast. 2005. Abl kinases regulate actin comet tail elongation via an N-WASP-dependent pathway. Mol. Cell. Biol. 25:8834-8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton, E. A., R. Plattner, and A. M. Pendergast. 2003. Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J. 22:5471-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell, K. N., G. L. Anderson, P. L. Williams, and L. R. Beuchat. 2003. Attraction of a free-living nematode, Caenorhabditis elegans, to foodborne pathogenic bacteria and its potential as a vector of Salmonella poona for preharvest contamination of cantaloupe. J. Food Prot. 66:1964-1971. [DOI] [PubMed] [Google Scholar]
- 12.Chang, S. L., G. Berg, N. A. Clarke, and P. W. Kabler. 1960. Survival, and protection against chlorination, of human enteric pathogens in free-living nematodes isolated from water supplies. Am. J. Trop. Med. Hyg. 9:136-142. [DOI] [PubMed] [Google Scholar]
- 13.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242-248. [DOI] [PubMed] [Google Scholar]
- 14.Darby, C., and S. Falkow. 2001. Mimicry of a G protein mutation by pertussis toxin expression in transgenic Caenorhabditis elegans. Infect. Immun. 69:6271-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng, X., E. R. Hofmann, A. Villanueva, O. Hobert, P. Capodieci, D. R. Veach, X. Yin, L. Campodonico, A. Glekas, C. Cordon-Cardo, B. Clarkson, W. G. Bornmann, Z. Fuks, M. O. Hengartner, and R. Kolesnick. 2004. Caenorhabditis elegans ABL-1 antagonizes p53-mediated germline apoptosis after ionizing irradiation. Nat. Genet. 36:906-912. [DOI] [PubMed] [Google Scholar]
- 16.Echeverria, P., B. A. Harrison, C. Tirapat, and A. McFarland. 1983. Flies as a source of enteric pathogens in a rural village in Thailand. Appl. Environ. Microbiol. 46:32-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez, M. I., A. Thuizat, T. Pedron, M. Neutra, A. Phalipon, and P. J. Sansonetti. 2003. A newborn mouse model for the study of intestinal pathogenesis of shigellosis. Cell Microbiol. 5:481-491. [DOI] [PubMed] [Google Scholar]
- 18.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galan, J. E., and A. Collmer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322-1328. [DOI] [PubMed] [Google Scholar]
- 20.Goddard, J. M., J. J. Weiland, and M. R. Capecchi. 1986. Isolation and characterization of Caenorhabditis elegans DNA sequences homologous to the v-abl oncogene. Proc. Natl. Acad. Sci. USA 83:2172-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 22.Jennison, A. V., and N. K. Verma. 2004. Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol. Rev. 28:43-58. [DOI] [PubMed] [Google Scholar]
- 23.Kamath, R. S., and J. Ahringer. 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30:313-321. [DOI] [PubMed] [Google Scholar]
- 24.Kenney, S. J., G. L. Anderson, P. L. Williams, P. D. Millner, and L. R. Beuchat. 2006. Migration of Caenorhabditis elegans to manure and manure compost and potential for transport of Salmonella newport to fruits and vegetables. Int. J. Food Microbiol. 106:61-68. [DOI] [PubMed] [Google Scholar]
- 25.Khin Nwe, O., A. A. Sebastian, and T. Aye. 1989. Carriage of enteric bacterial pathogens by house flies in Yangon, Myanmar. J. Diarrhoeal Dis. Res. 7:81-84. [PubMed] [Google Scholar]
- 26.Kurz, C. L., and J. J. Ewbank. 2003. Caenorhabditis elegans: an emerging genetic model for the study of innate immunity. Nat. Rev. Genet. 4:380-390. [DOI] [PubMed] [Google Scholar]
- 27.Labrousse, A., S. Chauvet, C. Couillault, C. L. Kurz, and J. J. Ewbank. 2000. Caenorhabditis elegans is a model host for Salmonella typhimurium. Curr. Biol. 10:1543-1545. [DOI] [PubMed] [Google Scholar]
- 28.Levine, O. S., and M. M. Levine. 1991. Houseflies (Musca domestica) as mechanical vectors of shigellosis. Rev. Infect. Dis. 13:688-696. [DOI] [PubMed] [Google Scholar]
- 29.Mahajan-Miklos, S., M. W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47-56. [DOI] [PubMed] [Google Scholar]
- 30.Mylonakis, E., and A. Aballay. 2005. Worms and flies as genetically tractable animal models to study host-pathogen interactions. Infect. Immun. 73:3833-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholas, H. R., and J. Hodgkin. 2004. Responses to infection and possible recognition strategies in the innate immune system of Caenorhabditis elegans. Mol. Immunol. 41:479-493. [DOI] [PubMed] [Google Scholar]
- 32.Sansonetti, P. J., D. J. Kopecko, and S. B. Formal. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 35:852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sifri, C. D., J. Begun, and F. M. Ausubel. 2005. The worm has turned—microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13:119-127. [DOI] [PubMed] [Google Scholar]
- 34.Singer, M., and P. J. Sansonetti. 2004. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J. Immunol. 173:4197-4206. [DOI] [PubMed] [Google Scholar]
- 35.Staskawicz, B. J., M. B. Mudgett, J. L. Dangl, and J. E. Galan. 2001. Common and contrasting themes of plant and animal diseases. Science 292:2285-2289. [DOI] [PubMed] [Google Scholar]
- 36.Sulston, J., and J. Hodgkin. 1988. Methods. In W. B. Wood (ed.), The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Swimm, A., B. Bommarius, Y. Li, D. Cheng, P. Reeves, M. Sherman, D. Veach, W. Bornmann, and D. Kalman. 2004. Enteropathogenic Escherichia coli use redundant tyrosine kinases to form actin pedestals. Mol. Biol. Cell 15:3520-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan, M. W., S. Mahajan-Miklos, and F. M. Ausubel. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenor, J. L., B. A. McCormick, F. M. Ausubel, and A. Aballay. 2004. Caenorhabditis elegans-based screen identifies Salmonella virulence factors required for conserved host-pathogen interactions. Curr. Biol. 14:1018-1024. [DOI] [PubMed] [Google Scholar]
- 40.Venkatesan, M. M., M. B. Goldberg, D. J. Rose, E. J. Grotbeck, V. Burland, and F. R. Blattner. 2001. Complete DNA sequence and analysis of the large virulence plasmid of Shigella flexneri. Infect. Immun. 69:3271-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walters, J. V., and R. R. Holcomb. 1967. Isolation of enteric pathogen from sewage-borne nematode. Nematology 13:155. [Google Scholar]
- 42.Wasilewska, L., and J. M. Webster. 1975. Free-living nematodes as disease factors of man and his crops. Int. J. Environ. Stud. 7:201-204. [Google Scholar]