Abstract
Yeast phosphatidylinositol transfer protein (Sec14p) is essential for Golgi function and cell viability. We now report a characterization of five yeast SFH (Sec Fourteen Homologue) proteins that share 24–65% primary sequence identity with Sec14p. We show that Sfh1p, which shares 64% primary sequence identity with Sec14p, is nonfunctional as a Sec14p in vivo or in vitro. Yet, SFH proteins sharing low primary sequence similarity with Sec14p (i.e., Sfh2p, Sfh3p, Sfh4p, and Sfh5p) represent novel phosphatidylinositol transfer proteins (PITPs) that exhibit phosphatidylinositol- but not phosphatidylcholine-transfer activity in vitro. Moreover, increased expression of Sfh2p, Sfh4p, or Sfh5p rescues sec14-associated growth and secretory defects in a phospholipase D (PLD)-sensitive manner. Several independent lines of evidence further demonstrate that SFH PITPs are collectively required for efficient activation of PLD in vegetative cells. These include a collective requirement for SFH proteins in Sec14p-independent cell growth and in optimal activation of PLD in Sec14p-deficient cells. Consistent with these findings, Sfh2p colocalizes with PLD in endosomal compartments. The data indicate that SFH gene products cooperate with “bypass-Sec14p” mutations and PLD in a complex interaction through which yeast can adapt to loss of the essential function of Sec14p. These findings expand the physiological repertoire of PITP function in yeast and provide the first in vivo demonstration of a role for specific PITPs in stimulating activation of PLD.
INTRODUCTION
Phosphatidylinositol transfer proteins (PITPs) are defined by their ability to transfer phosphatidylinositol (PtdIns) or phosphatidylcholine (PtdCho) monomers between membrane bilayers in vitro (Cleves et al., 1991a; Wirtz, 1991). PITP dependence has been observed in reconstitutions of constitutive exocytosis, regulated exocytosis, intra-Golgi membrane trafficking, and plasma membrane signaling in permeabilized mammalian cells. These data suggest that PITPs play important roles in regulating phosphoinositide production in vivo (Hay and Martin, 1993; Hay et al., 1995; Ohashi et al., 1995; Cunningham et al., 1996; DeCamilli et al., 1996; Jones et al., 1998; Simon et al., 1998).
The major PITP of Saccharomyces cerevisiae is encoded by SEC14 (Bankaitis et al., 1990; Cleves et al., 1991b). Sec14p is essential for protein transport from the Golgi complex and for cell viability (Novick et al., 1980; Bankaitis et al., 1989). Consistent with a role for Sec14p in stimulating Golgi secretory function, Sec14p localizes to yeast Golgi membranes in vivo (Cleves et al., 1991b). Information gained from a Sec14p crystal structure has guided the generation of Sec14p mutants specifically inactivated for PtdIns-transfer activity (Phillips et al., 1999). Interestingly, a mutant Sec14p deficient in PtdIns-transfer activity, and in its ability to stimulate phosphoinositide production in vivo and in vitro, fulfills essential Sec14p functions in yeast (Phillips et al., 1999).
Insights into the in vivo mechanism of Sec14p function have been derived from analyses of loss-of-function mutations that effect an efficient bypass of the essential Sec14p requirement for Golgi function and cell viability (Cleves et al., 1991a; Kearns et al., 1998a). One class of “bypass-Sec14p” loci define inactivating mutations that involve structural enzymes of the CDP-choline pathway for PtdCho biosynthesis (Cleves et al., 1991b; McGee et al., 1994), one of the two pathways for PtdCho biosynthesis in yeast (Figure 1). The evidence suggests that PtdCho-bound Sec14p down-regulates flux through this PtdCho biosynthetic pathway (McGee et al., 1994; Skinner et al., 1995). Another bypass-Sec14p locus defines loss-of-function mutations in SAC1 (Cleves et al., 1989; Figure 1). SAC1 encodes an integral membrane protein of the yeast endoplasmic reticulum and the Golgi complex (Sac1p; Whitters et al., 1993). SacIp regulates inositol phospholipid and PtdCho metabolism in vivo (Cleves et al., 1991a; Whitters et al., 1993; Kearns et al., 1997; Guo et al., 1999; Rivas et al., 1999; Stock et al., 1999; Hughes et al., 2000) and has intrinsic phosphoinositide phosphatase activity (Guo et al., 1999). The last characterized set of bypass-Sec14p mutations inactivate KES1, a gene encoding a yeast member of the oxysterol-binding protein family (Fang et al., 1996). These three classes of bypass-Sec14p mutations all require participation of the normally nonessential yeast phospholipase D (PLD) to manifest their bypass-Sec14p phenotypes (Sreenivas et al., 1998; Xie et al., 1998; Figure 1). In this regard, it is interesting to note that Sec14p deficiency evokes PLD activation (Patton-Vogt et al., 1997; Sreenivas et al., 1998; Xie et al., 1998), even though popular models posit that PITP stimulates PLD activation (Liscovitch and Cantley, 1995).
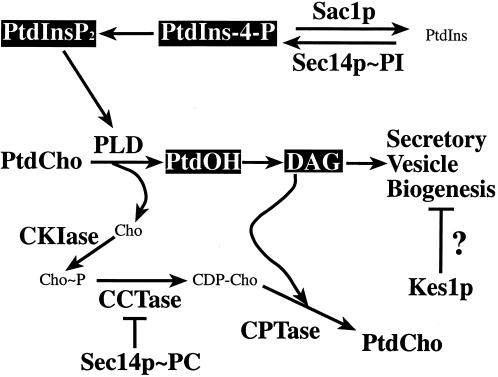
Figure 1.
Sec14p pathway in yeast. The PtdCho- and PtdIns-bound forms of Sec14p are proposed to independently regulate inositol and choline phospholipid metabolism (reviewed by Kearns et al., 1998a). PtdCho-bound Sec14p down-regulates flux through the diacylglycerol (DAG)-utilizing CDP-choline pathway for PtdCho biosynthesis. PtdIns-bound Sec14p stimulates PtdIns-4-P synthesis via the Pik1p pathway. PtdIns-4-P is a precursor for PtdIns-4,5-P2, which is required for activation of PLD. PLD hydrolyzes PtdCho to PtdOH and choline. PtdOH is dephosphorylated by PtdOH-phosphohydrolases to DAG. Evidence to support individual roles for PtdCho, DAG, PtdOH, PtdIns-4-P, and PtdIns-4,5-P2 in modulating yeast Golgi secretory function exists. However, the lipid regulators that directly interact with the Sec14p pathway are unclear. Bypass-Sec14p mutations used in this study inactivate choline kinase (CKIase; cki1), choline phosphate cytidylyltransferase (CCTase; pct1), the SacIp phosphoinositidase (sac1), and the Kes1p oxysterol-binding protein homologue (kes1). Known execution points for these enzymes/proteins are given. The precise execution point for Kes1p remains unclear.
In this report, we describe four novel yeast SFH (Sec Fourteen Homologue) proteins designated Sfh2p, Sfh3p, Sfh4p, and Sfh5p. These PITPs are atypical in that they exhibit PtdIns-transfer activity, but not PtdCho-transfer activity, in vitro. Individual overexpression of these novel PITPs rescues the growth and/or secretory defects associated with Sec14p deficiency. In all cases, however, PLD is either required for rescue or markedly facilitates the efficiency of rescue. We further demonstrate, by several independent assays, that bulk PLD activity is sensitive to en bloc disruption of the SFH genes and that SFH deficiencies phenocopy PLD defects. Collectively, these data expand the repertoire of PITPs and PITP-dependent functions in yeast and provide the first insights into the identities of proteins that contribute to PLD activation in this organism.
MATERIALS AND METHODS
Strains, Media, and Genetic Methods
Yeast complex and minimal media (Sherman et al., 1983), and methods for transformation of yeast with plasmids and linear DNA fragments (Ito et al., 1983; Rothstein, 1983; Sherman et al., 1983), have been described. Complete genotypes of yeast strains are listed in Table 1. Fine chemicals were purchased from Sigma Chemical (St. Louis, MO) unless specified otherwise. Fenpropimorph was purchased from Crescent Chemical (Hauppauge, NY), and restriction endonucleases were purchased from Promega (Madison, WI). [1,2-3H(N)]Inositol and [14C]PtdCho were supplied by American Radiolabeled Chemicals (St. Louis, MO). All other phospholipids were obtained from Avanti Polar Lipids (Birmingham, AL). [α-35S]dATP was from Amersham (Arlington Heights, IL).
Table 1.
Yeast strains
| Strain number | Genotype |
|---|---|
| CTY1-1A | MATa ura3-52 Δhis3-200 lys2-801 sec14-1ts |
| CTY100 | MATa ura3-52 Δhis3-200 lys2-801 sec14-1ts sac1-26cs |
| CTY159 | MATa ura3-52 Δhis3-200 lys2-801 sec14-1ts kes1-1 |
| CTY160 | MATa ura3-52 Δhis3-200 lys2-801 sec14-1ts cki1-1 |
| CTY182 | MATa ura3-52 Δhis3-200 lys2-801 SEC14 |
| CTY303 | MATa ura3-52 Δhis3-200 cki1-1 Δsec14::HISG |
| CTY808 | MATa ura3-52 Δhis3-200 cki1-1 Δsec14::HISG/YEplac195 |
| CTY965 | MATa ura3-52 Δhis3-200 lys2-801 sec14-1ts/YEp (SFH3) |
| CTY966 | MATa ura3-52 Δhis3-200 lys2-801 sec14-1ts/YEp (SFH4) |
| CTY967 | MATa ura3-52 Δhis3-200 lys2-801 sec14-1ts/YEp (SFH5) |
| CTY971 | MATa ura3-52 Δhis3-200 cki1-1 Δsec14::HISG/YEp (SFH4) |
| CTY972 | MATa ura3-52 Δhis3-200 cki1-1 Δsec14::HISG/YEp (SFH5) |
| CTY979 | MATa ura3-52 Δhis3-200 cki1-1 Δsec14::HISG/YEp (SFH3) |
| CTY1079 | MATa ura3-52 Δhis3-200 lys2-801 Δspo14::HIS3 sec14-1ts |
| CTY1099 | MATa ura3-52 Δhis3-200 lys2-801 Δspo14::URA3 sec14-1ts cki1-1 |
| CTY1106 | MATa ura3-52 Δhis3-200 lys2-801 sec14-1ts/YEp (SFH2) |
| CTY1109 | MATa ura3-52 Δhis3-200 cki1-1 Δsec14::HISG/YEp (SFH2) |
| CTY1118 | MATa ura3-52 Δhis3-200 sec14-1ts Δsfh2::URA3 Δsfh3::URA3 Δsfh4::URA3 Δsfh5::URA3 |
| CTY1289 | MATa Δhis3-200 ade2-101 leu2 cho3Δ::LEU2 opi3Δ::URA3 |
| CTY1344 | MATa ura3-52 Δhis3-200 lys2-801 sec14-3ts/YEp (SFH2::GFP) |
| CTY1427 | MATa ura3-52 Δhis3-200 sec14-1ts Δsfh2::URA3 Δsfh3::URA3 Δsfh4::URA3 Δsfh5::URA3 Δcki1::HIS3 |
| CTY1434 | MATa ura3-52 Δhis3-200 lys2-801 Δspo14::HIS3 sec14-1ts/YEp (SFH2) |
| CTY1435 | MATa ura3-52 Δhis3-200 lys2-801 Δspo14::HIS3 sec14-1ts/YEp (SFH3) |
| CTY1436 | MATa ura3-52 Δhis3-200 lys2-801 Δspo14::HIS3 sec14-1ts/YEp (SFH4) |
| CTY1437 | MATa ura3-52 Δhis3-200 lys2-801 Δspo14::HIS3 sec14-1ts/YEp (SFH5) |
Construction of SFH Expression Plasmids
The 1.25-kilobase (kb) coding region of SFH2 was amplified by PCR from plasmid pML103 (Lai et al., 1994) with the use of the primers identified in Tables 2 and 3. The PCR product was digested with the restriction endonucleases BsmBI and SphI and subcloned into the cognate restriction sites of plasmid pRE375. In this manner, the SFH2 coding sequence was positioned directly downstream of the 500-base pair promoter of the yeast SEC14 gene (PSEC14). The PSEC14::SFH2 cassette was recovered as a 1.75-kb EcoRI–SphI restriction fragment and subcloned into the unique EcoRI and SphI sites of the YEp(URA3) vector pSEY18. The resultant plasmid was designated pCTY201, and it drives constitutive overexpression of Sfh2p.
Table 2.
Designations of PCR primers used for the cloning and disruption of SFH genes
| Gene | PCR cloning | Disruption | Positive screening | Negative diagnosis |
|---|---|---|---|---|
| SFH2 | PML-5′, PML-3′ | URA3-E, URA3-F | PLM-A, URA3-F | PLM-B, SFH2-G |
| SFH3 | 5′-SFH3, 3′-SFH3 | SFH3-A, SFH3-B | SFH3-A, SFH3-C | 3′-SFH3, SFH3-D |
| SFH4 | 5′-SFH4, 3′-SFH4 | SFH4-A, SFH4-B | SFH4-B, SFH4-C | SFH4-C, SFH4-D |
| SFH5 | 5′-SFH5, 3′-SFH5 | SFH5-A, SFH5-B | SFH5-A, SFH5-C | 5′-SFH5, 3′-SFH5 |
Table 3.
Primer sequences
| Primer | Primer sequence (5′–3′) |
|---|---|
| PML5′ | aaaacgtctcaagcttatgtcctttgatagacagt |
| PML3′ | aaaagcatgcgcgtcgcgtggcttggct |
| URA3-E | cggaattcaagaagaaatcatcctggtttagcaagcttagcttttcaattcatcttt tttt |
| URA3-F | cggaattcaaaacaatcttggacgcgacgaccggatccagctttttctttccaatttttttt |
| PLM-A | gcggccctaaacgaggcggt |
| PLM-B | aaaggtaccggcctgtggtggcgttcgttcgttag |
| SFH2-G | caatctagacccgcaaacgaccaagaag |
| 5′-SFH3 | aaaagttaacatgttcaagagatttagcaaaaagaaggaggc |
| 3′-SFH3 | aaaaggatccggttaggaaaaagaagcgggaacgtg |
| SFH3-A | ggggtaccaatttgcctttacacggtactgctttccgagcttttcaattcatcttttttt |
| SFH3-B | ggggtaccgcaaaaagaaggaggctccagaagacccagctttttctttccaatttttttt |
| SFH3-C | ggttcatgcactatatgcgcatcccactc |
| SFH3-D | gtcggaggatgatcttaaaccc |
| 5′-SFH4 | aaaagttaacatgggtcttttttcaagaaaacgggacc |
| 3′-SFH4 | aaaaggatccgagtgcggatagccgattcagttg |
| SFH4-A | ggggtaccggatattttaattcgtcatgttggcccttgagcttttcaattcatcttttttt |
| SFH4-B | ggggtacccgggaccatacgcctgctgtgcctaaagagagctttttctttccaatttttttt |
| SFH4-C | gagtgcggatagccgattcagttgattc |
| SFH4-D | ccctcctgccaagtatgg |
| 5′-SFH5 | aaaagttaacatgaattcgacaatgacagtgagaagcagg |
| 3′-SFH5 | aaaaggatccggcggatctgatcgatctgtatccc |
| SFH5-A | cgggtacctacattctcgggtgtggctagaattgggcgagcttttcaattcatcttttttt |
| SFH5-B | cgggtacccccgccatacccttcatatgggcaatccttagctttttctttccaatttttttt |
| SFH5-C | ggcggatctgatcgatctgtatccc |
The coding sequences of the SFH3, SFH4, and SFH5 genes were amplified by PCR from genomic DNA with the use of the forward and reverse primers identified in Tables 2 and 3 (initiator codons are shown in boldface). The 1.15-kb SFH3, 1.2-kb SFH4, and 1.1-kb SFH5 PCR products were digested by HpaI and BamHI and inserted into the HpaI and BamHI sites of the YEp(URA3) plasmid pCTY335. These manipulations placed these genes under the transcriptional control of the powerful and constitutive yeast phosphoglycerate kinase promoter (PPGK) to yield plasmids pCTY344, pCTY345, and pCTY346, respectively.
Invertase Assays
Appropriate strains were grown to midlogarithmic phase in 2% glucose medium at 26°C with shaking. Cells were pelleted, washed with 2 volumes of water, resuspended in 2 volumes of prewarmed glucose (0.1%) YP medium, and incubated at 37°C for 2 h. The samples were adjusted to 10 mM NaN3, washed twice with ice-cold 10 mM NaN3, and resuspended in 0.5 ml of 10 mM NaN3. Each sample was divided into two aliquots, and the volumes were adjusted to 1.0 ml with 0.2% Triton X-100 and 10 mM NaN3 or 10 mM NaN3 to generate intact cell or permeabilized cell samples, respectively. External and total invertase activities were quantified as described previously (Salama et al., 1990).
Gene Disruptions
For each SFH gene, a DNA disruption cassette consisting of yeast URA3 as selectable marker, flanked by 25- to 35-base pair homologous sequences from the targeted gene, was created. Thus, upon recombination into the yeast genome, the individual disruption cassettes evoked replacement of the entire corresponding SFH coding region with URA3. Correctly targeted recombinant proteins were identified by positive PCR diagnosis with the use of one primer hybridizing to sequences within the disruption cassette and another primer hybridizing to sequences outside the deleted region of the targeted gene. As independent confirmation, a second PCR amplification was performed with the use of one primer hybridizing to sequences outside the disruption cassette and another primer hybridizing to SFH DNA deleted from the targeting cassette. These negative diagnoses were expected not to yield PCR product from genomic DNA templates prepared from correctly targeted yeast recombinant proteins. A comprehensive list of primers used in these experiments is presented in Tables 2 and 3.
Preparation of Yeast Cytosol and Phospholipid Transfer Assays
Appropriate strains were grown to midlogarithmic phase in medium lacking uracil and harvested by centrifugation. Cell pellets were resuspended in spheroplast buffer (1.1 M sorbitol, 10 mM Tris-HCl, pH 7.5), and 2-mercaptoethanol was added to a final concentration of 25 mM. Cells were incubated at room temperature for 10 min, pelleted, and resuspended in spheroplast buffer. Oxalyticase (Enzogenetics, Corvallis, OR) was added to a final concentration of 2 μg/ml, and cultures were incubated at 30°C for 60 min. Cells were pelleted (500 × g) and resuspended in modified spheroplast buffer (0.55 M sorbitol, 5 mM Tris-HCl, pH 7.5, 1 mM PMSF). Equal volumes of glass beads (0.1 mm, Sigma) were added, and the cells were shaken for a total of 6 min in 1-min bursts with 1-min rests on ice. Salt stripping of the membranes was then performed by adding KCl to a final concentration of 0.5 M and incubating the membranes on ice for 30 min. The broken spheroplast extracts were then clarified by serial centrifugation at 1000 × g, 13,000 × g, and 100,000 × g. The resulting cytosol fractions were assayed for protein concentration with the use of the bicinchoninic acid kit (Pierce, Rockford, IL) and BSA standards. PtdIns- and PtdCho-transfer assays were performed as described by Aitken et al. (1990).
Expression and Purification of SFH Proteins
To express full-length SFH proteins with hexahistidine epitopes at their amino termini, the coding regions of the corresponding SFH genes were subcloned into the Qiagen (Chatsworth, CA) His6 vector pQE-30. Primers were designed to amplify the SFH genes by PCR. These primers were designed to flank the SFH2 coding region with 5′-SalI and 3′-KpnI sites and to flank the SFH3, SFH4, and SFH5 coding regions with 5′-BamHI and 3′-KpnI sites. The forward and reverse primers used to amplify each gene are listed in Tables 2 and 3. The resulting PCR products were digested with the appropriate restriction enzymes and subcloned into the corresponding sites of pQE-30 (Qiagen). The final constructs were transformed into Escherichia coli strain KK2186. The SFH2, SFH3, SFH4, and SFH5 expression plasmids were designated pRE644, pRE743, pRE744, and pRE745, respectively. Recombinant His6-tagged SEC14 was expressed from pRE526 (Skinner et al., 1995). Preparation of cytosolic fractions from E. coli and purification of His6-tagged PITPs have been detailed by Kearns et al. (1998b).
Construction of SFH2::GFP Plasmid
The 1.9-kb SFH2 gene (including its own promoter region) was amplified by PCR from yeast genomic DNA with the use of the forward and reverse primers SFH2-C (5′-AAAAGAATTCCACTGTAAGAAGAACAAGAGATGATGC-3′) and SFH2-D (5′-AAAAACTAGTCTTAAAGTGGTTTTAAGGGCCTGTGGTG-3′). The fragment was digested with EcoRI and SpeI and cloned into the YEp vector pRS423 to yield YEp(SFH2). To subclone the EGFP gene into YEp(SFH2), a linker DNA fragment harboring XmaI and SphI sites was first inserted just upstream of the SFH2 stop codon in YEp(SFH2) by site-directed mutagenesis with the use of the following primers: SFH2-H (5′-AATGGTTCTCTAAAAGTTGCCCGGGCAGCATGCTGAGCCAAGCCACGCGAC) and SFH2-I (5′-GTCGCGTGGCTTGGCTCAGCATGCT GCCCGGGCAACTTTTAGAGAACCATT). The EGFP-coding region was then amplified by PCR from pEGFP-N1 (Clontech, Palo Alto, CA) with the use of primers EGFP-5′ (5′-AAAACCCGGGGCCACCATGGTGAGCAAGGGCGAG) and EGFP-3′ (5′-AAAAGCATGCCTTGTACAGCTCGTCCATGCC) and inserted into the XmaI and SphI sites (underlined in primer sequences) of the modified YEp(SFH2) plasmid. The resultant YEp(SFH2::GFP) plasmid was designated pCTY824.
Immunofluorescence Microscopy
The YEp(SFH2::GFP) plasmid was transformed into the sec14-1ts strain CTY1-1A (Table 1). Liquid cultures were subsequently grown overnight at 25°C in medium selective for the plasmid (i.e., uracil-free minimal medium). These cultures were subsequently diluted to an OD600 of 0.3–0.5 and were incubated as before for 2 to 3 h. Staining of yeast endosomes with the styryl dye FM 4-64 (Molecular Probes, Eugene, OR) was performed by incubating cells with FM 4-64 (40 μM final concentration from an 8 mM stock in DMSO) for 30–60 min at 0°C. Cells were washed twice with ice-cold YPD medium and incubated at 15°C for 15–25 min to chase FM 4-64 into endosomes (Vida and Emr, 1995). Stained cells were gently pelleted by centrifugation and mounted as suspensions in growth medium onto cold glass slides. Epifluorescence microscopy was performed with the use of a Nikon (Garden City, NY) Diaphot 300 microscope equipped with a Photometrics (Tucson, AZ) CH250 charge-coupled device camera. Excitation wavelengths for GFP and FM 4-64 were 495 and 545 nm, respectively, and fluorescence images were processed by IP Lab 2.0, Adobe Photoshop 5.0, and Adobe Pagemaker 6.0 software (Mountain View, CA).
Quantification of Choline Release
Choline was chemically measured in samples of media obtained from the appropriate yeast cultures. Appropriate yeast strains were grown to logarithmic growth phase at 26°C in minimal defined Wickerham's medium supplemented with inositol (100 μM) and lacking choline. Cells were pelleted, washed with water, and resuspended in fresh choline-free medium at 33.5°C. After 3 h, cells were pelleted by centrifugation, the culture supernatants were collected, and the supernatants were then further clarified by passage through a 0.45-μm (pore size) filter. The choline content of the supernatants was then determined with a choline oxidase–coupled assay. A coupled reaction was used in which H2O2 (produced via enzymatic oxidation of choline by choline oxidase) was reacted with aminoantipyrine and phenol to form quinoneimine (Warnick, 1986). Quinoneimine was then quantified spectrophotometrically at an absorbance wavelength of 490 nm. Standard curves relating choline concentration to quinoneimine production were used to relate A490 readings from media samples to absolute choline concentrations (Warnick, 1986).
Phosphatidic Acid Determinations
Yeast strains were grown to midlogarithmic growth phase (3 ml, OD600 = 0.8–1.0) in defined minimal medium containing 1 mM inositol and 1 mM choline and presented with [32P]orthophosphate (10 μCi/ml) for 20 h at 25°C with shaking. Cells were washed, resuspended in radio-label free medium, and incubated for 3 h at 33.5°C with shaking. Cell metabolism was terminated by the addition of TCA to 5%. One-tenth of the culture was removed after trichloroacetic acid (TCA) precipitation to assess incorporation of label. The cells were subsequently incubated for 20 min on ice in 5% TCA, and phospholipids were extracted by the method of Atkinson (1984). Briefly, cell pellets were resuspended in 1 ml of polar extraction solvent (McGee et al., 1994) for 20 min at 65°C. Lipids were recovered by the addition of 5 ml of CHCl3/CH3OH/butylated hydroxytoluene (2:1:0.005) and 0.5 ml of H2O, followed by vigorous shaking for 30 s. After phase separation at 4°C and a 5-min centrifugation, the organic phase was removed, dried under N2 gas, and reconstituted in 60 μl of CHCl3/CH3OH/butylated hydroxytoluene. Phospholipids were resolved by two-dimensional chromatography with the use of Whatman (Clifton, NJ) SG81 paper treated with EDTA (Steiner and Lester, 1972). The first-dimension solvent was CHCl3/CH3OH/NH4OH/H2O (22:9:1:0.26), and the second-dimension solvent was CHCl3/CH3OH/CH3COOH/H2O (8:1:1.25:0.25). Radiolabeled phospholipids were quantified with the use of the Phosphorimager 425 instrument (Molecular Dynamics, Sunnyvale, CA).
PLD Assays
PLD activity was assayed by measuring conversion of 1-palmitoyl-2-[6-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino]caproyl (NBD)- PtdCho to NBD-phosphatidic acid (PtdOH) with the use of clarified extracts prepared from appropriate yeast strains by vigorous disruption with glass beads (300–500 μm diameter). Assays were as described by Waksman et al. (1996) except that 100 μg of total protein was used per reaction and reactions were incubated at 30°C for 1 h. NBD-PtdCho and NBD-PtdOH were resolved in one dimension by TLC with the use of CHCl3/CH3OH/CH3COOH/H2O (8:1:1.25:0.25) as developing solvent. NBD phospholipids were visualized by UV illumination, and the corresponding NBD species were quantified by fluorescence counting with the use of the Storm apparatus (Molecular Dynamics).
RESULTS
Yeast Gene Products with Functional Relatedness to Sec14p
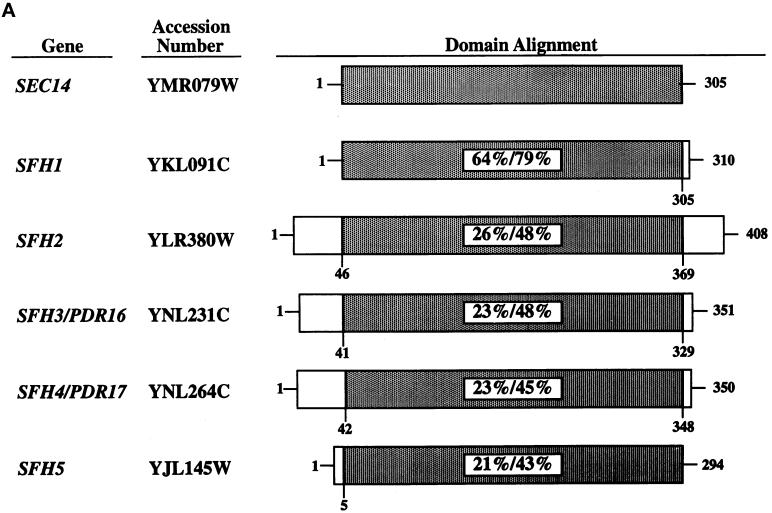
The yeast genome database identifies one ORF that potentially encodes a product sharing 64% primary sequence identity with Sec14p (Bankaitis et al., 1996). Four other ORFs potentially encode proteins sharing ∼25% identity and 45% similarity with Sec14p (Figure 2A). We designate these ORFs SFH1, SFH2, SFH3, SFH4, and SFH5 (Bankaitis et al., 1996; Phillips et al., 1999). The SFH3 and SFH4 genes have also been encountered in independent studies analyzing genes whose transcription is stimulated by challenge of yeast cells with inhibitors of sterol biosynthesis. In these studies, SFH3 and SFH4 have been designated PDR16 and PDR17, respectively (van den Hazel et al., 1999). We will use the SFH nomenclature here, both for purposes of conformity in referring to this class of proteins and because this nomenclature better reflects their structural and functional properties. Northern analyses indicated that SFH2, SFH3, SFH4, and SFH5 are all transcribed in vegetative cells, but no accumulation of SFH1 mRNA or protein was recorded under various growth conditions (our unpublished results).
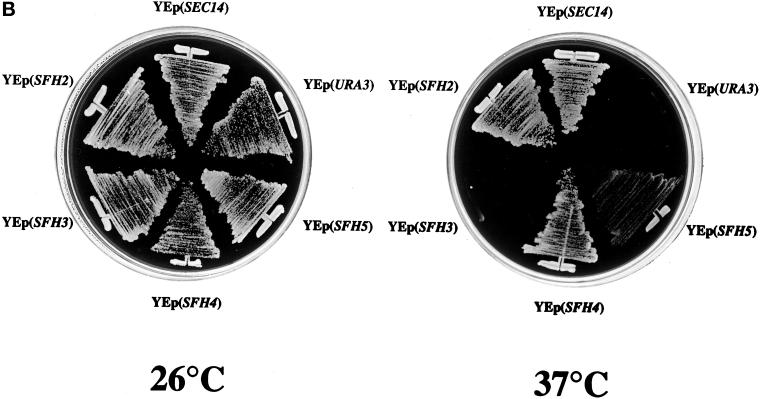
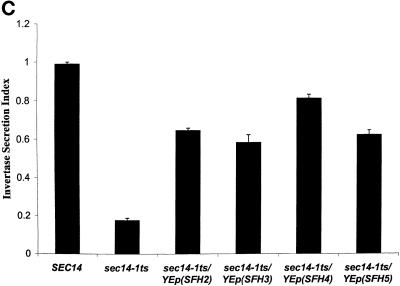
Figure 2.
SFH proteins can rescue growth and secretory defects associated with Sec14p dysfunction. (A) Schematic alignment of SFH gene products (also identified by their yeast genome database accession numbers) with Sec14p. Regions of homology are shaded, and the primary sequence identities/similarities shared by individual SFH gene products with Sec14p are given as percentages. (B) Expression of SFH2, SFH4, and SFH5 genes rescues the growth defects of sec14ts and Δsec14 yeast mutants. Growth properties of a sec14-1ts yeast strain (CTY1-1A) carrying YEp(SEC14) and YEp(URA3) as respective positive and negative controls for phenotypic complementation of the sec14-1ts growth defect at 37°C are as indicated. Growth properties of strain CTY1-1A carrying YEp(SFH2), YEp(SFH3), YEp(SFH4), or YEp(SFH5) are also shown. All yeast strains were streaked for isolation onto YPD plates and incubated at either a permissive (25°C) or a restrictive (37°C) temperature for sec14-1ts strains, as indicated. After 48 h of incubation, the growth results were recorded. YEp(SFH2) and YEp(SFH4) were nearly as effective as YEp(SEC14) in rescuing growth of the sec14-1ts strain at 37°C. YEp(SFH5) was less effective, and YEp(SFH3) was ineffective. (C) Expression of SFH2, SFH3, SFH4, and SFH5 increases invertase secretion efficiency in sec14ts strains at 37°C. Invertase secretion indices (i.e., measures of secretory function) were determined (see MATERIALS AND METHODS). Wild-type strains secrete invertase efficiently (secretion index ≥ 0.90), and Sec14p-insufficient cells secrete invertase poorly (secretion index ≤ 0.20). Each YEp(SFH) expression cassette markedly improved secretory pathway function under conditions of Sec14p deficiency. Values represent averages of at least three independent trials with triplicate determinations for each strain.
To assess whether any of these SFH gene products share significant functional redundancy with Sec14p, we generated appropriate expression vectors in both high-copy episomal (YEp) and low-copy centromeric configurations. These vectors were designed such that the powerful promoter of the yeast phosphoglycerate kinase structural gene drives the constitutive transcription of each SFH gene. SFH2 was an exception. For technical reasons, the YEp(SFH2) plasmid used the constitutive SEC14 promoter. Each expression plasmid was subsequently introduced into a sec14-1ts yeast strain (CTY1-1A), and the ability of each expression construct to effect phenotypic rescue of the signature growth defects of sec14-1ts strains at 37°C was subsequently assessed. Only the SFH4 expression cassette was able to support rescue of sec14-1ts–associated growth defects when configured on a centromeric plasmid (our unpublished results). However, individual expression of SFH2, SFH4, or SFH5 from YEp expression plasmids clearly rescued sec14-1ts–associated growth defects (Figure 2B). Of these plasmids, YEp(SFH2) and YEp(SFH4) were efficient in restoring growth to sec14-1ts strains at 37°C, whereas YEp(SFH5) was less effective. In independent plasmid shuffle experiments, these same three YEp(SFH) plasmids were also able to rescue the unconditional lethality associated with Δsec14 alleles (our unpublished results).
The phenotypic rescue of sec14-associated growth defects was accompanied by alleviation of the dramatic secretory pathway dysfunction that is associated with Sec14p insufficiency in yeast. In these experiments, secretion of the glycoprotein invertase was monitored as a measure of the efficiency of secretory pathway function. As shown in Figure 2C, wild-type yeast induced for invertase synthesis for 2 h at 37°C exhibited a secretion index of 0.90 ± 0.02, indicating that >90% of the invertase synthesized during that 2-h period was efficiently transported through the secretory pathway to the cell surface. In contrast, sec14ts strains incubated under the same conditions exhibited a dramatically reduced secretion index (0.18 ± 0.01). This reduced secretion index suggests accumulation of a large intracellular invertase pool that is arrested in its progression from the Golgi complex in Sec14p-deficient mutants. In accord with the results gleaned from phenotypic rescue experiments, YEp(SFH2), YEp(SFH4), and YEp(SFH5) dramatically improved the secretion index of the sec14-1ts strain from 0.18 ± 0.01 to 0.64 ± 0.01, 0.80 ± 0.02, and 0.64 ± 0.04, respectively.
In contrast, neither YEp(SFH1) (our unpublished results) nor YEp(SFH3) expression plasmids effected any detectable phenotypic rescue of sec14 growth defects at restrictive temperatures (Figure 2B). YEp(SFH1) also had no effect on the abnormally low secretion index of the sec14 host strain (our unpublished results). The failure of YEp(SFH1) to rescue sec14 growth and secretory defects was surprising because SFH1 shares a high degree of similarity with the SEC14 gene itself. SFH1 expression was not a problem, as demonstrated by immunoblotting experiments that clearly showed that YEp(SFH1) drove robust accumulation of a single polypeptide of the correct mass (35 kDa) that exhibited strong immunoreactivity to Sfh1p-directed antibodies (our unpublished results). The collective results indicate that Sfh1p is not a functional PITP (see below). Interestingly, SFH3 expression elicited a significant improvement in the invertase secretion index of the sec14 mutant strain, an improvement that was almost as large as that recorded for cells carrying either YEp(SFH2) or YEp(SFH4) (Figure 2C). Yet, YEp(SFH3) failed to recapitulate the phenotypic rescue of sec14 defects that was recorded for YEp(SFH2) and YEp(SFH4).
We conclude that Sfh2p, Sfh4p, and Sfh5p share some functional relatedness with Sec14p. The invertase data further suggest that Sfh3p does as well. Although we have not determined why Sfh3p expression fails to rescue sec14-associated growth defects, previous work has indicated that secretion indices of ∼0.50 are near the threshold for cell viability (Kearns et al., 1998b). Perhaps Sfh3p simply fails to accumulate to the levels achieved by overproduction of Sfh2p, Sfh4p, or Sfh5p and thereby does not effect a sufficiently efficient alleviation of the sec14-1ts Golgi secretory block to sustain cell growth.
SFH2, SFH3, SFH4, and SFH5 Proteins Are Nonclassic PITPs
Overexpression of mammalian or plant PITPs in yeast is sufficient to complement sec14-1ts–associated growth and secretion phenotypes (Skinner et al., 1993; Kearns et al. 1998a,b). Because individual expression of the SFH2, SFH3, SFH4, and SFH5 proteins rescues growth and/or secretion defects of sec14-1ts mutants at 37°C, and because the SFH proteins share primary sequence homology with Sec14p, we determined whether these SFH proteins define a novel class of yeast PITPs. Previous work by van den Hazel et al. (1999) demonstrated modest reductions in bulk PtdIns-transfer activity in crude yeast membrane fractions prepared from Sfh3p- and Sfh4p-deficient yeast strains. Although suggestive, those experiments lend themselves to multiple interpretations and do not yield clear conclusions regarding the biochemical properties of any of the SFH gene products. To characterize these proteins in more detail, we first expressed individual SFH proteins in the cki1, Δsec14 yeast strain CTY303 and assayed cytosol prepared from these strains for PtdIns- and PtdCho-transfer activity. The Sec14p-deficient CTY303 is a useful host strain for these experiments because it lacks detectable endogenous PtdIns- and PtdCho-transfer activity. CTY303 retains viability in the face of Sec14p deficiency because cki1 effects bypass of the essential Sec14p requirement (Cleves et al. 1991).
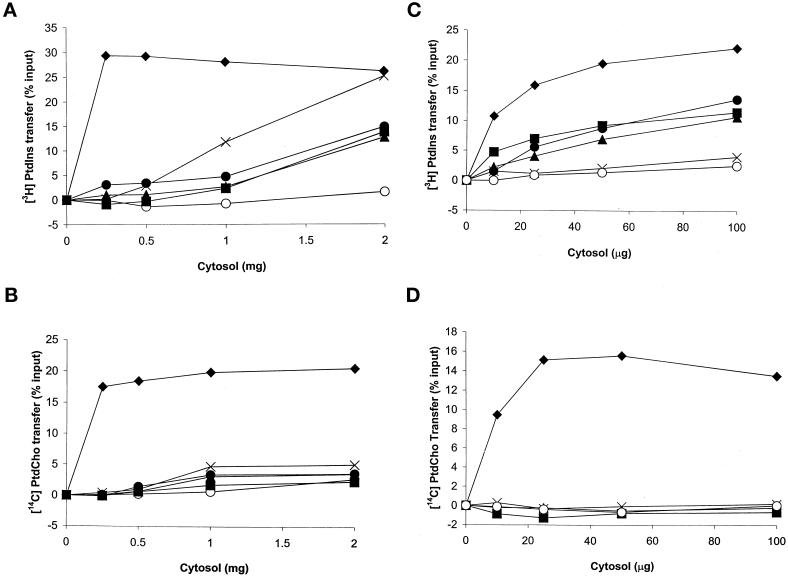
The PtdIns- and PtdCho-transfer profiles for yeast cytosol containing Sec14p, or each individual SFH protein, are shown in Figure 3, A and B, respectively. Wild-type Sec14p cytosol served as a positive control, and it exhibited robust PtdIns- and PtdCho-transfer activities. Wild-type cytosol catalyzed the in vitro transfer of ∼23 and 20% of the total input [3H]PtdIns- and [14C]PtdCho-transfer substrate at saturation, respectively. For in vitro transfer assays that used cytosol prepared from a CTY303 derivative expressing SFH proteins, the assay conditions for both PtdIns and PtdCho transfer were in the linear range from 0 mg to at least 2 mg of input cytosol. Under these conditions, we were unable to record any detectable PtdIns-transfer activity in cytosol containing Sfh1p (our unpublished results). However, cytosol fractions prepared from yeast strains individually overproducing Sfh2p, Sfh3p, Sfh4p, or Sfh5p all exhibited significant PtdIns-transfer activity (Figure 3A). At the highest concentrations of cytosol tested (2 mg/ml), the Sfh2p, Sfh3p, and Sfh4p cytosol preparations catalyzed the transfer of 13% of total input [3H]PtdIns substrate from donor to acceptor membranes in vitro. Sfh5p-containing cytosol effected a robust transfer of 25% of the input [3H]PtdIns substrate when 2 mg of cytosol was assayed, a value similar to that obtained for Sec14p at saturation. However, the concentration of cytosol required to saturate the PtdIns-transfer assay was quite different for Sec14p and Sfh5p. The PtdIns-transfer assay saturated at 0.25 mg of input Sec14p cytosol, whereas 2 mg of Sfh5p cytosol was required to achieve the same effect (Figure 3A).
Figure 3.
SFH proteins are atypical PITPs. The ability of each SFH protein to transfer [3H]PtdIns (A and C) or [14C]PtdCho (B and D) was determined in either cytosol fractions prepared from salt-stripped yeast membranes (A and B) or cytosol prepared from E. coli strains expressing recombinant SFH gene product (C and D). Activity is represented as the percentage of total input [3H]PtdIns or [14C]PtdCho transferred from donor membranes to unlabeled acceptor membranes during the course of the experiment (see MATERIALS AND METHODS). Cytosol values are presented as amounts of protein added to the assay cocktail. For the yeast cytosol experiments, the proteins of interest were expressed in strain CTY303 (Table 1), which has no detectable endogenous PtdIns- or PtdCho-transfer activity. CTY303/YEp(URA3) cytosol was prepared and used as a negative control. Assay blanks represent addition of buffer alone to the transfer assay reactions. ♦, Sec14p; ▴, Sfh2p; ▪, Sfh3p; ●, Sfh4p; ×, Sfh5p; ○, vector control. In experiments that used yeast cytosol, 14,155–17,319 cpm of input [3H]PtdIns and 14,963–22,035 cpm of input [14C]PtdCho was used in each assay. Background values for these PtdIns- and PtdCho-transfer assays were in the range of 521–697 and 76–159 cpm, respectively. All samples contained <170 mM KCl, a salt concentration that itself had no effect on background or signal in these assays. In experiments that used E. coli cytosol, 15,034–15,411 cpm of input [3H]PtdIns and 18,950–22,531 cpm of input [14C]PtdCho was used in each assay. Background values for assays that used recombinant SFH proteins were in the range of 501–601 for the PtdIns-transfer assays and 547–578 for the PtdCho-transfer assays. No KCl was present in any assays that used E. coli cytosol. These data are representative of at least five independent experiments.
During the course of these experiments, we found that detection of PtdIns-transfer activity in all cases, with the exception of Sec14p and Sfh4p, required that yeast membranes be stripped with 0.5 M KCl during preparation of the cytosol fraction (see MATERIALS AND METHODS). These data indicate that Sfh2p, Sfh3p, and Sfh5p are rather tightly associated with membranes, whereas a significant fraction of Sfh4p is either cytosolic or more loosely membrane bound under the overexpression conditions used in these experiments (our unpublished results).
Assessments of PtdCho-transfer activity yielded unexpected results. Sec14p cytosol elaborated abundant PtdCho-transfer activity (20% of input [14C]PtdCho transferred during the course of the assay). Yet, we failed to detect PtdCho transfer in assays supplemented with high concentrations of cytosol prepared from any of the YEp(SFH) derivatives of CTY303 (Figure 3B), including those expressing Sfh1p to high levels (our unpublished results).
To gain independent confirmation of the biochemical data obtained from yeast cytosol, we individually expressed recombinant His6-tagged SFH proteins in E. coli and measured PtdIns- and PtdCho-transfer activities from crude E. coli cytosol fractions. His6-tagged Sec14p was used as a positive control. The data obtained with the recombinant proteins largely recapitulated those obtained from yeast cytosol. However, salt stripping of E. coli membranes was not required for detection of soluble phospholipid transfer activity. Recombinant Sec14p cytosol transferred 22% of the total input [3H]PtdIns at the highest concentration tested. Likewise, recombinant Sfh2p, Sfh3p, and Sfh4p cytosol catalyzed the in vitro transfer of 12, 11, and 14%, respectively, of input [3H]PtdIns from donor to acceptor membranes (Figure 3C). Sfh5p was an exception in that it catalyzed robust PtdIns-transfer activity when assayed in the context of yeast cytosol but failed to elaborate detectable transfer activity when expressed as a recombinant protein in E. coli cytosol. This inactivity was not the result of Sfh5p instability in E. coli, because immunoblotting experiments revealed significant levels of full-length recombinant Sfh5p in input cytosol (our unpublished results).
As illustrated in Figure 3D, the PtdCho-transfer data obtained for bacterial cytosol harboring recombinant SFH proteins were also in agreement with the yeast cytosol data. Whereas Sec14p catalyzed vigorous rates of PtdCho-transfer activity (14% of total input substrate transferred), none of the Sfhp-containing cytosols exhibited significant PtdCho-transfer activity. Recombinant Sfh1p was again inactive in both PtdIns- and PtdCho-transfer assays, even when added as purified protein at micromolar concentrations (our unpublished results).
These collective data, although not permitting direct comparisons of specific activities for PtdIns transfer between Sec14p and the SFH proteins, formally demonstrate that Sfh2p, Sfh3p, and Sfh4p are novel yeast PITPs that do not effect PtdCho transfer, at least under the assay conditions used. From this perspective, these proteins represent nonclassic PITPs. Although we believe it is likely that Sfh5p is also a PtdIns-transfer protein of this class, our inability to detect PtdIns-transfer activity with recombinant Sfh5p leaves the evidence short of what is needed for confirmation.
SFH Gene Products and Sec14p-independent Cell Growth
We considered the possibility that SFH gene expression could influence the phenotypes associated with Sec14p insufficiency in yeast. To address the possible roles of SFH gene products in the execution of Sec14p-dependent secretory events, we systematically disrupted SFH genes individually and in combination in various yeast genetic backgrounds.
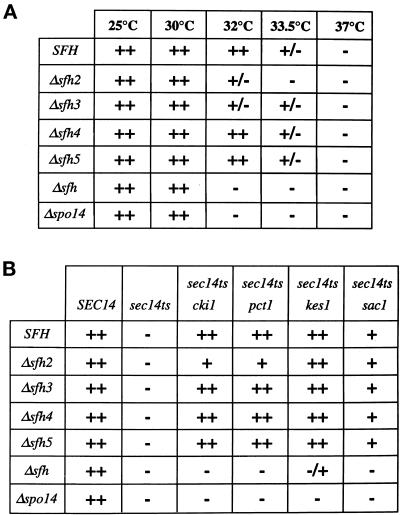
Initially, the SFH2, SFH3, SFH4, and SFH5 genes were individually disrupted in SEC14 strains to assess whether any single SFH gene was essential for cell growth. None of the individual disruption mutations exerted any dramatic effect on the viability of SEC14 strains, indicating that none of these gene products executes a unique essential function (Figure 4A). In the cases of the Δsfh3 and Δsfh4 mutants, discernible phenotypes do exist, and these provide some insight into biological function (see DISCUSSION). Yet, en bloc deletion of all four of these SFH genes in a SEC14 strain also had little effect on cell viability and vigor (Figure 4A). Thus, SFH-encoded PITPs are dispensable as a class in SEC14 yeast strains.
Figure 4.
Phenotypic consequences of SFH gene product insufficiency. (A) The sec14-1ts strain CTY1-1A (SFH), CTY1-1A derivatives carrying individual disruptions of each SFH gene as indicated at left, and a sec14-1ts strain collectively disrupted for SFH2, SFH3, SFH4, and SFH5 (Δsfh) were streaked for isolation on YPD agar and incubated at the indicated temperatures. Growth was scored after 48 and 72 h of incubation. Growth of SEC14 yeast was not affected by individual or aggregate disruption of SFH genes at any of the temperatures tested (our unpublished results). Disruption of SFH2 or SFH3 reduced the restrictive temperature of sec14-1ts strains, and en bloc deletion of the SFH genes exerted a more pronounced effect. Growth phenotypes of a sec14-1ts, Δspo14 mutant are presented for comparison. (B) Yeast strains (the sec14 and bypass-Sec14p genotypes indicated at top) carrying the designated individual sfh gene disruptions, or en bloc disruptions of SFH2, SFH3, SFH4, and SFH5 (Δsfh) (at left), were streaked for isolation on YPD agar and incubated at 37°C for 48 h. Growth phenotypes of Δspo14 derivatives of these bypass-Sec14p mutants are also presented for comparison. The Δspo14 and Δsfh genetic backgrounds were nonpermissive for bypass Sec14p.
If any of the SFH proteins exhibit authentic physiological redundancy with Sec14p, we would expect to record exacerbation of sec14-1ts defects upon ablation of SFH protein function. We did not find this to be the case for Δsfh4 or Δsfh5. Individual disruption of SFH4 or SFH5 failed to exert any negative effect on the growth of sec14-1ts strains, even at temperatures approaching the restrictive temperature of 34°C (Figure 4A). However, the Δsfh2 and Δsfh3 alleles did decrease the restrictive temperature of sec14-1ts strains to 33°C (Figure 4A). When Δsfh2, Δsfh3, Δsfh4, and Δsfh5 null alleles were combined in a sec14-1ts genetic background, no synthetic lethality was observed. Rather, a more severe reduction in the restrictive growth temperature of sec14-1ts strains was again recorded (Figure 4A). These data suggest that the SFH2, SFH3, SFH4, and SFH5 gene products collectively exert some influence on sec14-1ts growth phenotypes and that Sfh2p and Sfh3p perhaps play more prominent roles in this regard.
We also determined whether the Sec14p-independent viability of bypass-Sec14p mutants relied on SFH function. Individual Δsfh2, Δsfh3, Δsfh4, and Δsfh5 alleles were introduced into strains carrying cki1, kes1, or sac1 bypass-Sec14p alleles. Again, Δsfh3, Δsfh4, and Δsfh5 alleles had no obvious effects on the ability of any of these alleles to effect bypass Sec14p. The Δsfh2 allele visibly reduced the efficiency with which mutations in the CDP-choline pathway for PtdCho biosynthesis rescued sec14-1ts growth defects at 37°C but did not abolish this phenotypic rescue (Figure 4B). Thus, Sfh2p contributes to the bypass-Sec14p phenotype associated with CDP-choline pathway defects. Finally, aggregate incorporation of the Δsfh2, Δsfh3, Δsfh4, and Δsfh5 alleles into the cki1 or sac1 strain abolished their ability to effect bypass Sec14p (Figure 4C). Similarly, kes1-mediated bypass Sec14p was dramatically compromised by en bloc deletion of the SFH genes. Thus, Sec14p-independent growth exhibits a requirement for SFH gene product activity.
SFH protein involvement in facilitating Sec14p-independent growth in bypass-Sec14p mutants raised the possibility that SFH gene products are overproduced in these mutants. Northern analyses indicated no significant increase in SFH gene expression in Sec14p-deficient bypass-Sec14p strains relative to wild-type strains (our unpublished results).
Optimal PLD Activity Is Dependent on SFH Gene Product Function In Vivo
Yeast express a single PtdIns-4,5-P2–activated PLD that is encoded by the SPO14 gene (Rose et al., 1995; Waksman et al., 1996). PLD, which catalyzes the hydrolysis of PtdCho to PtdOH and free choline, is normally nonessential for vegetative growth of yeast, but this enzyme is obligatorily required for the viability of bypass-Sec14p mutants (Sreenivas et al., 1998; Xie et al., 1998). Moreover, PLD deficiency renders sec14-1ts strains much more temperature sensitive for growth, i.e., Δspo14 alleles reduce the restrictive temperature of sec14-1ts strains by several degrees, from 34 to ∼31°C (Rivas et al., 1999). Because the phenotypes associated with en bloc deletion of the SFH genes closely resembled those associated with PLD deficiency (Figure 4), we considered the possibility that SFH proteins contribute to PLD activation. This hypothesis predicts: (i) that PLD deficiency will compromise SFH overexpression–mediated rescue of sec14-1ts–associated growth defects, and (ii) that en bloc disruption of the SFH genes will result in reduced PLD activity in vivo.
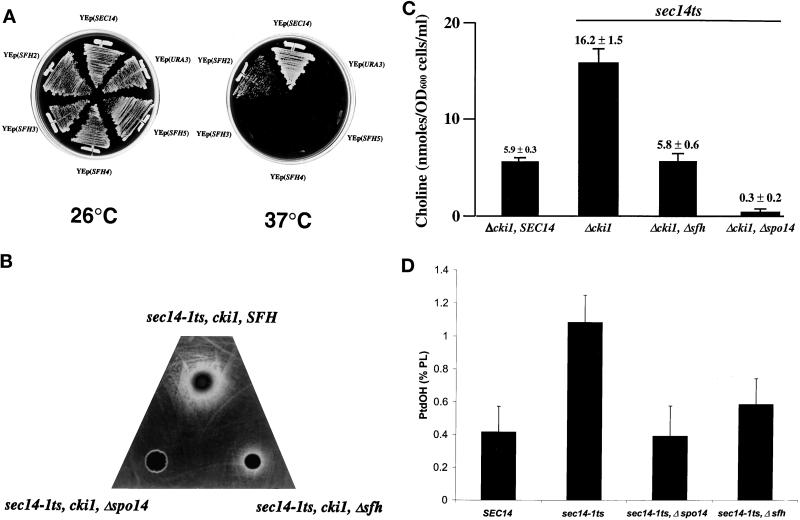
To determine whether the SFH proteins require PLD activity for stimulation of the Sec14p pathway in vivo, we tested the ability of YEp(SFH2), YEp(SFH4), and YEp(SFH5) to rescue sec14-1ts–associated growth defects in yeast strains deleted for SPO14. Only the sec14-1ts, Δspo14, YEp(SFH2) strain grew at all at 37°C, and it grew more poorly relative to the isogenic SPO14 strain (Figure 5A). Overexpression of either SFH4 or SFH5 failed to effect phenotypic rescue of Sec14p defects in the absence of a functional PLD (Figure 5A).
Figure 5.
SFH gene products contribute to PLD activity in vivo. (A) PLD requirement for YEp(SFH)-mediated rescue of sec14-associated growth defects. The sec14-1ts, Δspo14 strain CTY1079 was transformed with the indicated YEp(SFH) plasmids and YEp(SEC14) and YEp(URA3) plasmids as positive and negative controls, respectively. The corresponding transformants were streaked for isolated colonies on YPD agar and incubated at temperatures permissive (26°C) or restrictive (37°C) for the sec14-1ts, Δspo14 strain, as indicated. Results were scored after 48 h of incubation. YEp(SFH2) elicited a clear phenotypic rescue of sec14-1ts growth defects, whereas YEp(SFH3), YEp(SFH4), and YEp(SFH5) were unable to effect any relief of the sec14-associated growth defects in the absence of functional PLD activity. (B) PLD activation in Δsfh yeast strains as measured by choline excretion. Equal cell numbers of the sec14-1ts, cki1 strain CTY160 and its congenic sec14-1ts, cki1, Δsfh derivative CTY1427 were spotted on a lawn of the choline auxotrophic strain CTY1289 plated onto choline-free minimal agar. The plate was incubated at 30°C for 48 h. An inoculum of the isogenic Δspo14 derivative strain CTY1099 was also spotted onto the indicator lawn, and the absence of choline cross-feeding of the indicator establishes that the choline excreted by the CTY160 and CTY1289 cells was generated by the action of PLD. (C) Chemical quantification of choline excretion. The appropriate yeast strains were grown to midlogarithmic growth phase in minimal defined medium supplemented with inositol (100 μM) but lacking choline at 26°C. The cells were harvested, washed, and resuspended in fresh medium and incubated at 33.5°C for 3 h. A choline oxidase–coupled chemical assay was used to measure the choline concentration of culture supernatant (see MATERIALS AND METHODS). Relevant genotypes of the strains are given below the bars. Data represent triplicate determinations from at least three independent experiments. (D) PLD activation in Δsfh yeast strains as measured by PtdOH production. The appropriate yeast strains were radiolabeled to steady state with [32P]orthophosphate (10 μCi/ml) at 26°C in inositol- and choline-replete minimal medium (Xie et al., 1998). Cells were subsequently washed and incubated in radiolabel-free medium for 3 h at 33.5°C, and phospholipids were extracted and resolved (Xie et al., 1998). PtdOH was identified and quantified by phosphorimaging. Values are expressed as [32P (cpm) incorporated into PtdOH divided by total 32P (cpm) incorporated into extractable phospholipid] times 100%. The data were normalized by measuring relative rates of phosphate incorporation into phospholipid as a function of cell number for each strain. Under these experimental conditions, the following values of 32P (cpm) incorporation into extractable phospholipid per OD600 cells were obtained: CTY182 (SEC14, SFH), 8500 − 245; CTY1-1A (sec14-1ts, SFH), 17,000 − 1470; CTY1079 (sec14-1ts, Δspo14), 24,000 − 2400; CTY1118 (sec14-1ts, Δsfh), 15,000 − 645. These data represent the averages of at least three independent experiments.
To determine whether the SFH gene products collectively regulate PLD activity in vivo, we used two independent approaches to measure PLD activity in living cells. In vivo measurements were initially used because in vitro assays for PLD activity in yeast cell-free lysates do not always reliably reflect PLD activity in vivo. As an example of this discordance, deletion of the yeast PLD amino terminus yields a protein that is robustly active in vitro but that fails to hydrolyze PtdCho in vivo (Rudge et al., 1998; Xie et al., 1998; our unpublished results).
The first approach used an adapted version of a standard cross-feeding assay to crudely assess PLD-mediated production of choline in yeast cells blocked for choline salvage via the CDP-choline pathway (e.g., cki1 mutants) (Cleves et al., 1991). In these cki1 mutants, the choline generated via PLD-mediated hydrolysis of PtdCho is an inert metabolite that excreted from the cell. The excreted choline is then available for scavenging by an indicator strain that requires choline for growth (Patton-Vogt et al., 1997). As shown in Figure 5B, a cki1, sec14-1ts, SPO14 strain incubated at 30°C exhibited robust excretion of choline. This is shown by the large halo of indicator cells that grew around the focus at which the test cki1 cells were deposited. The PLD dependence of this choline excretion was indicated by the fact that the cki1, sec14-1ts, Δspo14 strain failed to support even a small halo of indicator growth. Examination of the cki1, sec14-1ts, Δsfh2, Δsfh3, Δsfh4, Δsfh5 mutant demonstrated that its choline excretion phenotype was strongly diminished relative to the cki1, sec14-1ts, SFH strain (Figure 5B). Little effect on choline excretion was recorded in strains carrying individual Δsfh2, Δsfh3, Δsfh4, or Δsfh5 alleles (our unpublished results).
To provide quantitative confirmation of the cross-feeding results, these same cki1 derivative strains were grown to early logarithmic growth phase in choline-free minimal medium at 26°C, washed, resuspended in fresh choline-free medium, and incubated at 33.5°C for 3 h. This incubation at 33.5°C results in partial inactivation of Sec14pts and concomitant activation of PLD (Patton-Vogt et al., 1997; Sreenivas et al., 1998; Xie et al., 1998). The medium was collected from each culture, and the choline content of each culture supernatant was measured by chemical assay (see MATERIALS AND METHODS). The quantitative data were consistent with the cross-feeding data. Sec14p-proficient cells accumulated only small amounts of choline in the culture supernatant (5.9 ± 0.3 nmol·OD600 cells−1·ml−1), whereas the isogenic cki1, sec14-1ts derivative excreted approximately threefold more choline per unit cell during the same 3 h of incubation at 33.5°C (16.2 ± 1.5 nmol·OD600 cells−1·ml−1) (Figure 5C). The cki1, sec14-1ts, Δsfh strain exhibited threefold reduction in excreted choline relative to its SFH partner and was no more proficient in choline release than was the SEC14 strain (5.8 ± 0.6 versus 5.9 ± 0.3 nmol·OD600 cells−1·ml−1). All of the excreted choline measured with this assay was attributable to PLD action, because choline levels were essentially undetectable in culture supernatants of the cki1, sec14-1ts, Δspo14 strain (0.3 ± 0.2 nmol·OD600 cells−1·ml−1) (Figure 5C). These data clearly demonstrate that PLD-dependent production of choline (and, by extension, PLD activity) is severely compromised in yeast deficient in SFH protein function.
The reduction of choline excretion in Δsfh strains was not simply a nonspecific consequence of further exacerbating Golgi dysfunction. Inactivation of the Pik1p PtdIns-4-kinase (by the pik1-101ts mutation that also blocks late Golgi secretory pathway function in yeast; Walch-Solimena and Novick, 1999) failed to recapitulate the Δsfh effect of reducing PLD activity in cki1, sec14-1ts strains at 33.5°C. Moreover, unlike sec14-1ts, the pik1-101ts allele failed to evoke PLD activation in a cki1 genetic background under the same conditions (our unpublished results). These data suggest that Pik1p is not a limiting component of an SFH-dependent pathway, or even of a Sec14p-dependent pathway, for PLD activation.
The choline excretion–based measurements of PLD activity required strains exhibiting specific defects in PtdCho metabolism for readout. To obtain independent assessments of PLD activity that did not rely on restrictions in PtdCho metabolism, we quantified PLD-dependent production of PtdOH in SFH-proficient and SFH-deficient strain pairs that were competent for PtdCho synthesis via the CDP-choline pathway. In these experiments, cells were labeled to steady state at 26°C with [32P]orthophosphate. Cells were then washed, resuspended in label-free medium, and incubated at 33.5°C for 3 h. This last incubation initiates a crude chase of 32P from PtdCho to PtdOH under conditions in which Sec14p deficiency is induced and PLD is activated (Patton-Vogt et al., 1997; Sreenivas et al., 1998; Xie et al., 1998). The PLD-mediated generation of PtdOH was then measured by comparing PtdOH levels in PLD-proficient and PLD-deficient strains after termination of the chase. Although this equilibrium 32P radiolabeling/chase regimen is a less sensitive measure of PLD-mediated metabolism of PtdCho (because the PtdOH product is both metabolically labile and actively generated by metabolic pathways other than PLD-mediated PtdCho hydrolysis), the contribution of PLD to PtdOH production is nonetheless readily discerned.
As shown in Figure 5D, PtdOH represented 0.42 ± 0.14% and 1.10 ± 0.16% of total extractable phospholipid in the SFH-proficient SEC14 and sec14-1ts strains, respectively. A Δspo14, sec14-1ts derivative served as a negative control, and PtdOH levels were reduced severalfold in this mutant relative to its SPO14, sec14-1ts partner (0.39 ± 0.18% versus 1.10 ± 0.16%). Thus, the 2.5-fold increase in PtdOH levels observed for the sec14-1ts strain (relative to its isogenic SEC14 partner) represented a measure of the PLD activation evoked by Sec14p deficiency in yeast. The SFH-deficient strain also exhibited marked reductions in PtdOH levels relative to its SFH-proficient partner, even though both strains expressed wild-type PLD (Figure 5D). In this case, the SFH-insufficient mutant was twofold reduced for PtdOH relative to the SFH-proficient strain (0.59 ± 0.16% versus 1.10 ± 0.16%).
These results were recapitulated when the cki1 yeast strains used in the choline excretion experiments were similarly analyzed. The cki1, sec14-1ts strain produced approximately twofold and sixfold more PtdOH during the chase incubation at 33.5°C than did the cki1, Δsfh and cki1, Δspo14 derivatives, respectively (our unpublished results). The collective data demonstrate that en bloc deletion of SFH2, SFH3, SFH4, and SFH5 compromises PLD activity in vivo, independent of CDP-choline pathway activity. Additional support for an in vivo link between Sfh2p and PLD function comes from an entirely independent Sfh2p-associated phenotype (see DISCUSSION).
PLD Activity and SFH Gene Product Function as Measured by Broken Cell Assay
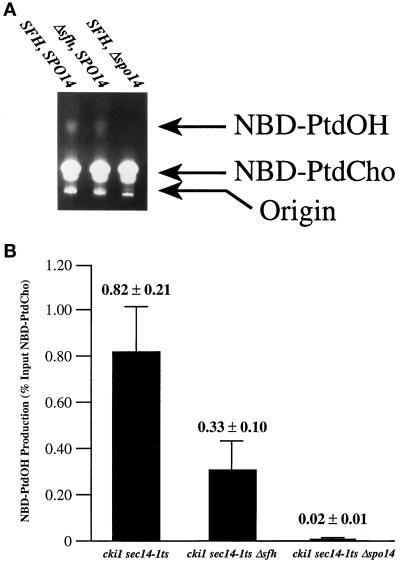
We also determined whether the stimulatory effects of SFH gene product function on PLD activity in vivo were discernible in broken cell assays that directly monitor the action of PLD on fluorescently labeled phospholipid substrate. To this end, the set of cki1, sec14-1ts derivative strains described above was cultured at 25°C to early logarithmic growth phase and shifted to 33.5°C for 3 h to induce activation of PLD, as described above. Clarified supernatants were subsequently prepared from broken cell preparations of these strains, and PLD activity in each lysate was measured by monitoring conversion of NBD-PtdCho substrate to NBD-PtdOH product (see MATERIALS AND METHODS). As shown in Figure 6A, the sec14-1ts strain exhibited clearly measurable PLD activity, as determined by conversion of NBD-PtdCho to NBD-PtdOH. Lysate prepared from the isogenic Δspo14 control failed to execute this conversion, thereby demonstrating that PLD is the source of this hydrolytic activity. The ability of lysate prepared from the sec14-1ts, Δsfh strain to convert NBD-PtdCho to NBD-PtdOH was significantly diminished in this assay relative to that of the sec14-1ts–positive control strain (Figure 6A).
Figure 6.
SFH gene products and PLD activity in broken cell preparations. The sec14-1ts, cki1 strain CTY160, its isogenic Δspo14 partner CTY1099, and its congenic Δsfh derivative CTY1427 were used in these experiments. These strains were cultured in YPD at 26°C until they reached early logarithmic growth phase. The cultures were then shifted to 33.5°C for 3 h, cells were harvested and broken by glass-bead lysis, and clarified lysates were prepared. PLD activity was assayed by monitoring conversion of input NBD-PtdCho substrate to NBD-PtdOH, as described by Waksman et al. (1996) (see MATERIALS AND METHODS). (A) TLC of PLD assay products. The products of PLD activity assays were resolved by one-dimensional TLC and visualized by illumination under UV light. The positions of input NBD-PtdCho substrate and NBD-PtdOH product are indicated at right. Identification of each species was made by comparison with the chromatographic properties of NBD-PtdCho and NBD-PtdOH standards. Relevant genotypes of yeast strains that served as sources of lysate for each corresponding assay are given above each lane. The sec14-1ts control lysate clearly supported conversion of NBD-PtdCho to NBD-PtdOH that was PLD dependent, as judged by the inability of Δspo14 lysate to support this reaction. Relative to the sec14-1ts, SFH control, lysate from the Δsfh strain effected only a weak, but detectable, conversion of NBD-PtdCho to NBD-PtdOH. (B) Quantification of in vitro PLD activity. Fluorometry was used to quantify NBD fluorescence in PtdCho and PtdOH species resolved from PLD activity assays by TLC. Relevant genotypes of the yeast strains from which the test lysates were prepared are given below each bar. The results are expressed as (NBD fluorescence recovered in PtdOH divided by NBD fluorescence recovered in PtdOH and PtdCho) times 100%. All fluorescence values obtained fell in the linear range of fluorometric detection, as assessed by fluorescence counting of serial dilutions of known quantities of NBD phospholipid standards. Measured values are given above each corresponding data bar (n ≥ 3).
Quantification of the data by fluorimetry confirmed the visual interpretation of the experimental results. In assays that used 100 μg of protein, lysate from the sec14-1ts control strain converted 0.82 ± 0.21% of total input NBD-PtdCho to NBD-PtdOH in 1 h at 30°C. This value was at least 40-fold greater than that measured for the Δspo14 control (0.02 ± 0.01% of NBD-substrate converted to product). Consistent with the in vivo data, the sec14-1ts, Δsfh strain exhibited a 2.5-fold reduction in PLD activity relative to the SFH-positive control (0.82 ± 0.21% versus 0.33 ± 0.10%; Figure 6B). These data are qualitatively and quantitatively consistent with the independent lines of in vivo evidence and establish a critical role for the SFH gene products in stimulating PLD activity in vivo.
Localization of Sfh2p and PLD in Endosomal Compartments
If SFH proteins regulate PLD activity in vivo, they should exhibit detectable colocalization with PLD in cells. To test this prediction, we expressed EGFP-tagged versions of SFH proteins in yeast. The fluorescent tags were configured at the carboxyl termini of these proteins because this orientation is predicted not to occlude the phospholipid-binding pocket of Sec14p-like proteins (Sha et al., 1998; Phillips et al., 1999). In further support of this notion, Yarrowia lipolytica Sec14p exhibits a large carboxyl-terminal extension that does not interfere with its PtdIns- and PtdCho-transfer activities (Lopez et al., 1994). The Sfh2p-GFP chimera met the dual criteria of being functional in yeast as a stable protein [as judged by immunoblotting and the ability of YEp(SFH2::GFP) to rescue sec14-1ts growth defects (our unpublished results)] and of generating a detectable fluorescence signal.
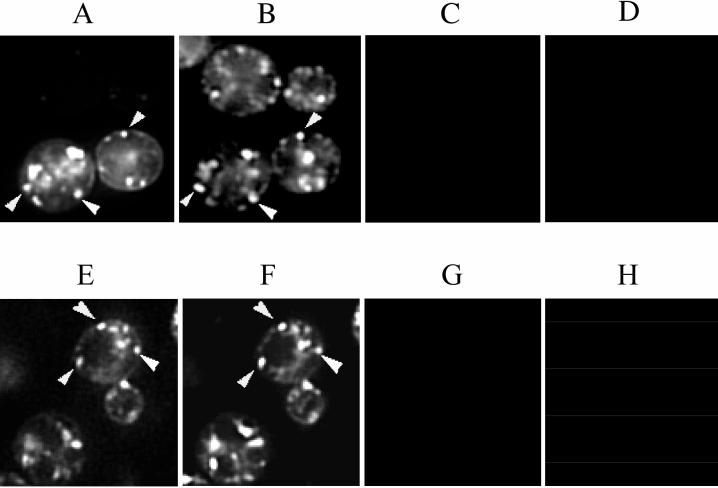
Sfh2p-GFP localized to 5–10 punctate structures dispersed in the cytoplasm (Figure 7A). This pattern was similar to the one observed for the fluorescent styryl dye FM 4-64 that was used to stain these same cells under conditions that capture the dye in endosomal compartments (Figure 7B). Indeed, comparison of the Sfh2p-GFP and FM 4-64 images demonstrated a substantial colocalization of Sfh2p staining with endosomes. We estimate that ∼60% of the Sfh2p-GFP–positive structures stain with FM 4-64, whereas some 80% of the FM 4-64–positive structures stain with Sfh2p-GFP. Finally, we used a functional GFP-PLD chimera (Rudge et al., 1998) to determine whether PLD and Sfh2p colocalize in yeast under conditions of Sfh2p overexpression. Comparisons of GFP-PLD and FM 4-64 staining profiles indicated that PLD exhibited predominantly endosomal staining (Figure 7, C and D). We estimate that ∼90% of the GFP-PLD–positive structures colocalized with FM 4-64–positive structures. These values are based on comparisons of the punctate aspects of Sfh2p-GFP, GFP-PLD, and FM 4-64 staining. Tubular structures also exhibit substantial costaining of Sfh2p-GFP or GFP-PLD and FM 4-64. Quantification of costaining in these structures is more difficult. Our finding that GFP-PLD localizes to endosomes differs from a previous report that GFP-PLD is diffusely distributed throughout the cytoplasm of vegetative cells (Rudge et al., 1998). We conclude that Sfh2p localizes to yeast endosomes when overexpressed and that Sfh2p substantially colocalizes with PLD under these conditions.
Figure 7.
Sfh2p and PLD partially localize to endosomes. Yeast cells expressing Sfh2p-GFP or PLD-GFP were stained with FM 4-64 under conditions in which the styryl dye is localized to endosomes (see MATERIALS AND METHODS). Separate images of GFP and FM 4-64 fluorescence were captured from the same sets of living yeast cells, and arrowheads indicate some examples of FM 4-64 structures (B and F) decorated with either Sfh2p-GFP (A) or PLD-GFP (E). Computer merge of these images confirms the colocalization of Sfh2p-GFP and GFP-PLD with FM 4-64 structures (our unpublished results). Because the Sfh2p-GFP fluorescence is rather dim, only the cells in the precise focal plane are apparent in A, whereas other cells in the approximate focal plane are also visible when the more intense FM 4-64 fluorescence is monitored in B. C, D, G, and H depict fluorescence bleed controls. C and G show the fluorescence pattern when the fields depicted in A and E, respectively, were excited for GFP fluorescence and viewed through the filter used to record FM 4-64 fluorescence. Reciprocally, D and H show the fluorescence pattern when the fields depicted in B and F, respectively, were excited for FM 4-64 fluorescence and viewed through the filter used to record GFP fluorescence. No fluorescence bleed was detected between the GFP and FM 4-64 channels.
DISCUSSION
Herein we characterize a novel family of PITPs in Saccharomyces cerevisiae that expand the repertoire of PITPs from one (Sec14p) to five in this yeast. These PITPs are encoded by the SFH2, SFH3, SFH4, and SFH5 genes, respectively. The corresponding gene products (collectively referred to as SFH proteins) share modest homology with Sec14p throughout their primary sequences (Figure 2A). The modest level of primary sequence homology shared by Sec14p and the SFH proteins assumes functional significance because individual overproduction of Sfh2p, Sfh4p, or Sfh5p rescues both the growth and the Golgi secretory defects associated with Sec14p dysfunction in vivo (Figure 2, B and C). Sfh3p overproduction also partially alleviates these Golgi secretory defects but is insufficient for restoration of growth to Sec14p-deficient yeast. Individual overproduction of any one of these four SFH proteins in yeast results in increased PtdIns-transfer activity in cytosol preparations (Figure 3A), and recombinant Sfh2p, Sfh3p, and Sfh4p exhibit PtdIns-transfer activity (Figure 3C). These data formally demonstrate that at least Sfh2p, Sfh3p, and Sfh4p are PITPs. Sfh5p overproduction evokes measurable PtdIns-transfer activity in yeast but not in E. coli (Figure 3, A and C). We cannot provide a precise rationale for these results. Sfh5p may misfold when expressed in bacteria, although we detect soluble full-length antigen in E. coli expressing Sfh5p. Alternatively, Sfh5p may be modified in yeast, and this modification may be essential for PtdIns-transfer activity. Finally, Sfh5p may not be a PITP but may regulate expression of SFH2, SFH3, or SFH4. Although we favor the idea that Sfh5p exhibits intrinsic PITP activity, a formal confirmation remains lacking.
The yeast SFH PITPs exhibit biochemical similarities to Sec14p. However, these proteins also exhibit properties that distinguish them from Sec14p. Whereas Sec14p conforms to a classic definition of a PITP (i.e., it is operationally a soluble protein that effects both PtdIns and PtdCho transfer in vivo), the SFH proteins exhibit PtdIns-transfer activity but not PtdCho-transfer activity (Figure 3, B and D). Moreover, these PITPs are membrane associated in yeast and must be stripped from membranes with salt for their activities to be measured in cytosol preparations. Thus, these SFH proteins represent atypical PITPs that more closely resemble the membrane-associated soybean Ssh2p, which exhibits PtdIns-transfer activity (but not PtdCho-transfer activity) in vitro and also binds phosphoinositides (Kearns et al., 1998b). Whether the yeast SFH proteins bind phosphoinositides remains to be determined. An additional point of interest in this regard is our inability to measure PITP activity in lysates prepared from the Δcki1, Δsec14 strain CTY303, a strain we have routinely used for biochemical characterization of both heterologous PITPs and the SFH gene products (see above). This strain expresses the full complement of functional SFH genes, but the aggregate SFH expression levels are too low to contribute meaningful signal in PtdIns-transfer assays.
The SFH proteins do not individually, or collectively, execute essential cellular functions (Figure 4A). Moreover, with the exception of Δsfh2 and Δsfh3, individual disruptions do not exacerbate sec14-1ts–associated growth defects, and en bloc deletion of SFH genes is not a lethal event when introduced into sec14-1ts genetic backgrounds. Rather, individual disruption of SFH2 or SFH3, or collective ablation of all SFH genes, decreases the restrictive temperature of sec14-1ts strains (Figure 4B). These data suggest that SFH proteins do not define a minor family of PITPs that share perfect functional redundancy with Sec14p, the YEp(SFH)-mediated rescue of sec14 defects notwithstanding. Rather, these SFH proteins exert other functions (see below).
Although we do not believe at present that SFH proteins simply represent minor Sec14p isoforms, these proteins do contribute to Sec14p-independent growth in bypass-Sec14p mutants. In particular, en bloc deletion of the SFH genes abolishes Sec14p-independent cell growth in bypass-Sec14p mutants defective in CDP-choline pathway or SacIp function and exerts a significant diminution on the efficiency with which kes1 mutations effect Sec14p-independent cell growth (Figure 4C). Our data provide four lines of evidence to indicate that a significant aspect of the mechanism by which the SFH gene products contribute to Sec14p-independent viability involves regulation of PLD, an enzyme whose catalytic activity is essential for bypass Sec14p. First, PLD is obligatorily required for phenotypic rescue of Sec14p defects by Sfh4p and Sfh5p overproduction, and PLD significantly improves the efficiency with which Sfh2p overproduction rescues sec14-1ts (Figure 5A). These data are consistent with a model in which Sfh4p and Sfh5p exert their effects through PLD activation and in which PLD activation constitutes a significant aspect of the means by which Sfh2p mediates rescue of Sec14p deficiency. Second, the dual effects of collective SFH protein deficiency on decreasing the restrictive temperature of sec14-1ts mutants and compromising bypass-Sec14p mechanisms (Figure 4C) closely mimic the phenotypic effects of PLD inactivation (Xie et al., 1998; Rivas et al., 1999). Third, in vivo measurements of PLD-dependent choline release and PLD-dependent PtdOH production demonstrate directly that en bloc deletion of SFH genes significantly reduces PLD activity in vivo (Figure 5, B–D). Fourth, the diminished PLD activity measured for Δsfh strains in vivo is recapitulated by measurements of PLD activity in cell-free preparations (Figure 6, A and B). Thus, SFH gene products cooperate with bypass-Sec14p mutations and PLD to define a complex mechanism by which yeast adapt to loss of essential Sec14p function. Sfh2p overproduction has a PLD-independent component for rescue of sec14-1ts defects, and this component likely operates through alteration in phosphoinositide metabolism (Cleves et al., 1991a; Whitters et al., 1993; Guo et al., 1999; Hama et al., 1999; Rivas et al., 1999; Stock et al., 1999; Hughes et al., 2000).
The requirement for the SFH proteins for efficient activation of PLD in vivo provides novel insight into how yeast regulate the activity of this PtdIns-4,5-P2–dependent enzyme. Liscovitch and Cantley (1995) postulated that PITP functions to activate PLD by stimulating synthesis of PtdIns-4,5-P2. Contrary to this proposal, Sec14p deficiency evokes PLD activation, and activated PLD is required for Sec14p-independent growth (Patton-Vogt et al., 1997; Sreenivas et al., 1998; Xie et al., 1998). This paradox is further emphasized by the demonstration that PLD remains activated when a Sec14p mutant functional as the sole source of Sec14p in vivo, yet incapable of binding/transferring PtdIns or of stimulating phosphoinositide synthesis in vitro or in vivo, is expressed in yeast (Phillips et al., 1999). Thus, PLD activation seemingly responds to loss of PtdIns-bound Sec14p function, and Pik1p deficiency does not evoke a similar activation of PLD (see RESULTS).
Our demonstration that the SFH gene products contribute to efficient PLD activation identifies the elusive link between PITPs and PLD activation in yeast. The colocalization of Sfh2p with PLD in endosomes further strengthens this link (Figure 7, B and C). Independent support for a link between Sfh2p and PLD function is provided by our observation that Sfh2p overexpression renders yeast resistant to the morpholine fungicide fenpropimorph. This fungicide targets sterol biosynthesis in yeast, and the Sfh2p-mediated fenpropimorph resistance phenotype is obligatorily dependent on both Sfh2p PtdIns-transfer activity and a functional PLD (our unpublished data). The mechanism for how SFH gene products stimulate PLD activity remains to be determined. However, because yeast PLD is a PtdIns-4,5-P2–activated enzyme and this activation is required for PLD function in vivo (Sciorra et al., 1999), a likely mechanism for SFH-mediated activation of PLD is through SFH-facilitated synthesis of PtdIns-4,5-P2.
What other physiological functions are executed by SFH proteins in yeast? SFH3 and SFH4 correspond to the PDR16 and PDR17 gene products, respectively. Transcriptional expression of these two genes is induced severalfold by challenge of yeast with azole inhibitors of sterol biosynthesis (van den Hazel et al., 1999). Simultaneous deletion of both of these genes exerts pleiotropic alterations in bulk membrane phospholipid and sterol composition and renders yeast hypersensitive to azole inhibitors of sterol biosynthesis (van den Hazel et al., 1999).
More penetrating insights into Sfh4p/Pdr17p function have been obtained from demonstration of a requirement for this PITP in stimulating decarboxylation of phosphatidylserine to phosphatidylethanolamine by a specific phosphatidylserine decarboxylase (Psd2p) that is localized to the yeast Golgi/vacuolar (endosomal?) system (Wu et al., 2000). Because the Stt4p PtdIns 4-kinase also stimulates this same metabolic reaction (Trotter et al., 1998), it is likely that Sfh4p and Stt4p synergize in generating a dedicated phosphoinositide (PtdIns-4-P or PtdIns-4,5-P2) pool that somehow regulates this process. Interestingly, Sec14p overproduction cannot fulfill the Sfh4p/Pdr17p requirement in the stimulation of this particular Psd2p-mediated metabolic reaction (Wu et al., 2000). The pleiotropic lipid metabolic defects recorded in sfh3/pdr16 and sfh4/pdr17 mutants are also manifested in the face of Sec14p expression. Thus, there is functional specificity that distinguishes these PITPs in vivo. A common thread linking SFH3/PDR16 and SFH4/PDR17/PSTB2 to Sec14p is their physiological involvement in regulating specific lipid metabolic pathways. The Sfh2p-mediated fenpropimorph resistance in yeast is also consistent with a role for Sfh2p in regulating sterol biosynthesis.
Finally, we find that the SFH gene product most similar to Sec14p (Sfh1p; Figure 2A) is nonfunctional as a PITP and cannot substitute for Sec14p in vivo. This is surprising because Sfh1p shares greater primary sequence similarity with Sec14p than do most other members of the Sec14p-like PITP family (Lopez et al., 1994; Bankaitis et al., 1996; Jouannic et al., 1998; Kearns et al., 1998b). Sfh1p conserves the recognized critical structural motifs characteristic of Sec14p-like PITP family members (Kearns et al., 1998b; Sha et al., 1998), so it remains unclear why it is inactive as a PITP in vivo and in vitro. In this regard, Sfh1p provides a platform for functional analysis. Mutations that resuscitate Sfh1p PITP activity would prove useful in identifying novel functional motifs of Sec14p-like PITPs.
ACKNOWLEDGMENTS
We thank JoAnne Engebrecht for generously providing YEp(GFP::SPO14) and for helpful discussions. We are also grateful to our University of Alabama at Birmingham colleagues Grace Zhai, Craig Garner, and Shaun Williams for providing us access to the Nikon microscope and for expert assistance with image processing. Finally, we thank two anonymous reviewers for helpful criticisms. This work was supported by National Institutes of Health grant GM44530 to V.A.B. M.B. was supported by National Institutes of Health grant AI38598.
REFERENCES
- Aitken JF, van Heusden GPH, Temkin M, Dowhan W. The gene encoding the phosphatidylinositol transfer protein is essential for cell growth. J Biol Chem. 1990;265:4711–4717. [PubMed] [Google Scholar]
- Atkinson KD. Saccharomyces cerevisiae recessive suppressor that circumvents phosphatidylserine deficiency. Genetics. 1984;108:533–543. doi: 10.1093/genetics/108.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Fry MR, Cartee RT, Kagiwada S. Molecular Biology Intelligence Unit. Austin, TX: RG Landes; 1996. Phospholipid transfer proteins: emerging roles in vesicle trafficking, signal transduction, and metabolic regulation; pp. 94–97. [Google Scholar]
- Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Bankaitis VA. Phospholipid transfer proteins: a biological debut. Trends Cell Biol. 1991a;1:30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991b;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves AE, Novick PJ, Bankaitis VA. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol. 1989;109:2939–2950. doi: 10.1083/jcb.109.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham E, Tan SK, Swigart P, Hsuan J, Bankaitis VA, Cockcroft S. The yeast and mammalian isoforms of phosphatidylinositol transfer protein can all restore phospholipase C-mediated inositol lipid signaling in cytosol-depleted RBL-2H3 and HL-60 cells. Proc Natl Acad Sci USA. 1996;93:6589–6593. doi: 10.1073/pnas.93.13.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCamilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MKY, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- Guo S, Stolz LE, Lemrow S, York JD. SAC1-like domains of yeast SAC1, INP52 and INP53, and human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald D. Direct involvement of phosphatidylinositol-4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34301. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TFJ. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Hay JC, Martin TFJ. Phosphatidylinositol transfer protein is required for ATP-dependent priming of Ca2+-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Hughes WE, Woscholski R, Cooke FT, Patrick RS, Dove SK, McDonald NQ, Parker PJ. SAC1 encodes a regulated lipid phosphoinositide phosphatase, defects in which can be suppressed by the homologous Inp52p and Inp53p phosphatases. J Biol Chem. 2000;275:801–808. doi: 10.1074/jbc.275.2.801. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkaline cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Alb JG, Jr, Phillips SE, Bankaitis VA, Howell KE. A phosphatidylinositol 3-kinase and phosphatidylinositol transfer protein act synergistically in formation of constitutive transport vesicles from the trans-Golgi network. J Biol Chem. 1998;273:10349–10354. doi: 10.1074/jbc.273.17.10349. [DOI] [PubMed] [Google Scholar]
- Jouannic N, Lepetit M, Vergnolle C, Cantrel C, Gardies A-M, Kader JC, Arondel V. Isolation of a cDNA from Arabidopsis thaliana that complements the sec14 mutant of yeast. Eur J Biochem. 1998;258:402–410. doi: 10.1046/j.1432-1327.1998.2580402.x. [DOI] [PubMed] [Google Scholar]
- Kearns BG, Alb JG, Jr, Bankaitis VA. Phosphatidylinositol transfer proteins: the long and winding road to physiological function. Trends Cell Biol. 1998a;8:276–282. doi: 10.1016/s0962-8924(98)01281-1. [DOI] [PubMed] [Google Scholar]
- Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. An essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns MA, Monks DE, Fang M, Rivas MP, Courtney PD, Chen J, Prestwich GD, Theibert AB, Dewey RE, Bankaitis VA. Novel developmentally regulated phosphoinositide binding proteins from soybean whose expression bypasses the requirement for an essential phosphatidylinositol transfer protein in yeast. EMBO J. 1998b;17:4004–4017. doi: 10.1093/emboj/17.14.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MH, Bard M, Pierson CA, Alexander JF, Goebl M, Carter GT, Kirsch DR. The identification of a gene family in the Saccharomyces cerevisiae ergosterol biosynthetic pathway. Gene. 1994;140:41–49. doi: 10.1016/0378-1119(94)90728-5. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Cantley LC. Signal transduction and membrane traffic: the PITP/phosphoinositide connection. Cell. 1995;81:659–662. doi: 10.1016/0092-8674(95)90525-1. [DOI] [PubMed] [Google Scholar]
- Lopez MC, Nicaud J-M, Skinner HB, Vergnolle C, Kader JC, Bankaitis VA, Gaillardin C. A phosphatidylinositol/phosphatidylcholine transfer protein is required for differentiation of the dimorphic yeast Yarrowia lipolytica from the yeast to the mycelial form. J Cell Biol. 1994;124:113–127. doi: 10.1083/jcb.125.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee TP, Skinner HB, Whitters EA, Henry SA, Bankaitis VA. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J Cell Biol. 1994;124:273–287. doi: 10.1083/jcb.124.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ohashi M, de Vries KJ, Frank R, Snoek G, Bankaitis V, Wirtz K, Huttner WB. A role for phosphatidylinositol transfer protein in secretory vesicle formation. Nature. 1995;377:544–547. doi: 10.1038/377544a0. [DOI] [PubMed] [Google Scholar]
- Patton-Vogt JL, Griac P, Sreenivas A, Bruno V, Dowd S, Swede MJ, Henry SA. Role for the yeast phosphatidylinositol/phosphatidylcholine transfer protein (Sec14p) in phosphatidylcholine turnover and INO1 regulation. J Biol Chem. 1997;272:20873–20883. doi: 10.1074/jbc.272.33.20873. [DOI] [PubMed] [Google Scholar]
- Phillips SE, et al. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol Cell. 1999;4:187–197. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- Rivas MP, Kearns BG, Xie Z, Guo S, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Pleiotropic alterations in lipid metabolism in yeast sac1 mutants: relationship to ‘Bypass Sec14p’ and inositol auxotrophy. Mol Biol Cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein RJ. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rose K, Rudge SA, Frohman MA, Morris AJ, Engebrecht J. Phospholipase D signaling is essential for meiosis. Proc Natl Acad Sci USA. 1995;92:12151–12155. doi: 10.1073/pnas.92.26.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge SA, Morris AJ, Engebrecht J. Relocalization of phospholipase D mediates membrane formation during meiosis. J Cell Biol. 1998;140:81–90. doi: 10.1083/jcb.140.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama SR, Cleves AE, Malehorn DE, Whitters EA, Bankaitis VA. Cloning and characterization of Kluyveromyces lactis SEC14, a gene whose product stimulates Golgi secretory function in Saccharomyces cerevisiae. J Bacteriol. 1990;172:4510–4521. doi: 10.1128/jb.172.8.4510-4521.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra VA, Rudge SA, Prestwich GD, Frohman MA, Engebrecht J, Morris AJ. Identification of a phosphoinositide binding motif that mediates activation of mammalian and yeast phospholipase D isoenzymes. EMBO J. 1999;18:5911–5921. doi: 10.1093/emboj/18.21.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha B, Phillips SE, Bankaitis VA, Luo M. Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol-transfer protein. Nature. 1998;391:506–510. doi: 10.1038/35179. [DOI] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1983. [Google Scholar]
- Simon J-P, Morimoto T, Bankaitis VA, Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DD. An essential role for phosphatidylinositol transfer protein in the scission of COPI-coated vesicles from the TGN. Proc Natl Acad Sci USA. 1998;95:11181–11186. doi: 10.1073/pnas.95.19.11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HB, Alb JG, Jr, Whitters EA, Helmkamp GM, Jr, Bankaitis VA. Phospholipid transfer activity is relevant to but not sufficient for the essential function of the yeast SEC14 gene product. EMBO J. 1993;12:4775–4784. doi: 10.1002/j.1460-2075.1993.tb06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HB, McGee TP, McMaster C, Fry MR, Bell RM, Bankaitis VA. Phosphatidylinositol transfer protein stimulates yeast Golgi secretory function by inhibiting choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci USA. 1995;92:112–116. doi: 10.1073/pnas.92.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivas A, Patton-Vogt JL, Bruno V, Griac P, Henry SA. A role for phospholipase D in growth, secretion, and regulation of membrane lipid synthesis in yeast. J Biol Chem. 1998;273:16635–16638. doi: 10.1074/jbc.273.27.16635. [DOI] [PubMed] [Google Scholar]
- Steiner MR, Lester RL. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972;260:222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Stock SD, Hama H, DeWald DB, Takemoto JY. SEC14-dependent secretion in Saccharomyces cerevisiae: non-dependence on sphingolipid-coupled diacylglycerol synthesis. J Biol Chem. 1999;274:12979–12983. doi: 10.1074/jbc.274.19.12979. [DOI] [PubMed] [Google Scholar]
- Trotter PJ, Wu W-I, Pedretti J, Yates R, Voelker DR. A genetic screen for aminophospholipid transport mutants identifies the phosphatidylinositol 4-kinase, Stt4p, as an essential component in phosphatidylserine metabolism. J Biol Chem. 1998;273:13189–13196. doi: 10.1074/jbc.273.21.13189. [DOI] [PubMed] [Google Scholar]
- van den Hazel HB, Pichler H, do Valle Matta MA, Leitner E, Goffeau A, Daum G. PDR16 and PDR17, two homologous genes of Saccharomyces cerevisiae, affect lipid biosynthesis and resistance to multiple drugs. J Biol Chem. 1999;274:1934–1941. doi: 10.1074/jbc.274.4.1934. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman M, Eli Y, Liscovitch M, Gerst JE. Identification and characterization of a gene encoding phospholipase D activity in yeast. J Biol Chem. 1996;271:2361–2364. doi: 10.1074/jbc.271.5.2361. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase Pik1 regulates secretion at the Golgi. Nat Cell Biol. 1999;1:523–555. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- Whitters EA, Cleves AE, McGee TP, Skinner HB, Bankaitis VA. SAC1p is an integral membrane protein that influences the cellular requirement for phospholipid transfer protein function and inositol in yeast. J Cell Biol. 1993;122:79–94. doi: 10.1083/jcb.122.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz KWA. Phospholipid transfer proteins. Annu Rev Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]
- Wu W-I, Routt S, Bankaitis V, Voelker DR. A new gene involved in the transport dependent metabolism of phosphatidylserine, PSTB2/PDR17, shares sequence similarity with the gene encoding the phosphatidylinositol/phosphatidylcholine transfer protein, SEC14. J Biol Chem. 2000;275:14446–14456. doi: 10.1074/jbc.275.19.14446. [DOI] [PubMed] [Google Scholar]
- Xie Z, Fang M, Rivas MP, Faulkner A, Sternweis PC, Engebrecht J, Bankaitis VA. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci USA. 1998;95:12346–12351. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]