Abstract
We examined how a dietary supplement affects the prevalence of antibiotic-resistant Escherichia coli on a dairy farm in Washington State. Between 2001 and 2004 the prevalence of fecal E. coli strains resistant to streptomycin, sulfadiazine, and tetracycline (SSuT strains) declined from 59.2% to 26.1% in the calf population. In 2003 the dairy discontinued use of a dietary supplement, and we hypothesized that the decline in prevalence of SSuT strains was related to this change in management. To test this we established three treatments in which calves received no supplement, the dietary supplement with oxytetracycline, or the dietary supplement without oxytetracycline. Calves receiving either dietary supplement had a significantly higher prevalence of SSuT E. coli than the no-supplement control group (≈37% versus 20%, respectively; P = 0.03). Importantly, there was no evidence that oxytetracycline contributed to an increased prevalence of fecal SSuT E. coli. We compared the growth characteristics of SSuT and non-SSuT E. coli in LB broth enriched with either the complete dietary supplement or its individual constituents. Both the complete dietary supplement and its vitamin D component supported a significantly higher cell density of SSuT strains (P = 0.003 and P = 0.001, respectively). The dry milk and vitamin A components of the dietary supplement did not support different cell densities. These results were consistent with selection and maintenance of SSuT E. coli due to environmental components independent of antibiotic selection.
Food animal producers rely on management methods to prevent disease outbreaks and increase production. Management can include controlling the environment (temperature, humidity, etc.), providing clean living space, providing good nutrition, using passive transfer if appropriate, and implementing biosecurity measures to block transmission of infectious diseases. Producers also use antimicrobial drugs for prophylactic treatment. Besides potential benefits for preventing disease, antimicrobial drugs have also been shown to increase average daily gains and feed conversion in some food animals (4, 8, 9, 13). Consequently, the potential disease and production benefits have led to widespread use of antimicrobial drugs in food animal production.
Unfortunately, the practice of using antimicrobial drugs for growth promotion and prophylaxis has probably contributed to an increase in the prevalence of antimicrobial-resistant bacteria in food animals, and it may contribute to increased prevalence of resistant bacteria in humans (2, 6, 26, 37). A worst-case scenario would occur if antimicrobial drug resistance genes could be maintained in the commensal flora from animals and these genes could be transferred to bacteria that are pathogenic to both animals and humans (14, 18, 27, 28, 30, 31, 35, 38, 39). This concern has prompted the European Union to ban the use of several antimicrobial drugs and the World Health Organization to suggest cessation of the use of all antimicrobial drugs for growth promotion (3, 17). In theory, the cessation of nontherapeutic drug use will result in lower selection pressure for emergence and maintenance of antimicrobial-resistant bacteria.
While prudent use of antimicrobial drugs has proven to be an effective way to curb rising levels of antimicrobial drug resistance (23, 24, 33), there are many recorded instances where withdrawal of antimicrobial drugs has not affected the prevalence of antimicrobial drug resistance in the population under study (11, 15, 22, 32, 36). One possible explanation for the maintenance of antimicrobial drug resistance in the absence of antimicrobial drug selection is close genetic linkage between resistance genes and other genes that provide significant adaptive advantages for specific niches. It is also possible that resistance genes may convey otherwise unrecognized benefits in a complex environment. Finally, if the resistance genes themselves convey little fitness cost, then only sporadic selection events would be needed to maintain the presence of these genes within a population, and we could expect a long half-life as resistance genes are lost by genetic drift (5).
Our research has focused on understanding the mechanisms that are responsible for maintaining antimicrobial resistance in commensal Escherichia coli at the Washington State University (WSU) dairy. In an earlier study we showed that a dietary supplement containing oxytetracycline was not necessary to maintain the most prevalent antimicrobial resistance phenotype (resistant to streptomycin, sulfadiazine, and tetracycline and susceptible to ampicillin, chloramphenicol, and nalidixic acid [SSuT]) in a dairy herd (22). We then generated null mutants for the antibiotic resistance genes, and using both in vitro and in vivo competition models we found that there was no apparent secondary fitness advantage attributable to the resistance genes themselves (21). Another explanation for maintenance of SSuT strains in the absence of antibiotic selection pressure would be that other closely associated genes convey a selective advantage in dairy calves. Selection in favor of these linked traits may be all that is necessary to maintain the SSuT strains in the population. The question then is, “What traits are likely to play this role?”
In 2003 the WSU dairy discontinued its use of a medicated dietary supplement, and we observed a low prevalence of SSuT strains in 2004 (21). This decline was contrary to our earlier predictions that oxytetracycline is not needed to maintain these strains at the dairy (22). If our earlier predictions were correct, then it is possible that the dietary supplement itself was providing a selective pressure to maintain SSuT strains in this population. The focus of the present study was to formally test this observation, with the implication that success of SSuT strains is most likely due to phenotypic traits that take direct or indirect advantage of the presence of the dietary supplement.
MATERIALS AND METHODS
Comparison of prevalences of antimicrobial drug resistance and patterns in 2001 and 2004.
The WSU dairy (Pullman, Wash.) maintains a closed dairy with all the replacement heifers raised on site. Holstein calves are housed in individual pens in a separate building at 24 to 48 h after birth. A calf ration includes milk (first 4 to 6 weeks of life) and grain supplement. A dietary supplement containing oxytetracycline (5.5 g/kg) was used until winter of 2003. The dietary supplement was composed of spray process grade A nonfat dry milk and vitamins (see below), and 15 to 20 g of dietary supplement was added directly to milk that was fed to the calves. The medicated dietary supplement was used for at least 12 years prior to its cessation in 2003. In two previous studies we examined the prevalences of the antimicrobial drug-resistant E. coli isolated from calves in 2001 and 2004 (21, 22). To examine changes following the withdrawal of the medicated dietary supplement, we used a Student t test to compare the prevalences of antibiotic-resistant E. coli in 2001 (22) and 2004 (21) (NCSS Statistical Software, Kaysville, UT).
Reintroduction of dietary supplement with and without oxytetracycline.
Supplement reintroduction experiments involved 27 neonatal calves that were sequentially assigned to one of the three groups. All calves originated from the WSU dairy. One group received the complete dietary supplement without oxytetracycline (Pennox-50), while a second group received the same dietary supplement with Pennox-50. A third group did not receive any supplement. Calves were housed in individual pens, but nose-to-nose contact with adjacent calves was possible. Treatment calves were intermixed so that there was no systematic interaction between treatments.
The dietary supplement was prepared by mixing 8.3 kg of dry skim milk, 156 g of vitamin D premix, and 241 g of vitamin A-30 premix (all from Thomas Products Inc., Hubbard, OR). The supplement with Pennox-50 also contained 412 g of Pennox-50 (oxytetracycline hydrochloride, equivalent to 110 g/kg; Pennfield Animal Health, Omaha, NE). Calves received 1 tablespoon (15 to 20 g) of dietary supplement per day, which was added directly to the milk at the morning feeding. The supplement was not stirred in the milk. Calves received 2.3 kg of milk at each feeding (twice daily). The final concentration of the oxytetracycline hydrochloride in the milk would be ≈35.6 mg/liter if it was fully dissolved.
Fecal sampling and antimicrobial drug resistance testing.
Each calf was sampled once a week for 3 months. Fecal samples (1 g) were collected with sterile tongue depressors from the floor or rectum and placed in sterile bags. Samples were returned to the laboratory, where they were streaked for isolation on three separate violet red bile agar (Remel Inc., Lenexa, KS)-4-methylumbelliferyl-β-d-glucuronide (MUG) (Biosyth Ag, Switzerland) plates within 4 h of collection and incubated overnight at 37°C. Twenty-one presumptive E. coli colonies (with pink coloration and fluorescence under UV light) per animal (seven per agar plate) were used to inoculate E. coli medium (Remel Inc.) with MUG (Biosyth Ag) broth (200 μl) in a 96-well plate format, leaving 8 to 24 noninoculated negative control wells. Each 96-well plate also included two positive control isolates, Q-89 and Q-90, which were resistant and susceptible to all tested antimicrobial drugs, respectively. The 96-well plates were then incubated (44.5°C, overnight), and the MUG reaction was confirmed under UV light.
Presumptive E. coli colonies were tested for antibiotic drug susceptibility by agar dilution at breakpoint concentrations. These methods have been described in detail and validated in previous work (21, 22). Antimicrobial drug (Sigma) susceptibilities were tested using Mueller-Hinton agar (Hardy Diagnostics) supplemented with ampicillin (16 μg/ml), tetracycline (10 μg/ml), chloramphenicol (16 μg/ml), streptomycin (12 μg/ml), sulfadiazine (512 μg/ml), or nalidixic acid (18 μg/ml). E. coli was inoculated on the antibiotic-supplemented Mueller-Hinton agar plates by using a 96-pin replicator. The replicator was flame sterilized, dipped into the culture plate one time, and then sequentially touched onto each antibiotic agar plate. A final antibiotic-free agar plate was used to confirm uniform inoculum delivery across all plates. The results of replicator assays were recorded after overnight incubation at 37°C. Results for antibiotic drug plates were coded with a dichotomous variable: zero for no growth and one for growth. These results were used to calculate the frequencies for different resistance patterns for each sample. Repeated-measures analysis of variance (ANOVA) was used to examine the prevalence of resistant bacteria among the three treatment groups. Individual calves represented the subject variable, with the sampling result of each week being the repeated measure nested within each calf. Planned comparisons were used to assess the significance of differences among the three treatment groups, where a P value of <0.05 was considered statistically significant (NCSS Statistical Software).
Growth curve analysis of SSuT and susceptible strains in vitro.
Growth curves were generated for SSuT and antimicrobial drug-susceptible E. coli in Luria-Bertani (LB) broth that included the dietary supplement or its components other than oxytetracycline. These included complete dietary supplement (10 g/liter), milk powder (10 g/liter), vitamin A (1 g/liter), or vitamin D (1 g/liter). The complete supplement and separate components were dissolved at 55°C in a water bath and filter sterilized (0.22 μm).
Each well of a 96-well microtiter plate contained 185 μl of appropriate medium and was inoculated with 1.2 μl of culture (prepared in LB at 37°C for 24 h). Each culture was inoculated into 6 or 12 replicate wells to calculate an average value for each time point. Each growth curve was independently replicated three times. The overnight cultures were comprised of a mixture of six different SSuT E. coli strains or a mixture of six different susceptible E. coli strains. The experiments were also repeated using individual SSuT and susceptible E. coli strains (no difference was noted between individual and mixed cultures). For these experiments the different strains were defined by unique macrorestriction patterns from pulsed-field gel electrophoresis, following the methods of Davis et al. (12). Some wells (between 16 and 24) were left uninoculated to control for contamination during the experiments (no contamination was observed). The plate was incubated (37°C) as a stationary culture in a SPECTRAmax 384 PLUS (Molecular Devices) plate reader. The culture was agitated before collection of absorbance (A600) values every 30 min. Absorbance results were plotted to observe growth differences, and a Student t test was used to compare end point absorbance (A600) values at 24 h for the SSuT and susceptible E. coli.
To confirm that absorbance was providing information about the density of viable bacteria, we calculated CFU for the SSuT and susceptible E. coli after 24 h of growth in LB and in LB enriched with dietary supplement. Samples from the cultures grown in the SPECTRAmax were diluted 10−6 and plated onto three replicate LB agar plates. Plates were incubated overnight at 37°C and CFU enumerated. A two-sample Student t test was used to compare the colony counts for SSuT and susceptible strains.
RESULTS
Comparison between 2001 and 2004.
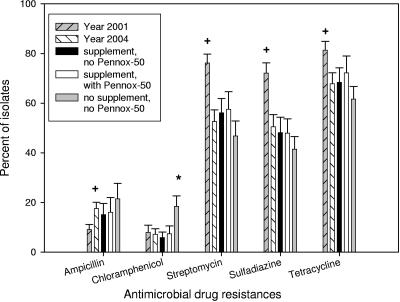
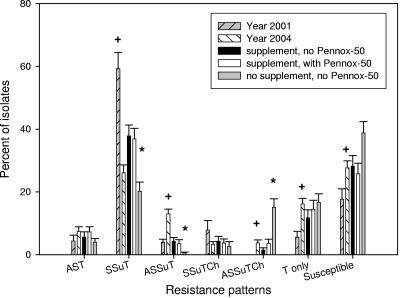
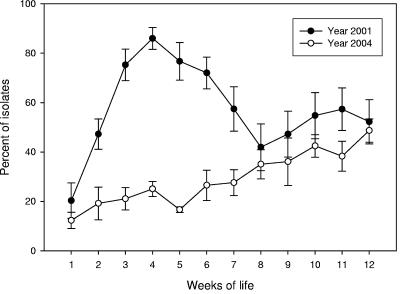
Between year 2001 (22) and year 2004 (21) study control groups there were statistically significant decreases in the prevalences of streptomycin (P < 0.001)-, sulfadiazine (P < 0.001)-, and tetracycline (P = 0.019)-resistant E. coli, but there was no significant change in the prevalence of chloramphenicol-resistant isolates (P = 0.84) and a significant increase in the prevalence of ampicillin-resistant isolates (P < 0.001) (Fig. 1). For this same period the prevalences of E. coli strains resistant to ampicillin, streptomycin, sulfadiazine, and tetracycline (ASSuT strains) (P < 0.001); to ampicillin, streptomycin, sulfadiazine, tetracycline, and chloramphenicol (ASSuTCh strains) (P = 0.002); and to tetracycline only (P < 0.001) and of susceptible strains (P = 0.014) increased significantly, and the prevalences of AST (P = 0.21) and SSuTCh (P = 0.075) E. coli strains did not change significantly, but there was a very significant decrease in the prevalence of SSuT (P < 0.001) E. coli strains (Fig. 2). The decrease in prevalence of SSuT E. coli for 2004 was most marked in the first 40 days of life, a period in 2001 that had the highest number of SSuT E. coli isolates (Fig. 3). The last observation is consistent with the close association of the SSuT E. coli with the presence (2001) or absence (2004) of the dietary supplement.
FIG. 1.
Frequency of antimicrobial drug resistance for all E. coli isolates shed from calves: year 2001 (n = 18 calves), year 2004 (n = 30), group receiving supplement without Pennox-50 (n = 9), group receiving supplement with Pennox-50 (n = 9), and group not receiving supplement or Pennox-50 (n = 9). + and *, statistically significant (P < 0.05 by Student's t test between years and repeated-measures ANOVA test for 2004 experiment, respectively); error bars indicate standard errors.
FIG. 2.
Frequency of antimicrobial drug resistance patterns for all E. coli isolates shed from calves: year 2001 (n = 18), year 2004 (n = 30), group receiving supplement without Pennox-50 (n = 9), group receiving supplement with Pennox-50 (n = 9), and group not receiving supplement or Pennox-50 (n = 9). + and *, statistically significant (P < 0.05 by Student's t test between years and repeated-measures ANOVA test for 2004 experiment, respectively). Resistance patterns are denoted by letters, where A is ampicillin, Ch is chloramphenicol, S is streptomycin, Su is sulfadiazine, and T is tetracycline. Susceptible isolates were susceptible to all above-mentioned antimicrobial drugs tested; error bars indicate standard errors.
FIG. 3.
Distribution of SSuT E. coli isolates over weeks of life for all the calves for year 2001 (n = 18) and year 2004 (n = 30); error bars indicate standard errors.
Results for reintroduction of dietary supplement at the dairy.
Calves were sequentially assigned to three groups that received either dietary supplement without oxytetracycline, dietary supplement with oxytetracycline, or no supplement. Among the three groups, there was no statistically significant difference in the levels of antimicrobial drug resistance to ampicillin, streptomycin, sulfadiazine, and tetracycline, except that the no-supplement group had a significantly higher level of chloramphenicol-resistant E. coli (P = 0.03) (Fig. 1). Comparison of the resistance patterns revealed significantly higher numbers of SSuT E. coli isolates in the two groups that received dietary supplement regardless of presence of oxytetracycline (Fig. 2). There was no statistically significant difference among the three groups for AST, SSuTCh, T-only, and susceptible E. coli, although the no-supplement group had significantly more ASSuTCh and fewer ASSuT E. coli isolates than the two calf groups receiving the dietary supplement.
Growth in LB enriched with dietary supplement.
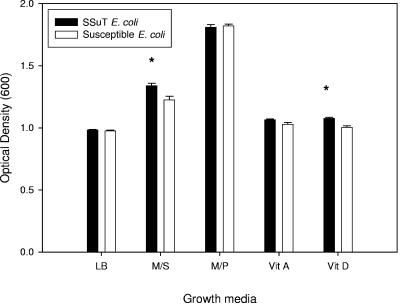
SSuT and susceptible E. coli strains had similar absorbance values at 24 h in LB broth (Student's t test, P = 0.44) (Fig. 4). In all cases the shapes of the growth curves for SSuT and susceptible E. coli were overlapping except in the stationary phase when LB was supplemented with either complete dietary supplement or vitamin D alone. In these cases, SSuT E. coli had a significantly higher absorbance value at 24 than to susceptible E. coli (Student's t test, P = 0.002 and P < 0.001, respectively) (Fig. 4). In all cases where LB included the complete dietary supplement or a supplement component, the absorbance values for both SSuT and susceptible E. coli increased over what was recorded for LB alone (Fig. 4). Thus, these compounds were not inhibitory, but rather the SSuT strains were able to attain a higher cell density than the susceptible strains when grown in LB with the complete dietary supplement or with the vitamin D component.
FIG. 4.
Absorbance values at 24 h of growth for SSuT and susceptible E. coli. LB, LB broth (six replicates for each resistotype); M/S, LB broth supplemented with dietary supplement (not containing oxytetracycline; three replicates for each resistotype); M/P, LB broth supplemented with milk powder (three replicates for each resistotype); Vit A, LB broth supplemented with vitamin A (three replicates for each resistotype); Vit D, LB broth supplemented with vitamin D (three replicates for each resistotype). Error bars indicate standard errors; *, statistically significant difference between SSuT and susceptible E. coli (P < 0.05 by Student's t test).
We compared the CFU at 24 h and verified that the average CFU for SSuT and susceptible E. coli did not differ significantly when the strains were grown in LB alone (1.3 × 109 and 1.3 × 109, respectively; Student's t test, P = 1.0). When grown in LB with complete dietary supplement, the average CFU for SSuT strains (9.7 × 108; 95% confidence interval, 9.0 × 108 to 1.0 × 109) was 19.8% higher than the CFU for susceptible E. coli (8.1 × 108; 95% confidence interval, 7.7 × 108 to 8.5 × 108) (Student's t test, P < 0.001).
DISCUSSION
We examined the effect of a dietary supplement on a population of antimicrobial-resistant, commensal E. coli strains in neonatal calves. The WSU dairy had used a dietary supplement containing oxytetracycline for at least 12 years prior to the winter of 2003, after which management chose to discontinue this practice. A comparison of prevalence data from 2001 (22) and 2004 (21) indicated a significant decline in SSuT strains between the two time points. A logical conclusion would be that removal of the oxytetracycline itself was most likely responsible for the decline in prevalence. This speculation, however, conflicts with an earlier conclusion that oxytetracycline is not necessary to maintain SSuT strains in this population (22). It is possible that the earlier conclusion was valid over the short time frame of that study but that over longer periods there is a selective effect from oxytetracycline. If, however, our earlier conclusion is correct and oxytetracycline was not having a significant impact, then the next obvious source of selection would be the dietary supplement itself. Consequently, the current study was designed to distinguish between effects from oxytetracycline-medicated and unmedicated dietary supplements.
In the current study we found a nearly twofold increase in the prevalence of SSuT strains in the two groups receiving the dietary supplement compared to the group that did not receive any supplement. Importantly, the addition of oxytetracycline had no apparent additive effect on the prevalence of SSuT strains relative to the group receiving the unmedicated supplement, which strengthens the earlier conclusion that oxytetracycline is not necessary to maintain a high prevalence of SSuT strains (22). The calves in each treatment group were housed in separate pens, but nose-to-nose contact was possible. Calves in all three treatment groups were housed within interspersed pens, so it is unlikely that the distribution of SSuT strains between the treatment groups could be explained by direct transmission between calves; otherwise, we would expect no difference between groups. In addition, our previous work at the dairy has shown, via pulsed-field gel electrophoresis, that calves in these interspersed pens can maintain distinct E. coli populations (21).
In vitro experiments suggest that the supplement provides a selective advantage to SSuT strains. The shapes of the growth curves (based on optical density values [data not shown]) showed that there was no obvious difference in the rate of growth, but differences arose as the cultures entered stationary phase. SSuT strains attained a 19.8% higher density of cells at stationary phase than non-SSuT strains. Non-SSuT strains were not inhibited by the presence of the dietary supplement (the optical density did not decline compared with that of the LB control), but they gained no significant advantage in the ability to sustain a higher density of cells. Presumably, the dietary supplement provided SSuT strains with the means to partially overcome an otherwise limiting factor in total population size, and the vitamin D component appears to be responsible for this effect. Interestingly, an inhibitory effect of vitamin D on E. coli and other bacteria in vitro has been documented, although the mechanism by which this occurred was not described (16). It is also possible that this beneficial effect observed in our study was due to unspecified components of the vitamin D additive. While our in vitro experiment demonstrated that SSuT strains can maintain a higher cell density, it is important to note that the in vivo effect may have no relation to this but instead may be associated with other physiological or community ecology changes associated with the dietary supplement.
The relationship between the prevalence of SSuT strains and the dietary supplement may be related to genetic linkage of the SSuT determinants to other genes that confer a selective advantage in the presence of the dietary supplement. Others investigators have reported examples of genetic linkage/association to antimicrobial drug resistance genes. For example, Calomiris et al. (10) found a close association between multiple antibiotic resistance traits and metal tolerance in bacteria isolated from drinking water. Kehrenberg and Schwarz (20) confirmed the physical linkage of three antimicrobial drug resistance genes, explaining the simultaneous occurrence of these resistances in Pasteurella and Manheimia isolates without direct selective pressure. Aarestrup (1) accounted for the persistence of glycopeptide resistance in enterococci from broilers and pigs by genetic linkage between ermB and vanA antibiotic resistance genes, resulting in coselection as a consequence of the continued use of macrolides.
While we found a close association between SSuT strains and withdrawal and introduction of the dietary supplement, this may be a unique case. Some resistance patterns increased between 2001 and 2004 (e.g., ASSuT and T only), although the overall prevalence of tetracycline resistance in fecal E. coli decreased about 10% between 2001 and 2004 (Fig. 1). This slow decay of other tetracycline resistance patterns compared to SSuT suggests either that there are other selective events maintaining antibiotic-resistant E. coli in the absence of oxytetracycline selection pressure or that we are observing a slow “decay” attributable to minimal fitness cost of the antimicrobial resistance genes. Several authors have reported very low “decay rates” after removal of antimicrobial drug pressure (19, 25, 34), and this makes intuitive sense for tetracycline resistance efflux genes because these genes are usually associated with a repressor gene that prevents expression in the absence of a tetracycline analog (7, 40). Thus, the biological cost of harboring tetracycline resistance genes may be small.
While we expect oxytetracycline to negatively affect the prevalence susceptible E. coli, it was interesting that the addition of oxytetracycline to the supplement had no measurable effect above what was observed with the supplement alone. Under idealized conditions, the final concentration of oxytetracycline in the milk should be 35.6 μg/ml, which is much higher than the threshold concentration for susceptible E. coli (≤4 μg/ml). We have demonstrated in vitro that the growth of antimicrobial drug-susceptible E. coli is suppressed in LB broth to which a similar ratio of dietary supplement (containing oxytetracycline) is added, but the growth of SSuT E. coli is uninhibited (data not shown), and given these results we would predict an additional selective advantage for the SSuT strains in the presence of oxytetracycline. In vivo, however, it is possible that the effectiveness of the tetracycline is much lower due to dilution in the intestinal content and chelation of the tetracycline by Ca2+ and Mg2+ cations present in the milk (29). More work is needed to address this issue, because we would expect a similar inactivation of oxytetracycline in vitro given that the supplement is largely dry milk.
In conclusion we have demonstrated that the dietary supplement, and specifically the vitamin D additive, is probably selecting for strains with a specific resistance pattern (SSuT) in the calves at a dairy. Even though the prevalence of SSuT E. coli was influenced significantly by the use of the dietary supplement, the overall prevalence of streptomycin, sulfadiazine, and tetracycline resistances remained relatively high, indicating that the mechanism that maintains the SSuT strains is not universal for other resistance phenotypes at the dairy. Previous experiments have also demonstrated that SSuT E. coli strains are more competitive in the calf intestinal environment than susceptible ones. This supports the hypothesis of a multifactor selective system that maintains a relatively constant level of antimicrobial-resistant bacteria. In the case of the SSuT strains, the primary mechanism for long-term maintenance is most likely related to the presence of a gene(s) that confers a direct and/or indirect selective advantage in the presence of a dietary supplement, and we speculate that this gene is closely linked to the antimicrobial resistance genes.
Acknowledgments
This project received financial support from the Agricultural Animal Health Program, College of Veterinary Medicine, Washington State University, Pullman, and from the USDA (grant NRI 2004-35201-14112).
Special thanks go to J. Swain, M. Krug, S. LaFrentz, E. Kuhn, M. Oatley, R. McClanahan, M. Soule, A. Ramsrud, the WSU Field Disease Investigation Unit, the WSU dairy, and the Animal Resource Unit, Pullman, Wash.
REFERENCES
- 1.Aarestrup, F. M. 2000. Characterization of glycopeptide-resistant Enterococcus faecium (GRE) from broilers and pigs in Denmark: genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J. Clin. Microbiol. 38:2774-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127-137. [DOI] [PubMed] [Google Scholar]
- 3.Aarestrup, F. M., A. M. Seyfarth, H. D. Emborg, K. Pedersen, R. S. Hendriksen, and F. Bager. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45:2054-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, K. L., T. G. Nagaraja, J. L. Morrill, P. G. Reddy, T. B. Avery, and N. V. Anderson. 1988. Performance and ruminal changes of early-weaned calves fed lasalocid. J. Anim. Sci. 66:806-813. [DOI] [PubMed] [Google Scholar]
- 5.Andersson, D. I., and B. R. Levin. 1999. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2:489-493. [DOI] [PubMed] [Google Scholar]
- 6.Aubry-Damon, H., K. Grenet, P. Sall-Ndiaye, D. Che, E. Cordeiro, M. E. Bougnoux, E. Rigaud, Y. Le Strat, V. Lemanissier, L. Armand-Lefevre, D. Delzescaux, J. C. Desenclos, M. Lienard, and A. Andremont. 2004. Antimicrobial resistance in commensal flora of pig farmers. Emerg. Infect. Dis. 10:873-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck, C. F., R. Mutzel, J. Barbe, and W. Muller. 1982. A multifunctional gene (tetR) controls Tn10-encoded tetracycline resistance. J. Bacteriol. 150:633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berge, A. C., P. Lindeque, D. A. Moore, and W. M. Sischo. 2005. A clinical trial evaluating prophylactic and therapeutic antibiotic use on health and performance of preweaned calves. J. Dairy Sci. 88:2166-2177. [DOI] [PubMed] [Google Scholar]
- 9.Braidwood, J. C., and N. W. Henry. 1990. Clinical efficacy of chlortetracycline hydrochloride administered in milk replacer to calves. Vet. Rec. 127:297-301. [PubMed] [Google Scholar]
- 10.Calomiris, J. J., J. L. Armstrong, and R. J. Seidler. 1984. Association of metal tolerance with multiple antibiotic resistance of bacteria isolated from drinking water. Appl. Environ. Microbiol. 47:1238-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaslus-Dancla, E., G. Gerbaud, M. Lagorce, J. P. Lafont, and P. Courvalin. 1987. Persistence of an antibiotic resistance plasmid in intestinal Escherichia coli of chickens in the absence of selective pressure. Antimicrob. Agents Chemother. 31:784-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, M. A., D. D. Hancock, T. E. Besser, and D. R. Call. 2003. Evaluation of pulsed-field gel electrophoresis as a tool for determining the degree of genetic relatedness between strains of Escherichia coli O157:H7. J. Clin. Microbiol. 41:1843-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donovan, D. C., S. T. Franklin, C. C. Chase, and A. R. Hippen. 2002. Growth and health of Holstein calves fed milk replacers supplemented with antibiotics or Enteroguard. J. Dairy Sci. 85:947-950. [DOI] [PubMed] [Google Scholar]
- 14.DuPont, H. L., and J. H. Steele. 1987. Use of antimicrobial agents in animal feeds: implications for human health. Rev. Infect. Dis. 9:447-460. [DOI] [PubMed] [Google Scholar]
- 15.Enne, V. I., D. M. Livermore, P. Stephens, and L. M. Hall. 2001. Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. Lancet 357:1325-1328. [DOI] [PubMed] [Google Scholar]
- 16.Feindt, E., and J. Stroder. 1977. Studies on the antimicrobial effect of vitamin D. Klin. Wochenschr. 55:507-508. [DOI] [PubMed] [Google Scholar]
- 17.Ferber, D. 2003. Antibiotic resistance. WHO advises kicking the livestock antibiotic habit. Science 301:1027. [DOI] [PubMed] [Google Scholar]
- 18.Hirsh, D. C., and N. Wiger. 1977. Effect of tetracycline upon transfer of an R plasmid from calves to human beings. Am. J. Vet. Res. 38:1137-1139. [PubMed] [Google Scholar]
- 19.Johnsen, P. J., J. I. Osterhus, H. Sletvold, M. Sorum, H. Kruse, K. Nielsen, G. S. Simonsen, and A. Sundsfjord. 2005. Persistence of animal and human glycopeptide-resistant enterococci on two Norwegian poultry farms formerly exposed to avoparcin is associated with a widespread plasmid-mediated vanA element within a polyclonal Enterococcus faecium population. Appl. Environ. Microbiol. 71:159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kehrenberg, C., and S. Schwarz. 2001. Occurrence and linkage of genes coding for resistance to sulfonamides, streptomycin and chloramphenicol in bacteria of the genera Pasteurella and Mannheimia. FEMS Microbiol. Lett. 205:283-290. [DOI] [PubMed] [Google Scholar]
- 21.Khachatryan, A. R., D. D. Hancock, T. E. Besser, and D. R. Call. 2006. Antimicrobial drug resistance genes do not convey secondary fitness advantages to calf-adapted Escherichia coli. Appl. Environ. Microbiol. 72:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khachatryan, A. R., D. D. Hancock, T. E. Besser, and D. R. Call. 2004. Role of calf-adapted Escherichia coli in maintenance of antimicrobial drug resistance in dairy calves. Appl. Environ. Microbiol. 70:752-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klare, I., D. Badstubner, C. Konstabel, G. Bohme, H. Claus, and W. Witte. 1999. Decreased incidence of vanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb. Drug Resist. 5:45-52. [DOI] [PubMed] [Google Scholar]
- 24.Langlois, B. E., K. A. Dawson, T. S. Stahly, and G. L. Cromwell. 1984. Antibiotic resistance of fecal coliforms from swine fed subtherapeutic and therapeutic levels of chlortetracycline. J. Anim. Sci. 58:666-674. [DOI] [PubMed] [Google Scholar]
- 25.Lee, C., B. E. Langlois, and K. A. Dawson. 1993. Detection of tetracycline resistance determinants in pig isolates from three herds with different histories of antimicrobial agent exposure. Appl. Environ. Microbiol. 59:1467-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy, S. B. 1978. Emergence of antibiotic-resistant bacteria in the intestinal flora of farm inhabitants. J. Infect. Dis. 137:689-690. [PubMed] [Google Scholar]
- 27.Levy, S. B., G. B. FitzGerald, and A. B. Macone. 1976. Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature 260:40-42. [DOI] [PubMed] [Google Scholar]
- 28.Marshall, B., D. Petrowski, and S. B. Levy. 1990. Inter- and intraspecies spread of Escherichia coli in a farm environment in the absence of antibiotic usage. Proc. Natl. Acad. Sci. USA 87:6609-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman, E. C., and C. W. Frank. 1976. Circular dichroism spectra of tetracycline complexes with Mg+2 and Ca+2. J. Pharm. Sci. 65:1728-1732. [DOI] [PubMed] [Google Scholar]
- 30.Nijsten, R., N. London, A. van den Bogaard, and E. Stobberingh. 1996. Antibiotic resistance among Escherichia coli isolated from faecal samples of pig farmers and pigs. J. Antimicrob. Chemother. 37:1131-1140. [DOI] [PubMed] [Google Scholar]
- 31.Oppegaard, H., T. M. Steinum, and Y. Wasteson. 2001. Horizontal transfer of a multi-drug resistance plasmid between coliform bacteria of human and bovine origin in a farm environment. Appl. Environ. Microbiol. 67:3732-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rollins, L. D., S. A. Gaines, D. W. Pocurull, H. D. Mercer, and L. T. Frobish. 1976. Persistence of transferable drug resistance in the lactose-fermenting enteric flora of swine following antimicrobial feeding. Can. J. Comp. Med. 40:175-183. [PMC free article] [PubMed] [Google Scholar]
- 33.Seppala, H., T. Klaukka, J. Vuopio-Varkila, A. Muotiala, H. Helenius, K. Lager, P. Huovinen, et al. 1997. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N. Engl. J. Med. 337:441-446. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu, M., T. Sakano, and S. Satoh. 1988. Linkage of K99 production and STa activity in a plasmid of an Escherichia coli porcine isolate. Microbiol. Immunol. 32:635-639. [DOI] [PubMed] [Google Scholar]
- 35.Shoemaker, N. B., H. Vlamakis, K. Hayes, and A. A. Salyers. 2001. Evidence for extensive resistance gene transfer among Bacteroides spp. and among Bacteroides and other genera in the human colon. Appl. Environ. Microbiol. 67:561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, H. W. 1975. Persistence of tetracycline resistance in pig E. coli. Nature 258:628-630. [DOI] [PubMed] [Google Scholar]
- 37.Threlfall, E. J., L. R. Ward, J. A. Frost, and G. A. Willshaw. 2000. The emergence and spread of antibiotic resistance in food-borne bacteria. Int. J. Food Microbiol. 62:1-5. [DOI] [PubMed] [Google Scholar]
- 38.van den Bogaard, A. E., and E. E. Stobberingh. 2000. Epidemiology of resistance to antibiotics. Links between animals and humans. Int. J. Antimicrob. Agents. 14:327-335. [DOI] [PubMed] [Google Scholar]
- 39.Winokur, P. L., D. L. Vonstein, L. J. Hoffman, E. K. Uhlenhopp, and G. V. Doern. 2001. Evidence for transfer of CMY-2 AmpC β-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob. Agents Chemother. 5:2716-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wray, L. V., Jr., R. A. Jorgensen, and W. S. Reznikoff. 1981. Identification of the tetracycline resistance promoter and repressor in transposon Tn10. J. Bacteriol. 147:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]