Abstract
Renewal of the gastrointestinal epithelium involves a coordinated process of terminal differentiation and programmed cell death. Integrins have been implicated in the control of apoptotic processes in various cell types. Here we examine the role of integrins in the regulation of apoptosis in gastrointestinal epithelial cells with the use of a rat small intestinal epithelial cell line (RIE1) as a model. Overexpression of the integrin α5 subunit in RIE1 cells conferred protection against several proapoptotic stimuli. In contrast, overexpression of the integrin α2 subunit had no effect on cell survival. The antiapoptotic effect of the α5 subunit was partially retained by a mutated version that had a truncation of the cytoplasmic domain. The antiapoptotic effects of the full-length or truncated α5 subunit were reversed upon treatment with inhibitors of phosphatidylinositol 3-kinase (PI-3-kinase), suggesting that the α5β1 integrin might interact with the PI-3-kinase/Akt survival pathway. When cells overexpressing α5 were allowed to adhere to fibronectin, there was a moderate activation of protein kinase B (PKB)/Akt, whereas no such effect was seen in α2-overexpressing cells adhering to collagen. Furthermore, in cells overexpressing α5 and adhering to fibronectin, there was a dramatic enhancement of the ability of growth factors to stimulate PKB/Akt; again, this was not seen in cells overexpressing α2 subunit and adhering to collagen or fibronectin. Expression of a dominant negative version of PKB/Akt in RIE cells blocked to ability of α5 to enhance cell survival. Thus, the α5β1 integrin seems to protect intestinal epithelial cells against proapoptotic stimuli by selectively enhancing the activity of the PI-3-kinase/Akt survival pathway.
INTRODUCTION
Integrin-mediated interactions with extracellular matrix components play crucial roles in many fundamental aspects of growth and differentiation (Aplin et al., 1998; Giancotti and Ruoslahti, 1999). For example, integrins are key regulators of the coordinated differentiation of many epithelial tissues. The functions of the β1 subfamily of integrins are particularly well understood in mammary development (Faraldo et al., 1998; Bissell et al., 1999) and in the differentiation of the epidermis (Watt, 1998; Zhu et al., 1999). However, integrins clearly play a role in other epithelia as well. The self-renewing cellular lining of the gastrointestinal tract is an interesting and important model for epithelial differentiation. Both in the small intestine and in the colon, epithelial renewal is accompanied by directed migration of differentiating cells away from the stem cell–rich crypts and ultimately results in apoptosis and shedding of terminally differentiated cells into the lumen of the gut (Stappenbeck et al., 1998; Karam, 1999). A variety of factors, including soluble hormones and cytokines, interactions with mesenchymal cells, and interactions with extracellular matrix, have been implicated in growth control mechanisms in the gastrointestinal tract (Burgess, 1998; Kedinger et al., 1998). Integrins are clearly involved in the regulation of intestinal cell function and differentiation (Pignatelli, 1993; Beaulieu, 1999); however, their role in this tissue is not as well understood as it is in mammary or epidermal epithelia. A variety of integrin subunits are detected in normal human small intestine (Beaulieu, 1992) and colon (Pignatelli, 1993), including α5β1 (Beaulieu, 1992), a key receptor for the matrix protein fibronectin (Ruoslahti, 1991).
Apoptosis plays a central role in the turnover of the cellular lining of the small intestine and colon (Stappenbeck et al., 1998). A number of recent studies have focused on the importance of integrins in the regulation of programmed cell death in various contexts, thus suggesting this possibility in the gastrointestinal tract as well. When epithelial cells are completely deprived of integrin-mediated anchorage to extracellular matrix, they undergo a form of apoptosis that has been termed “anoikis” (Frisch and Ruoslahti, 1997). Several signal transduction components have been implicated in the underlying mechanism of anoikis, including focal adhesion kinase (FAK) (Frisch et al., 1996b), phosphatidylinositol 3-kinase (PI-3-kinase) (Khwaja et al., 1997), and possibly c-Jun kinase (Frisch et al., 1996a; Khwaja and Downward, 1997). In addition to anoikis attributable to a general loss of cell–matrix contact, it is also clear that perturbation of specific integrins can contribute to programmed cell death. Thus, the α5β1 integrin seems to have a unique function in regulating apoptosis triggered by serum deprivation in both Chinese hamster ovary (CHO) cells (Zhang et al., 1995) and HT29 colonic carcinoma cells (O'Brien et al., 1996). This integrin has also been reported to protect neuronal-type cells against apoptosis triggered by β-amyloid peptide (Matter et al., 1998). In breast epithelial cells, the α6β1 integrin, whose ligand is laminin, has been shown to cooperate with insulin signaling pathways to prevent cells from becoming apoptotic (Farrelly et al., 1999). In endothelial cells, inhibition of the functions of the αvβ3 integrin can lead to programmed cell death (Brooks et al., 1994). Thus, several individual integrins have been specifically implicated in protection against apoptosis in various cell contexts.
Becoming apoptotic depends not only on the action of death-effector molecules but also on resistance mechanisms that counteract proapoptotic signals (Granville et al., 1998; Schulze-Osthoff et al., 1998). Signaling molecules known thus far that promote cell survival include FAK (Frisch et al., 1996b), MAPK (Berra et al., 1998), nuclear factor κB (Van Antwerp et al., 1998; Wang et al., 1998), Bcl-2 and Bcl-2–like proteins (Gajewski and Thompson, 1998), and PI-3-kinase and protein kinase B (PKB)/Akt (Downward, 1998). Conversely, proapoptotic signaling and effector molecules include c-Jun kinase (Ichijo et al., 1997; Yang et al., 1997) and p38 kinase (Berra et al., 1998), Bad and Bad-like proteins (Gajewski and Thompson, 1998), and the caspase family of proteases (Green and Kroemer, 1998; Slee et al., 1999). Recently, increasing evidence has emerged showing that PI-3-kinase and its downstream effector PKB/Akt, a serine–threonine kinase, play a key role in the regulation of cell survival (Downward, 1998). For example, in the case mentioned above, survival in mammary epithelial cells involved activation of PI-3-kinase and PKB/Akt by coordinated signaling of insulin and α6β1 integrin (Farrelly et al., 1999). In Madin-Darby canine kidney cells, PI-3-kinase signals through PKB/Akt to protect against apoptosis mediated by loss of cell anchorage or by radiation (Khwaja and Downward, 1997); PKB/Akt also protects fibroblasts from c-myc–mediated apoptosis (Kauffmann-Zeh et al., 1997).
After autophosphorylation of receptor tyrosine kinases by growth factor binding, PI-3-kinase is recruited to phosphotyrosine residues and then activated. The activated enzyme produces phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3 and phosphatidylinositol 3,4-bisphosphate (PtdIns(3,4)P2), which stimulate 3-phosphoinositide–dependent protein kinases 1 and 2; these kinases phosphorylate Thr-308 and Ser-473 on PKB/Akt, respectively (Alessi et al., 1997). PKB/Akt can also be phosphorylated at Thr-308 by Ca2+/calmodulin-dependent protein kinase kinase (Yano et al., 1998). Phosphorylated PKB/Akt in turn phosphorylates many different downstream substrates, including Bad, a death effector that functions in conjunction with Bcl-2 (Datta et al., 1997); phosphorylated Bad dissociates from Bcl-2 and binds to 14-3-3 proteins (Zha et al., 1996). Although the phosphorylation of Bad by PKB/Akt may play an important role in survival, it is likely that there are additional contributing factors. For example, in hematopoietic cells, PKB activation and Bad phosphorylation do not always correlate (Scheid and Duronio, 1998). Alternatively, activated PKB/Akt can phosphorylate caspase 9 and inhibit its protease activity, leading to cell survival (Cardone et al., 1998). Activated PKB/Akt can also phosphorylate FKHR, a Forkhead family transcription factor (Biggs et al., 1999; Guo et al., 1999; Kops et al., 1999; Rena et al., 1999). Phosphorylation results in retention of FKHR in the cytoplasm, reduced expression of proapoptotic genes such as Fas-ligand, and enhanced cell survival (Brunet et al., 1999; Tang et al., 1999). PKB/Akt was recently shown to induce nuclear factor κB, which also plays a role in cell survival (Kane et al., 1999). In addition, PKB/Akt was shown to prevent the release of cytochrome c from mitochondria by an unknown mechanism, thus contributing to cell survival (Kennedy et al., 1999).
In this study, we have examined the role of the α5β1 integrin in regulating apoptosis in intestinal epithelial cells. We have primarily used RIE1 cells, a rat nontransformed line of small intestinal origin that has been widely used as a model to study signal transduction processes relevant to the intestinal epithelium (DuBois et al., 1994; Oldham et al., 1996; Winesett et al., 1996). In addition, we have also extended our earlier studies with HT29 human colonic carcinoma cells (O'Brien et al., 1996). In both of these cell types, overexpression of the α5 integrin subunit provides dramatic protection against apoptosis induced by serum deprivation or by a variety of proapoptotic agents. This effect was not seen with overexpression of the α2 integrin subunit, indicating a specific role for α5β1. The antiapoptotic effects of α5β1 could be reversed by treatment with a selective PI-3-kinase inhibitor. In addition, cells expressing the α5β1 integrin displayed a dramatic enhancement of the ability of growth factors to activate PKB/Akt. Furthermore, a dominant negative version of PKB/Akt blocked the ability of the α5β1 integrin to promote cell survival in the presence of apoptotic stimuli. This suggests that the antiapoptotic effects of α5β1 seen in cells of the gastrointestinal epithelium may be due to a preferential interaction between α5β1 and the PI-3-kinase/Akt signaling pathway.
MATERIALS AND METHODS
Cell Cultures
Rat intestinal epithelial wild-type cells (RIE1 WT) were maintained in DMEM-H with 5% FBS. Stably transfected RIE1 cell lines such as pcDM control vector transfectant (RIE1 N12), α5-tailless mutant transfectants (α5/1-c3), full-length α5 transfectant (α5-c10), and full-length α2 transfectant (α2-P1) were maintained in DMEM-H with 5% FBS, 1 mg/ml G418, penicillin, and streptomycin. For serum deprivation experiments, cells were cultured in DMEM-H with 0.1% BSA for the indicated times. Wild-type colon carcinoma HT29 and α5 transfectant HT29 c28 cells have been described (O'Brien et al., 1996). Wild-type HT29 cells (HT29 WT) were cultured in DMEM-H including 10% FBS, penicillin, and streptomycin, whereas HT29 c28 transfectants were cultured in the medium for HT29 WT supplemented with 300 μg/ml G418. All cells were seeded at certain densities (RIE1 at 4 × 105 cells, HT29 at 2 × 106 cells per 60-mm dish) in normal serum–containing medium, and treatments were performed 24 h later.
Stable Transfectants
The full-length α5 cDNA sequence was subcloned as a NotI–XbaI insert into a pcDNA3.1 expression vector; the resulting vector was termed pcDMα5. An expression construct for α5/1 with a truncated cytoplasmic domain has been described (Bauer et al., 1993). An expression construct for the full-length α2 subunit has also been described (Aplin et al., 1999). For stable transfection of integrin subunit constructs into RIE1 cells, appropriate plasmids were transfected with the use of Lipofectamine Plus (Life Technologies, Gaithersburg, MD). In the case of the α5/1 construct, which lacks a neomycin selection marker, a pcDM plasmid with a neomycin gene was cotransfected to permit drug selection. Forty-eight hours after transfection, the transfectants were selected by culture in medium containing 2 mg/ml G418 for the first week, and then the concentration of G418 was reduced to 1 mg/ml and maintained in all cultures. In addition, one or two rounds of magnetic immunobead (Dynabeads M-450, Dynal A.S., Oslo, Norway) selections with the use of monoclonal anti-human α5 (P1D6) or α2 (P1E6) antibody were performed to accelerate the selection process. Western blot and/or flow cytometric analyses were done to check the expression levels of the integrin subunits in the transfectants. Clonal or pooled populations were chosen to have comparable levels of expression of the integrin subunits used.
Evaluating the Expression of Integrin Subunits
Western blots for integrin α5 subunits in stable transfectants were made in a standard way, with the use of either anti-human α5 mAb (Transduction Laboratories, Lexington, KY) or rabbit anti-α5 cytoplasmic tail polyclonal antibody (a gift from Dr. R.O. Hynes, Massachusetts Institute of Technology, Cambridge, MA). For α2 transfectants, lysates or immunoprecipitates (by anti-human α2 mAb P1E6) were used in a standard western blot with the use of rabbit anti-α2 cytoplasmic tail antibody (a gift from Dr. G. Tarone, University of Torino, Torino, Italy). Flow cytometric analyses for the expression of human α5 or α2 on the surface of the RIE1 cells were performed as follows. Cells were trypsinized, spun down, and counted. A half-million cells were washed with PBS/1% BSA. Pelleted cells were incubated on ice for 1 h with 100 μl of anti-human α5 antibody (P1D6) or anti-human α2 antibody (P1E6) solution diluted with PBS/1% BSA at a ratio of 1:50. Cells were then washed three times with PBS/1% BSA. Washed cells were incubated on ice for 45 min with 100 μl of R-phycoerythrin anti-mouse immunoglobulin G in PBS/1% BSA at a dilution of 1:100. After washes as described above, cells were fixed with 2% paraformaldehyde in PBS for 15 min at room temperature before analyses with the use of a flow cytometer (Becton Dickinson, San Jose, CA). As controls, cells were treated with only the secondary antibody.
TUNEL Assay by Flow Cytometry
A flow cytometry–based terminal deoxynucleotidyl transferase-mediated dUPT nick end-labeling (TUNEL) assay, which uses terminal transferase to label breaks in DNA strands, was performed with the use of the APO-BRDU kit (PharMingen, San Diego, CA). Sample preparations were made according to the manufacturer's protocols. In brief, cells on 60-mm dishes were used; in some cases, cells were treated with various drugs before analysis. Cells were trypsinized, collected, resuspended in PBS/1% BSA, and counted. One million to 1.5 million cells for each condition were spun down at 700 rpm at 4°C for 8 min. After one more wash with PBS/1% BSA, cells were fixed with 2% paraformaldehyde in PBS at 4°C for 15 min. Fixed cells were washed three times with PBS/1% BSA. The cells were then resuspended with 0.4 ml of PBS plus 4 ml of cold 70% ethyl alcohol and stored at −20°C (for at least 20 h) until all samples were prepared. All samples, including commercially available apoptosis-positive and apoptosis-negative control samples, were washed to remove alcohol. Washed cells were incubated with DNA-labeling solution (terminal deoxynucleotidyl transferase, Br-dUTP, reaction buffer, and distilled water) for 2 h at 37°C with occasional mixing. After incubation, cells were washed twice with a rinse buffer supplied by the manufacturer. Washed cells were incubated with FITC-labeled anti-Br-dUTP in the rinse buffer in the dark for 30 min. Then, 0.4 ml of propidium iodide/RNase solution supplied by the manufacturer was added before 30 min of additional incubation. The cells in propidium iodide/RNase solution were analyzed with the use of a flow cytometer (Becton Dickinson). The threshold for an event in cytometric analysis was kept at 5%. More than 2 × 104 events were counted for each sample.
Annexin V Assay
To further validate our apoptosis study, we also used annexin V staining followed by flow cytometric measurements. For this, we followed the manufacturer's protocols for the use of the annexin V–biotin kit (Trevigen, Gaithersburg, MD). Briefly, cells were treated and harvested as described above. One million cells for each condition were washed with PBS/1% BSA and then with ice-cold PBS. After centrifugation at 700 rpm for 8 min, cell pellets were resuspended with 100 μl of annexin V–binding buffer (10 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2) containing 5 μg/ml propidium iodide and annexin V–biotin reagent diluted 1:100. The suspension was kept in the dark for 15 min at room temperature. Controls such as propidium iodide alone or annexin V alone were also prepared. Cells incubated with annexin V–biotin were collected by spinning at 700 rpm at 4°C for 8 min. The pelleted cells were further incubated with streptavidin–FITC reagent at a dilution of 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA) for 15 min at room temperature in the dark. After incubation, 400 μl of the annexin V–binding buffer was added to each sample before flow cytometric analyses. The threshold for an event in cytometric analysis was kept at 5%. More than 4 × 104 events were counted for each sample.
Plating Cells on Fibronectin or Collagen, and Cell Lysis
Confluent cells were trypsinized, neutralized with soybean trypsin inhibitor (1 mg/ml), and spun down. Pelleted cells were washed with DMEM-H/1% BSA. Cells resuspended in DMEM-H/1% BSA were then held in suspension for 45 min at 37°C. Tissue culture dishes (60 or 100 mm) were precoated overnight with either human fibronectin (20 μg/ml; Collaborative Research, Bedford, MA) or rat tail collagen I (20 μg/ml; Biomedical Technologies, Stoughton, MA); these were washed twice and then blocked with DMEM-H/1% BSA for 30 min at 37°C. After incubation in suspension, cells were distributed equally to the dishes. Cells were then incubated at 37°C for the indicated times. In some cases, PDGF-BB (Upstate Biotechnologies, Lake Placid, NY), EGF (Upstate Biotechnologies), or LY294002 (Sigma Chemical, St. Louis, MO) was added at the indicated concentration to the culture medium. Immediately after treatments, dishes with cells were washed twice with 10 ml of ice-cold PBS. After removal of PBS, cell were lysed on ice with a lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 10% glycerol, 1% NP40, 10 mM NaF, 1 mM sodium orthovanadate, 10 μg/ml aprotinin, 1 mM serine protease inhibitors 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), 1 μM microcystin-LR; Calbiochem, San Diego, CA) (King et al., 1998). Lysates were left on ice for 20 min before centrifugation at 14,000 rpm for 15 min at 4°C. The supernatants were removed to new ice-cold tubes and stored at −80°C until use. Protein was determined by the bicinchoninic acid protein assay (Pierce, Rockford, IL) to allow normalization of protein amount in each sample.
Assay of PKB/Akt Activity by Immunoblotting
Proteins (15 μg/sample) in lysates were resolved by 8% SDS-PAGE, electroblotted to polyvinylidene difluoride membranes, blocked with 5% nonfat dried milk in PBS/0.05% Tween-20, and probed with primary antibodies. To assay PKB/Akt kinase activity, the membrane was probed with antiphospho-S473-PKB/Akt antibody (New England Biolabs, Beverly, MA) overnight at 4°C. To normalize for the activity of PKB/Akt in each condition, blots were probed for total PKB with the use of sheep polyclonal anti-rat PKB/Akt antibody (Upstate Biotechnologies) overnight at 4°C. After washes with PBS/0.05% Tween-20 for 1 h, membranes were incubated with fluorescein-conjugated antiimmunoglobulins for 1 h at room temperature. After washing as described above, membranes were further incubated with alkaline phosphatase–conjugated anti-fluorescein antibody for 1 h at room temperature. After washing again as described above, the signals from membranes were detected with the use of a chemifluorescence-based kit (ECF kit, Amersham, Arlington Heights, IL), and quantitation of the band intensities was done with the use of a chemifluorescence scanner on a phosphorimager with Image-QuaNT software (STORM 840, Molecular Dynamics, Sunnyvale, CA).
In Vitro PKB/Akt Assay
Confluent cells were serum starved overnight before trypsinization. Trypsinized cells were washed with DMEM-H/1% BSA and kept in suspension for 45 min before being replated on either collagen I–coated or fibronectin-coated culture dishes. Cells were treated with LY294002 in some cases. EGF at 40 ng/ml was used for the last 5 min of the experimental period of 60 min. Cells were harvested as described above. The protocol for the in vitro kinase assay was adapted from a previous study (Aplin et al., 1999). Equal amounts of protein in an equal volume of lysate were precleared with PBS-washed protein G–Sepharose beads for 30 min. Precleared lysates were used for immunoprecipitation of PKB/Akt with 4 μg/sample of sheep polyclonal anti-rat PKB/Akt (Upstate Biotechnologies) by rocking for 2 h at 4°C. Forty microliters of protein G–Sepharose slurry (50%) was added to each immunoprecipitate before 2 h of incubation at 4°C. The kinase reaction was started by adding 40 μl of the kinase reaction buffer (20 mM HEPES, pH 7.4, 1 mM DTT, 10 mM MnCl2, 10 mM MgCl2, 5 μM ATP, 10 μg/100 μl of histone 2B, and 10 μCi of [32P]ATP per assay) and incubating for 30 min at room temperature. The reactions were stopped by adding 14 μl of boiling 4× sample buffer. The samples were then boiled before being resolved by SDS-PAGE (15%). Autoradiography was performed with a dried gel, and the band intensities were quantitated with the use of a phosphorimager with Image-QuaNT software. A portion of the reaction mixture was also used for Western blotting with the sheep anti-rat PKB/Akt antibody to quantitate the amount of PKB/Akt in each reaction mixture.
Cell Survival Analysis, Effect of Dominant Negative PKB/Akt
Cells at 60∼80% confluence were cotransfected with β-galactosidase cDNA (0.67 μg/60-mm dish) and 1.0 μg/60-mm dish of either wild-type PKB/Akt or kinase-dead PKB/Akt cDNA (kind gifts from Dr. J. Channing Der, University of North Carolina, Chapel Hill, NC) with the use of Lipofectamine Plus reagent (Life Technologies). In addition, 1 μg of pcDM (a control vector) was also cotransfected. Transfection with DNA/lipid complexes was performed for 3 h, and then cells were maintained in normal culture medium. At 24 h after transfection, cells were either maintained in normal serum-containing culture medium or serum starved. After 40 h of culture in the presence or absence of serum, cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal) to count surviving β-galactosidase–positive cells in each condition. For each 60-mm dish, positive cells in 12 random grids (each grid was 2 mm) were counted. Values were compared between samples with the use of means and SDs.
RESULTS
Stable Expression of Integrin Subunits in RIE1 Cells
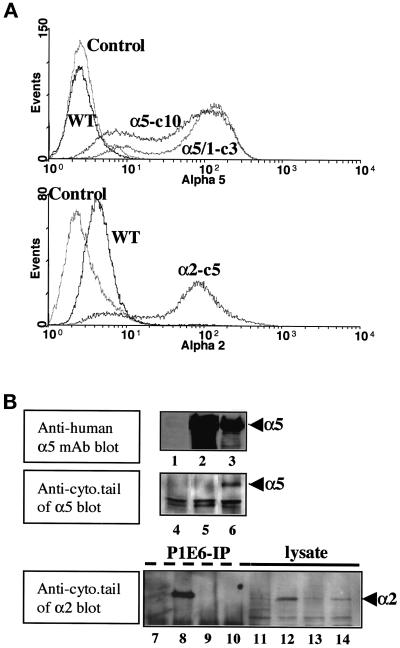
Intact human α5, a cytoplasmic tailless mutant of human α5 (termed α5/1), and intact human α2 integrin subunits were stably introduced into RIE1 cells. The transfectants underwent intensive G418 resistance and magnetic immunobead selections. Selected cells were checked for the expression of each integrin subunit by surface immunolabeling and flow cytometric measurements. Transfected cells showed significant but somewhat heterogeneous expression of each subunit compared with parental wild-type cells (Figure 1A). Western blots that used an anti-human α5 antibody recognizing an extracellular region of α5 showed significant expression of α5 subunit in lysates from cell lines expressing either full-length α5 or cytoplasmic tailless α5 (Figure 1B, upper panel, lanes 2 and 3). In contrast, blots that used an antibody to the α5 cytoplasmic domain reacted only with lysates from full-length α5 transfectants (Figure 1B, middle panel, lanes 5 and 6). As expected, the anti-human α5 subunit antibody did not cross-react with endogenous rat integrins in RIE1 WT cells (Figure 1B, lane 1), consistent with findings from flow cytometry analysis. Human α2 integrin transfectants (α2-P1) also showed significant expression, whereas RIE1 WT cells expressed a small amount of cross-reactive endogenous (rat) α2 (Figure 1B, bottom panel). Transfectants expressing each integrin subunit to a comparable degree were chosen and used for further experiments. Both clonally derived cell lines (designated in text and legends with a “c”) and pooled transfectants (designated with a “p”) for α5 and α2 were used in various experiments. Similar results were observed with pooled and cloned transfectants.
Figure 1.
Overexpression of integrin α subunits in RIE1 cells by stable transfection. (A) Flow cytometric histograms showing the expression of intact human α5 (α5-c10), its cytoplasmic tailless mutant (α5/1-c3), and an intact human α2 integrin subunit (α2-c5) in clonal RIE1 cell transformants. RIE1 WT cells and a negative control without primary antibody labeling (Control) are also shown. Anti-human α5 or α2 mAbs were used as primary antibodies. Other clones and pooled cell lines used in this study had similar levels of α5 or α2 expression as those shown here. (B) Western blot analyses of the stable transfectants for intact α5, a cytoplasmic tailless mutant of α5, and an intact human α2 integrin subunit. Lanes 1–3 show total cell lysates that were blotted with an anti-α5 mAb. Lanes 4–6 show cell lysates that were blotted with a polyclonal antibody that recognizes the α5 cytoplasmic tail across species. Lanes 7–14 show cell lysates that were blotted with an antibody that recognizes the α2 cytoplasmic tail across species: lanes 7–10 represent immunoprecipitates that use an anti-human α2 mAb, and lanes 11–14 represent total cell lysates. Lane 1, RIE1 WT; lane 2, clone 3, which is transfected with tailless α5; lane 3, clone 10, which is transfected with full-length α5; lanes 4–6, the same lysates as in lanes 1–3, respectively; lane 7, RIE1 WT control; lane 8, pool 1, which is transfected with full-length human α2; lanes 9 and 10, clones transfected with tailless and intact human α5, respectively; lanes 11–14, the same lysates as in lanes 7–10, respectively.
Ectopic Expression of the α5 Subunit Blocks the Apoptotic Effects of Serum Deprivation
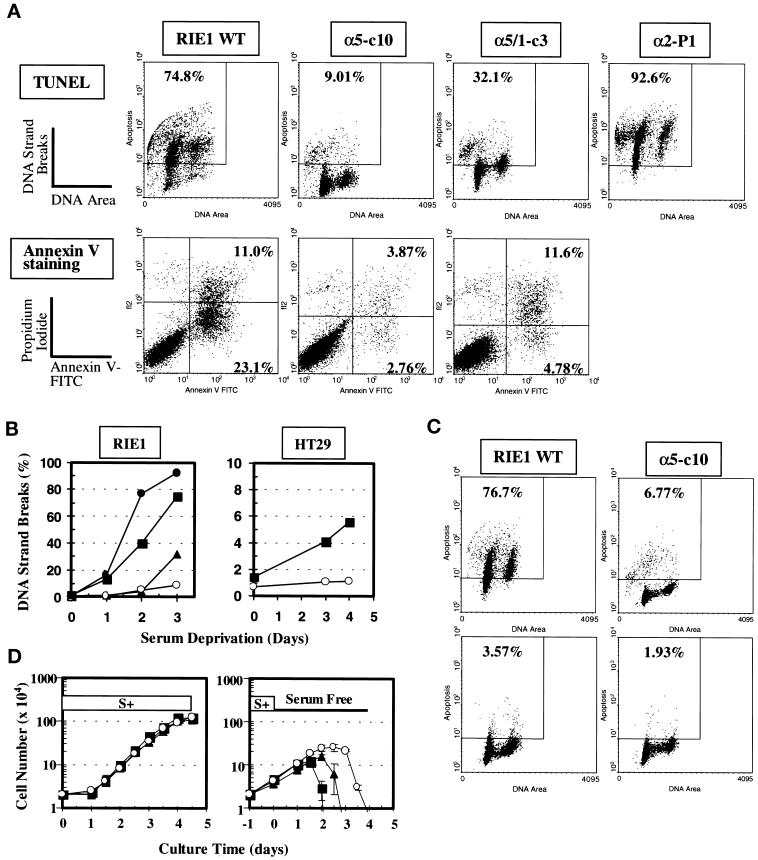
TUNEL assays and annexin V assays followed by flow cytometric measurements were performed to quantitatively evaluate the apoptotic population in RIE1 WT cells and transfectants under various experimental conditions. As shown in Figure 2A (upper panels), serum deprivation for 3 d resulted in significant DNA strand breaks in RIE1 WT cells and RIE1 α2-P1 cells (human α2 transfectants), indicating the occurrence of apoptosis. An increase in DNA strand breaks in RIE1 WT cells was evident as early as 24 h of serum deprivation and was strongly evident after 3 d of serum-free culture (Figure 2B, left panel). In contrast, cells overexpressing α5 (RIE1 α5-c10) showed remarkably reduced DNA strand breakage under the same conditions (Figure 2A, upper panels, and 2B, left panel). These effects were evident for up to 4 d of serum deprivation (Figure 2B, left panel; our unpublished results). This resistance to apoptosis was not an artifact in a single clone of cells, because other α5-positive clonal cell lines, as well as pooled cells expressing α5 subunit, also showed increased cell survival during serum deprivation (see Figure 4D; our unpublished results). When the apoptotic population was evaluated by a different method (annexin V staining), the result was consistent with the data from TUNEL assays. That is, RIE1 WT cells showed increased annexin V staining upon serum deprivation compared with RIE1 α5-c10 cells under the same conditions (Figure 2A, lower panels). Both the high level of TUNEL staining in WT cells and the lower level of staining in α5-c10 cells induced by serum deprivation were significantly reduced by treatment with a general caspase inhibitor, BDfmk (Figure 2C). This observation indicates that the results from the TUNEL and annexin V assays represent true apoptotic events involving activation of caspase(s).
Figure 2.
Apoptosis mediated by serum deprivation in RIE1 WT and its various transfectants. (A) Apoptosis measured by two different methods (TUNEL and annexin V staining). Cells were cultured for 72 h in serum-free conditions (DMEM-H with 0.1% BSA), harvested, and then measured for apoptosis. Upper panels, Apoptosis measured by the TUNEL method. The square in each histogram represents the area in which cells are considered apoptotic. The numerical percentage of apoptotic cells versus total cells is shown. Lower panels, Apoptosis analyzed by annexin V staining. The percentage values represent late apoptotic (upper right quadrant) and early apoptotic (lower right quadrant) populations versus total cells. α5-c10 is a clone expressing full-length α5; α5/1-c3 is a clone expressing a tailless α5; α2-P1 is a pool expressing full-length α2. (B) Trends for apoptosis attributable to serum deprivation in RIE1 cells (left) and HT29 cells (right). Apoptosis was analyzed by the TUNEL assay. (Left) ▪, RIE1 WT; ▴, α5/1-c3; ○, α5-c10; ●, α2-P1. (Right) ▪, HT29 WT; ○; HT29 c28, which expresses full-length α5. Data shown are representative of at least two independent experiments. (C) Block of apoptosis by a general caspase inhibitor, BDfmk. Cells were serum starved for 48 h without (upper panels) or with (lower panels) treatment with 100 μM BDfmk before evaluation of apoptosis with the TUNEL assay. The inhibitor was replenished every 24 h. (D) Growth curves of RIE1 transfectants in normal (left) or serum-free (right) culture conditions. In the right panel, cultures were switched to serum-free conditions after 1 d in normal serum-replete culture. Cell counts were done in triplicate by hemocytometry. ▪, RIE1 WT; ▴, α5/1-c3; ○, α5-c10.
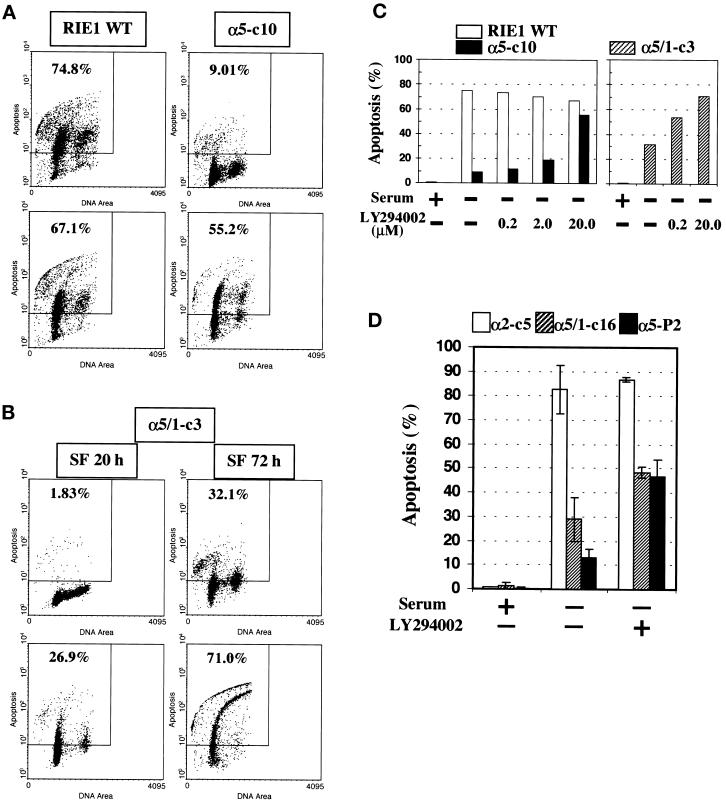
Figure 4.
Reversal of α5-mediated cell protection by PI-3-kinase inhibition. LY294002 was added to culture medium at the beginning of serum deprivation. The vehicle, DMSO, was maintained at <0.1%. (A) Reversal of α5-mediated antiapoptotic effects in α5-c10 cells by PI-3-kinase inhibition. Cells were serum starved for 72 h without (upper panels) or with (lower panels) 20 μM LY294002 before TUNEL assay. (B) Reversal of antiapoptosis effects in α5/1-c3 cells by PI-3-kinase inhibition. α5/1-c3 cells were serum starved for 20 h (left histograms) or 72 h (right histograms) without (upper panels) or with (lower panels) 20 μM LY294002 before TUNEL assay. Data shown are representative of at least two independent experiments. (C) Dose-response effect of LY294002 on apoptosis in serum-starved RIE1 cells. Open bars, WT cells; closed bars, α5-c10 cells; cross-hatched bars, α5/1-c3 cells. Data shown are representative of at least two independent experiments. (D) Serum deprivation–mediated apoptosis in additional cell lines. α2-c5, α5/1-c16, and α5-P2 cells were maintained in the presence of serum (+), or were serum starved (−) for 72 h, in the absence or presence of LY294002 (20 μM) before TUNEL assay. Means and SEs are shown.
Interestingly, RIE1 α5/1-c3 cells (expressing a cytoplasmic tailless mutant of α5) showed an intermediate level of apoptosis after serum deprivation for 3 d; the level was more than that seen in α5-expressing cells but less than seen in wild-type cells (Figure 2A). In addition, α5/1-c3 cells showed a delay in the initiation of apoptosis (Figure 2B, left panel; see also Figure 4B). At the beginning of the serum deprivation period (to 2 d), α5/1-c3 cells did not show significant apoptosis, but after 3 d of serum deprivation, considerable apoptosis was detected (Figure 2A and 2B, left panel). This observation indicates that the extracellular and/or transmembrane domain of the α5 integrin subunit can transduce a signal to support cell survival. However, the intracellular cytoplasmic tail of α5 also plays a role, particularly in survival during longer periods of serum deprivation.
As with the RIE1 cells, HT29 human colon carcinoma cells were also protected against the apoptotic effect of serum deprivation by ectopic overexpression of the α5 subunit, consistent with previous findings (O'Brien et al., 1996). After serum deprivation for 4 d, HT29 WT cultures contained 5∼10% apoptotic cells, but α5-expressing HT29 c28 cells showed a much lower level of apoptosis (<1.0%; Figure 2B, right panel). In general, the carcinoma cells showed less susceptibility to apoptosis mediated by serum withdrawal than the normal RIE1 cells; therefore, in subsequent experiments, we mainly used the RIE1 cell system.
These observations on apoptosis are consistent with survival and growth behavior in the presence or absence of serum, as shown in Figure 2D. Thus, α5-c10 cells survived longer in the absence of serum than WT or α5/1-c3 cells. However, RIE1 WT, α5-c10, and α5/1-c3 cells grew similarly in the presence of serum.
α5 Expression Protects Cells from Apoptosis Triggered by Different Cytotoxic Stimuli
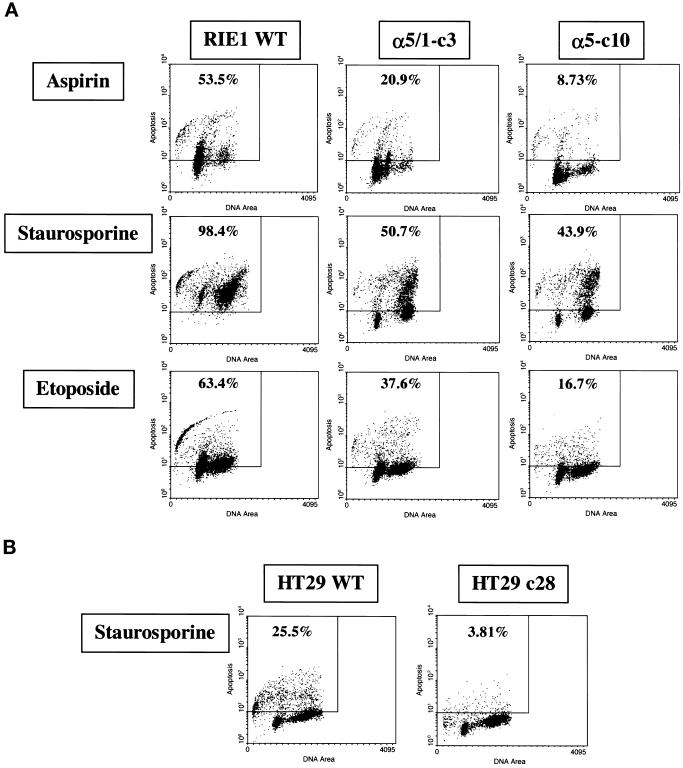
To generalize the concept that α5 subunit overexpression can protect gastrointestinal cells from apoptosis, analyses were performed after treatment with different cytotoxic agents in cultures of RIE1 WT, HT29 WT, and their α5 transfectants. The cytotoxic agents included aspirin (a cyclooxygenase inhibitor), staurosporine (a protein kinase inhibitor), and etoposide (a topoisomerase II inhibitor). As shown in Figure 3A, treatment with each agent resulted in significant levels of TUNEL staining in RIE1 WT cells but markedly lower levels of TUNEL staining in α5-expressing α5-c10 cells. The general antiapoptotic effect of α5 expression was also substantiated with the use of HT29 cells. HT29 c28 cells, which express α5, showed a low level of apoptosis after treatment with staurosporine (Figure 3B) and etoposide (our unpublished results), whereas the HT29 WT cells were substantially more apoptotic. Therefore, these observations indicate that α5 expression can protect cells from a variety of apoptotic stimuli, possibly by communicating with signaling molecule(s) important for cell survival and/or by antagonizing molecule(s) in proapoptotic signaling pathway(s). As in the case of serum deprivation, α5/1-c3 cells also showed intermediate levels of apoptosis as a result of each treatment with a cytotoxic agent (Figure 3A), indicating that a substantial part of the contribution of the α5 subunit to cell survival may be independent of the cytoplasmic domain.
Figure 3.
α5-mediated protection of cells from apoptosis triggered by various cytotoxic stimuli. Each cytotoxic reagent was added to culture medium at the beginning of the experiment. The vehicle, DMSO, was maintained at <0.1%. Data shown are representative of two independent experiments. (A) α5-mediated cell protection in RIE1 transfectants. Cells were treated with aspirin (upper panels) at 3.0 mM for 72 h, with staurosporine (middle panels) at 50 nM for 24 h, or with etoposide (lower panels) at 50 μM for 48 h before TUNEL analysis. α5-c10 is a clone expressing full-length α5; α5/1-c3 is a clone expressing a tailless α5. (B) α5-mediated protection in HT29 cells. Cells were treated with staurosporine for 48 h at 0.4 μM before TUNEL analysis. HT29 c28 is transfected with full-length α5.
Reversal of α5-mediated Antiapoptotic Effects by PI-3-Kinase Inhibition
In an effort to understand how the α5 subunit mediates cell survival and which signaling molecule(s) might be involved, cells were treated with LY294002, a specific inhibitor of PI-3-kinase. The increase in TUNEL staining in RIE1 WT cells induced by serum deprivation was not significantly affected by PI-3-kinase inhibition (Figure 4A, left panels). However, simultaneous treatment with LY294002 dramatically increased TUNEL staining in α5-c10 cells subjected to serum deprivation (Figure 4A, right panels). RIE1 α5/1-c3 also showed increased apoptosis in the presence of the PI-3-kinase inhibitor when cells were deprived of serum (Figure 4B). The effect of LY294002 was dose dependent in both the α5-c10 cells (Figure 4C, left panel) and the α5/1-c3 cells (Figure 4C, right panel). At early time points, the LY294002 effect in RIE1 α5/1-c3 cells was particularly clear (Figure 4B, lower left panel). The effect of LY294002 was also seen in additional independent clones and pools of α5 transfectants but not in α2 transfectants, indicating its generality in α5-expressing cells (Figure 4D). The LY294002 effect seen in RIE1 α5/1-c3 cells (Figure 4C) suggests that the contribution to cell survival that is independent of the cytoplasmic domain of α5 may also involve PI-3-kinase activity (however, the effect seen in α5/1-c16 cells was quite modest [Figure 4D]). Results similar to those seen with LY294002 were observed when α5-positive cells were treated with wortmannin, another PI-3-kinase inhibitor (our unpublished results). Reversal of α5-mediated antiapoptotic effects by PI-3-kinase inhibition was also seen in the HT29 cell system. HT29 c28 cells that are resistant to serum deprivation showed increased TUNEL staining upon PI-3-kinase inhibition (our unpublished results). Together, these observations suggest that the protection against apoptosis conferred by the α5 subunit involves a PI-3-kinase–dependent signaling pathway.
Specific Enhancement of PKB/Akt Activity in α5-expressing Cells
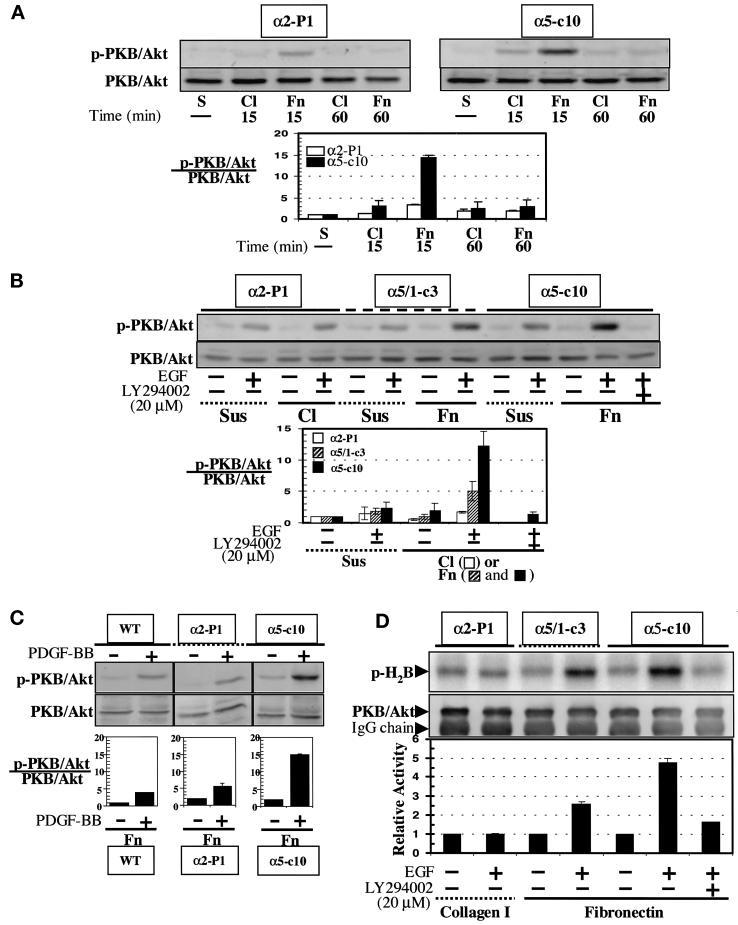
To determine whether α5 influences a PI-3-kinase signaling pathway, the activation of PKB/Akt, a downstream effector of PI-3-kinase, was measured upon adhesive interaction of RIE1 WT, α2-P1, α5/1-c3, and α5-c10 cells with extracellular matrix proteins. In initial experiments, we examined the phosphorylation of S473 with the use of immunoblotting with a specific antibody; phosphorylation of this residue is usually thought to indicate activation of the kinase (Chan et al., 1999). RIE cells plated on either fibronectin or collagen I attached well and had similar morphologies when observed by phase contrast microscopy; this was true for WT cells and for the various integrin subunit transfectants (our unpublished results). This finding suggests that endogenous integrins also contribute to the adhesion and spreading of WT and transfected RIE cells on both fibronectin and collagen substrata. When α5-c10 cells were plated on fibronectin (in the absence of soluble growth factors), PKB/Akt showed a peak of phosphorylation of S473 at early times (e.g., 15 min), which then declined to the basal level by 60 min (Figure 5A). Relatively little activation of PKB/Akt was noted in α5-c10 cells plated on collagen or in α2-P1 cells plated on either fibronectin or collagen. This suggests that direct engagement of α5β1 with fibronectin results in the activation of PKB/Akt. We also analyzed whether overexpression of α5β1 integrin could enhance PKB/Akt activity when cells were stimulated with growth factors. As shown in Figure 5B, treatment with EGF caused a dramatic activation of PKB/Akt in α5-c10 cells adherent to fibronectin; this effect was blocked by LY294002 and occurred to only a limited degree if the α5-c10 cells were held in suspension. Only a very modest increase in PKB/Akt activity was seen in α2-P1 cells when plated on collagen or held in suspension. RIE1 WT cells behaved in essentially the same manner as the α2-P1 cells when plated on either collagen or fibronectin (our unpublished results). An intermediate level of PKB/Akt phosphorylation was seen in α5/1-c3 cells (Figure 5B). Other growth factors, such as PDGF, were also able to preferentially activate PKB/Akt in the α5 transfectants (Figure 5C). In addition, Figure 5 shows that the WT and α2-expressing cells did not effectively activate PKB/Akt when plated on fibronectin. To confirm that the observed changes in S473 phosphorylation reflect changes in enzyme function, an in vitro kinase assay was used to evaluate PKB/Akt activity (Figure 5D). As shown, a high level of EGF-stimulated PKB/Akt activity was found in the α5-c10 cells on fibronectin, an intermediate level was found in the α5/1-c3 cells, and little activity was found in the α2-expressing cells; the activity was blocked by exposure to LY294002. These observations indicate again that α5β1, but not α2β1, is specifically implicated in signaling pathways that activate PKB/Akt. Thus, enhanced activation of PKB/Akt by direct signaling and cosignaling involving the α5β1 integrin may contribute to increased intestinal epithelial cell survival in the face of various proapoptotic influences.
Figure 5.
Enhancement of PKB/Akt specific activity by direct signaling or cosignaling of α5β1 integrin. (A) Direct signaling. Blots for S473-phosphorylated PKB/Akt (p-PKB/Akt) or total PKB/Akt from α5-c10 or α2-P1 total cell lysates are shown. Cells were maintained in suspension (S) or were distributed to culture dishes precoated with either fibronectin (Fn) or collagen I (Cl) and then incubated at 37°C for 15 or 60 min before harvesting. The bar graph illustrates the relative activity (p-PKB)/(total PKB). Means and SEs of three experiments are shown. (B) Cosignaling of α5 integrin receptor and tyrosine kinases. EGF (40 ng/ml) was added to culture medium for the last 5 min of a 60-min incubation period in the indicated samples. In some cases, the cells were preincubated with 20 μM LY294002. Cells were either maintained in suspension (Sus) or plated on fibronectin- (Fn) or collagen I– (Cl) coated dishes. Total cell lysates were Western blotted for S473-phosphorylated PKB/Akt or for total PKB/Akt. To determine the relative activity of PKB/Akt in each sample, the density of the phosphorylated S473 PKB/Akt band was divided by the density of the total PKB/Akt band for the corresponding sample. The resulting bar graph plot is shown below. Means and SEs of three experiments are shown. The open bars represent α2-P1 cells in suspension or on collagen; the hatched bars represent α5/1-c3 cells in suspension or on fibronectin; the closed bars represent α5-c10 cells in suspension or on fibronectin. The bands were visualized with a chemifluorescence scanner after development of blot membranes with an ECF kit (Amersham), as explained in MATERIALS AND METHODS. (C) Cosignaling. This experiment was done essentially as in B, except that cells were treated with PDGF-BB (40 ng/ml) and the WT and α2-expressing cells were plated on fibronectin rather than collagen. (D) In vitro PKB/Akt assay. Cells were serum starved overnight before replating and harvesting, as in B. Autoradiography was performed to measure 32P incorporation into histone 2B substrate (p-H2B) by PKB/Akt action. Total PKB/Akt in each assay was analyzed by Western blotting with the use of a polyclonal anti-rat PKB/Akt antibody, as explained in MATERIALS AND METHODS. The heavy chain of immunoglobulin G (IgG) is also shown in the blot for total PKB/Akt. The bar graph in the lower part of the figure represents the means of two experiments.
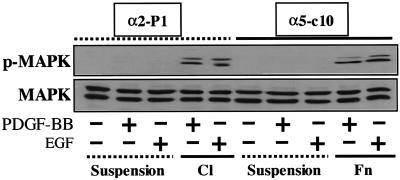
It is important to note that the α5-selective effect observed in connection with PKB/Akt activation did not extend to other aspects of signal transduction. Thus, as shown in Figure 6, RIE cells displayed a clear anchorage dependence of MAPK activation in response to growth factors. However, growth factor activation of MAPK occurred equally well in α5-positive cells plated on fibronectin and in α2-positive cells plated on collagen, indicating a lack of selectivity with regard to the transfected integrin subunit. This seems somewhat at variance with results in other cell types: α5 reportedly interacts with Shc to promote signaling to MAPK, whereas α2 does not (Wary et al., 1996).
Figure 6.
Signaling to MAPK. The experimental protocol was similar to that described in Figure 5B except that the lysates were blotted with either an antibody to activated phosphorylated MAPK (pMAPK) or an antibody to total MAPK. BB, XXXX; C1, collagen I; Fn, fibronectin.
PKB/Akt Is Essential for α5-mediated Enhanced Cell Survival
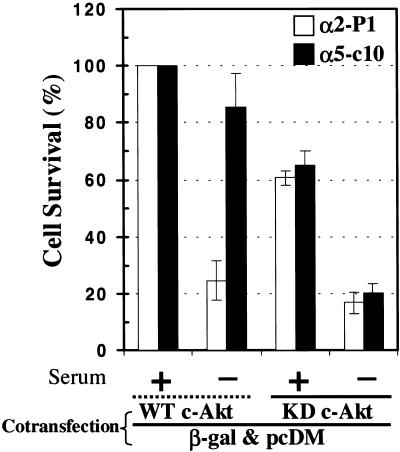
To further evaluate the potential role of PKB/Akt in the phenomenon of α5-mediated resistance to apoptosis, we used a dominant negative form of PKB/Akt. Both α5-c10 RIE cells and α2-expressing cells were transiently transfected with plasmids that expressed either wild-type PKB/Akt or a dominant negative (kinase-dead) version of the protein. The transfectants were marked by coexpression of β-galactosidase. The transfected cells were then cultured in serum-replete or serum-free conditions, and the percentage of surviving cells was determined for each experimental condition. As shown in Figure 7, wild-type PKB/Akt had little effect on the survival pattern of α5- or α2-expressing cell lines; thus, the α2 cells showed a sharp decline in survival in serum-free conditions, whereas the α5 cells did not. When cells were transfected with dominant negative PKB/Akt, there was a modest reduction in survival for both α2 and α5 cells in serum-replete medium. Moreover, the presence of the dominant negative PKB/Akt completely blocked the ability of α5 to enhance survival under serum-free conditions. Thus, PKB/Akt seems to play an important role in α5-mediated protection from apoptosis, as originally suggested by the experiments with LY294002.
Figure 7.
Effects of a dominant negative form of PKB/Akt. Cells were cotransfected with β-galactosidase (β-gal) plasmid and either WT PKB/Akt or kinase-dead (KD) PKB/Akt plasmids. After transfection and recovery, cells were maintained in serum-replete or serum-free conditions. After staining for β-galactosidase, the number of surviving cells was counted by visual observation. Percent survival was calculated in comparison with control cells cotransfected with β-galactosidase plasmid and WT PKB/Akt plasmid and maintained in serum. Means and SEs are shown.
DISCUSSION
In this study, we describe a role for the α5β1 integrin in regulating cell survival in normal rat intestinal epithelial cells (RIE1). We found that α5 subunit expression allows the RIE1 cells to be more resistant to proapoptotic stimuli, such as serum deprivation and treatment with various cytotoxic reagents, including aspirin, staurosporine, and etoposide. The antiapoptotic effects mediated by α5 subunit expression could be reversed by pharmacological inhibition of PI-3-kinase, indicating a role for this enzyme or its downstream effectors. Direct engagement of α5β1 integrin with fibronectin, as well as cosignaling upon stimulation with growth factors, specifically enhanced the activity of PKB/Akt, a downstream effector of PI-3-kinase known to be important in cell survival. Finally, expression of a dominant negative version of PKB/Akt blocked the ability of the α5 subunit to enhance cell survival. Thus, our findings indicate that expression of the α5β1 integrin in intestinal epithelial cells selectively modulates the PI-3-kinase/Akt pathway to promote cell survival in the face of general cytotoxic insults.
These observations may have some interesting implications for the biology of the normal intestinal epithelium. Renewal of the epithelial lining requires a coordinated process involving stem cell replication in the crypts, development of several differentiated lineages, cell migration, and ultimately terminal differentiation, including apoptosis and cell shedding (Stappenbeck et al., 1998; Karam, 1999). To sustain this process, intestinal cell populations need to be protected against premature apoptosis. We have shown that the α5β1 integrin can have an important antiapoptotic action in intestinal epithelial cells. This is consistent with the distribution of α5β1 and fibronectin, its major ligand, along the crypt–villus axis. Thus, α5β1 is found at the base of crypt and villus cells in the human small intestine in a patchy distribution (Beaulieu, 1992), whereas fibronectin is found along the crypt–villus axis except at the upper third of the villus in human and rat (Simon-Assmann et al., 1986; Beaulieu et al., 1991). This pattern of expression and the possible interaction of fibronectin and α5β1 along the crypt–villus axis parallels to some degree the pattern of terminal differentiation of intestinal cells. This may be somewhat similar to the situation observed in the growth and differentiation of the epidermis, in which pluripotent stem cells and replicating transit cells display high levels of β1 integrins, whereas terminally differentiating cells lose expression of α5β1 (Adams and Watt, 1990) and other β1 integrins (Watt, 1998). One cautionary note concerning our study is that we examined the effects of integrin subunits intentionally overexpressed by transfection. The impact of endogenous integrins, which are expressed at lower levels, may be less dramatic.
Because both small intestine and colonic cells display an effect of α5 subunit expression on the regulation of apoptosis, our results may have important ramifications for colon tumor biology. However, the role of α5β1 integrin in colon cancer is likely to be complex, and somewhat discordant results have been reported in the literature. In terms of the expression of α5β1, one group (Stallmach et al., 1992) has reported a progressive loss of integrin expression (including α5β1) with increasing neoplastic transformation, whereas another group (Gong et al., 1997) has found that highly invasive colon tumor lines express α5β1 but poorly invasive lines do not. In terms of integrin function, our group (Varner et al., 1995) and another group (Stallmach et al., 1994) have found that overexpression of α5β1 in HT29 colonic carcinoma cells resulted in a marked reduction in tumorigenicity. However, there is a report of increased tumorigenicity attributable to α5 transfection in the GEO colon cancer cell line (Gong et al., 1997). The divergent results in tumorigenesis studies are paralleled by somewhat conflicting studies in cell culture formats. Thus, we found that overexpression of α5β1 in HT29 cells led to reduced expression of genes associated with cell cycle traverse and increased expression of growth arrest–specific genes (Varner et al., 1995); however, full engagement of α5β1 by its ligand fibronectin could reverse the growth-inhibitory effects. In contrast, another report indicated that disruption of fibronectin binding to α5β1 would stimulate cell cycle–associated events in FET colon carcinoma cells (Gong et al., 1998). However, this same group found that overexpression of α5β1 in breast carcinoma cells led to up-regulation of type II TGFβ receptor expression, facilitating a negative regulatory pathway that led to reduced tumor growth (Wang et al., 1999). A negative role for α5β1 in tumor growth was also reported in early studies with CHO cells (Giancotti and Ruoslahti, 1990; Schreiner et al., 1991). The differences observed in the effects of α5β1 integrin on cell growth and tumor formation may indicate that signaling through this integrin is highly context dependent. It is important to note that α5β1 is not the only receptor for fibronectin in gastrointestinal epithelial cells; thus, the αvβ6 integrin (Munger et al., 1999) has been shown to play an important role in regulating metalloproteinase expression in colon cancer cells (Agrez et al., 1999). It is also interesting to note that colonic epithelial cells are exposed to a fibronectin-containing matrix within the crypt and that fibronectin deposits increase in colorectal cancers (Hauptmann et al., 1995; Pujuguet et al., 1996). Thus, the opportunity exists for integrin–fibronectin interactions that may contribute to the survival, growth, or differentiation of both normal and malignant colonic epithelia.
In several instances, overexpression of α5β1 integrin has been reported to reduce cell growth potential and to protect against apoptosis within the same cell type; this has been observed in CHO cells and in HT29 colon carcinoma cells (Giancotti and Ruoslahti, 1990; Varner et al., 1995; Zhang et al., 1995; O'Brien et al., 1996). These results are only superficially contradictory. Thus, it is well established that certain key signal transducers can have both progrowth and proapoptotic effects, depending on circumstances; good examples include the Ras and Myc oncogenes (Kauffmann-Zeh et al., 1997; Mayo et al., 1997; Prendergast, 1999). Conversely, it seems reasonable that integrin-dependent signals might have positive effects on cell survival in the face of stress but might also slow cell cycle progression under more favorable circumstances. In terms of colon tumor biology, our current observations, as well as previous findings (Varner et al., 1995), suggest that ectopic reexpression of α5β1 may lead to the emergence of carcinoma cells that grow relatively slowly but that are highly protected against stress. This may allow the colon tumor cells to infiltrate inappropriate environments outside of the mucosal lining and to survive stressors such as hypoxia. This could confer an overall advantage in terms of tumor growth and invasiveness.
That integrins can interact with the PI-3-kinase/Akt pathway now seems well established. For example, PI-3-kinase has been implicated in direct integrin-mediated activation of Raf-1 (King et al., 1998). In addition, as mentioned above, anoikis resulting from complete disruption of integrin-mediated adhesion involves reduced signaling through the PI-3-kinase/Akt pathway (Khwaja et al., 1997). Here we implicate α5β1 as having a selective effect on the PI-3-kinase/Akt pathway in intestinal epithelial cells, whereas other integrins, such as α2β1, are not involved. The evidence for a specific connection between α5β1 and the PI-3-kinase/Akt pathway includes the fact that α5-mediated antiapoptotic effects are reversed by pharmacological inhibition of PI-3-kinase or by expression of a dominant negative version of PKB/Akt, and that activation of PKB/Akt is specifically enhanced by direct signaling from α5β1 as well as by cosignaling through a growth factor receptor and α5β1 integrin. In a parallel set of observations, it was reported recently that mammary epithelial cells display α6β1-mediated cell survival that also requires the activities of PI-3-kinase and PKB/Akt (Farrelly et al., 1999). This finding suggests that there are integrin-specific linkages to the PI-3-kinase/Akt pathway but that the integrin specificity may vary in different cell types. Integrin α subunit–specific signaling to MAPK has been described elsewhere (Wary et al., 1996); however, as shown in RESULTS, in RIE1 cells activation of MAPK was observed in cells anchored on either fibronectin via α5β1 or on collagen via α2β1. Thus, in the intestinal epithelial cell system, the integrin specificity seems to be primarily directed toward the PI-3-kinase/Akt pathway, in agreement with results in mammary cells (Farrelly et al., 1999). Although α2β1 clearly does not interact effectively with the PKB/Akt survival pathway in intestinal cells, we cannot rule out the possibility that integrins in addition to α5β1 may also link to this pathway. However, both our current observations and previous studies from another group (Zhang et al., 1995) suggest that α5β1 may have a special role in protection against apoptosis. It should be noted that the current study, implicating PI-3-kinase/Akt in integrin regulation of apoptosis, is quite distinct from studies of “anoikis” (Frisch et al., 1996a,b; Khwaja and Downward, 1997; Khwaja et al., 1997). Here, both RIE WT cells and the α5 transfectants were attached and spread during the period in which apoptosis occurred. Thus, it is the presence of a particular integrin rather than the state of cell attachment that is important, as opposed to the situation in anoikis, in which attachment is the key factor.
An interesting result from the current study is the finding that expression of an α5 subunit with a cytoplasmic tail deletion conferred substantial protection against apoptosis. Furthermore, this antiapoptotic effect was at least partially reversed by PI-3-kinase inhibitors. Although the impact of the tailless mutant was less than that of full-length α5, these observations indicate that activation of the PI-3-kinase/Akt pathway and subsequent antiapoptotic effects may not strictly require the cytoplasmic domain. This stands in contrast to previous observations concerning the antiapoptotic effect of α5 in CHO cells (Zhang et al., 1995). In that study, truncation of the cytoplasmic domain abolished the effect, which seemed to be mediated through induction of Bcl-2. Because, in RIE1 cells, the cytoplasmic domain of α5 was not required for effects on the PI-3-kinase/Akt pathway, the integrin may be interacting with another transmembrane protein to mediate its effect. A number of transmembrane partner proteins for integrins have been described recently, including integrin-associated protein, caveolin, and members of the tetraspannin family (Hemler, 1998; Porter and Hogg, 1998); one of these may be involved in coupling α5 to the PI-3-kinase/Akt pathway.
At present, the precise mechanism(s) underlying integrin-specific activation of the PI-3-kinase/Akt pathway and subsequent effects on apoptosis remain unknown. The antiapoptotic effect of α5β1 in the context of serum deprivation might be simply due to increased cellular responsiveness to activation of PI-3-kinase/Akt via autocrine growth factors. However, the biochemical basis for α5β1 enhancement of growth factor signaling to PI-3-kinase/Akt is currently undefined. A concept consistent with emerging views in the literature (Aplin et al., 1998; Aplin and Juliano, 1999; Giancotti and Ruoslahti, 1999) is that integrins and/or integrin-associated cytoskeletal components assist in the formation of efficient signal transduction complexes. Nonetheless, it will be important to augment this general concept with specific details of the assembly of putative signaling complexes.
ACKNOWLEDGMENTS
The authors acknowledge our University of North Carolina colleagues Drs. Andrew E. Aplin and Sarah M. Short for insightful discussion and reading of the manuscript and Dr. Channing Der for the kind gift of PKB/Akt cDNAs. This work was supported by grant CA74966 from the National Institutes of Health to R.L.J.
REFERENCES
- Adams JC, Watt FM. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes α5β1 integrin loss from the cell surface. Cell. 1990;63:425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Agrez M, Gu X, Turton J, Meldrum C, Niu J, Antalis T, Howard EW. The alpha v beta 6 integrin induces gelatinase B secretion in colon cancer cells. Int J Cancer. 1999;81:90–97. doi: 10.1002/(sici)1097-0215(19990331)81:1<90::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Aplin A, Howe A, Alahari S, Juliano RL. Signal transduction by cell adhesion receptors. Pharmacol Rev. 1998;50:197–264. [PubMed] [Google Scholar]
- Aplin AE, Juliano RL. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J Cell Sci. 1999;112:695–706. doi: 10.1242/jcs.112.5.695. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Short SM, Juliano RJ. Anchorage-dependent regulation of the mitogen-activated protein kinase cascade by growth factors is supported by a variety of integrin alpha chains. J Biol Chem. 1999;274:31223–31228. doi: 10.1074/jbc.274.44.31223. [DOI] [PubMed] [Google Scholar]
- Bauer JS, Varner J, Schreiner C, Kornberg L, Nicholas R, Juliano RL. Functional role of the cytoplasmic domain of the integrin alpha 5 subunit. J Cell Biol. 1993;122:209–221. doi: 10.1083/jcb.122.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JF. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci. 1992;102:427–436. doi: 10.1242/jcs.102.3.427. [DOI] [PubMed] [Google Scholar]
- Beaulieu JF. Integrins and human intestinal cell functions. Front Biosci. 1999;4:D310–D321. doi: 10.2741/beaulieu. [DOI] [PubMed] [Google Scholar]
- Beaulieu JF, Vachon PH, Chartrand S. Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat Embryol. 1991;183:363–369. doi: 10.1007/BF00196837. [DOI] [PubMed] [Google Scholar]
- Berra E, Diaz-Meco MT, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 1998;273:10792–10797. doi: 10.1074/jbc.273.17.10792. ; Erratum 273, 16630. [DOI] [PubMed] [Google Scholar]
- Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757s–1763s. [PubMed] [Google Scholar]
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Burgess AW. Growth control mechanisms in normal and transformed intestinal cells. Philos Trans R Soc Lond B Biol Sci. 1998;353:903–909. doi: 10.1098/rstb.1998.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. Akt/PKB and other D3 phosphoinositide regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of Bad couples survival signal to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- DuBois RN, Awaad J, Morrow J, Roberts LJ, II, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-α and phorbol ester. J Clin Invest. 1994;93:493–498. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Perturbation of β1-integrin function alters the development of murine mammary gland. EMBO J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly N, Lee YJ, Oliver J, Dive C, Streuli CH. Extracellular matrix regulates apoptosis in mammary epithelium through a control on insulin signaling. J Cell Biol. 1999;144:1337–1348. doi: 10.1083/jcb.144.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Kelaita D, Sicks S. A role for Jun-N-terminal kinase in anoikis: suppression by bcl-2 and crmA. J Cell Biol. 1996a;135:1377–1382. doi: 10.1083/jcb.135.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996b;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Thompson CB. Apoptosis meets signal transduction: elimination of a BAD influence. Cell. 1998;87:589–592. doi: 10.1016/s0092-8674(00)81377-x. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gong J, Ko TC, Brattain MG. Disruption of fibronectin binding to the α5β1 integrin stimulates the expression of cyclin-dependent kinases and DNA synthesis through activation of extracellular signal-regulated kinase. J Biol Chem. 1998;273:1662–1669. doi: 10.1074/jbc.273.3.1662. [DOI] [PubMed] [Google Scholar]
- Gong J, Wang D, Sun L, Zborowska E, Willson JKV, Brattain MG. Role of α5β1 integrin in determining malignant properties of colon carcinoma cells. Cell Growth Differ. 1997;8:83–90. [PubMed] [Google Scholar]
- Granville DJ, Carthy CM, Hunt DWC, McManus BM. Apoptosis: molecular aspects of cell death and disease. Lab Invest. 1998;78:893–913. [PubMed] [Google Scholar]
- Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- Hauptmann S, et al. Extracellular matrix proteins in colorectal carcinomas. Lab Invest. 1995;73:172–182. [PubMed] [Google Scholar]
- Hemler ME. Integrin associated proteins. Curr Opin Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–D298. doi: 10.2741/karam. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signaling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- Kedinger M, Duluc I, Fritsch C, Lorentz O, Plateroti M, Freund JN. Intestinal epithelial-mesenchymal cell interactions. Ann NY Acad Sci. 1998;17:1–17. doi: 10.1111/j.1749-6632.1998.tb11107.x. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/protein kinase B inhibits cell death by preventing release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A, Downward J. Lack of correlation between activation of Jun-NH2-terminal kinase and induction of apoptosis after detachment of epithelial cells. J Cell Biol. 1997;139:1017–1023. doi: 10.1083/jcb.139.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops GJ, Ruiter ND, Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- Matter ML, Zhang Z, Nordstedt C, Ruoslahti E. The a5b1 integrin mediates elimination of amyloid-b peptide and protects against apoptosis. J Cell Biol. 1998;141:1019–1030. doi: 10.1083/jcb.141.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, Baldwin AS., Jr Requirement of NFκB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- Munger JS, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- O'Brien V, Frisch SM, Juliano RL. Expression of the integrin α5 subunit in HT29 colon carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp Cell Res. 1996;224:208–213. doi: 10.1006/excr.1996.0130. [DOI] [PubMed] [Google Scholar]
- Oldham SM, Clark GJ, Gangarosa LM, Coffey RJ, Jr, Der CJ. Activation of the Raf-1/MAP kinase cascade is not sufficient for Ras transformation of RIE-1 epithelial cells. Proc Natl Acad Sci USA. 1996;93:6924–6928. doi: 10.1073/pnas.93.14.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M. Models of colorectal tumor differentiation. Cancer Surv. 1993;16:3–13. [PubMed] [Google Scholar]
- Porter JC, Hogg N. Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol. 1998;8:390–396. doi: 10.1016/s0962-8924(98)01344-0. [DOI] [PubMed] [Google Scholar]
- Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18:2967–2987. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- Pujuguet P, Hammann A, Moutet M, Samuel JL, Martin F, Martin M. Expression of fibronectin ED-A+ and ED-B+ isoforms by human and experimental colorectal cancer. Am J Pathol. 1996;148:579–592. [PMC free article] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor Forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid MP, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/Akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner C, Fisher M, Hussein S, Juliano RL. Increased tumorigenicity of fibronectin receptor deficient Chinese hamster ovary cell variants. Cancer Res. 1991;51:1738–1740. [PubMed] [Google Scholar]
- Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P, Kedinger M, Haffen K. Immunocytochemical localization of extracellular-matrix protein in relation to rat intestinal morphogenesis. Differentiation. 1986;32:59–66. doi: 10.1111/j.1432-0436.1986.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Slee EA, et al. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspase-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallmach A, Lampe BV, Matthes H, Bornhoft G, Riecken EO. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumor transformation. Gut. 1992;33:342–346. doi: 10.1136/gut.33.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallmach A, Lampe BV, Orzechowski H-D, Matthes H, Riecken E-O. Increased fibronectin-receptor expression in colon carcinoma-derived HT29 cells decreases tumorigenicity in nude mice. Gastroenterology. 1994;106:19–27. doi: 10.1016/s0016-5085(94)94031-2. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Wong MH, Saam JR, Mysorekar IU, Gordon JI. Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr Opin Cell Biol. 1998;10:702–709. doi: 10.1016/s0955-0674(98)80110-5. [DOI] [PubMed] [Google Scholar]
- Tang ED, Nunez G, Barr FG, Guan K-L. Negative regulation of the Forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NFκB. Science. 1998;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Varner JA, Emerson DA, Juliano RL. Integrin α5β1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol Biol Cell. 1995;6:725–740. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NFκB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Wang D, Sun L, Zborowska E, Willson JK, Gong J, Verraraghavan J, Brattain MG. Control of type II transforming growth factor-beta receptor expression by integrin ligation. J Biol Chem. 1999;274:12840–12847. doi: 10.1074/jbc.274.18.12840. [DOI] [PubMed] [Google Scholar]
- Wary K, Mainiero F, Isakoff S, Marcantonio E, Giancotti F. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Watt FM. Epidermal stem cells: markers, patterning and the control of stem cell fate. Philos Trans R Soc Lond B Biol Sci. 1998;353:831–837. doi: 10.1098/rstb.1998.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winesett MP, Ramsey GW, Barnard JA. Type II TGF(β) receptor expression in intestinal cell lines and in the intestinal tract. Carcinogenesis. 1996;17:989–995. doi: 10.1093/carcin/17.5.989. [DOI] [PubMed] [Google Scholar]
- Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist Bad in response to survival factor results in binding to 14-3-3 not Bcl-XL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, Ruoslahti E. The α5β1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AJ, Haase I, Watt FM. Signaling via β1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci USA. 1999;96:6728–6733. doi: 10.1073/pnas.96.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]