Abstract
It has long been held that the malaria parasite, Plasmodium sp., is incapable of de novo fatty acid synthesis. This view has recently been overturned with the emergence of data for the presence of a fatty acid biosynthetic pathway in the relict plastid of P. falciparum (known as the apicoplast). This pathway represents the type II pathway common to plant chloroplasts and bacteria but distinct from the type I pathway of animals including humans. Specific inhibitors of the type II pathway, thiolactomycin and triclosan, have been reported to target this Plasmodium pathway. Here we report further inhibitors of the plastid-based pathway that inhibit Plasmodium parasites. These include several analogues of thiolactomycin, two with sixfold-greater efficacy than thiolactomycin. We also report that parasites respond very rapidly to such inhibitors and that the greatest sensitivity is seen in ring-stage parasites. This study substantiates the importance of fatty acid synthesis for blood-stage parasite survival and shows that this pathway provides scope for the development of novel antimalarial drugs.
Malaria is one of the world's most important infectious diseases in terms of both mortality and morbidity, causing over 1 million deaths and 300 to 500 million clinical infections annually (43). The rapid development of strains resistant to existing antimalarial compounds is frustrating efforts to combat the disease, and new chemotherapeutic targets are urgently required (43). The recent discovery of a plastid (plant chloroplast homologue) in Plasmodium falciparum and other apicomplexan parasites (18, 21, 22, 42) has offered a promising new avenue for this quest (2, 16, 31). Plastids ultimately derive from a prokaryotic endosymbiosis (1, 4), and plastid pathways that were inherited when the Plasmodium ancestor acquired this bacterial-type organelle (known as the apicoplast in these parasites) potentially offer specific targets for drugs because human cells lack a plastid. One such pathway found in plastids of plants and algae is de novo synthesis of fatty acids.
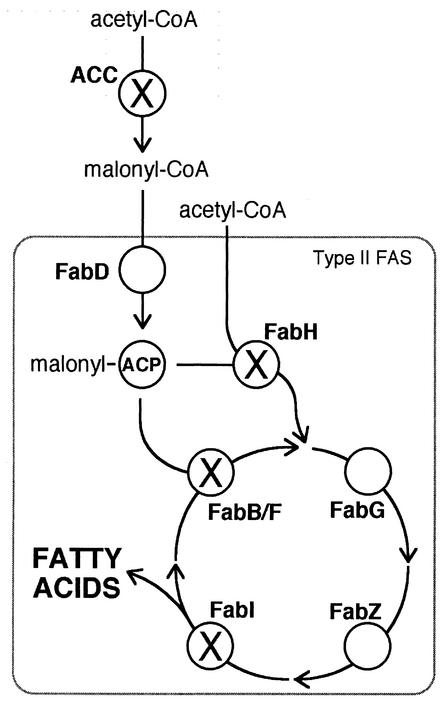
Fatty acids play a critical role in cells as metabolic precursors for biological membranes and energy stores. Their synthesis occurs as iterative elongations of acyl chains utilizing the 2-carbon donor malonyl coenzyme A (CoA) (Fig. 1). Fatty acid synthase (FAS) is the principal enzymatic unit of this process, and in bacterial systems separate proteins constitute the several enzyme activities of FAS. This system is known as the type II or dissociated pathway and is believed to represent the ancestral state (34). In animals, gene fusion events have resulted in FAS diverging as a single large multifunctional protein (34), and this cytosolic pathway is known as the type I or associated pathway. Plants utilize a plastid-based pathway, and this is of the bacterial type II system, suggesting that this pathway derived with the plastid in these organisms (12).
FIG. 1.
Pathway of type II de novo fatty acid biosynthesis showing key enzymes (boldface) and substrates. Enzymes belonging to the FAS complex are indicated by the box. The substrate for FAS, malonyl-CoA, is formed from acetyl-CoA by ACC. Fatty acid elongation consists of rounds of priming ACP with a malonyl moiety followed by condensation, reduction, dehydration, and reduction reactions that add two carbons to the growing acyl chain in each round. The first condensation reaction is catalyzed by FabH, and subsequent condensation reactions are catalyzed by FabB and/or FabF. Enzymes marked with an “X” are targets for inhibitory drugs that are the subjects of this investigation. FabD, malonyl-CoA:ACP transacylase; FabB, FabF, and FabH, β-ketoacyl-ACP synthases I, II, and III, respectively; FabG, β-ketoacyl-ACP reductase; FabZ, β-hydroxyacyl-ACP dehydratase; FabI, enoyl-ACP reductase.
Plasmodium spp. have long been believed to be unable to make their own fatty acids, depending instead on scavenged fatty acids from the host erythrocyte and serum (8, 14, 38). This notion is now being revised, with strong evidence for a type II pathway for de novo fatty acid synthesis occurring in apicoplasts. The first indication of fatty acid synthesis in apicoplasts was the discovery of nucleus-encoded FAS genes whose products are targeted to the apicoplast. These include acyl carrier protein (ACP), β-ketoacyl-ACP synthases III (FabH) and I/II (FabB/F) (39, 40), and enoyl-ACP reductase (FabI) (23, 35) (Fig. 1). Biochemical evidence for fatty acid synthesis in P. falciparum followed with 14C-labeled precursors (acetate and malonyl-CoA) incorporated into fatty acids (predominantly C10 to C14) in both in vivo and in vitro systems (35). Moreover, precursor incorporation is sensitive to triclosan, a known inhibitor of type II FAS, which is shown elsewhere, including by crystallography, to bind specifically to P. falciparum FabI (28, 35). This strongly implicates the apicoplast as the site of this fatty acid biosynthetic activity. In this study we further explore fatty acid synthesis as a target for chemotherapeutics by testing the antiparasite activity of several further compounds directed against type II fatty acid biosynthesis.
MATERIALS AND METHODS
Drug inhibition of in vitro-cultured P. falciparum.
Asynchronous P. falciparum cultures (strain W2mef) were cultured asexually in human erythrocytes (37). Parasites were resuspended in microtiter trays with 200 μl of hypoxanthine-free growth medium (to 0.5% parasitemia, 4% hematocrit) containing serial twofold dilutions of the compound being tested across the tray (plus no-compound controls) and 0.4 μCi of [3H]hypoxanthine. Parasites were incubated for 40 h, and label incorporation was measured as previously described (5). Triplicate (at least) experiments were performed. Thiolactomycin and its analogues were synthesized as described previously (41).
For life cycle stage-specific inhibition assays, P. falciparum (strain W2mef) cultures were synchronized by sorbitol treatment (9) and then allowed to complete one cycle and invade new erythrocytes at 3% parasitemia. Over the next 48-h cycle fresh aliquots of this culture were removed every 8 h for [3H]hypoxanthine incorporation assays that were performed over 16 h (instead of 40 h, to narrow the window of parasite stages for each assay). Inhibition was calculated relative to parasite aliquots without inhibitor (for maximal incorporation) and with 20 mM sodium azide (for minimal incorporation) to adjust for the differing rates of incorporation of [3H]hypoxanthine at different parasite stages. Each experiment was performed in triplicate.
RESULTS
Analogues of thiolactomycin show improved inhibition of P. falciparum.
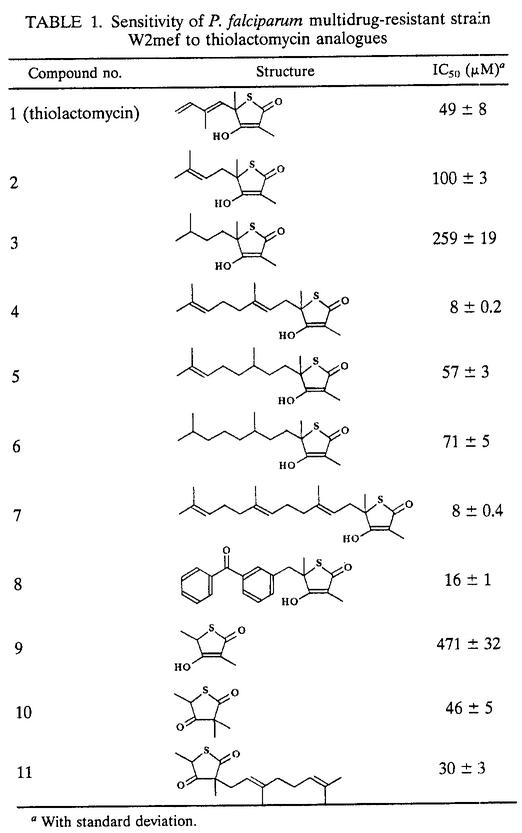
We have previously reported that the antibiotic thiolactomycin, an inhibitor of type II FAS-condensing enzymes β-ketoacyl-ACP synthases I, II, and III (FabB, FabF, and FabH, respectively), inhibits P. falciparum grown in vitro (39). To explore thiolactomycin sensitivity further, we have tested a series of analogues of thiolactomycin against parasite cultures. Half-maximal inhibition concentrations (IC50s) for each of these compounds were measured by a standard [3H]hypoxanthine uptake assay (5) (Table 1). These analogues were based on the thiolactone ring and mainly represent variations of the hydrophobic side group represented by a short isoprenoid moiety in thiolactomycin (Table 1). Several analogues showed efficacies equal to or greater than that of thiolactomycin, and the best two showed a sixfold improvement (analogues 4 and 7). These results indicate two conspicuous trends that relate the nature of the side chain to efficacy. One is that increased side chain length correlates with greater inhibition (compare analogues 9, 1, 4, and 7). The second trend is evident in compounds with equal side chain length but different saturation levels. Analogues 1 to 3 and 4 to 6 show that more double bonds correlate with greater inhibition. These two features suggest that the length and flexibility of this side group play an important role in antibiotic interaction with its target enzymes.
TABLE 1.
Sensitivity of P. falciparum multidrug-resistant strain W2mef to thiolactomycin analogues
With standard deviation.
Further inhibitors of fatty acid biosynthesis inhibit P. falciparum.
The fungal metabolite cerulenin is another inhibitor of β-ketoacyl-ACP synthases and forms a covalent bond at the active sites of synthases I and II (FabB and FabF). We tested cerulenin against P. falciparum and recorded an IC50 of 11 ± 4 μM. This concentration is equivalent to that seen in bacterial systems (27).
Fatty acid synthesis also presents drug targets upstream of FAS. The major substrate for FAS is malonyl-CoA, and its production from acetyl-CoA is often considered the first committed step of de novo fatty acid synthesis (Fig. 1) (3, 12). This reaction is catalyzed by acetyl-CoA carboxylase (ACC), and aryloxyphenoxypropionate herbicides specifically target certain forms of ACC. ACC activity in the related apicomplexan parasite Toxoplasma gondii has been shown elsewhere to be sensitive to this group of compounds (44, 45). We have tested equivalent compounds for inhibition of P. falciparum growth. We report that fenoxaprop, tralkoxydim, and diclofop inhibit P. falciparum with IC50s of 144 ± 22, 181 ± 6, and 210 ± 8 μM, respectively. These concentrations are equivalent to those shown elsewhere to inhibit T. gondii ACC (45) and are in a similar range as the IC50s for sensitive plants (5 to 35 μM) (44, 45).
P. falciparum shows the greatest sensitivity to fatty acid biosynthesis inhibitors in early-erythrocyte growth stages.
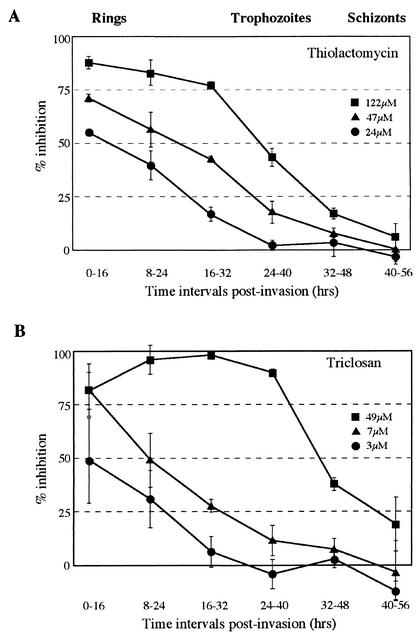
In order to examine growth stage-specific sensitivity of P. falciparum to fatty acid biosynthesis inhibitors, we synchronized parasite cultures and tested the effects of two such inhibitors throughout this cycle. The inhibitors used were thiolactomycin and triclosan. Triclosan is another specific inhibitor of type II FAS that binds to and inhibits enoyl-ACP reductase (FabI) (Fig. 1) (13, 20, 24). Triclosan has been recently shown to inhibit P. falciparum with an IC50 of 0.7 to 7 μM, depending on the parasite strain tested (23, 35).
Parasite inhibition with thiolactomycin and triclosan was measured over the blood-stage asexual life cycle of P. falciparum (approximately 40 h) for six time intervals, each starting 8 h apart. Each time interval was 16 h long, over which inhibition assays were conducted. Inhibitor concentrations that showed high, medium, and low levels of inhibition in asynchronous cultures were used. For each time interval culture smears were made at the beginning and end of the assays to monitor cell stages. At the beginning of the first assay all parasites were young rings (the first stage of infection) with no schizonts (dividing cells) remaining from the previous cycle. At the beginning of the last assay the vast majority of parasites were schizonts, although in some cases new rings had appeared. At the conclusion of these assays all parasites were either free merozoites or new rings. [3H]hypoxanthine uptake for healthy parasites (without inhibitors) varies according to parasite life cycle stage. The lowest uptake is in early rings (time interval 0 to 16 h), and the greatest is in trophozoites (time interval 24 to 40 h), where it is 30 times that of early rings. The schizont uptake rate (in time interval 40 to 56 h) was also higher than that of rings, by approximately threefold. This higher rate of incorporation of [3H]hypoxanthine into schizonts than into rings meant that the incorporation response for the last assay was chiefly from schizonts (and not from the newly invaded rings of this assay). Drug inhibition for each time interval was calculated as a percentage relative to maximal incorporation (treatments without inhibitor) and minimal incorporation (treatments with 20 mM sodium azide [36]), and these values are shown in Fig. 2. The sensitivity of both compounds was independent of parasite density (data not shown).
FIG. 2.
Life cycle-dependent sensitivities of P. falciparum to FAS inhibitors thiolactomycin and triclosan throughout asexual growth. Percentages of inhibition compared to those for parasites without drugs are recorded for six 16-h time intervals throughout the growth cycle of synchronized parasites in erythrocytes. Three concentrations were tested for each compound.
The results show that parasite sensitivity to thiolactomycin and triclosan is not constant throughout the growth cycle, with considerable differences seen between early- and late-stage parasites in erythrocytes (Fig. 2). For both compounds the greatest inhibition was seen in early stages after invasion (rings). A steady decrease in parasite sensitivity was seen as parasite growth progressed until almost complete parasite insensitivity was seen in the schizont stages at the concentrations used. Equivalent results with two different inhibitors suggested that the higher susceptibility of rings was not due to differential drug availability to parasites at different growth stages.
DISCUSSION
While the primary function of type I and type II FASs is conserved, their substantial conformational differences have created opportunities for inhibitors of FAS to select between them. An understanding of the precise mechanism for this discrimination between FASs awaits structural and drug-binding data from a type I FAS. Nevertheless, the utility of this discrimination has already been proven by the long use of triclosan as an antimicrobial agent in such products as toothpastes and shampoo (13, 20, 24) and by treatment of bacterial infections with thiolactomycin in mice (25). The apparent presence of a type II pathway for fatty acid biosynthesis in the P. falciparum apicoplast therefore presents an excellent potential target for parasite-specific inhibitors.
Since the initial report of P. falciparum inhibition by thiolactomycin (39), the crystal structure of thiolactomycin bound to Escherichia coli FabB has been solved (30). This structure shows that the thiolactone ring of thiolactomycin binds noncovalently to the protein's active site, apparently mimicking the thiolmalonate intermediate of the condensing reaction. Moreover, in conjunction with mutagenesis studies, several key residues (Phe 229, His 298, and His 333) have been identified that facilitate this protein-inhibitor interaction. Alignment of P. falciparum FabB/F with the E. coli FabB shows that these residues are all conserved in the P. falciparum sequence (Phe 232, His 306, and His 343; FabB/F accession number AF237575). This strongly implies that the P. falciparum FabB/F protein could be a target for thiolactomycin and could account for the antiparasite activity of this compound. FabH enzymes are also inhibited by thiolactomycin but to a lesser degree than are FabB or FabF enzymes (12, 30). This is due to replacement of the His 333 residue common to FabB/Fs with an Asn residue in FabHs, leading to reduced drug-binding affinity (30). This difference is also conserved in the P. falciparum FabH sequence. Hence, while FabH most probably offers a second target to thiolactomycin, we predict that this plays a lesser role than does FabB/F in parasite inhibition.
Price et al. (30) further observe that the isoprenoid moiety of thiolactomycin (Table 1) occupies a hydrophobic crevice in the active site that is distinct from the hydrophobic groove in this enzyme that ordinarily accommodates the elongating fatty acids. Within this crevice, Price et al. (30) identify two pairs of amino acids (Ala 271, Pro 272, Gly 391, and Phe 392) that sandwich the isoprenoid moiety by intercalated stacking. We note that these amino acid pairs occur as parts of two protein domains that are highly conserved throughout type II condensing enzymes FabB and FabF and that these four principal amino acids are identically represented by the P. falciparum FabB/F (Ala 274, Pro 275, Gly 403, and Phe 404; FabB/F accession number AF237575). Price et al. (30) also comment that the crevice is deeper than the length of the thiolactomycin side chain and is, therefore, not optimally filled by thiolactomycin. Our studies of thiolactomycin analogues support this assertion, where the length and flexibility of this side chain do influence the level of parasite inhibition. Moreover, it is the longer side chains, which may better occupy this cleft, that show the greatest inhibition. The subtle changes in thiolactomycin structure represented by these analogues suggest that an improved fit in the hydrophobic crevice offers greater enzyme inhibition. Studies of the pea (17) and mycobacteria (19) have also tested analogues of thiolactomycin in assays for fatty acid biosynthesis or activities of the individual condensing enzymes. These studies report similar increases in inhibition of fatty acid synthesis with longer side chains, as well as sensitivity to saturation level of this group. It is probable, therefore, that the hydrophobic crevice in the E. coli FabB, for which no specific function has yet been assigned, is a common feature of FabBs (as well as FabFs) and perhaps is responsible for the selectivity of thiolactomycin for type II FASs. Importantly, these results present scope for rational design of thiolactomycin-based compounds that could provide even greater antiparasite activity. Thiolactomycin has already been shown elsewhere to be nontoxic to mice and offers significant protection against urinary tract and intraperitoneal bacterial infections (25). This family of compounds now warrants greater investigation as potential antimalarial drugs.
Cerulenin provides an interesting parallel to thiolactomycin, given that it also possesses a long hydrophobic tail and is an inhibitor of FabB and FabF. The crystal structures of cerulenin bound to E. coli FabB and FabF have also been solved (26, 30). In this case, however, cerulenin's long hydrophobic side chain sits in the hydrophobic groove that accommodates the elongating fatty acids rather than the crevice used by the thiolactomycin tail. As for thiolactomycin, the key catalytic amino acid residues that interact with cerulenin have been identified (Cys 163, His 298, His 333, and Phe 392), and these are all conserved in the P. falciparum FabB/F (Cys 165, His 306, His 343, and Phe 404; FabB/F accession number AF237575). This suggests that the P. falciparum FabB/F is likely a target for cerulenin.
Aryloxyphenoxypropionate herbicides are a useful class of selective herbicides due to their specificity for the plastid ACCs of grasses. In most plants, and many algae, the plastid appears to have retained a prokaryotic form of ACC where each of its three catalytic domains is represented by a separate protein (11, 32, 33). However, grasses target a form of this enzyme, which consists of a single protein, to their plastids. This multidomain ACC in the plastids of grasses is sensitive to the aryloxyphenoxypropionate herbicides (44), while the multisubunit ACCs of other plastids, or indeed the ACCs in the cytosol of plants and animals, are not. Both P. falciparum and the related apicomplexan parasite T. gondii encode an equivalent single-protein-type ACC that is targeted to the apicoplast (10, 15). ACC activity assays and mutational analyses of the T. gondii ACC have demonstrated a specific inhibition of this enzyme by aryloxyphenoxypropionate herbicides (44, 45). P. falciparum has sensitivity to aryloxyphenoxypropionate herbicides equivalent to that of T. gondii. This most likely represents specific inhibition of the P. falciparum ACC, although confirmation of this awaits biochemical studies.
The differing sensitivity of P. falciparum to thiolactomycin and triclosan in different growth stages of intraerythrocytic parasites adds further insight into the action of these inhibitors. In T. gondii, apicoplast inhibition is reported elsewhere to be associated with a “delayed death” phenotype where parasite inhibition is seen only upon invasion of a subsequent host cell (6, 7, 29). However, the action of fatty acid synthesis inhibitors on P. falciparum has an immediate effect on parasite health without any such delay. These results indicate that nascent fatty acids are most important in the initial stages of erythrocyte infection when the extremely reduced merozoite begins to elaborate in order to consume its new host cell.
Conclusion.
A type II pathway for fatty acid biosynthesis in the apicoplast of P. falciparum is now strongly supported by both molecular and biochemical data. Multiple points in this pathway are known to be selectively targeted by drugs from both bacterial and plant studies. In this study we show that P. falciparum is also vulnerable at these points of attack. Collectively these data strongly support the critical nature of this pathway in the malaria parasite. Although this class of inhibitors shows efficacy only in the high nanomolar to micromolar range, this study provides an important proof of principle—that this newly discovered plant-type pathway can be targeted by selective inhibitors. Analogue studies indicate that scope exists for much higher efficacy with such inhibitors.
Acknowledgments
Triclosan was kindly provided by P. Lee of Ciba (Australia), and ACC inhibitors were provided by C. Preston and S. Powles.
R.F.W. and M.B.R. are recipients of NHMRC Peter Doherty Fellowships, and S.A.R. is a recipient of a Melbourne Research Scholarship. G.I.M. and A.F.C. are supported by the Australian Research Council, Australian National Health and Medical Research, and the Howard Hughes Medical Institute.
REFERENCES
- 1.Cavalier-Smith, T. 1982. The origins of plastids. Biol. J. Linn. Soc. 17:289-306. [Google Scholar]
- 2.Clough, B., K. Rangachari, M. Strath, P. R. Preiser, and R. Wilson. 1999. Antibiotic inhibitors of organellar protein synthesis in Plasmodium falciparum. Protist 150:189-195. [DOI] [PubMed] [Google Scholar]
- 3.Cronan, J. E., and C. O. Rock. 1996. Biosynthesis of membrane lipids, p. 612-636. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 4.Delwiche, C. 1999. Tracing the tread of plastid diversity through the tapestry of life. Am. Nat. 154:S164-S177. [DOI] [PubMed] [Google Scholar]
- 5.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fichera, M. E., M. K. Bhopale, and D. S. Roos. 1995. In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii. Antimicrob. Agents Chemother. 39:1530-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fichera, M. E., and D. S. Roos. 1997. A plastid organelle as a drug target in apicomplexan parasites. Nature 390:407-409. [DOI] [PubMed] [Google Scholar]
- 8.Fish, W. R. 1995. Lipid and membrane metabolism of the malaria parasite and the African trypanosome, p. 133-145. In J. J. Marr and M. Müller (ed.), Biochemistry and molecular biology of parasites. Academic Press, London, United Kingdom.
- 9.Freeman, R. R., and A. A. Holder. 1983. Light microscope morphology of Plasmodium falciparum. Ann. Trop. Med. Parasitol. 77:95-96. [DOI] [PubMed] [Google Scholar]
- 10.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gornicki, P., J. Faris, I. King, J. Podkowinski, B. Gill, and R. Haselkorn. 1997. Plastid-localized acetyl-CoA carboxylase of bread wheat is encoded by a single gene on each of the three ancestral chromosome sets. Proc. Natl. Acad. Sci. USA 94:14179-14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harwood, J. 1996. Recent advances in the biosynthesis of plant fatty-acids. Biochim. Biophys. Acta 1301:7-56. [DOI] [PubMed] [Google Scholar]
- 13.Heath, R. J., J. R. Rubin, D. R. Holland, E. L. Zhang, M. E. Snow, and C. O. Rock. 1999. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 274:11110-11114. [DOI] [PubMed] [Google Scholar]
- 14.Holz, G. G. 1977. Lipids and the malarial parasite. Bull. W. H. O. 55:237-248. [PMC free article] [PubMed] [Google Scholar]
- 15.Jelenska, J., M. J. Crawford, O. S. Harb, E. Zuther, R. Haselkorn, D. S. Roos, and P. Gornicki. 2001. Subcellular localization of acetyl-CoA carboxylase in the apicomplexan parasite Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 98:2723-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jomaa, H., J. Wiesner, S. Sanderbrand, B. Altincicek, C. Weidemeyer, M. Hintz, I. Turbachova, M. Eberl, J. Zeidler, H. K. Lichtenthaler, D. Soldati, and E. Beck. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573-1576. [DOI] [PubMed] [Google Scholar]
- 17.Jones, A. L., D. Herbert, A. J. Rutter, J. E. Dancer, and J. L. Harwood. 2000. Novel inhibitors of the condensing enzymes of the type II fatty acid synthase of pea (Pisum sativum). Biochem. J. 347:205-209. [PMC free article] [PubMed] [Google Scholar]
- 18.Köhler, S., C. F. Delwiche, P. W. Denny, L. G. Tilney, P. Webster, R. J. M. Wilson, J. D. Palmer, and D. S. Roos. 1997. A plastid of probable green algal origin in apicomplexan parasites. Science 275:1485-1488. [DOI] [PubMed] [Google Scholar]
- 19.Kremer, L., J. D. Douglas, A. R. Baulard, C. Morehouse, M. R. Guy, D. Alland, L. G. Dover, J. H. Lakey, W. R. Jacobs, Jr., P. J. Brennan, D. E. Minnikin, and G. S. Besra. 2000. Thiolactomycin and related analogues as novel anti-mycobacterial agents targeting KasA and KasB condensing enzymes in Mycobacterium tuberculosis. J. Biol. Chem. 275:16857-16864. [DOI] [PubMed] [Google Scholar]
- 20.Levy, C. W., A. Roujeinikova, S. Sedelnikova, P. J. Baker, A. R. Stuitje, A. R. Slabas, D. W. Rice, and J. B. Rafferty. 1999. Molecular basis of triclosan activity. Nature 398:383-384. [DOI] [PubMed] [Google Scholar]
- 21.McFadden, G. I., M. Reith, J. Munholland, and N. Lang-Unnasch. 1996. Plastid in human parasites. Nature 381:482.. [DOI] [PubMed] [Google Scholar]
- 22.McFadden, G. I., and R. F. Waller. 1997. Plastids in parasites of humans. Bioessays 19:1033-1040. [DOI] [PubMed] [Google Scholar]
- 23.McLeod, R., S. P. Muench, J. B. Rafferty, D. E. Kyle, E. J. Mui, M. J. Kirisits, D. G. Mack, C. W. Roberts, B. U. Samuel, R. E. Lyons, M. Dorris, W. K. Milhous, and D. W. Rice. 2001. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab I. Int. J. Parasitol. 31:109-113. [DOI] [PubMed] [Google Scholar]
- 24.McMurry, L. M., M. Oethinger, and S. B. Levy. 1998. Triclosan targets lipid synthesis. Nature 394:531-532. [DOI] [PubMed] [Google Scholar]
- 25.Miyakawa, S., K. Suzuki, Y. Harada, and H. Okazaki. 1982. Thiolactomycin, a new antibiotic. IV. Biological properties and chemotherapeutic activity in mice. J. Antibiot. 35:411-419. [DOI] [PubMed] [Google Scholar]
- 26.Moche, M., G. Schneider, P. Edwards, K. Dehesh, and Y. Lindqvist. 1999. Structure of the complex between the antibiotic cerulenin and its target, beta-ketoacyl-acyl carrier protein synthase. J. Biol. Chem. 274:6031-6034. [DOI] [PubMed] [Google Scholar]
- 27.Omura, S. 1976. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol. Rev. 40:681-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perozzo, R., M. Kuo, A. bir Singh Sidhu, J. T. Valiyaveettil, R. Bittman, W. R. Jacobs, Jr., D. A. Fidock, and J. C. Sacchettini. 2002. Structural elucidation of the specificity of the antibacterial agent triclosan for malarial enoyl ACP reductase. J. Biol. Chem. 277:13106-13114. [DOI] [PubMed] [Google Scholar]
- 29.Pfefferkorn, E. R., and S. E. Borotz. 1994. Comparison of mutants of Toxoplasma gondii selected for resistance to azithromycin, spiramycin, or clindamycin. Antimicrob. Agents Chemother. 338:31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price, A. C., Y. M. Zhang, C. O. Rock, and S. W. White. 2001. Structure of beta-ketoacyl-[acyl carrier protein] reductase from Escherichia coli: negative cooperativity and its structural basis. Biochemistry 40:12772-12781. [DOI] [PubMed] [Google Scholar]
- 31.Ralph, S. A., M. C. D'Ombrain, and G. I. McFadden. 2001. The apicoplast as an antimalarial drug target. Drug Resist. Updates 4:145-151. [DOI] [PubMed] [Google Scholar]
- 32.Reith, M., and J. Munholland. 1993. A high-resolution gene map of the chloroplast genome of the red alga Porphyra purpurea. Plant Cell 5:465-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulte, W., R. Topfer, R. Stracke, J. Schell, and N. Martini. 1997. Multi-functional acetyl-CoA carboxylase from Brassica napus is encoded by a multi-gene family: indication for plastidic localization of at least one isoform. Proc. Natl. Acad. Sci. USA 94:3465-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, S. 1994. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J. 8:1248-1259. [PubMed] [Google Scholar]
- 35.Surolia, N., and A. Surolia. 2001. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 7:167-173. [DOI] [PubMed] [Google Scholar]
- 36.Taverne, J., N. Matthews, P. Depledge, and J. Playfair. 1984. Malaria parasites and tumour cells are killed by the same component of tumour necrosis serum. Clin. Exp. Immunol. 57:287-292. [PMC free article] [PubMed] [Google Scholar]
- 37.Trager, W., and J. B. Jensen. 1978. Cultivation of malarial parasites. Nature 273:621-622. [DOI] [PubMed] [Google Scholar]
- 38.Vial, G. J., and M. L. Ancelin. 1992. Malarial lipids. An overview. Subcell. Biochem. 18:259-306. [PubMed] [Google Scholar]
- 39.Waller, R. F., P. J. Keeling, R. G. K. Donald, B. Striepen, E. Handman, N. Lang-Unnasch, A. F. Cowman, G. S. Besra, D. S. Roos, and G. I. McFadden. 1998. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 95:12352-12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waller, R. F., M. B. Reed, A. F. Cowman, and G. I. McFadden. 2000. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 19:1794-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, C.-L., and J. Salvino. 1984. Total synthesis of (+\−)-thiolactomycin. Tetrahedron Lett. 25:5243-5246. [Google Scholar]
- 42.Wilson, R. J. M., P. W. Denny, P. R. Preiser, K. Rangachari, K. Roberts, A. Roy, A. Whyte, M. Strath, D. J. Moore, P. W. Moore, and D. H. Williamson. 1996. Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 261:155-172. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 2000. The world health report 2000. World Health Organization, Geneva, Switzerland.
- 44.Zagnitko, O., J. Jelenska, G. Tevzadze, R. Haselkorn, and P. Gornicki. 2001. An isoleucine/leucine residue in the carboxyltransferase domain of acetyl-CoA carboxylase is critical for interaction with aryloxyphenoxypropionate and cyclohexanedione inhibitors. Proc. Natl. Acad. Sci. USA 98:6617-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuther, E., J. J. Johnson, R. Haselkorn, R. McLeod, and P. Gornicki. 1999. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. USA 96:13387-13392. [DOI] [PMC free article] [PubMed] [Google Scholar]