Abstract
Phytochelatin (PC) synthases are γ-glutamylcysteine (γ-Glu-Cys) dipeptidyl transpeptidases that catalyze the synthesis of heavy metal-binding PCs, (γ-Glu-Cys)nGly polymers, from glutathione (GSH) and/or shorter chain PCs. Here it is shown through investigations of the enzyme from Arabidopsis (Arabidopsis thaliana; AtPCS1) that, although the N-terminal half of the protein, alone, is sufficient for core catalysis through the formation of a single-site enzyme acyl intermediate, it is not sufficient for acylation at a second site and augmentative stimulation by free Cd2+. A purified N-terminally hexahistidinyl-tagged AtPCS1 truncate containing only the first 221 N-terminal amino acid residues of the enzyme (HIS-AtPCS1_221tr) is competent in the synthesis of PCs from GSH in media containing Cd2+ or the synthesis of S-methyl-PCs from S-methylglutathione in media devoid of heavy metal ions. However, whereas its full-length hexahistidinyl-tagged equivalent, HIS-AtPCS1, undergoes γ-Glu-Cys acylation at two sites during the Cd2+-dependent synthesis of PCs from GSH and is stimulated by free Cd2+ when synthesizing S-methyl-PCs from S-methylglutathione, HIS-AtPCS1_221tr undergoes γ-Glu-Cys acylation at only one site when GSH is the substrate and is not directly stimulated, but instead inhibited, by free Cd2+ when S-methylglutathione is the substrate. Through the application of sequence search algorithms capable of detecting distant homologies, work we reported briefly before but not in its entirety, it has been determined that the N-terminal half of AtPCS1 and its equivalents from other sources have the hallmarks of a papain-like, Clan CA Cys protease. Whereas the fold assignment deduced from these analyses, which substantiates and is substantiated by the recent determination of the crystal structure of a distant prokaryotic PC synthase homolog from the cyanobacterium Nostoc, is capable of explaining the strict requirement for a conserved Cys residue, Cys-56 in the case of AtPCS1, for formation of the biosynthetically competent γ-Glu-Cys enzyme acyl intermediate, the primary data from experiments directed at determining whether the other two residues, His-162 and Asp-180 of the putative papain-like catalytic triad of AtPCS1, are essential for catalysis have yet to be presented. This shortfall in our basic understanding of AtPCS1 is addressed here by the results of systematic site-directed mutagenesis studies that demonstrate that not only Cys-56 but also His-162 and Asp-180 are indeed required for net PC synthesis. It is therefore established experimentally that AtPCS1 and, by implication, other eukaryotic PC synthases are papain Cys protease superfamily members but ones, unlike their prokaryotic counterparts, which, in addition to having a papain-like N-terminal catalytic domain that undergoes primary γ-Glu-Cys acylation, contain an auxiliary metal-sensing C-terminal domain that undergoes secondary γ-Glu-Cys acylation.
Synthesized posttranslationally from the ubiquitous tripeptide glutathione (GSH; γ-Glu-Cys-Gly), phytochelatins (PCs), poly-(γ-Glu-Cys)n-Gly polymers, act as high-affinity metal chelators and promote the vacuolysosomal sequestration of heavy metals (Cobbett and Goldsbrough, 2002; Vatamaniuk et al., 2002; Rea et al., 2004). In the several years since the original cloning of genes encoding the enzyme, PC synthase (EC 2.3.2.15), responsible for the synthesis of PCs initially from the plants Arabidopsis (Arabidopsis thaliana) and wheat (Triticum aestivum) and from the fission yeast Schizosaccharomyces pombe (Clemens et al., 1999; Ha et al., 1999; Vatamaniuk et al., 1999), and subsequently from the model nematode Caenorhabditis elegans (Clemens et al., 2001; Vatamaniuk et al., 2001), considerable progress has been made in elucidating the basic enzymology of PC-dependent metal detoxification. Key advances in this research area include the following. (1) Determination of the capacity of cell-free extracts from Arabidopsis PCS1 (AtPCS1)- or S. pombe PCS (SpPCS)-transformed cells of Escherichia coli (Ha et al., 1999) and immunopurified epitope-tagged AtPCS1 for the heavy metal-activated synthesis of PCs from GSH in vitro (Vatamaniuk et al., 1999), so establishing that AtPCS1 and SpPCS, and by implication their equivalents from other sources, are not only necessary but also sufficient for PC biosynthesis. (2) Empirical verification that PC synthase is a dipeptidyl transferase that catalyzes the net transfer of a γ-Glu-Cys unit from one thiol peptide, GSH, or a previously synthesized PC to another or to a previously synthesized PC molecule to mediate chain extension in the N-to-C direction according to the general equation (γEC)nG + (γEC)mG→(γEC)n + 1G + (γEC)m − 1G (Vatamaniuk et al., 2004). (3) Recognition that, whereas PC synthase is able to bind heavy metals stoichiometrically, direct metal binding to the enzyme is not essential for core catalysis (Vatamaniuk et al., 2000). The kinetics of PC synthesis approximate a substituted enzyme mechanism in which micromolar concentrations of heavy-metal GSH thiolate, for instance, bis(glutathionato)cadmium (Cd.GS2), and millimolar concentrations of free GSH act, respectively, as low- and high-affinity cosubstrates. As indicated by the facility of PC synthase for the net synthesis of S-alkyl-PCs from S-alkylglutathiones in media devoid of metals, heavy metals are dispensable for catalysis, providing that the thiol groups on at least one of the substrates are blocked (Vatamaniuk et al., 2000). (4) Demonstration that the peptide fragment encompassing only the more sequence-conserved N-terminal half of the AtPCS1 polypeptide is sufficient for Cd2+-activated PC synthesis (Ruotolo et al., 2004), so establishing that this portion of the enzyme is responsible for core catalysis. (5) Isolation of a γ-Glu-Cys acyl-enzyme intermediate formed during catalysis by PC synthase and the tentative identification of one of the two sites of modification as a γ-Glu-Cys thioester of AtPCS1 Cys-56 by site-directed mutagenesis (Vatamaniuk et al., 2004). It is in light of these advances that it has been speculated that PC synthases catalyze a Cys protease-like reaction in which AtPCS1 Cys-56 and its equivalent in other members of this transpeptidase family satisfy the requirements of an active-site residue responsible for initial nucleophilic attack on the scissile bond of the GSH or PC γ-Glu-Cys donor such that PC synthase-mediated catalysis is initiated by the formation of a high-energy thioester intermediate (Rea et al., 2004; Vatamaniuk et al., 2004).
Although all studies of PC synthases have until very recently been concerned exclusively with the enzymes from eukaryotes, primarily those of the organisms in which PCs were first discovered (Kondo et al., 1984; Grill et al., 1989), systematic database searches not only disclose PC synthase sequences in plants and some other eukaryotes but also in some prokaryotes (e.g. Rea et al., 2004, and refs. therein). This in itself may not be surprising, except that all of the putative prokaryotic PC synthase homologs identified are one-half the length of their cognates from eukaryotes (220–237 compared to 421–506 residues) because they lack the more sequence-variable C-terminal domain. Crucial, therefore, is the finding, in agreement with the results obtained with N-terminal AtPCS1 peptide fragments (Ruotolo et al., 2004), that the one prokaryotic PC synthase homolog that has been assayed for activity, the alr0975 protein from Nostoc sp. PCC 7120 (NsPCS), bears a functional resemblance to its eukaryotic homologs. Despite its relatively low (22%–36%) overall sequence identity with the N-terminal domains of the eukaryotic enzymes, NsPCS catalyzes the deglycylation of GSH to γ-Glu-Cys at a high rate and the synthesis of PC2, albeit at a relatively low rate (Tsuji et al., 2004, 2005) and possibly only in vitro (Harada et al., 2004).
In this article, further insights into the catalytic mechanism of eukaryotic PC synthases are presented. Not only is it shown, in agreement with the findings of Ruotolo et al. (2004), that the N-terminal domain of AtPCS1 is sufficient for the Cd2+-dependent synthesis of PCs from GSH, but also that this domain, which encompasses Cys-56, undergoes Cd2+-independent γ-Glu-Cys acylation. Critically, however, this domain is not sufficient for Cd2+-dependent γ-Glu-Cys acylation at the second site or for the stimulation of S-methyl-PC synthesis from S-methylglutathione by micromolar concentrations of free Cd2+. These reactions require a contiguous C-terminal domain. Given the demonstrated dispensability of the C-terminal domain for core catalysis and single-site acylation, the distant homology between PC synthase family members and papain-like Cys proteases inferred from scanning the N-terminal sequences of the former for matches with the latter, the preliminary results of which were presented in Rea et al. (2004), is validated, which in turn substantiates the identification of AtPCS1 His-162, Asp-180, as well as Cys-56 as candidate residues of a Cys protease-like catalytic triad. This conclusion is verified by the results presented here showing the complete loss of function associated with substitution of any one of these residues. These findings complement, substantiate, and extend the seminal work by another group on the crystal structure of the cyanobacterial PC synthase homolog, NsPCS, in its unsubstituted and γ-Glu-Cys-acylated states (Vivares et al., 2005; for review, see Rea, 2006), while establishing that the C-terminal domain that distinguishes eukaryotic from prokaryotic PC synthases is necessary for two processes that are not essential for core catalysis, augmentative free Cd2+ sensing and γ-Glu-Cys acylation of the full-length enzyme at a second site.
RESULTS
Sufficiency of N-Terminal Domain for Core Catalysis and First-Site Acylation But Not for Direct Metal Sensing and Second-Site Acylation
Previous investigations have established that when native AtPCS1 is subjected to limited proteolysis with V8 protease, two N-terminal fragments ending at positions 372 and 283 are generated, both of which are competent in PC synthesis from GSH in the presence of Cd2+ at rates only about 5-fold lower than those of the full-length polypeptide (Ruotolo et al., 2004). Incisive as these investigations were in demonstrating the sufficiency of the N-terminal domain for catalysis, they did not address two other characteristics of the full-length enzyme. These are its susceptibility to direct augmentative stimulation by free Cd2+ (Vatamaniuk et al., 2000) and amenability to γ-Glu-Cys acylation at two sites when incubated in media containing both GSH and Cd2+ (Vatamaniuk et al., 2004). The key questions that remain are whether the N-terminal and/or C-terminal domains participate in metal sensing and/or are required for γ-Glu-Cys acylation at two sites.
To address these questions, full-length and C-terminally truncated N-terminally hexahistidinyl-tagged derivatives of AtPCS1, HIS-AtPCS1 and HIS-AtPCS1_221tr, respectively, were heterologously expressed in E. coli strain BL21 and purified by nickel nitrolotriacetic acid agarose (Ni-NTA) metal chelate chromatography. SDS-PAGE of the soluble extracts from cells expressing HIS-AtPCS1 (theoretical molecular mass 55.4 kD) and HIS-AtPCS1_221tr (theoretical molecular mass 25.8 kD) confirms that this procedure yields a single dominant 59,000-Mr band from the former and a single dominant 23,000-Mr band from the latter (Fig. 1). Moreover, as would be predicted for the N-terminal domain encompassed by HIS-AtPCS1_221tr, which was designed to span only the most sequence-conserved N-terminal residues of AtPCS1 (sufficient for core catalysis), the overall synthetic activities of this polypeptide and those of HIS-AtPCS1 are similar. When measured in standard PC synthase assay buffer containing 3.3 mm GSH and 50 μm CdCl2, or 3.3 mm S-methylglutathione alone, purified HIS-AtPCS1 is competent in the synthesis of PCs 2, 3, and 4 and S-methyl-PCs 2, 3, and 4 at aggregate rates of 14.8 ± 0.9 and 8.7 ± 0.4 μmol mg−1 min−1, respectively (Table I). The corresponding values for HIS-AtPCS1_221tr, which is also competent in the synthesis of PCs and S-methyl-PCs 2, 3, and 4, are 5.1 ± 0.7 and 6.4 ± 0.9 μmol mg−1 min−1, respectively (Table I). Because the properties of N-terminally hexahistidinyl-tagged full-length and truncated AtPCS1 after heterologous expression in E. coli closely approximate those of full-length C-terminally FLAG-tagged AtPCS1 after heterologous expression in yeast (Vatamaniuk et al., 2000, 2004), the former are suitable for probing the roles of the N-terminal and C-terminal domains in metal sensing and enzyme acylation.
Figure 1.
Purification of HIS-AtPCS1 and HIS-AtPCS1_221tr. Aliquots of the soluble fractions from crude lysates of E. coli BL21 (DE3) cells expressing HIS-AtPCS1 or HIS-AtPCS1_221tr before (C1 and C2, respectively; 20 μg protein) and after purification by Ni-NTA metal chelate chromatography (P1 and P2, respectively; 3 μg protein) were subjected to SDS-PAGE as described in “Materials and Methods.” The positions of the Mr markers (Mr × 103) are indicated.
Table I.
PC and S-methyl-PC synthetic activities and stoichiometries of γ-Glu-Cys acylation of purified HIS-AtPCS1 and HIS-AtPCS1_221tr
PC synthesis and S-methyl-PC synthesis were measured by incubating affinity-purified HIS-AtPCS1 or HIS-AtPCS1_221tr with GSH (3.3 mm) and CdCl2 (50 μm) or with S-methylglutathione (3 mm) in 100 mm HEPES-BTP buffer, pH 8.0, at 30°C for 10 min. The PCs or S-methyl-PCs generated were separated by HPLC and quantitated as described in “Materials and Methods.” Acylation of purified HIS-AtPCS1 or HIS-AtPCS1_221tr by GSH was assayed by incubating purified HIS-AtPCS1 or HIS-AtPCS1_221tr with [35S]Cys-labeled GSH (3 mm) in 100 mm HEPES-BTP buffer, pH 8.0, with or without CdCl2 (50 μm) for 15 min before terminating the reaction and measuring enzyme radioincorporation as described in “Materials and Methods.” Values shown are means ± se (n = 3–5).
| HIS-AtPCS1 | HIS-AtPCS1_221tr | |
|---|---|---|
| Catalytic Activity | μmol mg−1 min−1 | |
| PC synthesis | 14.8 ± 0.9 | 5.1 ± 0.7 |
| S-methyl-PC synthesis | 8.7 ± 0.4 | 6.4 ± 0.9 |
| γ-Glu-Cys Acylation | mol γ-Glu-Cys/mol polypeptide | |
| −Cd2+ | 1.1 ± 0.1 | 0.6 ± 0.1 |
| +Cd2+ | 1.7 ± 0.1 | 0.7 ± 0.1 |
At one level, HIS-AtPCS1_221tr's facility for the synthesis of S-methyl-PCs in the complete absence of heavy metals demonstrates that the C-terminal domain does not participate directly in core catalysis when the need for metal thiolates is obviated through the provision of S-alkylated substrate. At another level, the sufficiency of S-alkylglutathiones as substrates despite their inability to form metal thiolates offers the means of monitoring the effects of free heavy-metal ions on PC synthase activity through their interaction with the enzyme without the complications that attend heavy-metal-substrate thiol interactions. Specifically, the necessity or otherwise of the C-terminal domain for the interaction of Cd2+ with the enzyme and modulation of catalytic activity can be probed by measuring the initial rates of HIS-AtPCS1- and HIS-AtPCS1_221tr-catalyzed S-methyl-PC2 synthesis from S-methylglutathione in reaction media containing different concentrations of this metal ion.
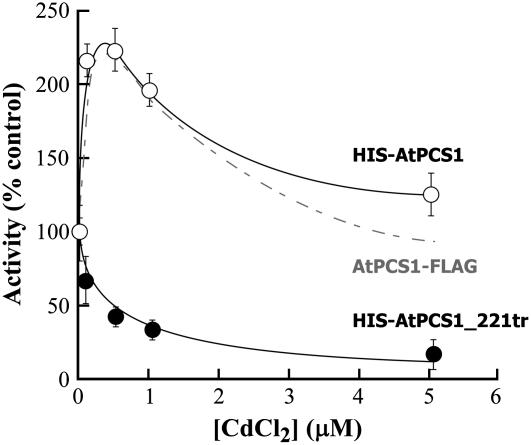
These manipulations demonstrated that the C-terminal domain, but not the N-terminal domain, is required for the augmentation of synthetic activity by free Cd2+. Whereas the S-methyl-PC2 synthetic activity of HIS-AtPCS1 is more than 2-fold stimulated by 0.25 to 1.00 μm Cd2+, the corresponding activity of HIS-AtPCS1_221tr is not stimulated but instead increasingly inhibited by increasing concentrations of this metal ion (Fig. 2). Evidently, direct metal binding to the N-terminal domain inhibits S-methyl-PC2 synthesis by both HIS-AtPCS1 and HIS-AtPCS1_221tr, but in the former, unlike the latter, metal binding to the C-terminal domain in the 0.25 to 1.00 μm range is more than sufficient to counteract the inhibition that would otherwise be seen. It is important to note that the Cd2+ activation and inhibition profiles reported here are not biased by the hexahistidinyl tag because the activation profile of HIS-AtPCS1 closely approximates that of purified C-terminally FLAG-tagged AtPCS1 (Fig. 2) and both are maximally stimulated by 0.5 μm Cd2+, a concentration commensurate with the ligand-binding constant for direct binding of this metal to the enzyme (Vatamaniuk et al., 2000). These findings reinforce the contention that the N-terminal domain is activated by metal thiolates (Cd.GS2 in this case), not by free metal ions, while at the same time demonstrating that, although the C-terminal domain is not essential for catalysis, it does participate in auxiliary metal sensing.
Figure 2.
Effect of different concentrations of Cd2+ on HIS-AtPCS1- and HIS-AtPCS1_221tr-catalyzed synthesis of S-methyl-PC2 from S-methylglutathione. The reaction conditions were as described in Table I, except that CdCl2 was added at the concentrations indicated. The values shown (±se for n = 3–5) are percentage activities versus purified enzyme assayed in reaction media lacking Cd2+. For comparison, the Cd2+ activation profile for S-methyl-PC2 synthesis by purified AtPCS1-FLAG (dashed line) is also shown (from Vatamaniuk et al., 2000).
The results of direct comparisons of the stoichiometries of γ-Glu-Cys acylation of HIS-AtPCS1 and HIS-AtPCS1_221tr by GSH in the absence and presence of Cd2+ also simplify considerations of the core catalytic mechanism. HIS-AtPCS1 undergoes acylation by 3 mm [35S]Cys-labeled GSH at a stoichiometry of approximately 1 (1.08 ± 0.070 mol γ-Glu-Cys/mol polypeptide) and a stoichiometry nearer 2 (1.66 ± 0.11 mol γ-Glu-Cys/mol polypeptide) when 50 μm Cd2+ is added to the reaction medium (Table I), as is the case for AtPCS1-FLAG (Vatamaniuk et al., 2004). HIS-AtPCS1_221tr, on the other hand, is acylated at only one site regardless of whether Cd2+ is added to the reaction medium. When incubated in media containing [35S]Cys-labeled GSH alone or [35S]Cys-labeled GSH plus Cd2+, HIS-AtPCS1_221tr is labeled at a stoichiometry of 0.59 ± 0.11 and 0.66 ± 0.06 mol γ-Glu-Cys/mol polypeptide, respectively (Table I). It is therefore apparent that, because HIS-AtPCS1_221tr is competent in PC synthesis, Cd2+-independent γ-Glu-Cys acylation at a single site on the N-terminal domain is sufficient for core catalysis.
Identification of Second and Third Residues of a Putative Papain-Like Catalytic Triad
Having determined the sufficiency of HIS-AtPCS1_221tr for the synthesis of PCs and S-methyl PCs from GSH and S-methylglutathione, respectively, the necessity of γ-Glu-Cys acylation at only one site for PC synthesis, and the dispensability of the C-terminal half of AtPCS1, except for augmentative metal sensing and secondary γ-Glu-Cys acylation, it is reasonable to assume that it is the more sequence-conserved N-terminal half of eukaryotic PC synthases that is exclusively responsible for core catalysis. Hence, to maximize the validity of insights into the structure-function requirements for core catalysis gained from the application of sensitive methods capable of detecting homologies between members of the PC synthase family and other proteins of known structure on the basis of sequence and protein fold recognition, the analyses were directed at the first 221 n-terminal residues of the enzyme.
Despite the inability of standard PSI-BLAST (Altschul et al., 1997) and many of the fold recognition methods implemented at the META server (Bujnicki et al., 2001a) to detect distant PC synthase homologs, regardless of the span or origin of the sequences submitted, the FFAS03 method for sequence profile matching, a high-sensitivity method whose sole input is sequence information (Rychlewski et al., 2000), consistently yielded matches with scores of up to −7.4 for Clan CA Cys proteases (Barrett and Rawlings, 2001; Rawlings et al., 2004). FFAS03 is a method for the detection of distant homologies wherein a PSI-BLAST profile (alignment) for the target protein is first generated by database searching and similar profiles are precalculated for all of the proteins of known structure identified in the searches. Using this input, FFASO3 then aligns the target profile with each of those derived from the proteins of known structure, while optimizing the scoring function for sensitivity and reliability (Rychlewski et al., 2000). When applied to the extended PC synthase family, this algorithm reveals clear matches with Clan CA Cys proteases as exemplified by the archetype papain from Carica papaya fruit and latex; staphopain A, an extracellular enzyme from Staphylococcus aureus; and several of the lysosomal cathepsins, for instance, cruzain, from animal sources (Fig. 3).
Figure 3.
Sequence alignment of six maximally divergent representatives of the PC synthase family, AtPCS1, A. yokoscense PCS1 (AyPCS1), CePCS1, SpPCS, NsPCS, and M. degradans PC synthase homolog (shown with standard gene product acronym or species name and GenBank ID) with three papain superfamily Cys proteases, papain, cruzain, and staphopain (shown with PDB code). The Cys proteases are shown in structural alignment. Within the groups of Cys protease and PC synthase family members, highly conserved positions are shown in bold type and invariant residues are shown on a gray background. Positions conserved across all of the sequences are shown in white type on a black background. The amino acid residue numbering and actual secondary structure for staphopain A are shown above the alignment. The residue numbering and predicted secondary structure for AtPCS1 are shown below the alignment. Cylinders, α-helix; arrows, β-sheet. Significant structural insertions present in papain and cruzain, versus staphopain A, are shown as X, where X is the insertion length (individual residues not shown). Shown here is the complete alignment of which portions were depicted schematically in Figure 2 of Rea et al. (2004).
Whereas caution might be warranted in that three higher FFAS03 scores have been reported for false positives in the latest complete LiveBench study (http://bioinfo.pl/LiveBench; Bujniski et al., 2001b), the notion of a distant homology between PC synthases and Clan CA Cys proteases is reinforced by several independent findings and considerations. First, there is a fundamental catalytic equivalence between PC synthases, dipeptidyl transferases, and Cys proteases in terms of the partial reactions they catalyze. Second, both classes of enzyme have a strict requirement for a nucleophilic Cys residue that undergoes peptidyl acylation during catalysis. In AtPCS1, this residue corresponds to Cys-56 (Vatamaniuk et al., 2004); in staphopain, it corresponds to Cys-24 (Barrett and Rawlings, 2001). Third, the nucleophilicity of the active-site Cys residues of Cys proteases is contingent on their immediate proximity to a His residue in the native enzyme, and it is the Cys and His residues of the papain-like peptidases, residues 24 and 120 in staphopain A, that precisely align with the conserved Cys and His residues of PC synthases, residues 56 and 162, respectively, in AtPCS1 (Fig. 3). Fourth, the third Cys protease catalytic triad residue, an Asn at position 141 in staphopain A, aligns with a conserved Asp residue in the PC synthase family, residue 180 in AtPCS1 (Fig. 3). The relevance of this alignment is that replacement of the triad Asn in proteases with an Asp in PC synthases is in principle acceptable functionally because the third catalytic residue can, as is the case for several members of Clan CA (Barrett and Rawlings, 2001), be either Asp or Asn. In short, the key residues conserved in the papains and PC synthases are in register and in the same absolute order in both sets of sequences. A subsidiary observation in support of the alignments, admittedly a less robust one, is the approximate agreement between the known secondary structure of Clan CA peptidases and the predicted structure of PC synthases in the corresponding regions in terms of the distribution and spans of α-helix, β-sheet, and random coil (Fig. 3).
Necessity of Cys-56, His-162, and Asp-180, But None of the Other Potentially Nucleophilic N-Terminal Domain Residues, for Core Catalysis
To test the hypothesis that Cys-56, His-162, and/or Asp-180 of AtPCS1 and, by implication, their equivalents in other PC synthases might form a papain-like peptidase catalytic triad, these residues were subjected to substitution by site-directed mutagenesis. So as not to overlook other conserved Cys, His, or Asp residues that might participate in catalysis and/or other residues whose side chains have the potential for forming oxyanions capable of initiating nucleophilic attack on peptidyl carbonyl atoms, not only Cys-56, His-162, and Asp-180, but also all of the other Cys, His, and Asp residues plus all of the Ser, Thr, and Tyr residues in the N-terminal catalytic half of AtPCS1 that are conserved among all or most of the sequenced eukaryotic PC synthases, were singly substituted. The Cys residues were substituted with Ser or Ala; all of the other residues were substituted with Ala. To assess the necessity of one or more of these residues for catalysis, the Cd2+-hypersensitive yeast ycf1Δ strain DTY167 was transformed with pYES3-AtPCS1∷FLAG constructs containing wild-type or mutant AtPCS1 and screened for suppression of the Cd2+-hypersensitive phenotype (Vatamaniuk et al., 2000, 2004). In those cases in which Cd2+ tolerance was not conferred by the heterologous expression of AtPCS1-FLAG, whole-cell soluble extracts were prepared from the transformants and assayed for PC synthesis in vitro to determine whether an impairment of catalytic activity was the cause.
The results of these manipulations were in remarkably close agreement with the predictions from FFAS03-based sequence profile matching. Of a total of 19 residues that were substituted, only three—Cys-56, His-162, and Asp-180—are essential for catalysis (Figs. 4 and 5). None of the Cys-to-Ser (or Ala), Ser-to-Ala, Thr-to-Ala, or Tyr-to-Ala substitutions, except for the C56S (and C56A) substitutions described previously (Vatamaniuk et al., 2004), decrease the capacity of heterologously expressed AtPCS1-FLAG to confer Cd2+ tolerance and abolish its facility for yielding cell-free extracts competent in the net synthesis of PCs from GSH (Figs. 4 and 5). C90S, C91S, C109S, C113S, S21A, S164A, T158A, Y7A, and Y186A mutant AtPCS1-FLAG confer similar degrees of Cd2+ tolerance on DTY167 cells as their wild-type equivalent (Figs. 4 and 5), and in those cases examined—namely, the Cys mutant series for which there was one that did not confer Cd2+ tolerance—the facility for conferring Cd2+ tolerance was associated with near-wild-type Cd2+-dependent in vitro PC synthetic activities in cell-free extracts prepared from the transformants (Fig. 5). In marked contrast, C56S-mutated AtPCS1-FLAG confers a degree of Cd2+ tolerance only marginally greater than that of empty-vector pYES3 controls, and cell-free extracts prepared from these transformants exhibit negligible PC synthetic activities (Fig. 5). It is notable that substitution of each of the five conserved Cys residues in the N-terminal domain with Ser or Ala yields the same results (data not shown). This implies that seryl hydroxyl groups cannot substitute for cysteinyl sulfhydryl groups at the same positions.
Figure 4.
Effect of heterologously expressed wild-type and Ser-to-Ala, Thr-to-Ala, or Tyr-to-Ala substituted AtPCS1-FLAG on the Cd2+ hypersensitivity of mutant Saccharomyces cerevisiae ycf1Δ strain DTY167. Strain DTY167 was transformed with pYES3-AtPCS1∷FLAG vector encoding wild-type AtPCS1-FLAG (○), or mutant AtPCS1-FLAG in which the Ser residues at positions 21 (□) or 164 (▪), Thr residue at position 158 (⋄), or Tyr residues at positions 7 (♦) or 186 (▵) were substituted with Ala. Strain DTY167 transformed with empty-vector pYES3 (•) was employed as the negative control. To assess Cd2+ hypersensitivity, the transformants were grown at 30°C to an OD600 nm of approximately 1.8 in AHC medium supplemented with Glc and Trp before inoculating aliquots into 2-mL volumes of the same medium containing the indicated concentrations of CdCl2. OD600 nm was measured after growth for 24 h. Values shown are means ± se (n = 3–5).
Figure 5.
Effect of heterologously expressed wild-type and singly Cys-, His-, or Asp-substituted AtPCS1-FLAG on the Cd2+ hypersensitivity (top) and PC synthase activity (bottom) of S. cerevisiae ycf1Δ mutant strain DTY167. Strain DTY167 was transformed with pYES3-AtPCS1∷FLAG vector encoding wild-type AtPCS1-FLAG (○), or mutant AtPCS1-FLAG in which the conserved Cys residues at positions 56 (□), 90 (▪), 91 (⋄), 109 (♦), or 113 (▵) had been substituted with Ser residues, or the His residues at positions 162 (□), 189 (▪), or 220 (⋄), or the Asp residues at positions 71 (□), 84 (▪), 89 (⋄), 174 (♦), 180 (▵), or 204 (▴) had been substituted with Ala residues. Strain DTY167 transformed with empty-vector pYES3 (•) was employed as the negative control. Cd2+ hypersensitivity was assessed as described in the legend to Figure 4. PC synthase activity was assessed by assaying aliquots of the soluble fractions from the transformants for Cd2+-dependent PC synthesis from GSH in media containing 3.3 mm GSH, 50 μm CdCl2, and 100 mm BTP-HEPES buffer, pH 8.0, at 30°C for 10 min before the fractionation and quantitation of PCs by RP-HPLC and reaction with DTNB, respectively. Values shown are means ± se (n = 3). Also shown are the results or SDS-PAGE and western analysis of wild-type and mutant AtPCS1-FLAG after heterologous expression. Aliquots (20 μg) of the soluble fractions from the transformants were electrophoresed, electrotransferred to nitrocellulose membranes, and probed with anti-FLAG M2 monoclonal antibody. The 58,000-Mr polypeptide was the only anti-FLAG immunoreactive band detected in the extracts.
The same basic pattern was seen for the His and Asp mutants. Of the three His residues that were substituted with Ala—His-162, which is fully conserved, and His-189 and His-220, which are conserved between AtPCS1 and CePCS1 but not SpPCS1—only His-162 is essential for Cd2+ tolerance and PC synthetic activity (Fig. 5). Whereas the effects at the level of both Cd2+ tolerance and enzyme activity of H162A-mutated pYES3-AtPCS1∷FLAG are indistinguishable from those of empty-vector pYES3, the Cd2+ tolerance conferred by H189A- or H220A-mutated pYES3-AtPCS1∷FLAG is slightly enhanced versus wild type, and catalytic activity is diminished by only 75% and 49%, respectively (Fig. 5). Likewise, of the six Asp substitutions, D71A, D84A, D89A, D174A, D180A, and D204A, only one (the one at position 180) abolishes Cd2+ tolerance and PC synthetic activity (Fig. 5). All of the Asp substitutions, except D180A, confer Cd2+ tolerance and PC synthetic activity, albeit at rates 80%, 14%, 43%, 81%, and 85% lower than wild type for D71A, D84A, D89A, D174A, and D204A, respectively (Fig. 5). There is, of course, a possibility that Asp-71, Asp-174, and/or Asp-204 contribute to catalysis or secondarily influence the scaffolding of the active site because of their charge characteristics, hence the attenuations of activity associated with their substitution, but they, unlike Asp-180, do not appear to be essential for catalysis.
The near-complete losses of activity associated with the C56S (or C56A), H162A, and D180A substitutions are not attributable to a decrease in the amount, stability, or integrity of the translation product. The transformants containing these mutant forms of pYES3-AtPCS1∷FLAG contain wild-type levels of the FLAG-tagged 58,000-Mr polypeptide as determined by SDS-PAGE and western analysis of whole-cell extracts with anti-FLAG M2 antibody (Fig. 5). In no case is there a change in the electrophoretic mobility of the anti-FLAG antibody-reactive band (Fig. 5), implying that mutagenesis does not impair biogenesis or confer increased susceptibility to partial proteolysis. As judged by the ease with which all of the mutant forms of AtPCS1-FLAG can be sampled from the cell-free extracts for such analyses without differential losses, appreciable changes in protein solubility are not responsible for the decreases in enzymic activity detected.
DISCUSSION
Assignment of eukaryotic PC synthases and their prokaryotic homologs to the papain Cys protease family, the first transpeptidases to be assigned to this family, and conservation of the catalytic triad of the latter in the former is in complete agreement with the crystal structure of NsPCS (Vivares et al., 2005; Rea, 2006). The results of the mutagenic analyses based on the FFAS03-generated alignments (Fig. 3) presented previously as preliminary results (Rea et al., 2004) and here in detail identify essential residues Cys-56, His-162, and Asp-180 (Fig. 5) as the catalytic triad of AtPCS1. The results of the independent crystallographic analyses of NsPCS performed by Vivares et al. (2005) identify the equivalent triad residues, Cys-70, His-183, and Asp-201, also tentatively identified as such by the FFAS03 method (Fig. 3), in the active site of the prokaryotic PC synthase homolog.
Implicit in the finding that the time and concentration dependence of PC synthesis approximates substituted-enzyme kinetics (Vatamaniuk et al., 2000) and that AtPCS1 forms a γ-Glu-Cys acyl intermediate during catalysis (Vatamaniuk et al., 2004) is the principle that initiation of the overall biosynthetic reaction is by cleavage of the Cys-Gly bond of the first substrate. Pivotal, therefore, is the convergence of two basic findings. The first is the crystal structure of the γ-Glu-Cys-acylated derivative of NsPCS, generated by crystallizing the enzyme in acidic media containing GSH, which unequivocally demonstrates acylation at a single site, the NsPCS equivalent of AtPCS1 Cys-56, Cys-70 (Vivares et al., 2005). The second is the demonstration that acylation of the HIS-AtPCS1_221tr truncate is at a single site regardless of whether or not Cd2+ is included in the reaction medium, whereas acylation of full-length HIS-AtPCS1 is at one site in the absence and at two sites in the presence of Cd2+ (Table I). These findings simplify considerations of the mechanism of core catalysis. They establish that the Cd2+-independent γ-Glu-Cys acylation of Cys-56 on the N-terminal domain of AtPCS1 is sufficient for core catalysis, thus establishing a strict parallelism with the Cd2+-independent acylation of NsPCS (Vivares et al., 2005) and presumably other prokaryotic PC synthases (Fig. 6). They provide compelling evidence to indicate that Cd2+-dependent acylation of AtPCS1 and presumably other eukaryotic PC synthases, which is not essential for core catalysis, is at a second site encompassed by the C-terminal domain or at a second site on the N-terminal domain whose acylation depends on the C-terminal domain (Fig. 6). When account is taken of the susceptibility of HIS-AtPCS1_221tr to inhibition rather than stimulation by free Cd2+ when synthesizing S-methyl-PCs from S-methylglutathione, under conditions in which the need for the metal thiolate Cd.GS2 as a substrate is obviated (Fig. 2), it is evident that the C-terminal domain of AtPCS1 participates directly in metal sensing associated with the augmentation of PC synthesis from GSH derivatives containing blocked thiols (Fig. 6).
Figure 6.
Schematic diagram depicting full-length AtPCS1 and its C-terminally truncated derivative, AtPCS1_221tr, and the reactions they catalyze. The approximate positions of all of the Cys residues are indicated by white vertical bars and of the conserved His and Asp residues in the N-terminal domain (dark gray) by blue and red bars, respectively. AtPCS1 residues Cys-56, His-162, and Asp-180, which when mutated abolish core catalysis, are the three residues that are conserved in all known PC synthases and align with the catalytic residues of NsPCS and members of the papain family of Cys proteases. Numbers on right denote total number of residues in each polypeptide. Full-length AtPCS1 undergoes γ-Glu-Cys acylation at two sites. Acylation at the first site is on Cys-56 in the N-terminal domain. Acylation at the second site is either within the C-terminal domain (light gray) as depicted or at a site within the N-terminal domain whose acylation depends on the C-terminal domain. Free Cd2+ stimulates the activity of full-length AtPCS1 by directly interacting with the C-terminal domain. Both full-length AtPCS1 and AtPCS1_221tr are competent in core catalysis, the synthesis of PCs and S-alkyl-PCs from GSH and Cd.GS2 and from S-alkylglutathione, respectively, where R = H+ or alkyl group and R′ = Cd or alkyl group (shown in black type). Full-length AtPCS1, unlike AtPCS1_221tr, mediates an auxiliary catalytic reaction (shown in gray) in which heavy metals, such as Cd2+, bind to the C-terminal domain to promote acylation at the second site and accelerate catalysis.
A reaction scheme capable of accounting for these findings, in conjunction with the necessity of His-162 and Asp-180, as well as Cys-56, for the activity of AtPCS1, the crystallographic data for NsPCS (Vivares et al., 2005), and the requirement that at least one of the thiol groups on one of the substrates must be blocked through metal thiolation or S-alkylation for the reaction to proceed to completion, is a revision and extension of the scheme presented previously (Rea et al., 2004). According to this scheme, the N-terminal domain of AtPCS1 catalyzes a dipeptidyl transpeptidation in the first phase of the reaction in which the γ-Glu-Cys donor, GSH or a PC, acylates the enzyme concomitant with the release of Gly (Fig. 6). By analogy with papain, nucleophilic attack on the scissile bond of the first substrate, the donor, by a Cys-derived thiolate anion, specifically that of Cys-56 in AtPCS1, and formation of a γ-Glu-Cys thioester, concomitant with the liberation of substrate Gly, is invoked. As in Cys proteases in general, and as stated in Rea et al. (2004) and amplified and refined by Vivares et al. (2005), Cys-56 and His-162, because of their immediate adjacency to each other and proximity to Asp-180 in the active site, would be expected to form a thiolate-imidazolium ion pair stabilized by the carboxylate group of the latter. Because both NsPCS and AtPCS1 are susceptible to acylation at this site in media devoid of heavy metals and the latter is competent in the synthesis of S-alkyl-PCs from S-alkylglutathione alone (Vatamaniuk et al., 2000), heavy-metal substrate complexes, for instance, Cd.GS2, are not required for this reaction (Fig. 6). In the next phase of the reaction, the Gly released from the first substrate is replaced by a second substrate. In the simplest case, the deglycylation of GSH or PCs, the predominant reaction catalyzed by NsPCS (Harada et al., 2004; Tsuji et al., 2004, 2005) and a major side reaction of AtPCS1 (Vatamaniuk et al., 2004), the second substrate is the solvent, water. Enhancement of the nucleophilicity of water consequent on the immediate proximity of His-162 and Asp-180 in the active site would serve to promote attack on the enzyme thioester bond concomitant with the liberation of free γ-Glu-Cys when the first substrate is GSH or a des(Gly)PCn, a simple (γ-Glu-Cys)n polymer, when the first substrate is a PC (PCn). In the case of net PC synthesis, the nucleophilicity of the N terminus of the acceptor GSH or PCn is considered to be enhanced by reconstitution of the thiolate-imidazolium ion pair upon cleavage of the thioester intermediate concomitant with the formation of a new peptide bond. The dissociation of PC2 or PCn + 1 from the enzyme and its replacement by a new molecule of donor GSH or PC then completes the catalytic cycle. Because appreciable net PC synthesis by AtPCS1 is contingent on the provision of S-alkylglutathione or Cd.GS2, this is probably the step that has an obligate or near-obligate requirement for substrate containing a blocked thiol group (Fig. 6).
Heavy metals are not crucial for core catalysis by the N-terminal domain of eukaryotic PC synthases other than through substrate thiolate formation (Vatamaniuk et al., 2000, 2004), but they are capable of augmenting synthetic activity in the presence of substrate-active S-alkyl derivatives. This they do predominantly, or exclusively, as indicated by the properties of HIS-AtPCS1_221tr (Fig. 2), through direct interaction with the C-terminal domain. The significance of the acylation of AtPCS1 at the second site consequent on the addition of Cd2+ to media containing GSH is not known, but it is not essential because its ablation (Fig. 1C) does not abolish core catalysis (Figs. 2 and 6).
In assigning a papain-like fold to PC synthases, it is also intriguing to note that it also offers fresh insight into the identity of subsidiary catalytic residues, specifically AtPCS1 residue Gln-50, the equivalent of staphopain residue Gln-18 (Fig. 3) and NsPCS residue Gln-64 (Vivares et al., 2005), in stabilization of the acyl intermediate. The side chain of Gln-18 in the papain family, along with the main chain of the catalytic Cys residue, is responsible for stabilizing the tetrahedral oxyanion transition state of the substrate during the acylation and deacylation reactions (Menard et al., 1991), a function that is served by NsPCS Gln-64 (Vivares et al., 2005) and presumably by AtPCS1 Gln-50.
The key difference between the catalytic mechanism of eukaryotic PC synthases and those of their prokaryotic counterparts and fellow papain superfamily members is that during PC synthesis nucleophilic attack on the thioester intermediate is not by water but instead by a second thiol derivative, thus resulting in transpeptidation rather than net hydrolysis. With respect to the role played by the prokaryotic homologs, it is likely, when account is taken of the overall degree of sequence similarity in the PC synthase family in combination with conservation of the catalytic triad, that, even if the bacterial enzymes are not true PC synthases (Harada et al., 2004), their substrates resemble those of their eukaryotic relatives. One possibility other than PC synthesis per se is that the bacterial enzymes serve to cleave GS conjugates. Knowing that GS-conjugate cleavage is a major auxiliary capability of eukaryotic PC synthases (Beck et al., 2003; Vatamaniuk et al., 2004), pathways for the processing of bacterial GS conjugates entailing the same deglycylation reaction as that catalyzed by eukaryotic PC synthases (Beck et al., 2003; Vatamaniuk et al., 2004) and proposed to function in the detoxification of GS-conjugable xenobiotics in plants (Beck et al., 2003) might be envisaged. The properties of NsPCS (Harada et al., 2004; Tsuji et al., 2004, 2005) are consistent with this and with the notion that the prokaryotic enzymes are more rudimentary, more peptidase-like than transpeptidase-like, in their action.
With respect to eukaryotic PC synthases, this difference from their prokaryotic homologs makes it plain, given its sufficiency for PC synthesis, primary γ-Glu-Cys acylation, and interaction with substrate active substrates, that crystallographic data will be required for the N-terminal domain of this group of enzymes if the mechanistic basis of PC synthesis per se is to be determined. There must be unique features of the N-terminal domains of eukaryotic PC synthases, quite distinct from and independent of the role played by the C-terminal domains of these enzymes in metal sensing and secondary acylation, which enhance their capacity to utilize a second incoming GSH derivative, one with a blocked thiol, over water as an acceptor of the γ-Glu-Cys unit derived from the first substrate. Now that the crystal structure of NsPCS in the native and acylated condition is known (Vivares et al., 2005), the onus is to obtain three-dimensional structural information of a similar quality for one or more eukaryotic PC synthases either as C-terminal truncates or full-length molecules before and after primary acylation, ideally before and after interaction with the second substrate (Rea, 2006).
MATERIALS AND METHODS
Heterologous Expression of Full-Length and C-Terminally Truncated N-Terminally Hexahistidinyl-Tagged AtPCS1 in Escherichia coli
For the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible heterologous expression of full-length and C-terminally truncated N-terminally hexahistidinyl-tagged AtPCS1, HIS-AtPCS1, and HIS-AtPCS1_221tr, respectively, in Escherichia coli, the coding sequences for full-length and the first 221 amino acid residues of AtPCS1 were PCR amplified from the yeast-E. coli shuttle vector pYES3-AtPCS1∷FLAG (Vatamaniuk et al., 1999) and subcloned into the E. coli expression vector pET28a. The primer pairs used for the amplification of full-length and truncated AtPCS1 were 5′-dGCTATGGCTAGCTTATATCGGCGA-3′ (forward primer, Nhe1 restriction site underlined) and 5′-dCTGGATCCCTAATAGGCAGGAGC-3′ (reverse primer, BamH1 site underlined, stop codon in bold), and 5′-dGCTATGGCTAGCTTATATCGGCGA-3′ (forward primer, Nhe1 site underlined) and 5′-TTTGGATCCCTATCTGTGTGGTCTAGATATGAGC-3′ (reverse primer, BamH1 site underlined, stop codon in bold), respectively. PCR reactions were performed using proofreading Pfx platinum polymerase (Invitrogen), according to the manufacturer's recommendation, and the gel-purified amplification products were digested with Nhe1 and BamH1 for ligation into Nhe1-BamH1-digested pET28a by standard procedures. After confirming the fidelity of the constructs by sequencing, they were transformed into E. coli strain BL21 (DE3; Novagen) and selected on kanamycin plates to yield E. coli BL21-PCS transformants competent in the IPTG-inducible expression of full-length HIS-AtPCS1, and E. coli BL21-PCS_221tr transformants competent in the expression of HIS-AtPCS1_221tr.
Purification of HIS-AtPCS1 and HIS-AtPCS1_221tr
HIS-AtPCS1 and HIS-AtPCS1_221tr were purified from the E. coli BL21-PCS and BL21-PCS_221tr transformants, respectively, after overnight growth at 37°C in 5 mL Luria-Bertani medium containing 50 μg/mL kanamycin. Following subinoculation of 4-mL aliquots of the primary cultures into 100 mL of fresh Terrific Broth medium (1.2% [w/v] tryptone, 2.4% [w/v] yeast extract, 0.4% [v/v] glycerol, 72 mm KH2PO4, 17 mm K2HPO4) and incubation at 37°C to an OD600 nm of 0.5 to 0.8, the cultures were induced by the addition of 0.6 mm IPTG and incubated for a further 9 h at 18°C in the case of HIS-AtPCS1 and for a further 4 h at 30°C in the case of HIS-AtPCS1_221tr. For lysis and fractionation, the IPTG-treated cells were pelleted by centrifugation and resuspended in 7.5 mL ice-cold lysis buffer (1% [w/v] Tween, 20 mm imidazole, 10% [w/v] glycerol, 500 mm NaCl dissolved in 50 mm Tris-HCl buffer, pH 8.0) and disrupted by sonication with a probe-type sonicator (Fisher model 550 sonic dismembranator). The resulting lysate was clarified by centrifugation for 40 min at 40,000g and applied to a 0.2-mL Ni-NTA metal chelate column pre-equilibrated with lysis buffer. Elution of HIS-AtPCS1 or HIS-AtPCS1_221tr was with 1 mL of elution buffer (250 mm imidazole dissolved in lysis buffer minus Tween) after washing the column with 10 mL of 10 mm 2-mercaptoethanol dissolved in lysis buffer minus Tween. The eluates were used immediately or frozen in liquid nitrogen and stored at −80°C.
Sequence Comparisons
Sequences homologous to AtPCS1 were sought in the GenPept and Unfinished Microbial Genome Databases at the National Center for Biotechnology Information (NCBI) using BLAST and PSI-BLAST (Altschul et al., 1997), and the resulting sequence set was aligned using T-COFFEE (Notredame et al., 2000). The alignments of the six maximally disparate representatives of the PC synthase family, those from Arabidopsis (Arabidopsis thaliana; AtPCS1), Athyrium yokoscense (AyPCS1), Caenorhabditis elegans (CePCS), Schizosaccharomyces pombe (SpPCS), Nostoc (NsPCS), and Microbulbifer degradans, were visualized using Jalview (Clamp et al., 2004). ESPRIPT (Gouet et al., 1999) was used for mapping conserved sequences onto structures. The region common to all of the PC synthase homologs retrieved, corresponding to the N-terminal half of eukaryotic PC synthases, was deployed for fold recognition scans. For these, the region from diverse homologous sequences, corresponding to the N-terminal half of AtPCS1, was submitted to the META server (Bujnicki et al., 2001a), a portal to the leading fold recognition methods for consensus predictions of maximized reliability (see Bujnicki et al., 2001b, and refs. therein). The most informative results in this case came from the FFAS03 method, a sensitive sequence-only-based procedure that serves to align sequence profiles pairwise (Rychlewski et al., 2000). Secondary structures were predicted using PSI-PRED (Jones, 1999).
Site-Directed Mutagenesis of AtPCS1-FLAG
Site-directed mutagenesis of AtPCS1 was performed directly on a pYES3-AtPCS1∷FLAG vector as described (Vatamaniuk et al., 2004). In all cases, the mutagenic oligonucleotides were designed to singly substitute each conserved Cys codon with a Ser codon and each conserved Asp, His, Ser, Thr, or Tyr codon with an Ala codon in the N-terminal catalytic half of AtPCS1. Mutagenesis was confirmed by sequencing the coding sequence encompassing the mutation before yeast transformation.
Yeast Cd2+-Hypersensitivity Suppression Assays
For assays of the capacity of wild-type and mutant heterologously expressed AtPCS1-FLAG for suppressing Cd2+ hypersensitivity, Saccharomyces cerevisiae strain DTY167 (MATα ura3-52 leu2-3,-112 his-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 ycf1∷hisG), deficient in vacuolar Cd2+ sequestration (Li et al., 1997), was transformed with wild-type or mutant pYES3-AtPCS1∷FLAG and grown at 30°C to an OD600 nm of approximately 1.8 in AHC medium supplemented with Glc and Trp before inoculating aliquots into 2-mL volumes of the same medium containing different concentrations of CdCl2. OD600 nm was measured after growth of the subcultures for 24 h. To ensure that the effects seen were attributable to the AtPCS1 inserts, empty-vector (pYES3) controls were employed throughout.
Measurement of PC Synthase Activity
The PC and S-methyl-PC synthase activities of affinity-purified HIS-AtPCS1 and HIS-AtPCS1_221tr were assayed in media containing the indicated concentrations of GSH, S-methylglutathione, and/or CdCl2 as described (Vatamaniuk et al., 2000). For the quantitation of PCs, thiols were estimated spectrophotometrically in the reverse phase (RP)-HPLC-separated fractions after reaction with 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB; Vatamaniuk et al., 2000). For the estimation of S-methyl-PCs, free amino groups were estimated in the RP-HPLC-separated fractions fluorimetrically after reaction with fluorescamine (Vatamaniuk et al., 2000). For in vitro assays of PC synthetic activity and estimation of the amount of AtPCS1-FLAG translation product, soluble fractions from the yeast DTY167 transformants were prepared by the disruption and fractionation of spheroplasts by differential centrifugation, and PC synthase activity and AtPCS1-FLAG polypeptide levels were assayed as described (Vatamaniuk et al., 1999, 2000). The rates of PC synthesis from unsubstituted GSH are expressed in units of micromoles of thiol incorporated into PCs. The rates of S-methyl-PC synthesis from S-methylglutathione are expressed in units of micromoles of S-methyl-PC2 synthesized.
Measurement of Acyl-Enzyme Intermediate Formation
Enzyme acylation was monitored as the incorporation of 35S label into HIS-AtPCS1 or HIS-AtPCS1_221tr after incubation in media containing [35S]Cys-labeled GSH plus or minus 50 μm CdCl2. Radioincorporation was estimated by a modification of the membrane filter-binding procedure of Chaparian and Evans (1991) as described (Vatamaniuk et al., 2004), except that the reaction was terminated and the acyl intermediate was stabilized with 6 m guanidinium-HCl dissolved in 100 mm sodium citrate buffer, pH 3.0, instead of sodium acetate buffer, pH 4.0. The stoichiometries of γ-Glu-Cys acylation of HIS-AtPCS1 and HIS-AtPCS1_221tr are expressed as mol γ-Glu-Cys/mol HIS-AtPCS1 or HIS-AtPCS1_221tr polypeptide, respectively.
SDS-PAGE and Western Analyses
To assess the homogeneity of the purified preparations of HIS-AtPCS1 and HIS-AtPCS1_221tr, protein samples were dissolved in denaturation buffer and subjected to one-dimensional SDS-PAGE on 12% (w/v) slab gels in a Bio-Rad minigel apparatus (Kim et al., 1995). For protein detection, the gels were stained with colloidal Coomassie Blue G-250 (Amersham Biosciences). To enumerate the steady-state levels of wild-type and mutant AtPCS1-FLAG translation products in the yeast transformants, aliquots of the cell extracts were separated by SDS-PAGE and subjected to western analysis with anti-FLAG M2 antibody (Sigma-Aldrich) by standard procedures (Vatamaniuk et al., 1999). Immunoreactive bands were visualized by ECL (Amersham-Pharmacia).
Protein Estimations
Protein was estimated routinely by the dye-binding method (Bradford, 1976).
Chemicals
[35S]Cys-labeled GSH (30–40 Ci/mmol) was purchased from Perkin-Elmer Life Sciences. Unless indicated to the contrary, all other reagents were purchased from Fisher Scientific, Research Organics, or Sigma-Aldrich.
This work was supported by the National Science Foundation (NSF; grant no. MCB–0077838 to P.A.R. and a Research Experience for Undergraduates Award to A.L.), the U.S. Department of Energy (grant no. DE–FG02–91ER20055 to P.A.R.), the NSF-North Atlantic Treaty Organization (a postdoctoral fellowship to N.D.R.), and the U.S. Environmental Protection Agency (grant no. EPA–X–83220101 to J.M.J.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Philip A. Rea (parea@sas.upenn.edu).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.082131.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AJ, Rawlings ND (2001) Evolutionary lines of cysteine proteases. Biol Chem 382: 727–733 [DOI] [PubMed] [Google Scholar]
- Beck A, Lendzian K, Oven M, Christmann A, Grill E (2003) Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates. Phytochemistry 62: 423–431 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bujnicki JM, Elofsson A, Fischer D, Rychlewski L (2001. a) Structure prediction meta server. Bioinformatics 17: 750–751 [DOI] [PubMed] [Google Scholar]
- Bujnicki JM, Elofsson A, Fischer D, Rychlewski L (2001. b) LiveBench-1: continuous benchmarking of protein structure prediction servers. Protein Sci 10: 352–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparian MG, Evans DR (1991) The catalytic mechanism of the amidotransferase domain of the Syrian hamster multifunctional protein CAD. Evidence for a CAD-glutamyl covalent intermediate in the formation of carbamyl phosphate. J Biol Chem 266: 3387–3395 [PubMed] [Google Scholar]
- Clamp M, Cuff J, Searle SM, Barton GJ (2004) The Jalview Java alignment editor. Bioinformatics 20: 426–427 [DOI] [PubMed] [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI (1999) Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J 18: 3325–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Schroeder JI, Degenkolb T (2001) Caenorhabditis expresses a functional phytochelatin synthase. Eur J Biochem 268: 3640–3643 [DOI] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Gouet P, Courcelle E, Stuart DI, Metoz F (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15: 305–308 [DOI] [PubMed] [Google Scholar]
- Grill E, Löffler S, Winnacker E-L, Zenk MH (1989) Phytochelatins, the heavy metal-binding peptides of plants are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86: 6838–6842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S-B, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsbrough PB, Cobbett CS (1999) Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada E, von Roepenack-Lahaye E, Clemens S (2004) A cyanobacterial protein with similarity to phytochelatin synthases catalyzes the conversion of glutathione to γ-glutamylcysteine and lacks phytochelatin synthase activity. Phytochemistry 65: 3179–3185 [DOI] [PubMed] [Google Scholar]
- Jones DT (1999) Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 292: 195–202 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Zhen R-G, Rea PA (1995) Site-directed mutagenesis of vacuolar H+-pyrophosphatase: necessity of Cys634 for inhibition by maleimides but not catalysis. J Biol Chem 270: 2630–2635 [DOI] [PubMed] [Google Scholar]
- Kondo N, Imai K, Isobe M, Goto T, Murasugi A, Wada-Nakagawa C, Hayashi Y (1984) Cadystin A and B, major unit peptides comprising cadmium binding peptides induced in a fission yeast—separation, revision of structures and synthesis. Tetrahedron Lett 25: 3869–3872 [Google Scholar]
- Li Z-S, Lu Y-P, Thiele DJ, Rea PA (1997) A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-mediated transport of bis(glutathionato)cadmium. Proc Natl Acad Sci USA 94: 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard R, Carriere J, Laflamme P, Plouffe C, Khouri HE, Vernet T, Tessier DC, Thomas DY, Storer AC (1991) Contribution of the glutamine 19 side chain to transition-state stabilization in the oxyanion hole of papain. Biochemistry 30: 8924–8928 [DOI] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol 302: 205–217 [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Tolle DP, Barrett AJ (2004) MEROPS: the peptidase database. Nucleic Acids Res 32: D160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea PA (2006) Phytochelatin synthase, papain's cousin, in stereo. Proc Natl Acad Sci USA 103: 507–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea PA, Vatamaniuk OK, Rigden DJ (2004) Weeds, worms and more: papain's long-lost cousin, phytochelatin synthase. Plant Physiol 136: 2463–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotolo R, Peracchi A, Bolchi A, Infusini G, Amoresano A, Ottonello S (2004) Domain organization of phytochelatin synthase. Functional properties of truncated enzyme species identified by limited proteolysis. J Biol Chem 279: 14686–14693 [DOI] [PubMed] [Google Scholar]
- Rychlewski L, Jaroszewski L, Li W, Godzik A (2000) Comparison of sequence profiles. Strategies for structural predictions using sequence information. Protein Sci 9: 232–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji N, Nishikori S, Iwabe O, Matsumoto S, Shiraki K, Miyasaka H, Takagi M, Miyamoto K, Hirata K (2005) Comparative analysis of the two-step reaction catalyzed by prokaryotic and eukaryotic phytochelatin synthase by an ion-pair liquid chromatography assay. Planta 222: 181–191 [DOI] [PubMed] [Google Scholar]
- Tsuji N, Nishikori S, Iwabe O, Shiraki K, Miyasaka H, Takagi M, Hirata K, Miyamoto K (2004) Characterization of phytochelatin synthase-like protein encoded by alr0975 from a prokaryote, Nostoc sp. PCC 7120. Biochem Biophys Res Commun 315: 751–755 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Bucher EA, Rea PA (2002) Worms take the ‘phyto’ out of ‘phytochelatins’. Trends Biotechnol 20: 61–64 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Bucher EA, Ward JT, Rea PA (2001) A new pathway for heavy metal detoxification in animals: phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J Biol Chem 276: 20817–20820 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lang A, Chalasani S, Demkiv L, Rea PA (2004) Phytochelatin synthase, a dipeptidyltransferase that undergoes multisite acylation with γ-glutamycysteine during catalysis. Stoichiometric and site-directed mutagenic analysis of Arabidopsis thaliana PCS1-catalyzed phytochelatin synthesis. J Biol Chem 279: 22449–22460 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu Y-P, Rea PA (1999) AtPCS1, a phytochelatin synthase from Arabidopsis thaliana: isolation and in vitro reconstitution. Proc Natl Acad Sci USA 96: 7110–7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu Y-P, Rea PA (2000) Mechanism of heavy metal activation of phytochelatin (PC) synthase: blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. J Biol Chem 275: 31451–31459 [DOI] [PubMed] [Google Scholar]
- Vivares D, Arnoux P, Pignol D (2005) A papain-like enzyme at work: native and acyl-enzyme intermediate structures in phytochelatin synthesis. Proc Natl Acad Sci USA 102: 18848–18853 [DOI] [PMC free article] [PubMed] [Google Scholar]