Abstract
Ascorbic acid (vitamin C) is a major antioxidant and redox buffer, but is also involved in other critical processes of plants. Recently, the hypothesis has been proposed that legume nodules are unable to synthesize ascorbate and have to import it from the shoot or root, thus providing a means by which the plant regulates nodule senescence. The last step of ascorbate biosynthesis in plants is catalyzed by l-galactono-1,4-lactone dehydrogenase (GalLDH). The mRNAs encoding GalLDH and three other enzymes involved in ascorbate biosynthesis are clearly detectable in nodules. Furthermore, an active membrane-bound GalLDH enzyme is present in nodule mitochondria. Biochemical assays on dissected nodules reveal that GalLDH activity and ascorbate are correlated in nodule tissues and predominantly localized in the infected zone, with lower levels of both parameters (relative to the infected tissues) in the apex (87%) and senescent region (43%) of indeterminate nodules and in the peripheral tissues (65%) of determinate nodules. In situ RNA hybridization showed that the GalLDH mRNA is particularly abundant in the infected zone of indeterminate and determinate nodules. Thus, our results refute the hypothesis that ascorbate is not synthesized in nodules and lend support to a previous conclusion that ascorbate in the infected zone is primarily involved in the protection of host cells against peroxide damage. Likewise, the high ascorbate and GalLDH activity levels found in the apex of indeterminate nodules strongly suggest a participation of ascorbate in additional functions during symbiosis, possibly related to cell growth and division and to molecular signaling.
l-Ascorbic acid (vitamin C) is a major soluble antioxidant and cellular reductant in plants and animals (Arrigoni and De Tullio, 2002). However, humans and other primates, guinea pigs, bats, and some birds and fish are unable to synthesize ascorbate and need to incorporate it in the diet. This is because they lack a functional gene encoding l-gulono-1,4-lactone oxidase, the enzyme that catalyzes the last step of ascorbate synthesis in animals (Valpuesta and Botella, 2004). In contrast, the last step of the d-Man/l-Gal (Wheeler et al., 1998) and d-GalUA (Valpuesta and Botella, 2004) pathways for ascorbate synthesis in plants is catalyzed by l-galactono-1,4-lactone dehydrogenase (GalLDH), which is localized in the mitochondrial inner membrane (Siendones et al., 1999; Bartoli et al., 2000). Alternative, or simultaneous, pathways for ascorbate synthesis can also be operative in plants (Davey et al., 1999; Lorence et al., 2004; Valpuesta and Botella, 2004).
In plant cells, ascorbate acts as an antioxidant molecule in its own right by directly scavenging reactive oxygen species and by regenerating α-tocopherol (Smirnoff, 2000), but also because it is the substrate of cytosolic ascorbate peroxidase (cAPX) and the corresponding organellar isoforms, which are critical components of the ascorbate-glutathione pathway for hydrogen peroxide (H2O2) detoxification (Nakano and Asada, 1981; Dalton et al., 1986). It is now evident, however, that ascorbate plays additional key roles in plants. Ascorbate is a cofactor of violaxanthin de-epoxidase, prolyl-hydroxylase, 1-aminocyclopropane-1-carboxylate oxidase, and 9-cis-epoxycarotenoid dioxygenase, and is therefore involved in photoprotection, cell expansion, and plant development (Smirnoff, 2000; Conklin, 2001; Arrigoni and De Tullio, 2002). More recently, ascorbate has been found to modulate the expression of genes involved in the plant defense response to biotic stress (Pastori et al., 2003). This multiplicity of functions has led some authors to perceive that ascorbate may be, in addition to a powerful antioxidant and redox buffer, a signaling molecule involved in the regulation of complex processes such as the senescence of plants and their response to ozone, photooxidative conditions, or pathogen attack (Smirnoff, 2000; Pastori et al., 2003; Conklin and Barth, 2004; Puppo et al., 2005).
Legume nodules are symbiotic structures (formed after infection of roots with soil rhizobia) that are able to reduce (fix) atmospheric nitrogen to ammonia. Nitrogen fixation is a highly beneficial process for agriculture and the environment but is limited by the lifespan of nodules and stress conditions. An interesting hypothesis has just been proposed concerning the role of ascorbate in nodules (Groten et al., 2005; Puppo et al., 2005). These authors conclude that mature nodules cannot synthesize ascorbate and have to import it from the parent plant, thus providing a way by which ascorbate, probably in conjunction with abscisic acid and other hormones, orchestrates nodule senescence. This hypothesis is based primarily on two observations in pea (Pisum sativum) plants. First, feeding experiments with the GalLDH substrate l-galactono-1,4-lactone (l-GalL) showed that the capacity of nodules to synthesize ascorbate is very low in comparison with leaves or roots. Second, western analysis with an antibody against maize (Zea mays) GalLDH showed that the protein was present in leaves and roots but not in nodules.
The implications of these findings, if proven to be correct, are important. For example, if nodule activity is dependent on the ascorbate supplied by the plant, it would be feasible to delay the senescence of nodules by manipulating their ascorbate content or their molecular cross talk with the shoot or root. However, there are some drawbacks in the hypothesis of Groten et al. (2005). First, GalLDH activity was not measured, and the absence of the enzyme was presumably inferred from the inability of the authors to detect an immunoreactive protein on western blots. Second, feeding experiments were only done in duplicate, and most of the ascorbate was degraded during incubation of excised nodules, which makes this procedure to detect synthesized ascorbate unreliable. Third, only pea nodules were examined, which leaves open the possibility that the capacity of nodules to synthesize ascorbate depends on the legume species or the nodule type.
We therefore decided to investigate in detail whether legume nodules are capable of de novo ascorbate biosynthesis. To test this, molecular analyses and biochemical assays of the GalLDH enzyme were performed using legumes that produce indeterminate or determinate nodules. The two types of nodules differ in important structural and biochemical features (Sprent, 1980; Vasse et al., 1990; Hirsch, 1992). Indeterminate nodules, such as those of alfalfa (Medicago sativa) and pea, have a persistent meristem, are elongate or club-shaped with an age gradient from the apex to the base, and usually export fixed nitrogen to the shoot in the form of amides or amino acids. In contrast, determinate nodules, such as those of bean (Phaseolus vulgaris) and Lotus (Lotus japonicus), lack a persistent meristem, are generally spherical, and export ureides to the shoot.
In this work, we characterize the structure and function of the GalLDH gene of legumes and show that indeterminate and determinate nodules express a highly active mitochondrial GalLDH in the infected tissue. In indeterminate nodules, there is also abundant enzyme activity in the apex (meristematic) region. Our results refute the hypothesis that ascorbate in nodules needs to be imported from the plant and hence that the ascorbate supply from the leaves or roots is a signal regulating nodule senescence.
RESULTS AND DISCUSSION
The GalLDH Gene Is Expressed in Leaves, Roots, and Nodules of Lotus
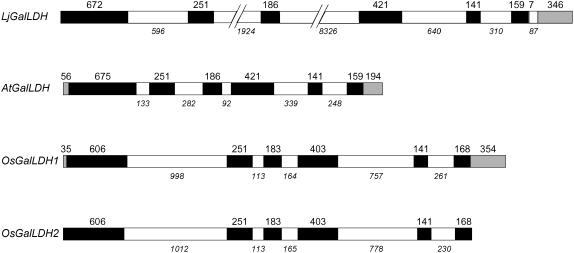
The first step of our study on ascorbate synthesis was to isolate and characterize a gene(s) encoding GalLDH in Lotus, a model legume for genetic and molecular analyses (Handberg and Stougaard, 1992). Because no homologous expressed sequence tags (ESTs) were available, genomic libraries of Lotus were screened using a tentative consensus sequence of Medicago truncatula (TC89371) and an EST of Glycine max (san59d11). As a result, a clone (LjT22C22) encoding a putative GalLDH was identified in a transformation-competent artificial chromosome (TAC) library. This gene was mapped, using a simple sequence repeat marker, at 44.2 cM on chromosome 5. Southern analysis revealed that there is a single copy of the GalLDH gene in the genomes of Lotus and other legumes such as pea and bean (data not shown). To elucidate the exon-intron composition of the Lotus GalLDH gene (LjGalLDH), total RNA was extracted from roots, gene-specific primers were designed, and cDNA clones were isolated and sequenced. The structure of the LjGalLDH gene was compared with other known or deduced GalLDH gene structures (Fig. 1). The sizes of exons 2 and 5 are identical in all four genes and those of exons 1, 3, 4, and 6 (excluding untranslated regions [UTRs]) are similar. However, LjGalLDH shows two significant differences with respect to the other genes. It contains an additional intron (intron 6) in the 3′-UTR and two unusually large introns (introns 2 and 3). A detailed search for transposon sequences in these introns revealed that, most likely, a class II element (MULE-type) is inserted in intron 2 and a class I (LTR-type) retroelement is present in intron 3. Interestingly, work in progress reveals that the transposon density in the genome is much higher in Lotus than in Arabidopsis (Arabidopsis thaliana) and that the class I and II transposons found in the GalLDH gene also occur in additional loci of the Lotus genome. Little is known about the possible functions of transposons in plants, but they are considered to be main contributors of genome size expansion and to play a role in genome rearrangement (Feschotte et al., 2002).
Figure 1.
Structure of the GalLDH gene of Lotus. Comparison of the GalLDH genes of Lotus (LjGalLDH), Arabidopsis (AtGalLDH, locus At3g47930), and Oryza sativa (OsGalLDH1, locus Os11g04740; OsGalLDH2, locus Os12g04520). Lengths of exons (open reading frames, black boxes; UTRs, gray boxes) and introns (white boxes) are given in base pairs and are drawn to scale, except for introns 2 and 3 of LjGalLDH.
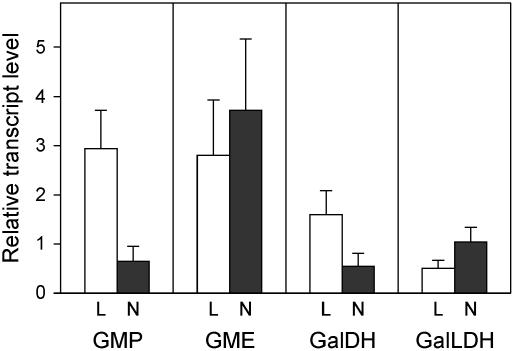
The expression of LjGalLDH in leaves, roots, and nodules was first studied by conventional reverse transcription (RT)-PCR using gene-specific primers designed to nucleotide sequences encompassing exons 4 to 6. A single amplicon of the expected 403 bp was found when total RNA from the three plant tissues was used as the template for the RT-PCR reactions (data not shown). Real-time quantitative RT-PCR (qRT-PCR) was then used to quantify mRNA levels with a second set of optimized primers, based on the sequence of exon 1 (Fig. 2). In addition, the mRNAs of three other genes involved in the main pathway for ascorbate biosynthesis (Wheeler et al., 1998) were also quantified in leaves, roots, and nodules. Results clearly showed that the genes encoding GDP-Man pyrophosphorylase (GMP), GDP-Man-3′,5′-epimerase (GME), and l-Gal dehydrogenase (GalDH) are transcribed in nodules (Fig. 2).
Figure 2.
Expression of four genes involved in the d-Man/l-Gal pathway for ascorbate biosynthesis in Lotus. The steady-state mRNA levels of GMP, GME, GalDH, and GalLDH were quantified in leaves, roots, and nodules by qRT-PCR. Values of leaves (L) and nodules (N) were expressed relative to roots of the same plants (which were arbitrarily given a value of 1) and are means ± se of four to five different extracts from at least two series of plants grown independently.
Legume Nodule Mitochondria Contain a Functional GalLDH
The LjGalLDH gene encodes a protein of 609 amino acids with a theoretical mass of 68.8 kD and a pI value of 8.66, which are similar to those of other deduced GalLDH proteins from higher plants. The remarkable homology among the GalLDH amino acid sequences of plants (>74% identity and >85% similarity) points out a high degree of conservation of the protein, which is probably associated with its critical function in metabolism. Prediction algorithms for protein topology (TMpred, http://www.ch.embnet.org/software/TMPRED_form.html) and subcellular localization (MitoProt, http://ihg.gsf.de/ihg/mitoprot.html; and TargetP 1.1, http://www.cbs.dtu.dk/services/TargetP/) indicated that the enzyme has several transmembrane helices and contains a transit peptide of 50 amino acids for mitochondrial targeting.
These predictions, along with the finding that the GalLDH gene is expressed in nodules, prompted us to investigate whether nodule mitochondria contain an active GalLDH. Bean nodules were used because they are amenable for purification of mitochondria, and we had hypothesized that they contain GalLDH to sustain the ascorbate-glutathione cycle of the cytosol (Iturbe-Ormaetxe et al., 2001). Indeed, this enzyme was extracted from mitochondria with an optimized medium containing 0.15% Triton X-100 and was assayed with l-GalL as the substrate and cytochrome c as the electron acceptor. The activity was 22.1 ± 1.6 nmol of ascorbate produced min−1 mg−1 of protein (mean ± se of three independent mitochondria preparations) and could not be detected when the detergent was omitted from the extraction medium or when the true substrate was replaced by its isomer d-GalL or by l-gulono-1,4-lactone. This demonstrates that the enzyme is a genuine GalLDH and is associated with the mitochondrial membranes. This finding is fully consistent with previous localizations of GalLDH in the inner membrane of mitochondria from bean hypocotyls (Siendones et al., 1999) and potato (Solanum tuberosum) leaves (Bartoli et al., 2000). However, in the former case, l-gulono-1,4-lactone was found to be 23% as effective as the genuine substrate l-GalL in the in vitro assay of GalLDH (Siendones et al., 1999).
Nodules Have Lower Ascorbate Concentrations Than the Leaves But Higher GalLDH and cAPX Activities
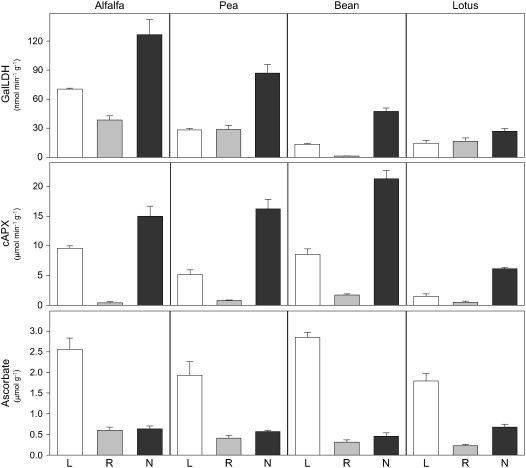
Because our results in bean nodules were in sharp contrast with the reported absence of GalLDH activity in pea nodules (Groten et al., 2005), we investigated whether these differences could be attributed to the different growth pattern of nodules. Thus, we determined the GalLDH activity and ascorbate content in leaves, roots, and nodules of two legumes (alfalfa and pea) with indeterminate nodules and two legumes (bean and Lotus) with determinate nodules (Fig. 3). Concomitantly, we measured the activity of cAPX because it is a very abundant protein and a major sink for ascorbate in nodules (Dalton et al., 1987).
Figure 3.
Distribution of GalLDH activity, cAPX activity, and ascorbate in legumes. Leaves (L), roots (R), and nodules (N) of alfalfa and pea (indeterminate nodulation) and Lotus and bean (determinate nodulation) were simultaneously harvested at the plant ages indicated under “Materials and Methods.” Values are means ± se of three to eight different extracts from at least two series of plants grown independently. Units of enzyme activity and ascorbate content are per gram of fresh weight.
The assay of GalLDH activity, under the same conditions and with the same controls as for mitochondria, demonstrated that the enzyme is present in the nodules of all legumes tested (Fig. 3). Furthermore, the activity was considerably greater in the nodules than in the corresponding leaves and roots. Interestingly, for all legume species, maximal extractable activities of GalLDH were obtained only when fresh leaves and roots were immediately processed, whereas the activity was preserved in frozen nodules. In particular, GalLDH activity was virtually lost in pea and bean leaves or in alfalfa and Lotus roots that had been frozen in liquid nitrogen and kept at −80°C for a few weeks (data not shown).
Early studies (Dalton et al., 1986; Matamoros et al., 1999a) had shown that nodules contain high levels of ascorbate (0.5–2 mm) and cAPX (up to 0.9% of the total soluble protein). These estimates are obviously dependent on nodule age and legume species but can be roughly confirmed in this work. Assuming uniform distribution of ascorbate and a water content of 90% in nodules (Dalton et al., 1986), ascorbate concentration in legume nodules is in the range of 0.5 to 0.8 mm. Also, the cAPX activity in nodules is 2- to 7-fold and 12- to 38-fold greater than those in the corresponding leaves and roots, respectively, which testifies to the abundance of this APX isoform in nodules (Fig. 3). However, leaves also contain chloroplast APXs that are rapidly inactivated by H2O2 in the absence of ascorbate (Nakano and Asada, 1981). This may also be the case for peroxisomal (Bunkelmann and Trelease, 1996) and mitochondrial (De Leonardis et al., 2000) APXs, although the lability of these two isoforms may vary with plant tissue. In fact, by extracting the enzymes in the presence (total APX activity) or absence (cAPX activity) of ascorbate, we found that the chloroplastic enzyme is highly labile and that its relative abundance is dependent on the legume species (data not shown). Ascorbate can reach 20 to 50 mm in the chloroplasts (Smirnoff, 2000), a very high concentration that may be required to maintain APX activity and hence to efficiently remove H2O2 through the ascorbate-glutathione cycle in the chloroplasts. Because cAPXs are insensitive to the lack of ascorbate (Dalton et al., 1987; Mittler and Zilinskas, 1991), the lower concentrations of this metabolite in nodules relative to leaves are probably sufficient to keep the cycle operational in the host cytoplasm of infected cells.
The finding that the nodules have higher expression levels (mRNA and activity) of GalLDH, but lower amounts of ascorbate, than the leaves (Figs. 2 and 3) is intriguing and may reflect differences between the two plant organs in the transport, degradation, or utilization of ascorbate. Also, expression of GalLDH in the leaves may depend on light intensity or be inhibited through a feedback mechanism by high ascorbate concentrations, as suggested for tobacco (Nicotiana tabacum) suspension cells (Tabata et al., 2002). Additional explanations may be differences in the stability of the mRNA or in the enzyme activity from leaves and nodules, or the existence in leaves of alternative routes for ascorbate biosynthesis (Davey et al., 1999; Lorence et al., 2004; Valpuesta and Botella, 2004). Interestingly, other authors also failed to find any correlation between GalLDH activity and ascorbate content in the leaves of various plants (Bartoli et al., 2005).
In contrast, the distribution of mitochondrial GalLDH and cAPX activities in the three organs of each legume species follow a similar pattern, which strongly suggests a close association between both enzyme activities (Fig. 3). We can envisage that a large part of the ascorbate produced in mitochondria is exported to the surrounding cytosol, where it fuels the ascorbate-glutathione cycle but may also serve additional purposes related to cell growth and molecular signaling. Moreover, the ascorbate synthesized on the inner membrane would be used, among other purposes, for the operation of the mitochondrial ascorbate-glutathione cycle (Dalton et al., 1993; Jiménez et al., 1997). Likewise, the finding of an active GalLDH in nodule mitochondria lends further support to our previous model for the functioning of antioxidant enzymes in bean nodules (Iturbe-Ormaetxe et al., 2001).
The GalLDH mRNA Is Primarily Localized in the Infected Zone of Nodules
To further investigate GalLDH expression in nodules, we localized the corresponding mRNA by fluorescence in situ hybridization (FISH) and the enzyme activity by biochemical assays of dissected nodules. For the histological description of nodules, we followed the terminology of Vasse et al. (1990) and Hirsch (1992).
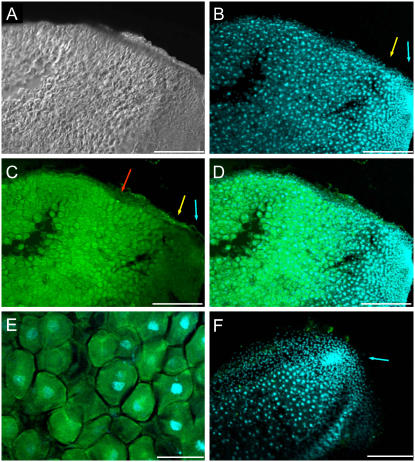
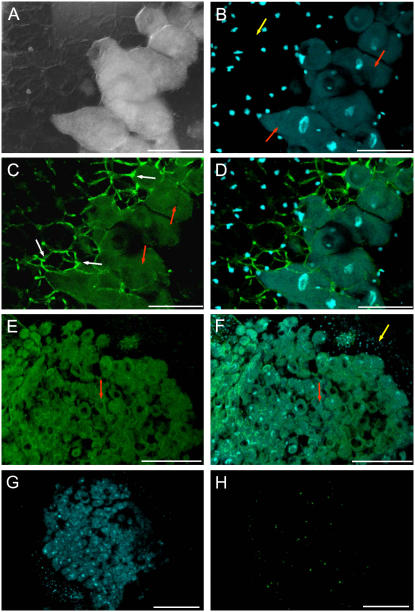
FISH detection was performed using confocal laser scanning microscopy after excitation of fluorochromes with different laser channels. The green fluorescence emission corresponded to the GalLDH mRNA and the blue fluorescence emission of 4′,6-diamidine-2-phenylindol (DAPI) to the nuclei. Images of alfalfa nodule sections using differential interference contrast (DIC) to delimit nodule structure (Fig. 4A) were also captured. The meristem (I) and invasion (II) zones of alfalfa nodules were discerned by the very intense DAPI staining (Fig. 4B). The differential GalLDH expression signal throughout the nodule was evident in the FISH (Fig. 4C) and merged DAPI/FISH (Fig. 4D) images. The central, infected region of the nodule (zone III) showed very intense green fluorescence, reflecting high GalLDH expression, whereas the nodule apex (zones I + II) generally displayed low or moderate fluorescence signal (Fig. 4C). The infected cells, which generally exhibited polygonal shape with a central nucleus, displayed intense fluorescence in the cytoplasm (Fig. 4E). Control nodule sections incubated with a sense GalLDH probe and DAPI for nuclei labeling did not show a significant green fluorescent signal (Fig. 4F).
Figure 4.
Localization of GalLDH mRNA in alfalfa (indeterminate) nodules. A, DIC image of a longitudinal section of a nodule. B, DAPI staining of nuclei (blue fluorescence) in the same nodule section as A. Note the high nuclei density in the meristematic zone (I; blue arrow) and invasion zone (II; yellow arrow). C, FISH of A with GalLDH antisense probe (green fluorescence). Note the very intense fluorescence in the infected zone (III; red arrow) and much lower fluorescence in zones I (blue arrow) and II (yellow arrow). D, Merged image of DAPI and FISH fluorescence signals. E, High magnification of the infected region of the nodule, showing an overlay of DAPI and FISH signals. Transcript labeling (green) is mostly observed in the host cell cytoplasm. F, Overlaid images of a nodule longitudinal section with DAPI and FISH using a GalLDH sense probe (control). Note the virtual absence of green fluorescence as well as the very intense labeling of the nuclei in the meristem zone (I; blue arrow). Magnification bars = 300 μm in A to D and F; 75 μm in E.
The determinate nodules of Lotus were also examined to localize GalLDH expression. In addition to FISH images with an antisense GalLDH probe (Fig. 5, C and E), DIC (Fig. 5A), DAPI (Fig. 5, B, D, F, and G), and merged DAPI/FISH (Fig. 5, D and F) images were captured and processed. As occurred with the indeterminate nodules of alfalfa, the green fluorescence signal, marking GalLDH mRNA localization, was more intense in the infected zone than in the peripheral tissues (Fig. 5, C and E). According to Hirsch (1992), these peripheral tissues comprise the nodule cortex, endodermis, and nodule parenchyma. Control sections incubated with a sense probe showed no significant green fluorescent signal (Fig. 5H).
Figure 5.
Localization of GalLDH mRNA in Lotus (determinate) nodules. A, DIC image showing the peripheral tissues and infected region of a nodule. Note the large polygonal, infected cells. B, DAPI staining (blue fluorescence) of the same nodule section as A. The nuclei of infected cells (red arrows) are larger than those of cortical cells (yellow arrow) and are localized toward the center of the cells. C, FISH signal in the same nodule section as A with GalLDH antisense probe. The green fluorescence is localized in the polygonal, large infected cells, with poor signal in the peripheral tissue. Note also the bright green autofluorescence of cell walls at the peripheral cell layers adjacent to infected cells (white arrows). Autofluoresecence was often observed at high magnifications but can be easily distinguished from the FISH fluorescence signal of the host cell cytoplasm (red arrows). D, Merged image of DAPI and FISH fluorescence signals. E, FISH signal in the central, infected region of nodules (red arrows). F, Merged image of DAPI and FISH signals. Note the labeling of nuclei in the cortical cells (yellow arrow), which display a low fluorescence signal compared with the infected cells (red arrows). G, Low magnification view of a nodule after DAPI labeling. H, The same nodule section as G incubated with a GalLDH sense probe as a negative control. Note the virtual absence of green fluorescence signal. Magnification bars = 75 μm in A to D; 300 μm in E to H.
Ascorbate Content Is Correlated to GalLDH Activity in Nodule Tissues
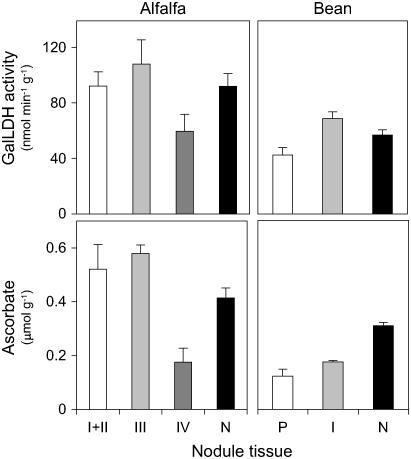
Dissection studies of alfalfa (indeterminate) and bean (determinate) nodules (Fig. 6) showed a significant correlation (r2 = 0.91; n = 5) between the extractable GalLDH activity and the ascorbate content, considering the mean values of the three fractions of alfalfa together with the two fractions of bean nodules. The highest GalLDH activity and ascorbate concentration were found in the infected tissue (zone III of alfalfa nodules and central zone of bean nodules) of the two types of nodules. The apex (zones I + II) of alfalfa nodules and the peripheral tissues of bean nodules also contained moderate or high levels of GalLDH activity and ascorbate (65%–90% relative to infected tissue), whereas the senescent region (zone IV) of alfalfa nodules had lower levels of both parameters (30%–55% relative to infected tissue). Whole nodules, processed simultaneously to nodule fractions, served as controls for extraction of GalLDH and ascorbate. Values of both parameters in whole nodules were, as expected, intermediate to those found in the nodule fractions, except for the ascorbate concentration of bean nodules. This was markedly greater than that in the corresponding peripheral and infected tissues, indicating a loss of ascorbate during dissection. The lower recovery of ascorbate in bean nodule fractions is probably due to their slower dissection compared to alfalfa nodules and occurred despite nodule fractions being directly collected into liquid nitrogen and dithioerythritol being added prior to the assay to ensure that all ascorbate was in the reduced state.
Figure 6.
Distribution of GalLDH activity and ascorbate in tissues of alfalfa (indeterminate) and bean (determinate) nodules. Both parameters were measured in zones I + II (meristem + invasion), III (infected), and IV (senescent) of alfalfa nodules, and in the peripheral tissues (P) and infected zone (I) of bean nodules. Whole nodules (N) were extracted in parallel as controls for recovery of GalLDH activity and ascorbate. Values are means ± se of three to five different extracts from nodules of at least two series of plants grown independently. Units of GalLDH activity and ascorbate content are per gram of fresh weight.
The enhanced GalLDH activity and ascorbate content in the infected zone of alfalfa and bean nodules (Fig. 6) are consistent with the primary localization of GalLDH mRNA in the same nodule tissue (Figs. 4 and 5). However, the apex of alfalfa nodules show lower levels of GalLDH mRNA than the infected zone (Fig. 4), yet similar GalLDH activity (Fig. 6), which suggests that the enzyme may be regulated, at least in part, at the posttranscriptional level. Interestingly, a posttranslational regulation of the enzyme has also been invoked to account for the lack of correlation between GalLDH protein and activity in wheat (Triticum aestivum) leaves (Bartoli et al., 2005).
Ascorbate Can Be Synthesized and May Have Multiple Functions in Nodules
We have shown so far that the genes encoding GMP, GME, GalDH, and GalLDH are expressed in nodules and that there is an active GalLDH in nodule mitochondria. Although the first two enzymes participate early in the ascorbate biosynthetic pathway and may be shared for other processes related to carbohydrate metabolism, GalDH and GalLDH catalyze the two last committed steps of the d-Man/l-Gal pathway (Wheeler et al., 1998). Therefore, the nodules are competent in ascorbate synthesis. This conclusion is further supported by the strong correlation between GalLDH activity and ascorbate content in alfalfa and bean nodule tissues.
Our results are in sharp contrast with the conclusions drawn by Groten et al. (2005), who suggested that young pea nodules may synthesize ascorbate but that this capacity is lost early in nodule development. In a follow-up publication, they further stated, without providing any additional proof, that nodules do not produce ascorbate (Groten et al., 2006). We have found that GalLDH is functional in mature nodules of four legumes, including pea (Fig. 3). Furthermore, in bean, the GalLDH activities of mitochondria from 5-week-old nodules (flowering plants) and 6-week-old nodules (fruiting plants) are still 96% and 58% of the activity found in 3-week-old nodules (young plants), respectively (data not shown). Thus, nodule aging decreases the potential for ascorbate synthesis, but this does not explain the negative results of Groten et al. (2005). Incubation of excised nodules with buffer for 8 h causes >90% degradation of endogenous ascorbate in nodules of pea (Groten et al., 2005) and bean (data not shown). This observation may reflect a general deterioration of excised nodules and leads us to conclude that the infiltration method is unreliable to detect de novo synthesis of ascorbate. It also casts doubts about the validity of some results reported using vacuum infiltration of nodules with l-GalL (Groten et al., 2005). For the same reason, prolonged incubation of excised nodules in buffer solution cannot be used for feeding experiments with isotopically labeled ascorbate precursors. On the other hand, the failure of Groten et al. (2005) to detect immunoreactive bands in pea nodule extracts using an antibody against maize GalLDH does not necessarily preclude the presence of the enzyme activity (or even the protein).
The distribution of ascorbate in the various tissues of alfalfa and bean nodules (Fig. 6) closely parallels that previously found for glutathione and homoglutathione in pea and bean nodules (Matamoros et al., 1999b). The abundance of ascorbate and thiols in the nodule-infected tissue strongly suggest that both types of redox metabolites cooperate in scavenging harmful (and controlling useful) concentrations of H2O2 in host cells by fueling the ascorbate-glutathione cycle. This conclusion is supported by previous findings that cAPX, a major enzyme of the ascorbate-glutathione cycle, is also predominantly localized to the infected region (Dalton et al., 1993, 1998). The protective role of ascorbate, thiols, and associated enzymes may be crucial because nitrogenase, leghemoglobin, and other abundant nodule proteins are particularly prone to oxidation by reactive oxygen species (Dalton et al., 1986). However, ascorbate (Fig. 6) and thiols (Matamoros et al., 1999b) are also present at high concentrations in the nodule apex, which is indicative that they fulfill additional roles in the symbiosis. In other plant systems, it has been shown that ascorbate and glutathione are required for cell division and elongation (Vernoux et al., 2000; Potters et al., 2004) and that they also modulate stress perception and signaling (Foyer and Noctor, 2005). In nodules, the high mitotic activity in zone I (meristem) and the growth of infection threads in zone II (invasion) are therefore expected to require ascorbate and thiols. For instance, Hyp-rich glycoproteins are a major component of the lumen of infection threads (Rathbun et al., 2002), and this hydroxylation is catalyzed by prolyl-hydroxylases using ascorbate as a cofactor (Arrigoni et al., 1977). This explanation is also fully consistent with previous observations in G. max that Hyp-rich proteins are especially abundant in walls of cortical cells and in meristematic cells (Ye and Varner, 1991) and that monodehydroascorbate reductase is primarily localized in nodule cell walls, where it may regenerate the ascorbate consumed in reactions such as the hydroxylation of Pro residues, maintenance of redox status of cell wall proteins, and lignification (Dalton et al., 1993). On the other hand, the low concentrations of ascorbate and thiol compounds (Matamoros et al., 1999b) in the senescent zone are consistent with the proposed relationship between the decrease in antioxidant defenses and the onset of nodule senescence (Matamoros et al., 1999a; Puppo et al., 2005). Whether this decline only modulates the senescence process (Foyer and Noctor, 2005; Groten et al., 2005) or may also lead to the oxidative damage of cellular components (Evans et al., 1999; Alesandrini et al., 2003; Rubio et al., 2004) merits further investigation.
MATERIALS AND METHODS
Biological Material
Nodulated plants of alfalfa (Medicago sativa L. cv Aragón × Sinorhizobium meliloti strain 102F78), pea (Pisum sativum L. cv Lincoln × Rhizobium leguminosarum biovar. leguminosarum strain NLV8), common bean (Phaseolus vulgaris L. cv Contender × Rhizobium leguminosarum biovar. phaseoli strain 3622), and Lotus (Lotus japonicus accession 'Miyakojima' MG-20 × Mesorhizobium loti strain NZP2235) were grown for 60, 35, 30, and 45 d, respectively, under controlled environmental conditions (Matamoros et al., 1999b, 2003). Leaves, roots, and nodules of the four legume species were harvested directly either into liquid nitrogen (molecular and biochemical analyses) or into fixative (confocal laser scanning microscopy). For determination of ascorbic acid and GalLDH activity in nodule tissues, alfalfa and bean nodules were dissected under a binocular microscope and the resulting portions were immediately immersed in liquid nitrogen.
Isolation, Characterization, and Mapping of the LjGalLDH Gene
Fragmental information on the genomic sequence of LjGalLDH was identified in the random genome sequence database of Lotus (S. Sato and S. Tabata, unpublished data) by a tblastx search using an EST encoding Glycine max GalLDH (BQ133574) as a query. Candidate TAC clones containing LjGalLDH were screened from a three-dimensional pool by PCR with primer sets (5′-GTCCACCATCACAACACCTG-3′ and 5′-ACTGTCAGGCTGGTGGAAAT-3′) designed on the genome fragment. The nucleotide sequence of one of the candidate TAC clones (LjT22C22) was determined according to the bridging shotgun method (Sato et al., 2001).
The LjGalLDH gene was mapped using a simple sequence repeat marker found on the bacterial artificial chromosome clone (LjB14G21), overlapping with LjT22C22. A primer set (5′-ACATGATATTTTACCCTCCC-3′ and 5′-AAACAGTTACGCCTCCGGTC-3′) that amplified 140-bp and 154-bp products from Lotus accessions Gifu B-129 and Miyakojima MG-20, respectively, was used for genotyping of the F2 mapping population of the B-129 × MG-20 cross, as described (Sato et al., 2001).
Expression Analysis of LjGalLDH in Plants
Leaves, roots, and nodules were harvested from Lotus plants directly into liquid nitrogen. Total RNA was isolated from approximately 50 to 100 mg of plant material using the RNAqueous isolation kit (Ambion). First-strand cDNA was synthesized from DNase-treated RNA using oligo(dT)17 and Moloney murine leukemia virus reverse transcriptase (Promega). The qRT-PCR analysis was performed with the iCycler iQ machine and iQ SYBR-Green Supermix reagents (Bio-Rad) using specific primers, based on Lotus sequences, for GMP (TC14556; 5′-CCGTCTGTTTTGGACCGAATT-3′, 5′-TCCAGGCAGAACCATTGCA-3′), GME (TC8218; 5′-GAAGGCTCCTGCTGCTTTTTG-3′, 5′-GGCTCACGGAAGTCGGATTTA-3′), GalDH (TC10248; 5′-TCAAGCTGCTGCAACCCATT-3′, 5′-TGCCAACAAGCACCGATGT-3′), and GalLDH (5′-GAGATGCTGAGAGCGCTGG-3′, 5′-GTGATGGTGGACACGGAGG-3′). The PCR program consisted of a first step of denaturation and Taq activation at 95°C for 5 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. To determine the specificity of the PCR, the amplified products were subjected to melt curve analysis according to the machine's standard protocol. For each PCR reaction, the ubiquitin (5′-TTCACCTTGTGCTCCGTCTTC-3′, 5′-AACAACAGCACACACAGACAA-3′) and β-actin (5′-GCATTGTTGGTCGTCCTCGT-3′, 5′-TGTGCCTCATCCCCAACATA-3′) genes of Lotus were used for normalization. Relative gene expression was determined using the 2−ΔΔCt method, where Ct is the threshold cycle. Before cDNA synthesis, all RNA samples were tested for the absence of contaminating genomic DNA.
Biochemical Assays
Enzyme extractions were performed at 0°C to 4°C and enzyme assays at 25°C. GalLDH was extracted from 0.1 to 0.5 g of plant tissue with 0.5 to 2.5 mL of 50 mm Tris-HCl, pH 8.0, and 0.15% Triton X-100. The extract was centrifuged (13,000g × 10 min) and GalLDH activity determined following the reduction of cytochrome c at 550 nm (ɛ = 17.3 mm−1 cm−1), essentially as described by Bartoli et al. (2000). The medium consisted of 50 mm Tris-HCl, pH 8.0, 0.15% Triton X-100, 10 μm KCN, 4.25 mm l-GalL, and 60 μm cytochrome c. Enzyme activity was calculated taking into account that 2 nmol of cytochrome c are reduced per nmol of l-GalL oxidized (Tabata et al., 2001).
cAPX was extracted from 0.1 g of plant tissue with 1 mL of 50 mm potassium phosphate buffer, pH 7.0, containing 0.5% (w/v) PVP-10. The activity in the soluble fraction (13,000g × 10 min) was assayed following the decrease in A290 (ɛ = 2.8 mm−1 cm−1). The assay medium comprised 50 mm potassium phosphate buffer, pH 7.0, 0.5 mm ascorbate, and 0.5 mm H2O2. Controls omitting the extract to correct for nonenzymatic ascorbate oxidation and controls omitting H2O2 to correct for APX-independent ascorbate oxidation were run in parallel.
Ascorbate content was determined following published protocols (Bartoli et al., 2000) with minor modifications. Ascorbate was extracted from 0.1 g of plant tissue with 0.5 mL of HClO4 in an ice-cold Eppendorf tube, and the extract was cleared by centrifugation (13,000g × 10 min) at 4°C. The supernatant was neutralized with 1 m K2CO3 and incubated for 15 min with 0.4 mm dithioerythritol at room temperature. Ascorbate concentration was then determined in 90 mm HEPES, pH 5.6, as the decrease of A265 (ɛ = 14.1 mm−1 cm−1) produced after addition of 0.05 units of ascorbate oxidase (Sigma).
Subcellular Localization of GalLDH
All steps were performed at 0°C to 4°C. Bean nodules (10 g) were ground in a mortar with a medium containing 30 mm MOPS, pH 7.2, 0.35 m mannitol, 2 mm EDTA, 10 mm KH2PO4, 2% (w/v) polyvinylpolypyrrolidone, and 0.4% (w/v) bovine serum albumin (BSA). The homogenate was filtered through four layers of cheesecloth and centrifuged (twice at 4,000g × 5 min, then at 12,000g × 15 min). The mitochondria-enriched pellet was resuspended in a washing medium comprising 20 mm MOPS, pH 7.2, 0.3 m mannitol, and 1 mm EDTA. After a new centrifugation, the pellet was resuspended in 1.8 mL of washing medium and loaded on top of a gradient composed of four layers containing 10%, 15%, 35%, and 50% (v/v) Percoll (Iturbe-Ormaetxe et al., 2001). After centrifugation (13,000g × 35 min), the mitochondria, which banded between the 15% and 35% Percoll layers, were removed and washed twice (17,000g × 10 min). The final pellet, containing the purified mitochondria, was resuspended in a hypoosmotic buffer containing 50 mm Tris-HCl, pH 8.0, and 0.15% Triton X-100. The broken mitochondria were cleared by centrifugation (13,000g × 10 min) and the supernatant used for the GalLDH activity assay.
Fluorescence in Situ mRNA Hybridization and Confocal Laser Scanning Microscopy
Nodules were fixed in 4% formaldehyde in phosphate-buffered saline (PBS) overnight at 4°C, washed in PBS, and directly cut into 30-μm sections with a vibratome. Nodule sections were placed on 3-aminopropyltriethoxysilane-coated slides and prepared for FISH analysis as described (Massonneau et al., 2005) with minor modifications. Sections were dehydrated in a series of 30%, 50%, 70%, and 100% methanol-water, rehydrated in a series of 70%, 50%, and 30% methanol-water, and finally washed in PBS (5 min for each step). Sections were then treated with 2% (w/v) cellulase (Onozuka R-10) in PBS for 1 h at room temperature in a humid chamber, washed in PBS and subsequently in water, dehydrated in a series of 30%, 50%, 70%, and 100% methanol-water, and stored dry until hybridization.
For FISH analysis, antisense and sense (control) digoxigenin-labeled RNA probes were synthesized by in vitro transcription using digoxigenin-UTP according to the manufacturer's specifications (Roche). A 1:25 dilution of the probe was prepared in fresh hybridization solution (50% formamide, 10% dextran sulfate, 10 mm Tris-HCl, pH 7.5, 1 mm EDTA, 300 mm NaCl, 200 μg mL−1 yeast tRNA) and was placed over the sections overnight at 50°C. Sections were washed four times (2 min each, room temperature) in 4× SSC, four times (2 min each, room temperature) in 2× SSC, and twice in 0.1× SCC (15 min each, 50°C). After washing in PBS, sections were incubated in 5% BSA for 10 min and with mouse anti-digoxigenin antibody (at 1:5,000 dilution in PBS containing 3% BSA) for 90 min at room temperature. This was followed by incubation with fluorescent anti-mouse Alexa Fluor 488 antibody (Molecular Probes), applied at a dilution of 1:25 in PBS for 1 h at room temperature and in the dark. Sections were washed in PBS, counterstained with DAPI, mounted in Mowiol, and observed. Confocal images of nodule sections were acquired on a Leica TCS-SP microscope (Leica) equipped with plan achromatic objectives. Images were captured as a z-series of optical sections. Color images of DAPI fluorescence were acquired with excitation at 351 nm (argon laser line) and emission at 440 ± 30 nm, and FISH fluorescence with excitation at 488 nm (helium-neon-krypton laser line) and emission at 520 ± 20 nm. Data processing and merging of signals were performed with Leica software (LCS version 2.61 Build 1537).
Sequence data from this article can be found in the GenBank data library under accession number DQ455608.
Acknowledgments
We thank Prof. M.C. Risueño for advice on confocal microscopy and helpful comments on the manuscript. This work is part of the Ph.D. thesis of J.L. (supervised by M.A.M. and M.B.).
This work was supported by the Ministerio de Educación y Ciencia-Fondos Europeos de Desarrollo Regional (grant no. AGL2005-01404) and by Gobierno de Aragón-Fondo Social Europeo (group E33 and grant no. PIP137/2005). We also acknowledge postdoctoral contracts of the “Ramon y Cajal” (M.A.M.) and “Juan de la Cierva” (J.R., M.J.C.) programs from the Ministerio de Educación y Ciencia, and a predoctoral fellowship (J.L.) from Gobierno de Aragón.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Manuel A. Matamoros (manumat@eead.csic.es).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.081463.
References
- Alesandrini F, Mathis R, Van de Sype G, Hérouart D, Puppo A (2003) Possible roles for a cysteine protease and hydrogen peroxide in soybean nodule development and senescence. New Phytol 158: 131–138 [Google Scholar]
- Arrigoni O, Arrigoni-Liso R, Calabrese G (1977) Ascorbic acid requirement for biosynthesis of hydroxyproline-containing proteins in plants. FEBS Lett 82: 135–138 [DOI] [PubMed] [Google Scholar]
- Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than an antioxidant. Biochim Biophys Acta 1569: 1–9 [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Guiamet JJ, Kiddle G, Pastori GM, Di Cagno R, Theodoulou FL, Foyer CH (2005) Ascorbate content of wheat leaves is not determined by maximal L-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. Plant Cell Environ 28: 1073–1081 [Google Scholar]
- Bartoli CG, Pastori GM, Foyer CH (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123: 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunkelmann JR, Trelease RN (1996) Ascorbate peroxidase. A prominent membrane protein in oilseed glyoxysomes. Plant Physiol 110: 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL (2001) Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ 24: 383–394 [Google Scholar]
- Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27: 959–970 [Google Scholar]
- Dalton DA, Baird LM, Langeberg L, Taugher CY, Anyan WR, Vance CP, Sarath G (1993) Subcellular localization of oxygen defense enzymes in soybean (Glycine max [L.] Merr.) root nodules. Plant Physiol 102: 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Hanus FJ, Russell SA, Evans HJ (1987) Purification, properties, and distribution of ascorbate peroxidase in legume root nodules. Plant Physiol 83: 789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Joyner SL, Becana M, Iturbe-Ormaetxe I, Chatfield JM (1998) Antioxidant defenses in the peripheral cell layers of legume root nodules. Plant Physiol 116: 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA 83: 3811–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MW, Gilot C, Persiau G, Østergaard J, Huan Y, Bauw GC, Van Montagu MC (1999) Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiol 121: 535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leonardis S, Dipierro N, Dipierro S (2000) Purification and characterization of an ascorbate peroxidase from potato tuber mitochondria. Plant Physiol Biochem 38: 773–779 [Google Scholar]
- Evans PJ, Gallesi D, Mathieu C, Hernandez MJ, de Felipe MR, Halliwell B, Puppo A (1999) Oxidative stress occurs during soybean nodule senescence. Planta 208: 73–79 [Google Scholar]
- Feschotte C, Jiang N, Wessler SR (2002) Plant transposable elements: where genetics meets genomics. Nature Rev Genet 3: 329–341 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28: 1056–1071 [Google Scholar]
- Groten K, Dutilleul C, van Heerden PDR, Vanacker H, Bernard S, Finkemeier I, Dietz KJ, Foyer CH (2006) Redox regulation of peroxiredoxin and proteinases by ascorbate and thiols during pea root nodule senescence. FEBS Lett 580: 1269–1276 [DOI] [PubMed] [Google Scholar]
- Groten K, Vanacker H, Dutilleul C, Bastian F, Bernard S, Carzaniga R, Foyer CH (2005) The roles of redox processes in pea nodule development and senescence. Plant Cell Environ 28: 1293–1304 [Google Scholar]
- Handberg K, Stougaard J (1992) Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J 2: 487–496 [Google Scholar]
- Hirsch AM (1992) Developmental biology of legume nodulation. New Phytol 122: 211–237 [DOI] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Matamoros MA, Rubio MC, Dalton DA, Becana M (2001) The antioxidants of legume nodule mitochondria. Mol Plant Microbe Interact 14: 1189–1196 [DOI] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, del Río LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea (Pisum sativum L.) leaves. Plant Physiol 114: 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence A, Chevone BI, Mendes P, Nessler CL (2004) Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol 134: 1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massonneau A, Coronado MJ, Audran A, Bagniewska A, Mòl R, Testillano PS, Goralski G, Dumas C, Risueño MC, Matthys-Rochon E (2005) Multicellular structures developing during maize microspore culture express both endosperm and embryo-specific genes and show different embryogenic potentialities. Eur J Cell Biol 84: 663–675 [DOI] [PubMed] [Google Scholar]
- Matamoros MA, Baird LM, Escuredo PR, Dalton DA, Minchin FR, Iturbe-Ormaetxe I, Rubio MC, Moran JF, Gordon AJ, Becana M (1999. a) Stress-induced legume root nodule senescence. Physiological, biochemical, and structural alterations. Plant Physiol 121: 97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros MA, Clemente MR, Sato S, Asamizu E, Tabata S, Ramos J, Moran JF, Stiller J, Gresshoff P, Becana M (2003) Molecular analysis of the pathway for the synthesis of thiol tripeptides in the model legume Lotus japonicus. Mol Plant Microbe Interact 16: 1039–1046 [DOI] [PubMed] [Google Scholar]
- Matamoros MA, Moran JF, Iturbe-Ormaetxe I, Rubio MC, Becana M (1999. b) Glutathione and homoglutathione synthesis in legume root nodules. Plant Physiol 121: 879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Zilinskas B (1991) Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiol 97: 962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22: 867–880 [Google Scholar]
- Pastori G, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15: 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, Horemans N, Bellone S, Caubergs J, Trost P, Guisez Y, Asard H (2004) Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiol 134: 1479–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Lucas MM, De Felipe MR, Harrison J, Vanacker H, Foyer CH (2005) Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165: 683–701 [DOI] [PubMed] [Google Scholar]
- Rathbun EA, Naldrett MJ, Brewin NJ (2002) Identification of a family of extensin-like glycoproteins in the lumen of Rhizobium-induced infection threads in pea root nodules. Mol Plant Microbe Interact 15: 350–359 [DOI] [PubMed] [Google Scholar]
- Rubio MC, James EK, Clemente MR, Bucciarelli B, Fedorova M, Vance CP, Becana M (2004) Localization of superoxide dismutases and hydrogen peroxide in legume root nodules. Mol Plant Microbe Interact 17: 1294–1305 [DOI] [PubMed] [Google Scholar]
- Sato S, Kaneko T, Nakamura Y, Asamizu E, Kato T, Tabata S (2001) Structural analysis of a Lotus japonicus genome. I. Sequence features and mapping of fifty-six TAC clones which cover the 5.4-Mb regions of the genome. DNA Res 8: 311–318 [DOI] [PubMed] [Google Scholar]
- Siendones E, González-Reyes JA, Santos-Ocaña C, Navas P, Córdoba F (1999) Biosynthesis of ascorbic acid in kidney bean. L-Galactono-γ-lactone dehydrogenase is an intrinsic protein located at the mitochondrial inner membrane. Plant Physiol 120: 907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci 355: 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent JI (1980) Root nodule anatomy, type of export product and evolutionary origin in some Leguminosae. Plant Cell Environ 3: 35–43 [Google Scholar]
- Tabata K, Oba K, Suzuki K, Esaka M (2001) Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase. Plant J 27: 139–148 [DOI] [PubMed] [Google Scholar]
- Tabata K, Takaoka T, Esaka M (2002) Gene expression of ascorbic acid-related enzymes in tobacco. Phytochemistry 61: 631–635 [DOI] [PubMed] [Google Scholar]
- Valpuesta V, Botella MA (2004) Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant. Trends Plant Sci 9: 573–577 [DOI] [PubMed] [Google Scholar]
- Vasse J, De Billy F, Camut S, Truchet G (1990) Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol 172: 4295–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld J-P, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, et al (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-H, Varner JE (1991) Tissue-specific expression of cell wall proteins in developing soybean tissues. Plant Cell 3: 23–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393: 365–369 [DOI] [PubMed] [Google Scholar]