Abstract
Centrosome assembly is important for mitotic spindle formation and if defective may contribute to genomic instability in cancer. Here we show that in somatic cells centrosome assembly of two proteins involved in microtubule nucleation, pericentrin and γ tubulin, is inhibited in the absence of microtubules. A more potent inhibitory effect on centrosome assembly of these proteins is observed after specific disruption of the microtubule motor cytoplasmic dynein by microinjection of dynein antibodies or by overexpression of the dynamitin subunit of the dynein binding complex dynactin. Consistent with these observations is the ability of pericentrin to cosediment with taxol-stabilized microtubules in a dynein- and dynactin-dependent manner. Centrosomes in cells with reduced levels of pericentrin and γ tubulin have a diminished capacity to nucleate microtubules. In living cells expressing a green fluorescent protein-pericentrin fusion protein, green fluorescent protein particles containing endogenous pericentrin and γ tubulin move along microtubules at speeds of dynein and dock at centrosomes. In Xenopus extracts where γ tubulin assembly onto centrioles can occur without microtubules, we find that assembly is enhanced in the presence of microtubules and inhibited by dynein antibodies. From these studies we conclude that pericentrin and γ tubulin are novel dynein cargoes that can be transported to centrosomes on microtubules and whose assembly contributes to microtubule nucleation.
INTRODUCTION
Centrosomes and other microtubule-organizing centers represent a structurally diverse class of organelles that share the common ability to nucleate and organize microtubules and play an important role in many fundamental cellular processes (for review, see Kellogg et al., 1994; Zimmerman et al., 1999). In interphase cells, centrosome-anchored microtubules serve as tracks for molecular motor-based transport and positioning of vesicles and organelles (see Karki and Holzbaur, 1999). Centrosomes also serve to anchor important regulatory molecules such as protein kinase A, which has been shown to regulate spindle function (Schmidt et al., 1999; Takahashi et al., 1999; Witczak et al., 1999; Diviani et al., 2000; D. Diviani, J. Langeberg, A. Purohit, A. Young, S. Doxsey, and J. Scott, unpublished results). Moreover, an increasing number of molecules that regulate cellular processes such as cell cycle progression and centrosome duplication are localized to centrosomes (see Doxsey, 1998; Zimmerman et al., 1999). In mitotic cells, centrosomes play an important role in the assembly and function of mitotic spindles and thus in the fidelity of chromosome segregation (Merdes and Cleveland, 1997; Waters and Salmon, 1997; see Compton, 1998; Hyman and Karsenti, 1998). In tumor cells, centrosome structure, number, and function are altered, suggesting that centrosome defects may contribute to tumorigenesis as first hypothesized by Boveri (1914) (also see Wilson, 1925; Chial and Winey, 1999; Pihan and Doxsey, 1999; Salisbury et al., 1999).
Centrosomes in most animal cells are structurally complex organelles that comprise of a pair of centrioles surrounded by a protein matrix. Centrioles are microtubule barrels that seem to serve as templates for recruitment of the centrosome matrix components (Bobinnec et al., 1998; Marshall and Rosenbaum, 1999). The centrosome matrix is an organized lattice-like structure that serves as the site of centrosome-mediated microtubule nucleation (Gould and Borisy, 1990; Thompson-Coffe et al., 1996; Dictenberg et al., 1998; Moritz et al., 1998; Schnackenberg et al., 1998). In higher eukaryotes, microtubule nucleation at the centrosome appears to be mediated by a complex of γ tubulin and associated proteins, which is organized into a ring-like structure (Moritz et al., 1998; Schnackenberg et al., 1998). A γ tubulin complex with a similar organization (the γ tubulin ring complex [γ TuRC]) is present in Xenopus and Drosophila extracts and when purified is able to mediate microtubule nucleation in vitro (Zheng et al., 1995; Oegema et al., 1999). For microtubule nucleation to take place, however, the soluble nucleating proteins must first be recruited to and assembled onto centrosomes (Stearns and Kirschner, 1994; Felix et al., 1994; Dictenberg et al., 1998; Moritz et al., 1998; Schnackenberg et al., 1998). Because purified γ TuRCs lack the ability to assemble onto centrosomes in vitro (Moritz et al., 1998), other components appear to be required.
Several candidate proteins have recently been identified that appear to be involved in the assembly of γ tubulin onto centrosomes and spindle poles. Pericentrin is a centrosome protein that plays a role in centrosome and spindle organization (Doxsey et al., 1994; Dictenberg et al., 1998). It forms an ∼3 MDa-complex together with γ tubulin and can be dissociated into a pericentrin subcomplex whose function is unknown and a γ tubulin subcomplex that appears to be the γ TuRC based on its biochemical properties. These observations have led to the hypothesis that the co-complex containing pericentrin and γ tubulin is the assembly-competent form of the microtubule nucleating complex and that pericentrin is an important player in γ tubulin assembly at centrosomes. Two other centrosome–spindle pole proteins have recently been implicated in the organization of γ tubulin into functional microtubule nucleating centers. Drosophila abnormal spindle protein (do Carmo Avides and Glover, 1999) is present at spindle poles where it plays a role in recruitment of γ tubulin complexes and pole focusing during mitosis. The Drosophila protein centrosomin is required for spindle pole recruitment of CP60, CP190, and γ tubulin, for generating astral microtubules, and for proper spacing of spindles during mitosis (Megraw et al., 1999). The precise mechanism by which pericentrin, abnormal spindle protein, centrosomin, and other proteins contribute to the assembly and organization of functional γ tubulin complexes at the centrosome remains to be determined.
Another protein implicated in centrosome assembly is the minus end-directed microtubule motor cytoplasmic dynein. Dynein binds directly to dynactin (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995), a protein complex that plays a role in dynein-mediated transport in vitro (Gill et al., 1991) and is believed to be required for most, if not all, of dynein-mediated cellular activities (see Schroer, 1996; Karki and Holzbaur, 1999). Dynein and dynactin are localized to numerous cellular structures, and they play important roles in mitotic spindle organization and orientation (Vallee and Sheetz, 1996; for review see Karki and Holzbaur, 1999). For example, dynein and dynactin together with the spindle pole protein NuMA are involved in focusing microtubules at the poles of mitotic spindles (Heald et al., 1996; Merdes et al., 1996; Gaglio et al., 1997). Dynein also plays a role in spindle orientation apparently through an interaction with microtubules at the cell cortex (Carminati and Stearns, 1997; Cottingham and Hoyt, 1997; Gonczy et al., 1999). Recent evidence indicates that dynein is involved in centrosome separation and centrosome binding to the nucleus (Gonczy et al., 1999; Robinson et al., 1999), and that dynactin is involved in microtubule anchoring at centrosomes (Quintyne et al., 1999).
The role of dynein and dynactin in the recruitment and assembly of centrosome proteins is less clear. The duplication of centrosomes requires microtubules (Kuriyama, 1982), suggesting a role for dynein in the assembly and organization of centrosome proteins. Assembly of the centrosome matrix protein PCM1 onto centrosomes in pharmacologically treated cells is impaired in the absence of microtubules, and binding of the protein to microtubules in vitro appears to be dynactin dependent (Balczon et al., 1999). In a more recent study (Purohit et al., 1999), ectopic expression of pericentrin was shown to mislocalize dynein and induce spindle defects indistinguishable from those caused by overexpression of the dynamitin subunit of dynactin (Echeverri et al., 1996). Further investigation of this phenomenon demonstrated that pericentrin interacted directly with the dynein light intermediate chain, suggesting a role for this interaction in pericentrin assembly and spindle function (Purohit et al., 1999; S. Tynan, A. Purohit, S. Doxsey, and R. Vallee, unpublished results). Although indirect, these studies implicate microtubules and dynein/dynactin in the centrosome assembly process.
Microtubule-independent mechanisms for protein assembly onto centrosomes have also been described. In embryonic systems, assembly of γ tubulin onto centrioles can occur in the absence of microtubules (Stearns and Kirschner, 1994; Felix et al., 1994; Moritz et al., 1998; Schnackenberg et al., 1998). In somatic cells constitutively expressing γ tubulin fused to green fluorescent protein (GFP), γ tubulin-GFP was recruited to centrosomes in the absence of microtubules, presumably in a dynein-independent manner (Khodjakov and Rieder, 1999). In this manuscript, we directly address the role of dynein in recruitment of centrosome proteins in both embryonic and somatic cell systems. Our results demonstrate that dynein can mediate recruitment of pericentrin and γ tubulin to centrosomes. We discuss our results in terms of models that are consistent with both dynein-dependent and -independent recruitment mechanisms.
MATERIALS AND METHODS
Cell Culture, Transfection, and Synchrony
COS-7 and Chinese hamster ovary (CHO) cells were grown as described (American Type Culture Collection, Manassas, VA). For experiments in Figures 1 and 4, cells were grown to 40% (COS) and 80% (CHO) confluency and transfected with cDNAs (2 μg/35-mm plate and 8 μg/100-mm plate, respectively) as described (Dictenberg et al., 1998) (LipofectAMINE; Life Technologies, Gaithersburg, MD). Cells were then incubated 24 h before live imaging (Figure 1, A and B), fixation (Figure 1, D and E), or biochemical analysis (see Figure 4C). For cell synchronization studies (Figure 2, A–F), highly enriched fractions of mitotic CHO cells were isolated in large numbers as described (Sparks et al., 1995). Briefly, mitotic CHO cells were collected by forced pipetting, pelleted at 200 × g, and plated on 12-mm coverslips at 50% confluency. Synchronized cells (85–90% mitotic by flow cytometry) were incubated at 37°C for 2 h to allow entry into G1, transferred to medium with nocodazole (10 μg/ml) or cytochalasin D (10 μg/ml) for 14 h, and processed for immunofluorescence. Microtubule depolymerization and actin disruption were confirmed by immunofluorescence (our unpublished results).
Figure 1.
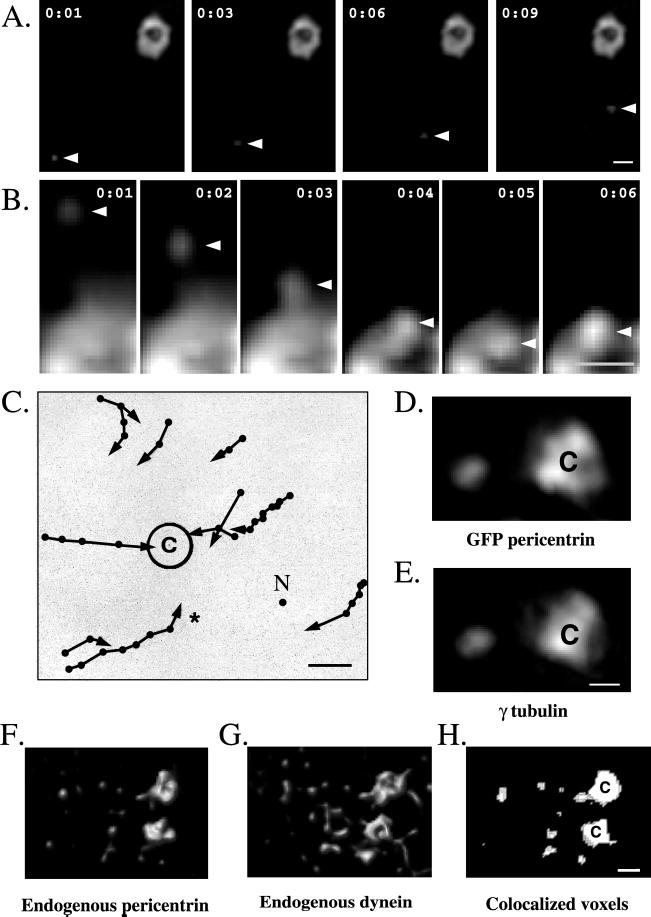
GFP-pericentrin particles move toward and assemble onto centrosomes. (A) Time-lapse images of a GFP-pericentrin particle (arrowhead) in an interphase COS-7 cell moving toward a GFP-pericentrin–labeled centrosome (upper right). Time elapsed between images is shown in seconds. (B) Time-lapse images of a GFP-pericentrin particle (arrowhead) moving toward and joining a section of a GFP-pericentrin–labeled centrosome (bottom). Note that magnification is threefold higher than in A. (C) Schematic representation of several GFP-pericentrin particle movements (arrows) and their relationship to the centrosome (c). Time elapsed between points is 1 s. No movement was detected in the presence of nocodazole (N represents a summary of movements recorded in the presence of nocodazole; n = 10). The asterisk shows tracing of a particle imaged in A. (D and E) Immunofluorescence localization of endogenous γ tubulin (E) and a GFP-pericentrin particle (D, left) near the centrosome (C). (F–H) Immunofluorescence localization of endogenous pericentrin (F) and cytoplasmic dynein (G) in pericentriolar satellites surrounding prophase centrosomes (c). (H) Colocalized voxels (see Dictenberg et al., 1998). Note that nearly all pericentrin-staining satellites (F, left side) colocalize with dynein (G). (A–E) COS cells; (F–H) CHO cells. Bars, 1 μm (bar in E for D and E, bar in H for F–H).
Figure 4.
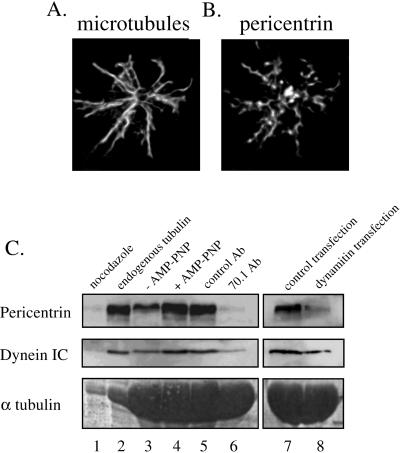
Pericentrin associates with microtubules in a dynein- and dynactin-dependent manner. Immunofluorescence staining is shown for microtubules (A) and pericentrin (B) in CHO cells treated with nocodazole for one cell cycle and then released from the drug for 20 min to promote microtubule polymerization. Nearly all noncentrosomal pericentrin colocalizes with microtubules. Bar in B, 1 μm (for A and B). (C) Taxol was used to assemble microtubules in CHO cell lysates (see MATERIALS AND METHODS). Other reagents were added as indicated, microtubules were centrifuged through a 20% sucrose cushion, and proteins in the microtubule pellet were analyzed by Western blot for pericentrin, dynein intermediate chain (IC), or α tubulin (to control for microtubule loading). Unless otherwise indicated, all lysates contained GTP, exogenous tubulin, taxol, and AMP-PNP. In addition, lysates contained nocodazole without taxol (lane 1), no exogenous tubulin (lane 2), the absence (lane 3) or presence (lane 4) of AMP-PNP, control antibody (β galactosidase, lane 5), or 70.1 antibody (lane 6). Lanes 7 and 8, lysates from cells expressing control protein (GFP) or dynamitin, respectively. Similar results were obtained with endogenous or exogenous tubulin (compare lanes 2 and 4).
Figure 2.
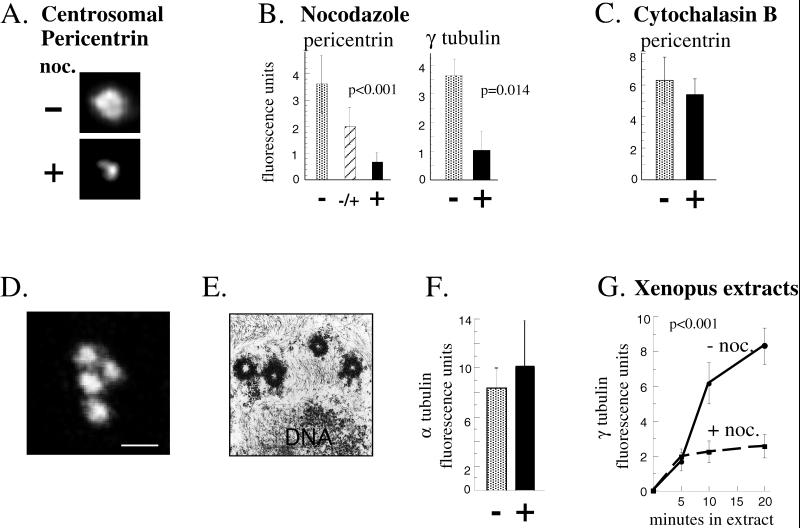
Centrosome assembly of pericentrin and γ tubulin but not centrioles requires microtubules. For the experiments described in A–F, cells were treated as indicated at G1, and assays were performed in the following metaphase (see MATERIALS AND METHODS). (A) Immunofluorescence images showing pericentrin staining at centrosomes in cells incubated for one cell cycle in the presence (+) or absence (−) of nocodazole (noc.) to depolymerize microtubules. Depolymerization of microtubules was confirmed by immunofluorescence staining with anti-α tubulin antibodies. (B) Quantity of centrosome-associated pericentrin and γ tubulin assembled in the absence of microtubules as described in A. An intermediate level of pericentrin is observed when centrosomes are allowed to assemble for half of the cell cycle then exposed to nocodazole for the remaining half (middle bar in first graph). Shown are the mean ± SE of representative experiments; p values (nonparametric Wilcoxon rank sum test) reflect statistical differences between nocodazole-treated (pericentrin, n = 134; γ tubulin, n = 146) and DMSO-treated controls (pericentrin, n = 61; γ tubulin, n = 71) determined from four independent experiments. (C) Centrosome-associated levels of pericentrin in the presence (+) or absence (−) of cytochalasin B. Values represent mean ± SE for cytochalasin-treated (n = 38) and DMSO-treated control cells (n = 19) from two independent experiments. For A–C, G1 levels of centrosome fluorescence were subtracted (∼15% of total; see MATERIALS AND METHODS). (D) Confocal microscope image of α tubulin immunostaining structures representing centrioles in cells treated with nocodazole for one cell cycle. (E) Transmission electron microscopic image of a single cell treated as in D showing four centriole profiles. More than three centrioles were detected in serial sections of seven cells. (F) Quantification of α tubulin immunofluorescence in cells treated as in D (black bar; n = 18) compared with control mitotic cells (stippled bar; n = 10). Values are mean ± SE from three independent experiments. Control cells represented by the stippled bar were briefly treated with nocodazole (10 μg/ml, 60 min, 37°C) to depolymerize cytoplasmic microtubules and thus reveal centriolar α tubulin fluorescence. The values are not significantly different, further demonstrating that the centriole pair duplicated from G1 to M in the absence of cytoplasmic microtubules as in control cells. (A–F) CHO cells. (G) Microtubule-enhanced assembly of γ tubulin in Xenopus extracts. Extracts and sperm nuclei were incubated with or without nocodazole (noc.) for the times indicated (see MATERIALS AND METHODS for details). Each time point represents mean ± SE of at least 13 measurements from one experiment; p values (multiple regression with interaction term) were determined by averaging all values from three independent experiments. Values shown are ×10−5. Background centriole levels of γ tubulin (<12.2% of experimental values) were subtracted.
Microinjections
COS cells were used for microinjection, because they were easier to inject than CHO cells given their larger size (footprint). Mitotic COS cells were located on gridded coverslips (Cellocate; Eppendorf, Madison, WI) and allowed to enter G1. They were microinjected into the nucleus with plasmid DNAs (10 μg/ml in Tris-EDTA; Micropipette, Life Technologies) or into the cytoplasm with 70.1 ascites fluid or control immunoglobulin G (mouse IgG; Sigma, St. Louis, MO; both 10 mg/ml in PBS). FITC-dextran (10 kDa; Molecular Probes, Eugene, OR) was added to all reagents to identify injected cells. Ascites fluid containing 70.1 antibody was a gift from J. Burkhardt (University of Chicago, Chicago, IL) and was used at 1:5 in PBS for microinjections as described (Burkhardt et al., 1997). Cells entering the next mitosis, initially identified by phase-contrast microscopy, were fixed in −20°C methanol and processed for immunofluorescence to detect γ tubulin or pericentrin, injected IgG or overexpressed protein, and mitotic chromosomes (DAPI).
Live Cell Imaging
We previously demonstrated that GFP-pericentrin localized to centrosomes and caused no detectable changes in centrosome-mediated microtubule nucleation (Dictenberg et al., 1998; Young et al., 1999). To examine centrosome assembly of GFP-pericentrin, three-dimensional immunofluorescence images of GFP-pericentrin were captured in live cells with or without nocodazole (Young et al., 1999) and processed as described (Dictenberg et al., 1998). Briefly, images of GFP-pericentrin particles were captured on a Nikon (Melville, NY) microscope with rapid image acquisition cameras, a step motor, and a 60× water immersion objective (Young et al., 1999). For each image, 10 optical sections (z-planes) were collected at 100-nm intervals in a 17-μm2 region (x, y). Each three-dimensional series was captured every second and restored (Carrington et al., 1995) and displayed as maximum intensity projections.
Quantitative Immunofluorescence on Fixed Cells
For immunofluorescence studies in Figure 2, synchronized CHO cells were extracted with 0.1% Triton-X-100 in microtubule stabilizing buffer and fixed in 3.7% formaldehyde and then in −20°C methanol; similar results were obtained in nonextracted cells (our unpublished results). Microinjected COS and control cells were fixed in −20°C methanol. Centrosomes were immunostained for γ tubulin (Sigma) and pericentrin (Dictenberg et al., 1998), or microtubule asters were stained for α tubulin (Sigma). Cy3 and/or FITC secondary antibodies were used as described (Dictenberg et al., 1998). The fluorescence intensity (integrated optical density) was measured within 2-μm2 (pericentrin and γ tubulin) or 1.25-μm2 (α tubulin) regions around the centrosome using a Silicon Graphics (Mountain View, CA) workstation as described (Dictenberg et al., 1998). To monitor net assembly of pericentrin and γ tubulin at the centrosome, we subtracted baseline G1 staining levels of these proteins (Dictenberg et al., 1998) from the final metaphase values.
Centrosome Assembly in Xenopus Extracts
Xenopus sperm nuclei centrifuged onto 12-mm glass coverslips (2 × 103, 10,000 × g, 10 min, 4°C) were incubated with high-speed supernatants of cytostatic factor-arrested Xenopus extracts (20 μl) (Dictenberg et al., 1998) at room temperature for the indicated times. Nocodazole (2 μg/ml), anti-dynein IC (70.1; Sigma), or anti-β-galactosidase antibodies (Boehringer Mannheim, Indianapolis, IN) were added to extracts in Xenopus buffer (Dictenberg et al., 1998) as indicated (0.18 μg/ml final concentration, 30% dilution of extract). Coverslips were washed briefly, fixed in methanol (−20°C), and processed for immunofluorescence (Dictenberg et al., 1998) using an antibody to γ tubulin (a gift from T. Stearns, Stanford University, Stanford, CA) and α tubulin (Sigma). Fluorescence intensity values (integrated optical density) from representative experiments are shown in Figures 2G and 3C.
Figure 3.
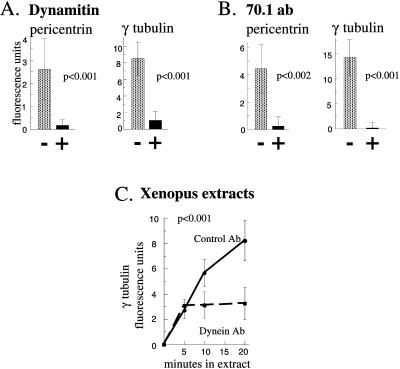
Assembly of pericentrin and γ tubulin at the centrosome requires cytoplasmic dynein and dynactin. (A) When dynamitin overexpression is induced by microinjection of cDNA into G1 cell nuclei (see MATERIALS AND METHODS), centrosome levels of pericentrin and γ tubulin are reduced (black bars) compared with untreated control cells (stippled bars). Shown are the mean ± SE of a representative experiment. p values reflect differences between dynamitin-expressing cells (n = 20) and control cells (β galactosidase cDNA injected, n = 10; uninjected, n = 177) from seven independent experiments. Values obtained from β galactosidase cDNA-injected cells did not deviate >5.3% from uninjected cells and were not statistically different from uninjected cells (p < 0.87). Dynamitin and β galactosidase expression were confirmed by immunofluorescence staining and were roughly similar (our unpublished results). (B) When G1 cells are microinjected with ascites fluid containing 70.1 antibody, pericentrin and γ tubulin levels are reduced up to 50-fold (black bars) compared with control uninjected cells (stippled bars). Shown are the mean ± SE of a representative experiment. p values reflect statistical differences between 70.1 injected (n = 14) and control cells (control mouse IgG injected, n = 10; uninjected, n = 90) from six independent experiments. Values obtained from cells injected with control mouse IgG were not statistically different from uninjected cells (p ∼ 0.95) and did not vary >6.5% from uninjected controls. (A and B) COS cells. For A and B, G1 levels of centrosome fluorescence were subtracted. (C) Centrosome assembly of γ tubulin in Xenopus extracts in the absence of nocodazole and in the presence of anti-dynein antibody or control antibody (β-galactosidase). Values shown are ×10−5. Background centriole levels of γ tubulin (<6.7% of experimental values) were subtracted.
Microtubule Nucleation
Cells treated with nocodazole for 14 h or control cells treated with nocodazole for 1.5 h were washed free of the drug to allow microtubule nucleation (3–5 min), fixed in 2% glutaraldehyde for 10 min, and immunostained for α tubulin. Nucleation was quantified by measuring α tubulin fluorescence in a 5-μm2 region centered on the aster.
Microtubule Binding Assay
Cells were lysed at 4°C in 100 mM 1,4-piperazinediethanesulfonic acid, pH 6.8, 1 mM MgCl2, 1 mM EGTA, and 1% Triton X-100 and spun 13,000 × g for 30 min. Microtubule affinity experiments were performed as described (Schroer and Sheetz, 1991; Balczon et al., 1999) with some modifications. Unless otherwise indicated, to the cleared lysates we added purified calf brain tubulin (200 μg), GTP (0.5 mM), taxol (10 μM), and adenylyl 5′-imidodiphosphate (AMP-PNP; 0.5 mM) and incubated for 20 min at 37°C. Lysates were layered over a 20% sucrose cushion in the above buffer containing GTP with (see Figure 4, lanes 2–8) or without (see Figure 4, lane 1) taxol and spun at 20,000 × g for 30 min at room temperature. Microtubule pellets were collected after removing lysate and cushion. Antibodies to β galactosidase (control) and 70.1 were used for Western blots.
RESULTS
Microtubule-based Movement of GFP-Pericentrin Particles toward Centrosomes
To visualize centrosome assembly, a GFP-pericentrin fusion protein was expressed transiently in COS cells (Dictenberg et al., 1998; see MATERIALS AND METHODS) and imaged by high-speed, high-resolution immunofluorescence microscopy (Carrington et al., 1995; Young et al., 1999). GFP-pericentrin localized to centrosomes and to tiny particles that moved toward the minus ends of microtubules and joined with centrosomes (Figure 1, A–C). These movements were commonly observed (64.7% of all imaging sessions; n = 34); they often exceeded 1 μm/s, and they were abolished when microtubules were depolymerized with nocodazole (n = 10; Figure 1C). Immunofluorescence microscopy on fixed cells demonstrated that endogenous γ tubulin colocalized with GFP-pericentrin particles (Figure 1, D and E), suggesting that centrosomal transport of both GFP-pericentrin and endogenous γ tubulin was mediated by a microtubule-based mechanism. The speed and direction of transport was consistent with the activity of the molecular motor cytoplasmic dynein.
To study the role of dynein in the transport process, we initially examined the spatial relationship of the motor and centrosome proteins in nontransfected cells. High-resolution immunofluorescence microscopy demonstrated that dynein was found at nearly all sites labeled with pericentrin, including centrosomes and material surrounding centrosomes known as pericentriolar satellites (Figure 1, F–H) (Brinkley and Stubblefield, 1970; Rieder and Borisy, 1982). The pericentriolar satellites also contained γ tubulin (Dictenberg et al., 1998) but not dynamitin (our unpublished results) or the centrosome protein centrin (Lingle et al., 1998). The molecular composition, position, and size of the satellites were strikingly similar to the motile GFP-pericentrin particles, suggesting that they represented higher order assemblies of centrosome protein complexes in transit to the centrosome.
Depolymerization of Microtubules Inhibits Centrosomal Recruitment of Endogenous Pericentrin and γ Tubulin
To examine recruitment of endogenous pericentrin and γ tubulin onto centrosomes, we quantified centrosomal immunofluorescence in fixed cells (see MATERIALS AND METHODS for details). Our previous studies showed that centrosome levels of both pericentrin and γ tubulin increase five- to sevenfold from early G1 to metaphase (M) (Dictenberg et al., 1998). When synchronized cells were allowed to progress from G1 to M in the presence of nocodazole to depolymerize microtubules, centrosomal recruitment of both proteins was inhibited (Figure 2, A and B). Because assembly was unaffected by disruption of actin filaments (Figure 2C), the process appeared to be microtubule specific. To rule out the possibility that microtubule depolymerization induced disassembly of pericentrin rather than inhibited its assembly, we showed that the fraction of pericentrin assembled onto centrosomes remained centrosome associated after microtubule depolymerization (Figure 2B, middle bar). Similar results were observed at several different cell cycle stages (e.g., late G1, S, and G2). We also ruled out the possibility that nocodazole blocked assembly of centrioles, microtubule barrels within centrosomes that duplicate every cell cycle (Hinchcliffe et al., 1999; Lacey et al., 1999) and appear to serve as templates for recruitment of centrosome proteins (Bobinnec et al., 1998; see Marshall and Rosenbaum, 1999). Centrioles assembled normally in cells treated with nocodazole, demonstrating that the number of centriole templates was the same as in untreated cells (Figure 2, D–F) and emphasizing the specificity of the microtubule-dependent assembly of pericentrin and γ tubulin. From these results, we conclude that centrosome recruitment of pericentrin and γ tubulin is microtubule dependent.
In extracts from embryonic cells, recruitment of γ tubulin onto sperm centrioles has been shown to occur in the absence of microtubules, although the contribution of microtubule-based transport was not examined (Stearns and Kirschner, 1994; Felix et al., 1994; Moritz et al., 1998; Schnackenberg et al., 1998). We found that γ tubulin recruitment in the presence of microtubules in Xenopus extracts was at least threefold greater than that assembled in their absence (Figure 2G; see MATERIALS AND METHODS). This result shows that both microtubule-dependent and -independent mechanisms operate during centrosome assembly in early Xenopus development and suggests that microtubule-mediated assembly may be required for proper centrosome function in early embryogenesis.
Cytoplasmic Dynein Antibody and Dynamitin Overexpression Inhibit Centrosomal Recruitment of Endogenous Pericentrin and γ Tubulin
We next examined the role of dynein in the centrosomal recruitment of pericentrin and γ tubulin in both somatic cells and Xenopus extracts. Dynactin is a protein complex that interacts with dynein and plays a role in motor localization and function (see Schroer, 1996; Karki and Holzbaur, 1999). Overexpression of the dynamitin subunit of dynactin disrupts the dynactin complex and impairs dynein-mediated functions (Echeverri et al. 1996; Burkhardt et al., 1997; see Karki and Holzbaur, 1999). Overexpression of dynamitin in CHO cells during the period from G1 to M caused a significant reduction in centrosome levels of pericentrin and γ tubulin compared with untreated cells or cells overexpressing β galactosidase (Figure 3A). To block dynein function by an independent mechanism, we microinjected an anti-dynein intermediate chain antibody previously shown to inhibit dynein-mediated processes (70.1) (see Karki and Holzbaur, 1999). The dynein antibody also caused a significant reduction in centrosome levels of pericentrin and γ tubulin compared with cells microinjected with control IgG or uninjected cells (Figure 3B). Similarly, addition of the dynein antibody to Xenopus extracts blocked microtubule-dependent assembly of γ tubulin onto sperm centrioles, whereas control IgG had no effect (Figure 3C). Based on these observations, we conclude that pericentrin and γ tubulin can be transported to centrosomes by dynein-mediated movements along microtubules.
Centrosomes with Reduced Pericentrin and γ Tubulin Nucleate Fewer Microtubules
To determine the functional consequence of inhibiting assembly of pericentrin and γ tubulin, we examined the ability of centrosomes to nucleate microtubules. When cells were treated from G1 to M with nocodazole (as in Figure 2, A and B) and washed free of the drug, microtubule nucleation from individual centrosomes was 16.6% of control centrosomes as measured by α tubulin fluorescence of nucleated microtubules surrounding the centrosome (n = 76; p < 0.001). Upon removal of nocodazole, we unexpectedly found that the pericentrin staining pattern was coincident with nucleated microtubules (Figure 4, A and B). This distribution was particularly striking because pericentrin was almost never detected on microtubules in control cells at any cell cycle stage (Doxsey et al., 1994; Dictenberg et al., 1998). The amount of microtubule-associated pericentrin increased rapidly after nocodazole washout, reached a maximum level at ∼15 min, and was barely detectable by 30 min. During this period, pericentrin accumulation at centrosomes increased rapidly (97 ± 4.2% increase), suggesting that the protein was undergoing rapid microtubule-dependent assembly onto centrosomes.
Pericentrin Binds Microtubules in a Dynein-dependent Manner
The data presented thus far suggested that pericentrin associated with microtubules through an interaction with dynein. To test this directly, taxol-stabilized microtubules were incubated with cell lysates, pelleted by centrifugation, and assayed for the presence of pericentrin. Pericentrin cosedimented with microtubules under these conditions (Figure 4C, lanes 2 and 4) and was not detected when microtubule polymerization was inhibited by addition of nocodazole (Figure 4C, lane 1). When AMP-PNP was added to enhance motor binding to microtubules, we observed a twofold increase in the association of pericentrin with microtubules (Figure 4C, lanes 3 and 4). The association of both dynein and pericentrin with microtubules was inhibited when the 70.1 dynein antibody was added to cell lysates and when lysates from cells overexpressing dynamitin were used (Figure 4C, lanes 5–8). Because 70.1 recognizes an epitope on the dynein intermediate chain that interacts with dynactin p150glued (Vaughan and Vallee, 1995; Steffen et al., 1997), the antibody probably disrupts the dynein–dynactin interaction, dissociating dynein from microtubules by a mechanism similar to that observed in cells overexpressing dynamitin (Echeverri et al., 1996). In contrast, control antibody or lysates expressing a control construct (GFP) had no effect on microtubule binding. From this analysis, we conclude that pericentrin binds along the length of microtubules through an interaction with dynein and/or dynactin.
DISCUSSION
A Role for Cytoplasmic Dynein in the Recruitment of Pericentrin and γ Tubulin onto Centrosomes
Using several approaches to examine centrosome protein assembly in both somatic cells and embryonic systems, we demonstrate that pericentrin and γ tubulin can be recruited to centrosomes by a dynein-driven mechanism. We show that the cell cycle-dependent increase in centrosomal accumulation of these proteins (Dictenberg et al., 1998) was inhibited by treatments that disrupt dynein-mediated cellular events in vivo and in vitro, including depolymerization of microtubules, addition of dynein antibodies, and elevation of dynactin levels (Vaisberg et al., 1993; Echeverri et al., 1996; Gaglio et al., 1996; Heald et al., 1996; Merdes et al., 1996; Burkhardt et al., 1997; Ahmed et al., 1998). We provide the first demonstration that pericentrin associates with microtubules both in vivo and in vitro, and we show that microtubule binding of pericentrin requires dynein and dynactin. We visualized pericentrin expressed as a GFP fusion protein and found that it moved toward centrosomes in a microtubule-dependent manner at speeds consistent with those of dynein. These movements probably represent direct transport of pericentrin by dynein, because pericentrin has been shown to interact directly with the dynein light intermediate chain 1 (Purohit et al., 1999; S. Tynan, A. Purohit, S. Doxsey, and R. Vallee, unpublished results).
A Model for Recruitment of Centrosome Proteins
Based on the data described above, we propose a model for the assembly of microtubule nucleating proteins. In our model, pericentrin binds to dynein through the light intermediate chain (Purohit et al., 1999) and to the γ TuRC (Dictenberg et al., 1998) through specific subunits of this complex (W. Zimmerman and S. Doxsey, unpublished observations). Dynein would mediate binding of the large pericentrin-γ TuRC complex to microtubules and direct transport of the complex to centrosomes. At the centrosome, pericentrin-γ TuRC complexes would be anchored, whereas dynein could be released for additional rounds of transport or anchored to perform additional roles. Dynactin may facilitate microtubule association or processivity of dynein (Schroer and Sheetz, 1991; Schroer, 1996) and may contribute to centrosomal anchoring of γ tubulin (Quintyne et al., 1999). Our work raises the possibility that pericentrin mediates centrosome and spindle function through dynein-dependent assembly of microtubule nucleating complexes and other activities (see below).
Alternative Mechanisms for Recruitment of Centrosome Proteins
There is now good evidence for microtubule-dependent (Kuriyama, 1982; Balczon et al., 1999; this manuscript) and microtubule-independent (Felix et al., 1994; Stearns and Kirschner, 1994; Moritz et al., 1998; Schnackenberg et al., 1998; Khodjakov and Rieder, 1999) mechanisms for recruitment of proteins onto centrosomes. These studies support the idea that dynein-mediated and passive diffusion mechanisms represent parallel pathways for centrosome assembly. It is possible that one pathway predominates over the other in certain biological systems or at different stages of the cell cycle. In embryonic systems, for example, high levels of centrosome proteins (Gard et al., 1990) may be sufficient to drive the initial stages of microtubule-independent recruitment onto centrioles, although dynein-mediated transport becomes a major contributor at later times (Figure 3). Alternative mechanisms could also account for centrosome protein recruitment. Spontaneously assembled microtubules could be capped by γ tubulin (and pericentrin) complexes (Zheng et al., 1995), and these small microtubule fragments could be transported toward the minus ends of microtubules by dynein as described during spindle assembly in Xenopus extracts (Heald et al., 1996). Our data do not distinguish between this microtubule fragment mechanism and our model in which presumably inactive centrosome proteins are transported to centrosomes and become active for microtubule nucleating activity. Another possibility is that centrosome-nucleated microtubules are released (Keating et al., 1997) but remain tethered to the centrosome, perhaps through an interaction with dynactin (Quintyne et al., 1999), and they provide new minus ends for binding of γ tubulin-pericentrin complexes after passive diffusion to these sites. Although this mechanism could account for the microtubule dependency of centrosome protein recruitment, it is inconsistent with our kinetic data showing directed movement of GFP-pericentrin toward centrosomes.
Centrosome Assembly and Cancer
Regulation of the dynein-mediated centrosome protein assembly process is likely to be important in the control of microtubule nucleation at centrosomes. One candidate regulatory molecule is protein kinase A (PKA). PKA has recently been identified as a pericentrin-interacting protein, and disruption of the pericentrin–PKA interaction induces a subset of spindle defects observed in cells overexpressing pericentrin (Diviani et al., 2000; D. Diviani, L. Langeberg, A. Purohit, A. Young, S. Doxsey, and J. Scott, unpublished results). Misregulation of the centrosome assembly process could induce ectopic organization of structurally aberrant centrosomes observed in malignant tumors (Lingle et al., 1998; Pihan et al., 1998). Through the organization of dysfunctional spindles that missegregate chromosomes, abnormal centrosomes could contribute to genomic instability and tumorigenesis (Doxsey, 1998; Pihan and Doxsey, 1999).
Supplementary Material
ACKNOWLEDGMENTS
We thank W. Theurkauf, C. Sparks, R. Vallee, and Y.-L. Wang for critical reading, J. Burkhardt for 70.1 antibodies, R. Vallee for p50 cDNA and β-galactosidase cDNA, J. Wuu and H. Chung for statistical analysis, and Walter Carrington, Kevin Fogarty, and Larry Lifschitz for image preparation and analysis. This work was supported by National Institutes of Health grant GM-51994 (S.J.D.). S.J.D. is an Established Investigator of the American Heart Association and by National Science Foundation grants DBI 9200027, DBI 9724611 (R.T.).
Footnotes
Online version of this article contains video material for fig. 1. Online version available at www.molbiolcell.org.
REFERENCES
- Ahmed FJ, Echeverri CJ, Vallee RB, Bass PW. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J Cell Biol. 1998;140:139–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balczon R, Varden CE, Schroer TA. Role for microtubules in centrosome doubling in Chinese hamster ovary cells. Cell Motil Cytoskeleton. 1999;42:60–72. doi: 10.1002/(SICI)1097-0169(1999)42:1<60::AID-CM6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Edde CL, Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveri T. Zur frage der entstehung maligner tumoren. Jena: Fischer Verlag; 1914. . English translation (1929) by M. Boveri reprinted as The Origin of Malignant Tumors, Baltimore: Williams & Wilkins. [Google Scholar]
- Brinkley BR, Stubblefield E. Microtubule organizing centers. Annu Rev Cell Biol. 1970;1:119–172. doi: 10.1146/annurev.cb.01.110185.001045. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati JL, Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J Cell Biol. 1997;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington W, Lynch RM, Moore ED, Isenberg G, Fogarty KE, Fay FS. Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science. 1995;268:1483–1486. doi: 10.1126/science.7770772. [DOI] [PubMed] [Google Scholar]
- Chial HJ, Winey M. Mechanisms of genetic instability revealed by analysis of yeast spindle pole body duplication. Biol Cell. 1999;91:439–450. [PubMed] [Google Scholar]
- Compton DA. Focusing on spindle poles. J Cell Sci. 1998;111:1377–1481. doi: 10.1242/jcs.111.11.1477. [DOI] [PubMed] [Google Scholar]
- Cottingham F, Hoyt A. Mitotic spindle positioning in Saccharomyces cerivisiae is accompanied by antagonistically acting microtubule motors. J Cell Biol. 1997;138:1041–1053. doi: 10.1083/jcb.138.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg J, Zimmerman W, Sparks C, Young A, Vidair C, Zheng Y, Carrington W, Fay F, Doxsey SJ. Pericentrin and gamma tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviani D, Langeberg LK, Doxsey SJ, Scott JD. Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr Biol. 2000;10:417–420. doi: 10.1016/s0960-9822(00)00422-x. [DOI] [PubMed] [Google Scholar]
- do Carmo Avides M, Glover DM. Abnormal spindle protein, ASP, and the integrity of mitotic centrosomal microtubule organizing centers. Science. 1999;283:1733–1735. doi: 10.1126/science.283.5408.1733. [DOI] [PubMed] [Google Scholar]
- Doxsey SJ. The centrosome—a tiny organelle with big potential. Nat Genet. 1998;20:104–106. doi: 10.1038/2392. [DOI] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco P, Kirschner M. Pericentrin, a highly conserved protein of centrosomes involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M-A, Antony C, Wright M, Maro B. Centrosome assembly in vitro: role of γ tubulin recruitment in Xenopus sperm aster formation. J Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio, T., Dionne, M.A., and Compton, D. (1997). Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 1055–1066. [DOI] [PMC free article] [PubMed]
- Gaglio T, Saredi A, Bingham J, Hasbani MJ, Gill S, Schroer TA, Compton DA. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D, Hafezi S, Zhang T, Doxsey SJ. Centrosome duplication continues in cycloheximide-treated Xenopus blastulae in the absence of a detectable cell cycle. J Cell Biol. 1990;110:2033–2042. doi: 10.1083/jcb.110.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P, Pichler S, Kirckham M, Hyman A. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in one cell stage Caenorhabditis elegans embryo. J Cell Biol. 1999;147:135–150. doi: 10.1083/jcb.147.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RR, Borisy GG. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J Cell Biol. 1990;73:601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- Hyman A, Karsenti E. The role of nucleation in patterning microtubule networks. J Cell Sci. 1998;111:2077–2083. doi: 10.1242/jcs.111.15.2077. [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur ELF. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur ELF. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr Opin Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Peloquin JG, Rodonov VI, Momcilovic D, Borisy GG. Microtubule release from centrosomes. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DR, Moritz M, Alberts BM. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. The sudden recruitment of γ tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle do not require microtubules. J Cell Biol. 1999;146:585–596. doi: 10.1083/jcb.146.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R. The effect of colcemid on the centriole cycle in Chinese hamster ovary cells. J Cell Biol. 1982;53:155–171. doi: 10.1242/jcs.53.1.155. [DOI] [PubMed] [Google Scholar]
- Lacey KR, Jackson P, Stearns T. Cyclin-dependent kinase control of centrsome duplication. Proc Natl Acad Sci USA. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Rosenbaum JL. The renaissance of the centriole. Curr Biol. 1999;25:218–220. doi: 10.1016/s0960-9822(99)80133-x. [DOI] [PubMed] [Google Scholar]
- Megraw TL, Li K, Kao LR, Kaufman TC. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development. 1999;126:2829–2839. doi: 10.1242/dev.126.13.2829. [DOI] [PubMed] [Google Scholar]
- Merdes A, Cleveland DW. Pathways of spindle pole formation: different mechanisms: conserved components. J Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Moritz M, Zheng Y, Alberts BM, Oegema K. Recruitment of the γ tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J Cell Biol. 1998;142:775–786. doi: 10.1083/jcb.142.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema K, Wiese C, Martin OC, Milligan RA, Iwamatsu A, Mitchison TJ, Zheng Y. Characterization of two related Drosophila γ tubulin complexes that differ in their ability to nucleate microtubules. J Cell Biol. 1999;144:721–723. doi: 10.1083/jcb.144.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihan G, Purohit A, Knecht H, Woda B, Quesenberry P, Doxsey SJ. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 1998;58:3974–3985. [PubMed] [Google Scholar]
- Pihan GA, Doxsey SJ. The mitotic machine as a source of genetic instability in cancer. Semin Cancer Res. 1999;9:289–302. doi: 10.1006/scbi.1999.0131. [DOI] [PubMed] [Google Scholar]
- Purohit A, Tynan S, Vallee RB, Doxsey SJ. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J Cell Biol. 1999;147:481–491. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA. Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol. 1999;147:1–14. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Borisy GG. The centrosome in PtK2 cells: asymmetric distribution and structural changes in the pericentriolar material. Biol Cell. 1982;44:117–132. [Google Scholar]
- Robinson J, Wojcik E, Sanders M, McGrail M, Hays T. Cytoplasimc dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J Cell Biol. 1999;146:597–608. doi: 10.1083/jcb.146.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury J, Whittehead CM, Lingle WL, Barrett SL. Centrosomes and cancer. Biol Cell. 1999;91:451–460. [PubMed] [Google Scholar]
- Schmidt PH, Dransfield DT, Claudio JO, Hawley RG, Trotter KW, Migram SL, Goldenring JR. AKAP 350, a multiply spliced proteins kinase A-anchoring protein associated with centrosomes. J Biol Chem. 1999;274:3055–3066. doi: 10.1074/jbc.274.5.3055. [DOI] [PubMed] [Google Scholar]
- Schnackenberg BJ, Khodjakov A, Rieder CL, Palazzo RE. The disassembly and reassembly of functional centrosomes in vitro. Proc Natl Acad Sci USA. 1998;95:9295–9300. doi: 10.1073/pnas.95.16.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA. Structure and function of dynactin. Semin Cell Biol. 1996;7:321–328. [Google Scholar]
- Schroer TA, Sheetz MP. Two activators of microtubule-based vesicle transport. J Cell Biol. 1991;115:1309–1318. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks CA, Fey E, Vidair C, Doxsey SJ. Phosphorylation of NuMA occurs during nuclear breakdown not spindle assembly. J Cell Sci. 1995;108:3389–3396. doi: 10.1242/jcs.108.11.3389. [DOI] [PubMed] [Google Scholar]
- Stearns T, Kirschner M. Reconstitution of centrosome assembly, role of γ tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Steffen W, Karki S, Vaughan KT, Vallee RB, Holzbaur ELF, Weiss DG, Kuznetsov SA. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol Biol Cell. 1997;8:2077–2088. doi: 10.1091/mbc.8.10.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Shibata H, Shimakawa M, Miyamoto M, Mukai H, Ono Y. Chracterization of a novel giant scaffolding protein, cg-NAP, that anchors multiple signaling enzymes to centrosome and the Golgi apparatus. J Biol Chem. 1999;274:17267–17274. doi: 10.1074/jbc.274.24.17267. [DOI] [PubMed] [Google Scholar]
- Thompson-Coffe C, Coffee G, Schatten H, Mazia D, Schatten G. Cold-treated centrosome: isolation of centrosomes from mitotic sea urchin eggs, production of an anticentrosomal antibody and novel ultrastructural imaging. Cell Motil Cytoskeleton. 1996;33:197–207. doi: 10.1002/(SICI)1097-0169(1996)33:3<197::AID-CM4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Vaisberg EA, Koonce MP, McIntosh JR. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R, Sheetz MP. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150-glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Salmon ED. Pathways of spindle assembly. Curr Opin Cell Biol. 1997;9:37–43. doi: 10.1016/s0955-0674(97)80149-4. [DOI] [PubMed] [Google Scholar]
- Wilson EB. The Cell in Development and Heredity. New York: Macmillan; 1925. [Google Scholar]
- Witczak O, Skalhegg S, Keryer G, Bornens M, Tasken K, Jahnsen T, Orstavik K. Cloning and characterization of a cDNA encoding an A-kinase achoring protein located in the centrosome, AKAP450. EMBO J. 1999;18:1858–1868. doi: 10.1093/emboj/18.7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Tuft R, Carrington W, Doxsey SJ. Centrosome dynamics in living cells. Methods Cell Biol. 1999;58:223–238. doi: 10.1016/s0091-679x(08)61958-5. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wong M, Alberts B, Mitchision T. Nucleation of microtubule assembly by a γ tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman W, Sparks C, Doxsey SJ. Amorphous no longer: the centrosome comes into focus. Curr Opin Cell Biol. 1999;11:122–128. doi: 10.1016/s0955-0674(99)80015-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.