Abstract
Pollen tube growth depends on the differential distribution of organelles and vesicles along the tube. The role of microtubules in organelle movement is uncertain, mainly because information at the molecular level is limited. In an effort to understand the molecular basis of microtubule-based movement, we isolated from tobacco pollen tubes polypeptides that cosediment with microtubules in an ATP-dependent manner. Major polypeptides released from microtubules by ATP (ATP-MAPs) had molecular masses of 90, 80, and 41 kD. Several findings indicate that the 90-kD ATP-MAP is a kinesin-related motor: binding of the polypeptide to microtubules was enhanced by the nonhydrolyzable ATP analog AMP-PNP; the 90-kD polypeptide reacted specifically with a peptide antibody directed against a highly conserved region in the motor domain of the kinesin superfamily; purified 90-kD ATP-MAP induced microtubules to glide in motility assays in vitro; and the 90-kD ATP-MAP cofractionated with microtubule-activated ATPase activity. Immunolocalization studies indicated that the 90-kD ATP-MAP binds to organelles associated with microtubules in the cortical region of the pollen tube. These findings suggest that the 90-kD ATP-MAP is a kinesin-related microtubule motor that moves organelles in the cortex of growing pollen tubes.
INTRODUCTION
Microtubule motor proteins are an important class of microtubule-associated proteins that transport specific cargo structures in a process driven by ATP hydrolysis. Microtubule- based motors are classified into two main superfamilies: kinesin, which comprises conventional kinesin and kinesin- like proteins (KLPs), and dynein; both superfamilies include several members that play important roles in such cellular mechanisms as organelle transport and mitosis. Motor proteins transport proteins, lipids, and other cell components to different parts of the cell at suitable velocities in membranous organelles. Intracellular transport is therefore essential for cellular morphogenesis and function (Hirokawa et al., 1998). Microtubule-based motor proteins share some biochemical and functional features, such as nucleotide-dependent microtubule binding, microtubule-activated ATPase activity, and motor-driven microtubule translocation (Hirokawa, 1998). Although microtubule-based motor proteins are characterized primarily in animal cells, a large kinesin family has also been characterized in plant cells (Asada and Collings, 1997). The biochemical and structural properties of KLPs in plant cells are similar to those in animal cells. They have microtubule-stimulated ATPase activity (Mitsui et al., 1994), promote gliding of microtubules in motility assays in vitro (Song et al., 1997), and bind to microtubules in a nucleotide-dependent manner (Mitsui et al., 1996). Although most KLPs identified in plants are probably involved in cell division (Asada and Shibaoka, 1994; Liu et al., 1996; Mitsui et al., 1996; Asada et al., 1997), genetic analysis of Arabidopsis mutants having structural alterations of trichomes suggests that KLPs also take part in cell morphogenesis (Oppenheimer et al., 1997), which implies transport of molecules to specific cell regions.
Organelle transport in plant cells is important, for example, in construction of the cell plate during cytokinesis, maintenance of an even distribution of subcellular components in very large cells, and asymmetrical delivery of membrane material in cells that grow in a polarized way (Williamson, 1993). One of the best examples of a tip-growing cell is the pollen tube, a specialized plant cell with the biological function of conveying sperm cells to the ovary (Mascarenhas, 1993). The pollen tube is characterized by impressive movement of organelles, which is sustained principally by the acto-myosin system (Cai et al., 1997). Although many data on the role of actin filaments are now becoming available (Cai et al., 1997), there is little information on the function of vegetative microtubules during pollen tube growth. Pollen tube microtubules are thought to play a role in controlling the apical transport of secretory vesicles (Cai et al., 1993), in the positioning of organelles (Joos et al., 1994), and in pulsed growth (Geitmann et al., 1995), but indications of these activities are preliminary. Microtubules do not seem to control the deposition of cellulose fibrils (Li et al., 1997) but may play an indirect role in the translocation of sperm cells and the vegetative nucleus (Åström et al., 1995). Although the structure and localization of microtubules in the pollen tube are well known (Derksen et al., 1985; Pierson et al., 1986; Raudaskoski et al., 1987; Del Casino et al., 1993), much less is known about their involvement in the translocation of organelles along the tube. Two microtubule-based motor proteins, a pollen kinesin homolog (PKH) (Tiezzi et al., 1992) and dynein-related polypeptides (Moscatelli et al., 1995), have been identified in the vegetative cytoplasm of pollen tubes and localized in association with membrane structures (Liu et al., 1994; Moscatelli et al., 1998). The PKH is presumably involved in the process of tip growth, whereas the dynein-related polypeptide or polypeptides probably take part in the translocation of membrane-bounded organelles. Although these motor proteins have been identified by using specific antibodies and are therefore confirmed by immunological criteria, their functional properties are still unknown.
Previous experiments with microtubule inhibitors have suggested that microtubules are not involved in the flow of organelles along the tube (Heslop-Harrison et al., 1988). They may nevertheless participate in other transport activities not yet fully clarified (such as endocytosis, fine-tuning of vesicle delivery, and microtubule-dependent deposition of specific cell wall components) and in the establishment and control of cell polarity. Furthermore, evidence that microtubules and actin filaments cooperate functionally to move vesicles and organelles in different types of cells (Goode et al., 2000) suggests that the role of pollen tube microtubules in organelle transport could be complementary to actin-based transport.
Three aspects need to be investigated to understand the function of microtubule-based intracellular motility in the pollen tube: identification of novel motor proteins, biochemical and functional characterization of the proteins, and localization of the motor proteins in the pollen tube. The aim of this study, therefore, was to identify and characterize proteins that could be microtubule-based motors in the pollen tube. This was done by studying biochemical/functional aspects of these proteins, which required purification from their cells. We used the classic experimental approach of ATP-dependent microtubule binding to isolate specific polypeptides from the pollen tube of tobacco. The polypeptides were then analyzed by various methods (ATP-dependent microtubule binding, microtubule-enhanced ATPase activity, immunological cross-reactivity, ability to glide microtubules in motility assay in vitro, and immunolocalization in cells) to determine whether their properties were similar to those of motor proteins, especially kinesins. In brief, we found a 90-kD polypeptide with properties typical of the kinesin family that associated with the organelles localized along the microtubules in the pollen tube.
RESULTS
ATP-Dependent Release of Microtubule Binding Polypeptides from Tobacco Pollen Tubes
Microtubule-based motors usually bind to microtubules in an ATP-sensitive manner. Removal of nucleotides may increase the binding affinity of motor proteins to microtubules (Cole et al., 1992). We used this general approach to identify proteins that bind to microtubules in the absence of ATP and in the presence of its nonhydrolyzable analog, adenylylimido-diphosphate (AMP-PNP). We then released the proteins from the microtubules by adding ATP. A typical preparation started with 1 g of dry pollen, which yielded ∼40 ± 5 mg of proteins in the low-speed supernatant (LSS; i.e., supernatant obtained after low-speed centrifugation). The ATP-dependent microtubule-associated proteins (ATP-MAPs) fraction contained 25 ± 7 μg of proteins (mean ±se, n = 5). Polypeptides from a cytosolic homogenate of tobacco pollen tubes (Figure 1, lane 3) were induced to cosediment with taxol-stabilized microtubules in the presence of AMP-PNP. Polypeptides that bound to microtubules (Figure 1, lane 6) were released by washing with ATP and KCl. ATP-MAPs were then recovered in the final supernatant (Figure 1, lane 8). The ATP-MAPs fraction contained a few polypeptides, the most abundant having molecular masses of 90, 80, 53, and 41 kD. The relative intensities of the bands at 90, 80, and 41 kD were invariable during different experiments and often were present in an equimolar ratio. The 53-kD polypeptides were tubulin molecules, as demonstrated by a positive blot with a polyclonal anti-tubulin antibody (data not shown). Bands at 90 and 80 kD were also seen in the taxol pellet (lane 6) and were less intense in the ATP pellet (lane 7).
Figure 1.
Silver-Stained SDS Gel Showing Intermediate Fractions Obtained during Preparation of ATP-MAPs from a High-Speed Supernatant of Tobacco Pollen Tubes.
Lane 1, Mr standards (indicated at left in kilodaltons); lanes 2 and 3, the LSS and the high-speed supernatant (HSS), respectively; lane 4, the supernatant after hexokinase treatment; lane 5, the taxol supernatant; lane 6, the corresponding pellet; lane 7, the pellet obtained after ATP elution; and lane 8, the ATP-released polypeptides (ATP-MAPs). Polypeptides of 90, 80, and 41 kD are indicated. Protein loading was 3 μg in lanes 2 to 5 and 8. Volumes identical to that in lane 8 were loaded in lanes 6 and 7. T, tubulin.
This experimental protocol was also performed without exogenous tubulin, using taxol and GTP to induce polymerization of the endogenous tubulin pool and using AMP-PNP to enhance binding of kinesin family members. Under these conditions, only faint bands were visible in the final sample, even after extensive silver staining, and few polypeptides were observed in the corresponding ATP pellet (data not shown).
In other control experiments, AMP-PNP was replaced with an equal concentration of ATP during the binding step. After purification, the corresponding ATP-MAPs fraction contained only faint bands (Figure 2A), except for a couple of bands migrating in the position of tubulin.
Figure 2.
Differential Binding of Pollen Tube Proteins to Microtubules.
(A) Addition of 10 mM ATP instead of 10 mM AMP-PNP during the binding step prevents recovery of polypeptides in the final ATP supernatants (only the tubulin doublet is present; the lane is overloaded).
(B) Silver-stained SDS gel showing ATP-dependent release of pollen ATP-MAPs from microtubules. In this case, the taxol pellet was first washed with ATP and then centrifuged to yield the ATP supernatant (lane 1). The corresponding pellet was then washed with ATP/KCl to obtain a second supernatant (lane 2). Polypeptides of 90, 80, and 41 kD are indicated by the arrowheads. Identical volumes were loaded in each lane.
(C) AMP-PNP–sensitive binding of pollen ATP-MAPs to microtubules. Before addition of bovine brain microtubules, ATP-depleted HSS was divided into two parts, one of which was not supplemented with AMP-PNP. Both taxol pellets were then processed as reported. The silver-stained SDS gel (identical volumes loaded in each lane) shows the final ATP-MAPs fractions, obtained without AMP-PNP (lane 1) and with AMP-PNP (lane 2). T, tubulin.
We also investigated the capacity of ATP and KCl to trigger dissociation of ATP-MAPs from microtubules. The taxol pellet was first washed with ATP reagent only and then centrifuged. The resulting ATP supernatant (Figure 2B, lane 1) contained polypeptides at 90, 80, and 41 kD, in addition to the tubulin subunits. The corresponding pellet was treated with ATP and KCl and then centrifuged to obtain a new supernatant (Figure 2B, lane 2). The 90-kD polypeptide was no longer detected, and the 41-kD band was faint. Most of the 80-kD polypeptide was found in this sample. Densitometric analysis of three different preparations revealed that ATP released almost all of the 90-kD polypeptide, only 15 ± 6% (mean ±se, n = 3) of the 80-kD polypeptide, and 80 ± 8% (n = 3) of the 41-kD polypeptide.
We also investigated the effect of AMP-PNP on the affinity of ATP-MAPs for microtubules. The same pollen extract was divided and processed in two ways. As shown in Figure 2C, a similar polypeptide pattern was found in samples obtained without (lane 1) and with (lane 2) AMP-PNP. The polypeptide pattern was similar in both cases, but more ATP-MAPs were recovered after treatment with AMP-PNP. To quantify the intensity variation of each band, we analyzed the gel by scanning densitometry. Results from three different preparations indicated that the amount of the 80-kD polypeptide increased from 3.06 ± 0.65 to 20.6 ± 1.55 (mean ±se, n = 3) arbitrary quantitative units in the AMP-PNP–treated sample, whereas the 90-kD polypeptide ranged from only 5.18 ± 0.78 to 13.3 ± 1.85 (n = 3) arbitrary quantitative units. Tubulin concentrations were unchanged, and the relative quantity of the 41-kD polypeptide increased from 9.48 ± 1.01 to 19.03 ± 1.22 arbitrary quantitative units (n = 3) after AMP-PNP treatment.
Fractionation of ATP-MAPs and ATPase Activity Assay
Almost all microtubule-based motor proteins have microtubule-enhanced ATPase activity (Cohn, 1990). To establish whether pollen ATP-MAPs share this feature, we separated polypeptides in the ATP-MAPs fraction by using gel filtration chromatography and then determined ATPase activity. Typical elution profiles for relative protein concentration and ATPase activity are shown in Figure 3; the corresponding polypeptide composition of fractions from the preparation is shown in Figure 4. Five main peaks were detected after gel filtration chromatography (Figure 3A). The first peak (fractions 15 to 16) represented the void volume of the gel filtration column (and probably contained very high molecular mass proteins or aggregates). The 80-kD polypeptide was the main constituent of the second protein peak (fractions 23 to 25). The 90-kD ATP-MAP eluted in the third peak (fractions 27 to 28), separately from the 80-kD polypeptide. The fourth peak was essentially the 41-kD polypeptide (fractions 30 to 31). The fifth peak consisted of ATP (from the releasing buffer) and thus represented the bed volume of the column. The electrophoretic pattern shown in Figure 4A confirmed that individual ATP-MAPs did not coelute and therefore did not form a macromolecular complex. When the ATPase activity of each fraction was evaluated, the peak for the 90-kD polypeptide coincided with a peak of microtubule-stimulated ATPase activity (Figure 3B). In this case, ATPase activity is expressed as nmol Pi min−1 mL−1 because the assay was performed on samples of equal volume. A peak of ATPase activity associated with the 90-kD ATP-MAP was apparent only in the presence of microtubules. Analysis of the other fractions provided very low enzymatic activities. The specific ATPase activity in the peak fraction of the 90-kD was estimated as 7.3 ± 2.2 nmol Pi min–1 mg–1 (mean ±se, n = 3), which was stimulated approximately threefold by the addition of microtubules (to 22.5 ± 3.4 nmol Pi min–1 mg–1, n = 3).
Figure 3.
Gel Filtration Chromatography of the Pollen Tube ATP-MAPs and Quantitation of Mg-ATP Activity.
(A) The ATP-MAPs fraction was fractionated on a Superdex 200 HR 10/30 chromatography column. The column was eluted at 0.75 mL min−1, and 0.5-mL fractions were collected. The absorbance of the eluate was monitored at a wavelength of 280 nm and is expressed as absorbance units (mAU). Arrows indicate the position of protein standards: from left to right, catalase, IgG, BSA, and ovalbumin. Numbers in the graph indicate the main peaks.
(B) Mg-ATPase activity with and without microtubules was analyzed for the same fractions. Activity is expressed as nmol of Pi min−1 mL−1.
Figure 4.
Silver-Stained SDS Gels of the Gel Filtration Fractions from the Preparation Shown in Figure 3 and Fractionation of Pollen ATP-MAPs by Sucrose Gradient Centrifugation.
(A) Superdex 200 HR 10/30 gel filtration of pollen ATP-MAPs. The numbers and arrowheads at left indicate Mr standards (M) in kilodaltons. The numbers at top are fraction numbers. The numbers and arrowheads at right indicate 90-, 80-, and 41-kD ATP-MAPs, which peaked in fractions 27 to 28, 24, and 31, respectively. The column (1 × 30 cm) was eluted with HEMD buffer. Proteins eluted between fractions 15 and 38, but only fractions 17 to 34 are shown. L, ATP-eluted MAPs loaded onto the column.
(B) Fractionation of pollen ATP-MAPs by sucrose gradient centrifugation. The silver-stained SDS gel shows that the 90- and 41-kD polypeptides cosedimented in a single peak centered around fraction 13, whereas the 80-kD polypeptide eluted around fraction 14. In this experiment, 4.8 mL of 5 to 25% sucrose in HEEM buffer plus 2 mM DTT was overlaid with 200 μL of ATP-MAPs and centrifuged at 83,700g for 15 hr at 4°C in a Sorvall AH-650 swinging-bucket rotor. Arrows at bottom indicate positions of standard proteins: from left to right, thyroglobulin, catalase, and BSA. L, loaded sample; T, tubulin.
The molecular mass of ATP-MAPs was calculated by the Svedberg equation using average values of the diffusion and sedimentation coefficients. The diffusion coefficient of ATP-MAPs was measured by comparing the elution profiles on the gel filtration column of single ATP-MAPs with those for a series of proteins having known diffusion coefficients (catalase, BSA, and ovalbumin). The mean (±se) of three measurements indicated diffusion coefficients of 5.60 ± 0.22, 4.52 ± 0.35, and 7.56 ± 0.48 × 10–7 cm2 sec–1 for the 90-, 80-, and 41-kD ATP-MAPs, respectively. Sucrose gradient ultracentrifugation was used to determine the sedimentation coefficient of ATP-MAPs (Martin and Ames, 1961). A series of proteins with known sedimentation coefficients was used to calibrate the gradients. The elution positions of the protein standards are indicated by arrows in Figure 4B. Three individual measurements yielded sedimentation coefficients of 6.52, 5.91, and 7.13 for the 90-, 80-, and 41-kD ATP-MAP, respectively. Values of molecular mass were calculated by the Svedberg equation and determined to be 102, 115, and 83 kD for the 90-, 80-, and 41-kD polypeptides, respectively. Comparing these data with the SDS-PAGE results suggests that the 90- and 80-kD ATP-MAPs are monomeric proteins.
Interactions between Pollen Tube ATP-MAPs and Microtubules
After purification on the gel filtration column, isolated ATP-MAPs were probed for capacity to bind microtubules under different conditions. The results were compared with those for other known proteins. In a control sedimentation assay, MAP2 (a nonmotor microtubule-associated protein) bound to microtubules (Figure 5A, lane 2), but BSA did not (Figure 5A, lane 4). Neither of the two proteins pelleted in the absence of microtubules (Figure 5A, lanes 7 and 9). Bovine brain kinesin did not sediment in the absence of microtubules (Figure 5B, lanes 1 and 2) or in the presence of microtubules plus ATP (Figure 5B, lanes 7 and 8). When nucleotides were absent (lanes 3 and 4) or when AMP-PNP was added to the mixture (Figure 5B, lanes 5 and 6), bovine brain kinesin bound to microtubules and was sedimented into the pellet. The three major ATP-MAPs showed similar binding properties (Figures 5C to 5E). Under our assay conditions, the 90-, 80-, and 41-kD ATP-MAPs pelleted with microtubules in the absence of nucleotides (Figures 5C to 5E, lanes 1 and 2) and in the presence of AMP-PNP (Figures 5C to 5E, lanes 3 and 4). The three polypeptides exhibited different behavior in the presence of ATP and microtubules: the 90-kD band did not bind (Figure 5C, lanes 5 and 6), most of the 80-kD polypeptide bound to microtubules (Figure 5D, lanes 5 and 6), and a few 41-kD polypeptides remained attached to microtubules (Figure 5E, lanes 5 and 6). The nucleotide-sensitive microtubule binding properties of 90-kD ATP-MAP are identical to those of conventional bovine brain kinesin.
Figure 5.
Nucleotide-Sensitive Binding of Gel Filtration–Purified Pollen ATP-MAPs to Bovine Brain Microtubules by Sedimentation Assay.
(A) Control experiment showing that a known nonmotor microtubule binding protein (MAP2) binds to taxol-stabilized microtubules (pellet in lane 2), whereas BSA does not (pellet in lane 4). Lanes 5 and 6 show the pattern of microtubules. MAP2 alone did not sediment under the binding condition (supernatant and pellet in lanes 7 and 8). BSA alone did not pellet again (supernatant and pellet in lanes 9 and 10).
(B) Control experiment showing that bovine brain kinesin alone does not pellet (lanes 1 and 2). Lanes 3 and 4 show kinesin plus microtubules (kinesin pellets in the presence of microtubules). Lanes 5 and 6 show kinesin plus microtubules plus AMP-PNP (kinesin binds more efficiently to microtubules). Lanes 7 and 8 show kinesin plus microtubules plus ATP (now kinesin does not pellet). KHC, kinesin heavy chain; KLC, kinesin light chain.
(C) Binding assay of the 90-kD polypeptide. . The numbers and arrowheads at left indicate Mr standards (M) in kilodaltons. Lanes 1 and 2 show the 90-kD ATP-MAP plus microtubules (it binds to microtubules and pellets). Lanes 3 and 4 show that the binding affinity of the 90-kD polypeptide increases in the presence of AMP-PNP. Lanes 5 and 6 show the 90-kD ATP-MAP plus microtubules plus ATP (no pellet forms). Lanes 7 and 8 show that the 90-kD ATP-MAP alone does not pellet.
(D) Binding assay of the 80-kD polypeptide. Conditions are as described for the 90-kD polypeptide in (C); lanes 1 and 2 are 80-kD polypeptide plus microtubules; lanes 3 and 4 are 80-kD polypeptide plus microtubules plus AMP-PNP; lanes 5 and 6 are 80-kD polypeptide plus microtubules plus ATP; and lanes 7 and 8 show that the 80-kD polypeptide alone does not pellet.
(E) Binding experiment with the 41-kD polypeptide. Conditions are as described in (C). Lanes 1 and 2 are in the absence of nucleotides; lanes 3 and 4 are with AMP-PNP; lanes 5 and 6 are with ATP; and lanes 7 and 8 show that the 41-kD polypeptide alone does not pellet.
P, pellet; S, supernatant; T, tubulin.
In Vitro Motility Assay
As a key feature, motor proteins cause microtubules to glide in motility assays in vitro (Vale et al., 1985). The motor activity of ATP-MAPs was determined by gliding assays of microtubules on ATP-MAPs bound to cover slips. Microtubules were assembled from bovine brain tubulin polymerized at 35°C and stabilized with taxol. Single microtubules bound to the polypeptides in the ATP-MAPs fraction on the cover slip and glided in the presence of ATP. The ATP-MAPs fraction caused microtubules to glide at a speed of 0.03 μm sec–1 (data not shown). After purification of ATP-MAPs by gel filtration chromatography, the motility assay was focused on the 90-kD ATP-MAP, because this polypeptide showed microtubule-stimulated ATPase activity. Preliminary experiments performed on purified 80- and 41-kD polypeptides did not give positive results.
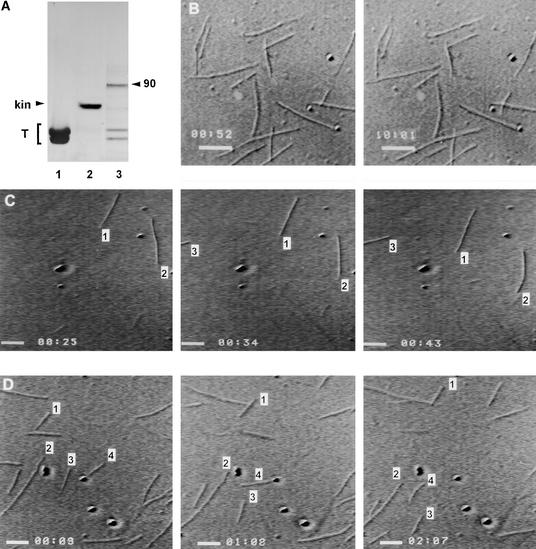
Proteins used in the assay are shown in Figure 6A. Lane 1 is tubulin, lane 2 contains recombinant kinesin, and lane 3 is fraction 27 obtained from gel filtration chromatography of ATP-MAPs. Fraction 27 contains the 90-kD ATP-MAP and tubulin, plus other minor contaminating proteins at 100 to 110 kD. Using scanning densitometry, we estimated that the 90-kD ATP-MAP corresponded to 30.9 ± 4.6% of the protein in the sample (mean ±se, n = 3), whereas the tubulin heterodimer accounted for 47.9 ± 5.7%. If tubulin is not taken into account, the concentration of the 90-kD polypeptide increases to 59.3 ± 6.8% of total protein content. Therefore, not only is the concentration of contaminating proteins very low, but these polypeptides did not cross-react with any of the anti-kinesin antibodies we used. Microtubules alone (without addition of motor proteins) did not show motor activity after ∼10 min of observation (Figure 6B). A sequence of frames of microtubules gliding on commercial recombinant kinesin (used as a positive control) is shown in Figure 6C. Velocity was determined for 30 microtubules bound to recombinant kinesin that adhered to the cover slip. Virtually 90 to 95% of microtubules glided on recombinant kinesin at a speed of 0.2 ± 0.04 μm sec–1 (mean ±se, n = 30) (Figure 6C). For comparison, microtubules that glided on purified 90-kD ATP-MAP are shown in Figure 6D. The translocation speed of purified 90-kD ATP-MAP was determined by examining the gliding movement of 40 microtubules bound to the motor protein. Most microtubules translocated actively at a velocity of 0.040 ± 0.008 μm sec–1 (n = 40) (Figure 6D). In the absence of ATP, no gliding was observed; the addition of 5 mM AMP-PNP to the motility buffer was sufficient to inhibit microtubule translocation. The time indicated in each figure (shown as min:sec) was generated by an Argus-20 image processor.
Figure 6.
Microtubules Gliding on Recombinant Kinesin and the 90-kD ATP-MAPs.
(A) Polypeptides used in the assay. Lane 1, tubulin (T); lane 2, commercial recombinant kinesin (kin); and lane 3, purified 90-kD ATP-MAP.
(B) Time-lapse sequences showing that microtubules alone do not move. Numbers above scale bars denote time (min:sec).
(C) and (D) Time-lapse sequences of microtubules gliding on recombinant kinesin (C) and purified 90-kD ATP-MAP bound to a cover slip (D). The time (min:sec) is indicated at bottom left and was generated automatically by Argus-20. In (C), microtubules are gliding on commercially available recombinant kinesin. Frames were captured every 9 sec. Three single microtubules are numbered. In (D), microtubules are moving on purified 90-kD ATP-MAP bound to a cover slip. Frames were captured every 59 sec. Numbers indicate some moving microtubules.
Bars in (B) to (D) = 2 μm, computed automatically by Argus-20.
Immunoblot Analysis with Pan-Kinesin Antibodies in Pollen Tube Extracts and Purified ATP-MAPs
Pollen ATP-MAPs were found to have properties that match those of motor proteins, such as ATP-sensitive AMP-PNP–enhanced microtubule binding, microtubule-stimulated ATPase activity, and microtubule motor activity. Anti-kinesin antibodies (especially antibodies to kinesin peptides) can be used as a first approach for analyzing the immunological and structural relationship between new motors and the kinesin superfamily (Cole et al., 1992; Sawin et al., 1992). To compare the pollen ATP-MAPs with kinesins, we used several different anti-kinesin antibodies: HD (directed against the head of Drosophila kinesin), SUK4 (against sea urchin kinesin), three anti-peptide antibodies (HYPIR, LAGSE, and MMR44, raised against highly conserved amino acid sequences found in most kinesins and KLPs), and a commercially available anti-kinesin antibody (Sigma). The antibodies were probed on purified bovine brain kinesin, on purified ATP-MAPs, on the crude extract, and on the cytosolic and membrane fractions from pollen tubes. As expected, all antibodies recognized the heavy chain of bovine brain kinesin (data not shown). When the antibodies were tested on purified ATP-MAPs and on protein fractions from pollen tubes, the results were negative. The only exception was the MMR44 antibody, discussed below.
SDS-PAGE and immunoblot analysis of protein extracts are shown in Figure 7. A 7.5% gel was loaded with bovine brain kinesin (Figure 7A, lane 2), bovine brain tubulin (lane 3), purified 80-kD ATP-MAP (lane 4), 90-kD ATP-MAP (lane 5), 41-kD ATP-MAP (lane 6), LSS (lane 7), cytosolic pollen tube fraction (lane 8), and membrane pollen tube fraction (lane 9). These proteins were transferred to polyvinylidene difluoride and immunoblotted with the MMR44 antibody (Figure 7B), which recognized the heavy chain of bovine brain kinesin (lane 11) but not purified tubulin (lane 12). The antibody did not stain purified 80-kD (lane 13) or 41-kD (lane 15) polypeptides, but it recognized the 90-kD ATP-MAP (lane 14). It also recognized a band at 90 kD in the tube extract (lane 16). The same band was weakly detected in the cytosolic fraction (lane 17) but more intensely in the membrane fraction (lane 18). A reactive band at 102 kD was also found in the LSS and in the cytosolic fraction (lanes 16 and 17). The MMR44 antibody recognized two polypeptides in the tube extract: the 102-kD polypeptide was only cytoplasmic, whereas the 90-kD ATP-MAP was detected in association with a membrane fraction from pollen tubes. We emphasize that the 90-kD ATP-MAP is not a proteolytic fragment of the 102-kD polypeptide because the latter is not detected in the membrane fraction. Moreover, detection of the 90-kD polypeptide in the cytosolic fraction was not a result of contamination by the membrane fraction.
Figure 7.
Anti-Kinesin Immunoblot Analysis.
(A) Silver-stained SDS gel of bovine brain kinesin (lane 2, 2 μg); purified bovine brain tubulin (lane 3, 20 μg); gel filtration–purified 80-kD ATP-MAPs (lane 4, 40 μL), 90-kD ATP-MAPs (lane 5, 40 μL), and 41-kD ATP-MAPs (lane 6, 40 μL); LSS from tobacco pollen tubes (lane 7, 40 μg); and cytosolic fraction (lane 8, 40 μg) and membrane fraction (lane 9, 40 μg) of tobacco pollen tubes. Lane 1 contains Mr standards, with masses indicated at left in kilodaltons. T, tubulin.
(B) MMR44 antibody recognized the kinesin heavy chain (KHC, lane 11) but not bovine brain tubulin (lane 12). The antibody did not recognize the 80-kD (lane 13) or the 41-kD polypeptide (lane 15), but it did label purified 90-kD ATP-MAP (lane 14). Note the band at ∼102 kD in LSS (lane 16) and in cytosolic fraction (lane 17). The MMR44 antibody labeled 90-kD ATP-MAP in the LSS (lane 16) and in the membrane fraction (lane 18). A faint band at the same molecular mass was also detected in the cytosolic fraction (lane 17). Lane 10 contains biotinylated marker proteins, with sizes indicated at left in kilodaltons.
Lanes 7, 8, 9, 16, 17, and 18 were overloaded to evaluate antibody specificity.
Immunolabeling with the MMR44 Antibody on Isolated Organelles
To determine whether the 90-kD ATP-MAP associated with all the membranous structures of the pollen tube, we affixed organelles extracted from the tube to glass slides and labeled the organelles with the MMR44 antibody. The phase contrast micrograph of Figure 8A is of a typical preparation of organelles after processing for immunofluorescence, and the fluorescence micrograph of Figure 8B of the same field shows labeling associated with individual organelles. The nature of the labeled organelles cannot be determined with certainty. However, these results confirm that the 90-kD ATP-MAP identified in blot analysis by the MMR44 antibody is associated with isolated organelles. The MMR44 antibody labeled only a small percentage (∼7%) of the organelles on the glass slide; moreover, the size of labeled organelles was not uniform (Figure 8C). Details of some organelles that react with the MMR44 antibody are shown in Figure 8D.
Figure 8.
Immunofluorescence Localization of MMR44-Labeled Polypeptide Associated with Organelles from Tobacco Pollen Tubes.
(A) DIC micrograph of organelle fraction from tobacco.
(B) Fluorescence microscopy of the field shown in (A). Only a small percentage of organelles were labeled.
(C) A detail of organelles immobilized on a poly-l-lysine–coated slide. Organelles labeled by the MMR44 antibody (arrows) were not uniform in size.
(D) Fluorescence microscopy of the field shown in (C).
Bars in (A) to (D) = 4 μm.
Staining with the MMR44 Antibody in Tobacco Pollen Tubes
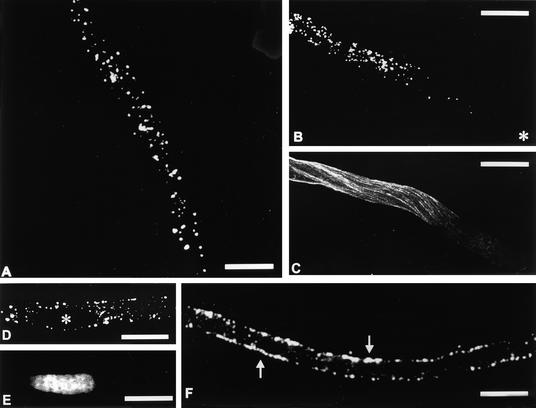
Distribution of MMR44-labeled organelles in growing pollen tubes was monitored by immunofluorescence microscopy techniques. Punctate staining of presumed organelles was observed with the MMR44 antibody (Figure 9) in chemically fixed pollen tubes. Staining was found to be uniform along the tube (Figure 9A), except in the apical region, where only a few faint spots could be seen (Figure 9B). The most intense region of MMR44 staining overlapped with the region of the pollen tube in which microtubules were most abundant (Figure 9C). Staining of the generative cell with the MMR44 antibody was not observed. Although some fluorescent spots were found at the edge of the generative cell, propidium iodide staining of nucleic acids revealed that the cytoplasm of the cell was not stained (Figures 9D and 9E). A confocal section through the middle of a pollen tube showed that the organelles labeled by the MMR44 antibody were confined to the cortical region (Figure 9F, arrows), where microtubules also happen to be concentrated. We emphasize that the MMR44 antibody did not stain the cytoplasmic microtubule array because the antibody did not recognize bovine brain tubulin in the immunoblot (Figure 7B).
Figure 9.
Immunolocalization of MMR44 Antibody in Tobacco Pollen Tubes.
(A) The MMR44 antibody labeled in a punctate fashion throughout the vegetative cytoplasm, indicating that the antigen it recognized is associated with organelles.
(B) The punctate pattern was not observed in the apical region of the pollen tube, where only a few, faint dots could be seen. The asterisk indicates the pollen tube apex.
(C) Typical immunostaining of microtubules in the pollen tube reveals that the distribution of MMR44-labeled organelles coincides with regions in which microtubules are abundant. (This is not the same pollen tube shown in [B].)
(D) and (E) In (D), the MMR44 antibody did not label the generative cell (asterisk) as compared by DNA staining in (E). Some fluorescent dots can be seen at the edge of the generative cell.
(F) A confocal section through the middle of a pollen tube. MMR44 staining is concentrated in the cortical region (arrows), and only faint spots can be seen in the central regions.
Bars in (A) to (F) = 20 μm.
Chemically fixed pollen tubes were doubly labeled with an antibody against the α-tubulin subunit and the MMR44 antibody (Figure 10). As shown in Figure 10A, the microtubules (green) extend along the tube in a longitudinal fashion, whereas the punctate staining pattern of MMR44 (red) occurs in rows, indicating alignment of the organelles along filamentous structures (arrow). Higher magnification of double-labeled pollen tubes probed with the antibody to α-tubulin and with MMR44 revealed several organelle-like fluorescent spots aligned along microtubules (Figure 10B, arrows). Figure 10C shows detail of an area of Figure 10B in which MMR44-stained organelles have colocalized specifically with microtubules (arrows). Colocalization of MMR44-stained organelles with microtubules was also confirmed by confocal analysis of cortical regions of the pollen tubes. Figure 10D shows a confocal section through the cortex of tobacco pollen tubes, where microtubules overlapped with several MMR44-labeled organelles (arrows).
Figure 10.
Double Immunolocalization of MMR44 and Anti-Tubulin Antibodies in Tobacco Pollen Tubes.
(A) Colocalization of organelles labeled with the MMR44 antibody along microtubules. Bundles of microtubules (green) extend along the longitudinal axis of the pollen tube. Rows of punctate dots stained by MMR44 (red) are aligned with microtubule bundles (arrow). Bar = 20 μm.
(B) Colocalization of organelles labeled with the MMR44 antibody (red) along microtubules (green). Organelles labeled with MMR44 are aligned in rows where microtubules are located (arrows). Bar = 10 μm.
(C) Magnification of the enclosed region in (B) showing organelles precisely aligned with microtubules (arrows). Bar = 2 μm.
(D) A confocal section through pollen tube cortex showing organelles (red, arrows) that colocalize with a couple of microtubule bundles (green). Bars in (A) to (D) = 5 μm.
DISCUSSION
In this article, we report the identification and functional characterization of nucleotide-dependent microtubule binding polypeptides from tobacco pollen tubes. One of these polypeptides (the 90-kD ATP-MAP) has microtubule-stimulated ATPase activity and can translocate microtubules in motility assays in vitro. Furthermore, a pan-kinesin antibody (MMR44), which recognized the 90-kD ATP-MAP, stained membrane-bounded organelles from pollen tubes that were found to colocalize with microtubules in the cortical region of the tube.
The biochemical and functional properties of ATP-MAPs are typical of microtubule-based motor proteins, matching those of kinesins and KLPs but differing from those of other nonmotor microtubule binding proteins. Several microtubule binding proteins have been identified in plants, but only motor proteins have microtubule-dependent ATPase activity. The 90-kD ATP-MAP has microtubule-stimulated ATPase activity, whereas the 80- and 41-kD polypeptides do not. The specific ATPase activity of the 90-kD ATP-MAP increased approximately threefold after the addition of microtubules. This enhancement is similar to that reported for tobacco TKRP125 (Asada and Shibaoka, 1994), Arabidopsis katC (Mitsui et al., 1994) and katD (Tamura et al., 1999), the PKH (Cai et al., 1993), and the 100-kD kinesin-related polypeptide of Corylus pollen (Liu et al., 1994). The microtubule binding properties of individual ATP-MAPs differ to some extent. ATP releases the 90-kD and almost all of the 41-kD ATP-MAPs, whereas the 80-kD polypeptide requires the presence of 200 mM KCl for its release from microtubules. The binding conditions of the 80-kD polypeptide were similar to those of other KLPs that were not completely released from microtubules in the presence of ATP (Hogan et al., 1993). These KLPs may have an additional ATP-independent microtubule binding site. The 90-kD ATP-MAP has microtubule binding properties almost identical to those of kinesins and does not pellet with microtubules in the presence of ATP, but it does pellet in the absence of nucleotides and in the presence of AMP-PNP.
Here, we used animal tubulin to isolate ATP-MAPs and to analyze ATPase activity and in vitro microtubule motility. Although endogenous pollen tube microtubules would have been more appropriate, our choice of exogenous tubulin was dictated by the fact that the structure of microtubules formed by taxol-induced polymerization of pollen tube tubulin differs from that of native microtubules (Tiezzi et al., 1987). Furthermore, the enzyme and motor activity of kinesins (including those from plant cells) has generally been analyzed using animal tubulin assembled into taxol-stabilized microtubules.
One of the strongest indications of the presence of motor proteins in the ATP-MAPs fraction was that the 90-kD ATP-MAP sustained microtubule translocation in motility assays in vitro. This was achieved after purification of the 90-kD ATP-MAP by gel filtration chromatography. The ATP-MAPs fraction sustained gliding of microtubules, and this activity was related to the 90-kD ATP-MAP; neither the 80- nor the 41-kD polypeptide glided microtubules in these assays. The 90-kD ATP-MAP sustained microtubule translocation at a speed of 0.040 ± 0.008 μm sec−1. Although low compared with animal kinesin (Saxton et al., 1988) and KLPs (Cole et al., 1993), the velocity compares favorably with that of motors such as the homotetrameric kinesin of sea urchin eggs (Cole et al., 1994) and AtKCBP of Arabidopsis (Song et al., 1997). Indeed, TKRP125 of tobacco BY-2 cells causes microtubule gliding at only ∼0.021 μm sec−1 (Asada and Shibaoka, 1994). The finding that Ca2+/calmodulin modulates both the ATPase activity (Deavours et al., 1998) and microtubule motility (Song et al., 1997) of a plant kinesin subfamily suggests that similar mechanisms of control might also exist in the pollen tube. Indeed, the pollen tube is characterized by a relatively high concentration of Ca2+ in the apex (Pierson et al., 1994), with calmodulin concentrated in a V-shaped region close to the tip (Moutinho et al., 1998). Although the 90-kD ATP-MAP is not distributed in the apical region of the pollen tube, understanding the modulation by Ca2+/calmodulin of the enzymatic and motility properties of this protein is an interesting topic for future research.
The diffusion and sedimentation coefficients suggest that the 90-kD ATP-MAP may be a monomeric protein. Most kinesins and KLPs identified in cells are dimeric proteins, by virtue of a coiled-coil stalk domain that forms dimers (Thormahlen et al., 1998). Monomeric, globular KLPs have also been reported, acting as proteins involved in organelle transport (Okada et al., 1995). The nucleotide sequence of katD, a KLP expressed in the floral tissue of Arabidopsis and among the few examples of monomeric KLPs in plant cells, suggests that this protein is monomeric (Tamura et al., 1999). A monomeric microtubule-based motor in the pollen tube, therefore, cannot be considered unusual.
The biochemical and in vitro motility data suggest that the 90-kD ATP-MAP could be a microtubule-based motor. Further correlations between pollen ATP-MAPs and the kinesin superfamily were found by using antibodies against different conserved kinesin motifs. Several motifs were recognized in each of the complete kinesin motor domain sequences, and additional motifs were found in most kinesin proteins (Vale and Fletterick, 1997). The MMR44 antibody is raised against two highly conserved amino acid sequences of the kinesin motor domain (Marks et al., 1994), which are also found in plant KLPs. Two KLPs from tobacco cells are KRP125 (Asada et al., 1997) and TCK1 (Wang et al., 1996). The MMR44 antibody also recognizes a KLP identified in Neurospora crassa (Steinberg and Schliwa, 1995). When probed in pollen tube extracts, that antibody recognized the 90-kD ATP-MAP, underlining the possibility that the 90-kD protein may be a kinesin. Although the MMR44 antibody was raised against a peptide sequence that contains and extends over the LAGSE motif, the LAGSE antibody (Sawin et al., 1992) failed to give a positive blot with 90-kD ATP-MAP. We also probed pollen fractions and purified ATP-MAPs with the SUK4 (Ingold et al., 1988), HD (Rodionov et al., 1993), and HYPIR antibodies (Sawin et al., 1992) without obtaining positive results (data not shown). Results were also negative with a commercial anti-kinesin antibody. Plant kinesins are not always recognized by anti-kinesins raised against sequences from other eukaryotic cells (Asada and Shibaoka, 1994).
Immunoblot analysis of pollen tube fractions revealed that the 90-kD ATP-MAP is concentrated in the membrane fraction. The differential distribution of the 90-kD ATP-MAP between the membrane and soluble protein pool was similar to the distribution of the 100-kD kinesin-related protein of Corylus pollen, which is concentrated in the membrane fraction but also occurs as a soluble protein (Liu et al., 1994). The MMR44 antibody cross-reacted with another cytoplasmic polypeptide of 102 kD that did not bind microtubules during the preparation of ATP-MAPs (data not shown) and that was not detected in the membrane fraction. The 102-kD polypeptide was therefore not a microtubule binding protein, nor could it be associated with the organelle-like pattern observed when the MMR44 antibody was probed on pollen tubes.
Reaction of the MMR44 antibody with organelle-coated slides showed that the 90-kD ATP-MAP was associated with organelles of different sizes. We have shown by protein blot assays that the MMR44 antibody also cross-reacted with a 102-kD polypeptide that does not pellet with the pollen tube organelles. Therefore, results obtained with the MMR44 antibody on organelle-coated slides are ascribed to the presence of the 90-kD polypeptide. Association of kinesins and KLPs with organelles has been demonstrated in several reports (Hirokawa, 1998). The MMR44 antibody also reacts with kinesin molecules associated with organelles in animal cells (Marks et al., 1994). We have not yet performed experiments to determine the type of organelle that associates with the 90-kD ATP-MAP. The punctate staining observed in pollen tubes with MMR44 can reasonably be ascribed to association of the 90-kD ATP-MAP with organelles in the cortical region of the tube. In this study, we did not observe staining of the generative cell or the apical region of the pollen tube, which implies that the 90-kD ATP-MAP is neither associated with the generative cell nor involved in translocation of vesicles in the tube apex. The localization of the MMR44-stained organelles is consistent with their interaction with microtubules: organelles are located in the cortical region of the pollen tube, where microtubules concentrate; organelles colocalize with microtubules. The staining pattern therefore indicates that the 90-kD ATP-MAP may participate in the translocation of organelles along the cortical microtubule cytoskeleton. However, it is unlikely to be the sole motor protein driving organelle motion in the pollen tube cortex. The staining pattern with MMR44 antibody differed from the patterns obtained with k71s23 anti-PKH (Tiezzi et al., 1992) and DY-1 anti-dynein (Moscatelli et al., 1998) in tobacco pollen tubes. The pollen tube therefore presumably contains different microtubule motors that convey specific organelles or cargoes. Nothing similar to this functional differentiation occurs in other plant cells, but animal cells show several such examples (Hirokawa, 1996). This differential distribution of KLPs is somewhat reminiscent of the different localization of myosin classes in the pollen tube (Miller et al., 1995).
The identification of a microtubule motor in the cortical region of the pollen tube suggests that this protein participates in the translocation of organelles in the tube cortex. Toxicological evidence (Cai et al., 1997; Li et al., 1997) indicates that actin–myosin interactions are the main force-generating system for organelle movement in the pollen tube. On the other hand, evidence also suggests that microtubules and actin filaments combine forces in many types of cells to deliver organelles to their final destination (Goode et al., 2000). Coordinated microtubule- and actin-based transport of organelles and vesicles occurs by many mechanisms in eukaryotic cells, including the presence of both motor classes on the same organelle (Fath et al., 1994), the involvement of kinesin and myosin in two transport steps that assemble vesicles in sites of exocytosis (Bi et al., 1997), and a physical interaction between myosins and kinesin-related proteins (Beningo et al., 2000). The only evidence of interactions between actin filaments and microtubules in the pollen tube is coalignment (Pierson et al., 1986; Lancelle and Hepler, 1991); there is no indication that the two systems cooperate functionally during vesicle and organelle transport. On the basis of the present results, we propose that the translocation of organelles and vesicles in the pollen tube cortex is the result of dynamic interactions between microtubules and motor proteins. For example, the actin network may be used for long-range transport of organelles, and the microtubule network for short-range transport or fine-tuning of vesicle delivery.
These data are evidence that the pollen tubes of tobacco contain a 90-kD polypeptide with motor activity along microtubules. The polypeptide has microtubule-stimulated ATPase activity, binds microtubules in a nucleotide-sensitive way, and cross-reacts with an anti-kinesin. The antibody localizes the motor protein on the surface of membrane-bound organelles, which associate with microtubules in the cortex of the pollen tube. These findings favor the hypothesis that the pollen tube contains a motor protein that sustains movement of subcellular components along microtubules.
METHODS
Chemicals and Antibodies
All chemicals (unless otherwise indicated) were obtained from Sigma (Milan, Italy). The following anti-kinesin antibodies were used: polyclonal antibody (HD) directed against the head of Drosophila kinesin (Rodionov et al., 1993); monoclonal antibody (SUK4) directed against the sea urchin egg kinesin heavy chain (Ingold et al., 1988); peptide antibodies LAGSE, HYPIR (Sawin et al., 1992), and MMR44 (Marks et al., 1994) directed against highly conserved regions of the kinesin motor domain; and anti-kinesin antibody from Sigma. Tubulin was probed with monoclonal antibodies against the α subunit (Amersham Pharmacia, Uppsala, Sweden).
Pollen Culture and Preparation of Subcellular Fractions
Anthers of tobacco (Nicotiana tabacum) were collected from plants grown in the botanical gardens of Siena University. After dehiscence, pollen was collected, dried on silica gel, and stored at −20°C. Before use, pollen was progressively acclimatized to room temperature and hydrated in a moist chamber. It was then germinated in BK medium (Brewbaker and Kwack, 1963) containing 15% (w/v) sucrose for 2 to 3 hr in a rotary incubator at 24°C, after which proteins were extracted from the germinated pollens.
To prepare the cytosolic and membrane fractions, we washed germinated pollen (∼0.1 g) twice with 10 mL of HEEM buffer (25 mM Hepes, pH 7.5, 2 mM EDTA, 2 mM EGTA, and 1 mM MgCl2) plus 15% sucrose, resuspended in an equal volume of extraction buffer (HEEM buffer containing 1 mM polymethylsulfonyl fluoride [PMSF], 0.1 mg mL–1 tosyl-arginine-methyl ester, 1 mM DTT, 10 μg mL–1 leupeptin, 8 μM antipain, 10 μg mL–1 pepstatin A, and 10% mannitol), and homogenized on ice with a motor-driven Potter-Elvehjem homogenizer. The homogenate was centrifuged at 10,000g for 10 min at 4°C to remove large cell debris. This low-speed supernatant (LSS) was partially processed for SDS-PAGE analysis, and the remainder was centrifuged again at 100,000g for 60 min at 4°C on a 15% sucrose cushion in the homogenization buffer. The high-speed supernatant (HSS; the cytosolic fraction) and the pellet (the membrane fraction) were processed for SDS-PAGE analysis. The membrane fraction was also used for the immunofluorescence assay of organelles dispersed on poly-l-lysine–coated slides (see below). In this case, the pellet was resuspended in HEEM buffer supplemented with 1 mM DTT, 1 mM PMSF, and 10% mannitol.
Purification of ATP-Sensitive Microtubule Binding Proteins from Pollen Tubes
ATP-dependent microtubule binding proteins from the HSS of pollen tubes were isolated by a combination of methods (Cole et al., 1992, 1994). After germination, pollen cells were washed in HEEM buffer containing 15% sucrose. Pollen was collected by centrifuging at 800g for 10 min and homogenized in 1 volume of cold extraction buffer (HEEM containing 2 mM DTT, 10 μg mL–1 leupeptin, 10 μg mL–1 pepstatin, 40 μM antipain, 1 mM PMSF, 0.1 mg mL–1 tosyl-arginine-methyl ester, 1 mM NaN3, and 15% sucrose). The sample was homogenized in a cold room by using a motor-driven Potter-Elvehjem homogenizer (15 strokes) and then centrifuged at 85,000g for 30 min at 4°C. The pellet and the superficial lipid layer were discarded. The LSS was frozen in liquid nitrogen and stored at −80°C. Before use, the LSS was thawed in cool water and then put on ice. Five to ten frozen extracts were used each time to increase protein recovery. Fresh protease inhibitors (PMSF and leupeptin) were added. The LSS was then centrifuged at 186,000g for 60 min at 4°C on a 20% (w/v) sucrose cushion in HEEM buffer plus 2 mM DTT, and the pellet was discarded. The HSS thus obtained was supplemented with hexokinase and d(+)-glucose to final concentrations of 10 units mL–1 and 50 mM, respectively. The sample was incubated for 30 min at room temperature and centrifuged at 110,000g for 30 min at 4°C on a 20% (w/v) sucrose cushion; again, the pellet was discarded. The supernatant was supplemented with 1 mg mL–1 bovine brain tubulin, 1 mM GTP, and 20 μM taxol, incubated for 30 min at 25°C, and mixed with adenylylimido-diphosphate (AMP-PNP) made up to 10 mM. After incubation for 30 min at 25°C, the sample was centrifuged at 27,000g for 30 min at 20°C. The supernatant was processed for SDS-PAGE analysis, and the pellet was washed with 0.3 mL of EDTA buffer (HEEM buffer without MgCl2 but containing 10 mM EDTA, 2 mM DTT, 5 μM taxol, and 1 mM AMP-PNP). After incubation for 10 min at 4°C, the sample was centrifuged at 27,000g for 30 min at 4°C. The pellet was resuspended in 0.3 mL of ATP buffer (HEEM buffer without EDTA but containing 10 mM Mg-ATP, 200 mM KCl, 2 mM DTT, and 5 μM taxol). The sample was incubated for ∼14 hr at 4°C and then centrifuged at 27,000g for 30 min at 4°C. The supernatant collected was thereafter referred to as the ATP-MAPs (ATP-dependent microtubule-associated proteins) fraction (Cole et al., 1992). The pellet was resuspended in 0.3 mL of HEEM buffer and processed for SDS-PAGE analysis.
As a control, the above protocol was also performed without addition of bovine brain tubulin. Taxol (20 μM) and GTP (1 mM) were added to ATP-depleted HSS of tobacco pollen tubes to induce polymerization of endogenous tubulin. The resulting taxol pellet was washed with EDTA buffer and incubated with the ATP buffer for ∼14 hr at 4°C. After centrifugation, the ATP supernatant and pellet were processed for SDS-PAGE analysis.
The binding of pollen polypeptides to microtubules was also evaluated by using AMP-PNP instead of ATP during the binding step. The rest of the protocol was the same.
The ability of ATP to dissociate ATP-MAPs from microtubules was determined by incubating the taxol pellet in KCl-depleted ATP buffer for 30 min at room temperature. The sample was centrifuged at 27,000g for 30 min at 15°C. The resulting ATP supernatant was processed for SDS-PAGE analysis. The ATP-extracted pellet was incubated with ATP/KCl mixture as reported above. After overnight incubation at 4°C, the sample was centrifuged to obtain the corresponding supernatant and pellet.
The effect of AMP-PNP on microtubule binding affinity was analyzed by omitting AMP-PNP during the binding step. The sample was still supplemented with tubulin, taxol, and GTP as described above, incubated for 30 min, and processed as already outlined.
Fractionation of ATP-MAPs by Gel Filtration Chromatography
The ATP-MAPs fraction was fractionated by gel filtration chromatography on a Superdex 200 HR 10/30 column (Amersham Pharmacia). The sample (0.3 mL) was centrifuged in a microfuge to remove insoluble aggregates and then loaded onto the column. An AKTA Purifier system (Amersham Pharmacia) was used for chromatographic analysis. A 1.5-column volume of HEMD buffer (25 mM Hepes, pH 7.5, 2 mM EGTA, 1 mM MgCl2, and 1 mM DTT ) was used for elution at an elution rate of 0.75 mL min−1. Eluting polypeptides were collected in 0.5-mL fractions. Absorbance was monitored at 254 and 280 nm. The chromatographic results were exported as text files and were processed using Microsoft Excel. Thyroglobulin and ATP were used to determine the void volume and total volume of the column, respectively. Catalase, BSA, and ovalbumin were used as standards to calculate the diffusion coefficient of ATP-MAPs. Aliquots from each fraction were analyzed by SDS-PAGE on 7.5% gels and stained with silver.
ATPase Activity
Samples (400 μL) were supplemented with taxol-polymerized tubulin at 1 mg mL–1. After a 10-min incubation, ATP was added to a final concentration of 2 mM. The assay volume was 600 μL. Test tubes were incubated at 35°C in a water bath. Aliquots of 50 μL were removed after 5, 10, 20, 30, and 40 min and analyzed to determine the linearity of enzyme activity. The quantity of free inorganic phosphate was calculated by photometric assay (Gonzales-Romo et al., 1992) and compared with a standard concentration curve for inorganic phosphate. The results were processed using Microsoft Excel.
Velocity Sedimentation
Centrifuging through continuous sucrose gradients without further processing was used to fractionate polypeptides of the ATP-MAPs fraction. Samples (∼200 μL) were loaded directly onto 4.8 mL of 5 to 25% sucrose gradient prepared by three freezing/thawing cycles (Baxter-Gabbard, 1972). Sucrose was dissolved in HEEM buffer containing 1 mM PMSF and 2 mM DTT. Thyroglobulin (19.1 S), catalase (11.3 S), and BSA (4.4 S) were used as standards to calculate the sedimentation coefficient (Martin and Ames, 1961). Samples were centrifuged at 80,000g for 15 hr at 4°C (Sorvall AH-650 rotor; Kendro Laboratory Products, Newtown, CT), and 20 fractions of ∼250 μL each were collected from the sample tube bottom. The samples were analyzed by silver-stained SDS-PAGE.
The molecular mass of ATP-MAPs was determined by substituting the calculated diffusion and sedimentation coefficients into the Svedberg equation:
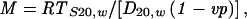 |
where R is the ideal gas constant (8.31 × 107 erg deg–1 mol–1) and T = 293K; we assumed a partial specific volume of υ = 0.725 cm3 g–1 and the density of water at 293K as P = 0.9982 g cm–3.
Microtubule Cosedimentation Assay
Gel filtration fractions that contained purified ATP-MAPs were pooled and analyzed by a microtubule cosedimentation assay (Goode and Feinstein, 1994). Briefly, 50 μL of sample was combined with 40 μL of taxol-stabilized microtubules (0.5 mg mL–1 tubulin concentration) and 10 μL of HEEM/taxol buffer (HEEM buffer plus 20 μM taxol). As a control, the HEEM/taxol buffer was supplemented with 100 mM ATP or 50 mM AMP-PNP to final concentrations of 10 and 5 mM, respectively. All samples were incubated for 30 min at room temperature and then centrifuged at 100,000g for 60 min at 20°C on a 40% (w/v) glycerol cushion in HEEM/taxol buffer. Supernatants were processed for SDS-PAGE analysis. The pellets were resuspended in an equal volume of HEEM/taxol buffer and then processed for SDS-PAGE analysis. All supernatants and pellets were analyzed on 7.5% gels and stained with silver.
In control experiments, a nonmotor microtubule binding protein (bovine brain MAP2), a protein unrelated to microtubule (BSA), and a known microtubule-based motor protein (bovine brain kinesin) were used to validate the microtubule sedimentation assay. As a further control, purified bovine brain tubulin was processed in the same way but in the absence of any other protein.
Purification and Sources of Other Proteins
Tubulin was isolated from bovine brain by three cycles of temperature-dependent polymerization–depolymerization (Williams and Lee, 1982). Tubulin was further purified through a Mono-Q HR5/5 column (Amersham Pharmacia) and desalted with a HiPrep 26/10 Desalting column (Amersham Pharmacia). Bovine brain kinesin was purified by cosedimentation with microtubules, followed by anion exchange chromatography on a Mono-Q column and gel filtration chromatography on a Sephacryl S-300 column (Amersham Pharmacia) (Kuznetsov et al., 1988). MAP2 was isolated from bovine brain by sedimentation with microtubules and heat denaturation (Hugdahl et al., 1993). Recombinant kinesin was obtained from Cytoskeleton, Inc. (Denver, CO).
Motility Assays
The motor activity of purified proteins was analyzed by in vitro motility assays (Cross, 1998). Microtubules were polymerized from monomeric tubulin (∼10 mg mL–1) in the presence of 1 mM GTP and 10% glycerol for 20 min at 35°C. The microtubule sample was diluted 1:25 in microtubule resuspension buffer (80 mM Pipes, pH 6.8, 1 mM MgCl2, 1 mM EGTA, 1 mM DTT, and 20 μM taxol), left at room temperature, and used for several motility assays. Two pieces of double-sided scotch tape were attached to thoroughly clean microscope slides, and clean cover slips were placed on these to form perfusion chambers of 10 to 15 μL. The motor solution was introduced into the perfusion chamber and incubated for 5 min in a humid atmosphere. Multiple perfusions of the 90-kD ATP-MAP (1-min incubation each) were needed to increase the concentration of motor protein on the cover slip surface. Because of the low concentration of motor protein, the cover slips were also precoated with 5 mg/mL casein. The microtubule solution was diluted 1:10 in motility buffer (80 mM Pipes, pH 6.8, 1 mM MgCl2, 1 mM EGTA, 1 mM DTT, 5 mM ATP, and 2 μM taxol) and added to the perfusion chamber. After a 1-min incubation, the slides were observed by video-enhanced differential contrast microscopy for gliding of microtubules (Weiss, 1986). In control experiments, the motor protein solution was substituted for a nonmotor protein such as casein. As further control, the motility buffer was depleted of ATP, or the ATP was replaced by an equal concentration of AMP-PNP. The equipment for the in vitro motility assays consisted of a Zeiss (Oberkochen, Germany) Axiophot microscope with a 100× Planapos 1.3 objective and phase contrast filter set. A Hamamatsu C2400-75i charge-coupled device (CCD) camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan) connected to an Argus-20 image enhancer (Hamamatsu) was used to visualize the microtubules. The Background Subtraction and Average commands of the instruments were used to enhance image quality. Video sequences were recorded on U-matic tapes (Sony, Tokyo, Japan). To print single video images, individual frames were captured with HPD-CP software (version 1.0; Hamamatsu) running on a PC interfaced to Argus-20 via a small computer system interface connection. To evaluate gliding velocity, single microtubules were tracked with the mouse cursor, and their velocity was calculated with the Speed command of Argus-20.
Immunofluorescence Assays
Staining of organelles adhering to poly-l-lysine–coated slides was performed as follows. Approximately 10 μL of organelle solution (prepared as described above) was dispersed on poly-l-lysine–coated slides, and the organelles were allowed to adhere for 10 min in a humidity chamber. After washing with blocking solution (HEEM buffer supplemented with 1 mM DTT, 1 mM PMSF, 10% mannitol, and 5% BSA), the sample was incubated for 30 min with the primary antibody (MMR44) diluted 1:200 in blocking solution. After extensive washing in blocking solution, the secondary antibody (rhodamine-conjugated goat anti–rabbit Ig diluted 1:300; Molecular Probes, Leiden, The Netherlands) was added and incubated for 30 min in a humidity chamber. After they were washed with blocking solution, the preparations were mounted for observation with a Zeiss Axiophot microscope with a 100× Planapos 1.3 objective and DIC filter set. Both DIC and fluorescent images were acquired by using the Hamamatsu C2400-75i CCD camera connected to the image processor. The Frint command of the Argus-20 was used to boost the sensitivity of the CCD camera, allowing observation of fluorescence phenomena. In controls, the primary antibody was omitted.
The standard protocol for immunofluorescence microscopy of chemically fixed pollen tubes was used (Del Casino et al., 1993). Nucleic acids were stained with propidium iodide diluted 1:7000 and added to samples immediately before microscopic observation. The primary antibodies (MMR44 and α-tubulin) were used at a dilution of 1:200. The secondary antibody Texas Red–conjugated goat anti–rabbit Ig (Molecular Probes) was diluted 1:300, and fluorescein isothiocyanate–conjugated goat anti–mouse Ig (Cappel Laboratories, Durham, NC) was diluted 1:400. A TCS 4D Leica confocal scanning light microscope with a 100× oil-immersion objective was used to collect images. In control experiments, the primary antibodies were omitted or were labeled with the unrelated secondary antibody.
Electrophoresis and Immunoblot Analysis
For SDS-PAGE analysis, we used a 7.5% linear acrylamide concentration (Laemmli, 1970). Electrophoresis reagents were from Bio-Rad Laboratories (Hercules, CA). All gels were stained with silver (Dunn, 1989). For immunoblot analysis, gels were transferred to polyvinylidene difluoride membranes (Amersham Pharmacia) by using a TE22 Transfer Unit (Amersham Pharmacia). Blotting conditions were according to Towbin et al. (1979). Primary antibodies were diluted as follows: 10 μg mL–1 for HD, SUK-4, LAGSE, and HYPIR; 1:2500 for MMR44; and 1:100 for commercial anti-kinesin. The secondary antibodies were donkey anti–mouse IgG peroxidase-linked (Amersham) and donkey anti–rabbit IgG peroxidase-linked (Amersham), each diluted 1:5000. Biotinylated molecular mass standards were from Amersham Pharmacia. Blots were developed according to the manufacturer's instructions with the enhanced chemiluminescence kit from Amersham Pharmacia. Gels and blots were scanned with a GS-710 densitometer and Quantity One software (both from Bio-Rad). Scans were performed for densitometric analysis to determine the molecular mass of polypeptides and to store images of gels and blots.
Determination of Protein Concentration
When required, the protein concentration was determined by a photometric method (Lowry et al., 1951), with BSA as the protein standard.
General Image Processing
Electronic images of gels, blots, immunofluorescence microscopy, and motility assays were recorded on Kodak Gold 100 ASA 35-mm films using a Polaroid (Bedford, MA) ProPalette 7000. Figures were mounted using Microsoft PowerPoint, photographed with the ProPalette, and printed on Agfa paper (Agfa Gevaert, Leverkusen, Germany).
Acknowledgments
We thank Dr. Mark McNiven (Center for Basic Research in Digestive Diseases, Mayo Clinic and Foundation, Rochester, MN) for the kind gift of the MMR44 antibody. We also thank Dr. Kenneth E. Sawin (Department of Pharmacology, University of California, San Francisco, CA) for kindly donating the LAGSE and HYPIR antibodies, Dr. Fatima K. Gyoeva (Institute of Protein Research, Russian Academy of Sciences, Moscow, Russia) for providing the HD antibody, and Dr. Jonathan M. Scholey (Section of Molecular and Cellular Biology, University of California at Davis, CA) for the SUK4 antibody. The LAGSE, HYPIR, HD, and SUK4 antibodies were all obtained with the generous help of Dr. Uday K. Tirlapur. We thank Prof. Ronald D. Vale (Department of Cellular and Molecular Pharmacology, University of California, San Francisco, CA), Dr. Clive Lloyd (Department of Cell Biology, The John Innes Centre, Colney, Norwich, UK), and Dr. Francesca Navone (Dipartimento di Farmacologia, Università di Milano, Milano, Italy) for helpful and critical discussion, and also Dr. Robert A. Cross (Marie Curie Molecular Motors Group, Oxted, UK), whose suggestions were important for setting up the motility assay. This study was financed in part by a grant from the University of Siena (Research Program 1999).
References
- Asada, T., and Collings, D. (1997). Molecular motors in higher plants. Trends Plant Sci. 2, 29–37. [Google Scholar]
- Asada, T., and Shibaoka, H. (1994). Isolation of polypeptides with microtubule-translocating activity from phragmoplasts of tobacco BY-2 cells. J. Cell Sci. 107, 2249–2257. [DOI] [PubMed] [Google Scholar]
- Asada, T., Kuriyama, R., and Shibaoka, H. (1997). TKRP125, a kinesin-related protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J. Cell Sci. 110, 179–189. [DOI] [PubMed] [Google Scholar]
- Åström, H., Sorri, O., and Raudaskoski, M. (1995). Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sex. Plant Reprod. 8, 61–69. [Google Scholar]
- Baxter-Gabbard, K.L. (1972). A simple method for the large-scale preparation of sucrose gradients. FEBS Lett. 20, 117–119. [DOI] [PubMed] [Google Scholar]
- Beningo, K.A., Lillie, S.H., and Brown, S.S. (2000). The yeast kinesin-related protein Smy1p exerts its effects on the class V myosin Myo2p via a physical interaction. Mol. Biol. Cell 11, 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, G.-Q., Morris, R.L., Liao, G., Alderton, J.M., Scholey, J.M., and Steinhardt, R.A. (1997). Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis. J. Cell Biol. 138, 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewbaker, J.L., and Kwack, B.H. (1963). The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 50, 859–865. [Google Scholar]
- Cai, G., Bartalesi, A., Del Casino, C., Moscatelli, A., Tiezzi, A., and Cresti, M. (1993). The kinesin-immunoreactive homologue from Nicotiana tabacum pollen tube: Biochemical properties and subcellular localization. Planta 191, 496–506. [Google Scholar]
- Cai, G., Moscatelli, A., and Cresti, M. (1997). Cytoskeletal organization and pollen tube growth. Trends Plant Sci. 2, 86–91. [Google Scholar]
- Cohn, S.A. (1990). The mechanochemistry of kinesin. Mol. Chem. Neuropathol. 12, 83–94. [DOI] [PubMed] [Google Scholar]
- Cole, D.G., Cande, W.Z., Baskin, R.J., Skoufias, D.A., Hogan, C.J., and Scholey, J.M. (1992). Isolation of sea urchin egg kinesin-related protein using peptide antibodies. J. Cell Sci. 101, 291–301. [DOI] [PubMed] [Google Scholar]
- Cole, D.G., Chinn, S.W., Wedaman, K.P., Hall, K., Vuong, T., and Scholey, J.M. (1993). Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature 366, 268–270. [DOI] [PubMed] [Google Scholar]
- Cole, D.G., Saxton, W.M., Sheehan, K.B., and Scholey, J.M. (1994). A “slow” homotetrameric kinesin-related motor protein purified from Drosophila embryos. J. Biol. Chem. 269, 22913–22916. [PMC free article] [PubMed] [Google Scholar]
- Cross, R.A. (1998). Microtubule motility assays. In Cell Biology: A Laboratory Handbook, J.E. Celis, ed (New York: Academic Press), pp. 317–326.
- Deavours, B.E., Reddy, A.S.N., and Walker, R.A. (1998). Ca2+/calmodulin regulation of the Arabidopsis kinesin-like calmodulin-binding protein. Cell Motil. Cytoskeleton 40, 408–416. [DOI] [PubMed] [Google Scholar]
- Del Casino, C., Li, Y., Moscatelli, A., Scali, M., Tiezzi, A., and Cresti, M. (1993). Distribution of microtubules during the growth of tobacco pollen tubes. Biol. Cell 79, 125–132. [Google Scholar]
- Derksen, J., Pierson, E.S., and Traas, J.A. (1985). Microtubules in vegetative and generative cells of pollen tubes. Eur. J. Cell Biol. 38, 142–148. [Google Scholar]
- Dunn, M. (1989). Determination of total protein concentration. In Protein Purification Methods: A Practical Approach, E.L.V. Harris and S. Angal, eds (Oxford, UK: IRL Press, Oxford University Press), pp. 29–40.
- Fath, K.R., Trimbur, G.M., and Burgess, D.R. (1994). Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J. Cell Biol. 126, 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitmann, A., Li, Y.-Q., and Cresti, M. (1995). The role of cytoskeleton and dictyosome activity in the pulsatory growth of Nicotiana tabacum and Petunia hybrida pollen tubes. Bot. Acta 109, 102–109. [Google Scholar]
- Gonzales-Romo, P., Sanchez-Nito, S., and Gavilanes-Ruiz, M. (1992). A modified colorimetric method for the determination of orthophosphate in the presence of high ATP concentrations. Anal. Biochem. 200, 235–238. [DOI] [PubMed] [Google Scholar]
- Goode, B.L., and Feinstein, S.C. (1994). Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J. Cell Biol. 124, 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode, B.L., Drubin, D.G., and Barnes, G. (2000). Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 12, 63–71. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison, J., Heslop-Harrison, Y., Cresti, M., Tiezzi, A., and Moscatelli, A. (1988). Cytoskeletal elements, cell shaping and movement in the angiosperm pollen tube. J. Cell Sci. 91, 49–60. [Google Scholar]
- Hirokawa, N. (1996). The molecular mechanism of organelle transport along microtubules: The identification and characterization of KIFs (kinesin superfamily proteins). Cell Struct. Funct. 21, 357–367. [DOI] [PubMed] [Google Scholar]
- Hirokawa, N. (1998). Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279, 519–526. [DOI] [PubMed] [Google Scholar]
- Hirokawa, N., Noda, Y., and Okada, Y. (1998). Kinesin and dynein superfamily proteins in organelle transport and cell division. Curr. Opin. Cell Biol. 10, 60–73. [DOI] [PubMed] [Google Scholar]
- Hogan, C.J., Wein, H., Wordeman, L., Scholey, J.M., Sawin, K.E., and Cande, W.Z. (1993). Inhibition of anaphase spindle elongation in vitro by a peptide antibody that recognizes kinesin motor domain. Proc. Natl. Acad. Sci. USA 90, 6611–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl, J.D., Bokros, C.L., Hanesworth, V.R., Aalund, G.R., and Morejohn, L.C. (1993). Unique functional characteristics of the polymerization and MAP binding regulatory domains of plant tubulin. Plant Cell 5, 1063–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold, A.L., Cohn, S.A., and Scholey, J.M. (1988). Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J. Cell Biol. 107, 2657–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos, U., van Aken, J., and Kristen, U. (1994). Microtubules are involved in maintaining the cellular polarity in pollen tubes of Nicotiana sylvestris. Protoplasma 179, 5–15. [Google Scholar]
- Kuznetsov, S.A., Vaisberg, Y.A., Shanina, N.A., Magretova, N.N., Chernyak, V.Y., and Gelfald, V.I. (1988). The quaternary structure of bovine brain kinesin. EMBO J. 7, 353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lancelle, S.A., and Hepler, P.K. (1991). Association of actin with cortical microtubules revealed by immunogold localization in Nicotiana pollen tubes. Protoplasma 165, 167–172. [Google Scholar]
- Li, Y.-Q., Moscatelli, A., Cai, G., and Cresti, M. (1997). Functional interactions among cytoskeleton, membranes, and cell wall in the pollen tube of flowering plants. Int. Rev. Cytol. 176, 133–199. [DOI] [PubMed] [Google Scholar]
- Liu, B., Cyr, R.J., and Palevitz, B.A. (1996). A kinesin-like protein, katAp, in the cells of Arabidopsis and other plants. Plant Cell 8, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G.Q., Cai, G., Del Casino, C., Tiezzi, A., and Cresti, M. (1994). Kinesin-related polypeptide is associated with vesicles from Corylus avellana pollen. Cell Motil. Cytoskeleton 29, 155–166. [DOI] [PubMed] [Google Scholar]
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- Marks, D.L., Larkin, J.M., and McNiven, M.A. (1994). Association of kinesin with the Golgi apparatus in rat hepatocytes. J. Cell Sci. 107, 2417–2426. [DOI] [PubMed] [Google Scholar]
- Martin, R.G., and Ames, B.N. (1961). A method for determining the sedimentation behavior of enzymes; application to protein mixtures. J. Biol. Chem. 236, 1372–1379. [PubMed] [Google Scholar]
- Mascarenhas, J.P. (1993). Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5, 1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, D.D., Scordilis, S.P., and Hepler, P.K. (1995). Identification and localization of three classes of myosins in pollen tubes of Lilium longiflorum and Nicotiana alata. J. Cell Sci. 108, 2549–2563. [DOI] [PubMed] [Google Scholar]
- Mitsui, H., Nakatani, K., Yamaguchi-Shinozaki, K., Shinozaki, K., Nishikawa, K., and Takahashi, H. (1994). Sequencing and characterization of the kinesin-related genes katB and katC of Arabidopsis thaliana. Plant Mol. Biol. 25, 865–876. [DOI] [PubMed] [Google Scholar]
- Mitsui, H., Hasezawa, S., Nagata, T., and Takahashi, H. (1996). Cell cycle–dependent accumulation of a kinesin-like protein, KatB/C in synchronized tobacco BY-2 cells. Plant Mol. Biol. 30, 177–181. [DOI] [PubMed] [Google Scholar]
- Moscatelli, A., Del Casino, C., Lozzi, L., Cai, G., Scali, M., Tiezzi, A., and Cresti, M. (1995). High molecular weight polypeptides related to dynein heavy chains in Nicotiana tabacum pollen tubes. J. Cell Sci. 108, 1117–1125. [DOI] [PubMed] [Google Scholar]
- Moscatelli, A., Cai, G., Ciampolini, F., and Cresti, M. (1998). Dynein heavy chain–related polypeptides are associated with organelles in pollen tubes of Nicotiana tabacum. Sex. Plant Reprod. 11, 31–40. [Google Scholar]
- Moutinho, A., Love, J., Trewavas, A.J., and Malhó, R. (1998). Distribution of calmodulin protein and mRNA in growing pollen tubes. Sex. Plant Reprod. 11, 131–139. [Google Scholar]
- Okada, Y., Yamazaki, H., Sekine-Aizawa, Y., and Hirokawa, N. (1995). The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81, 769–780. [DOI] [PubMed] [Google Scholar]
- Oppenheimer, D.G., Pollock, M.A., Vacik, J., Szymanski, D.B., Ericson, B., Feldmann, K., and Marks, M.D. (1997). Essential role of a kinesin-like protein in Arabidopsis trichome morphogenesis. Proc. Natl. Acad. Sci. USA 94, 6261–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson, E.S., Derksen, J., and Traas, J.A. (1986). Organization of microfilaments and microtubules in pollen tubes grown in vitro or in vivo in various angiosperms. Eur. J. Cell Biol. 41, 14–18. [Google Scholar]
- Pierson, E.S., Miller, D.D., Callaham, D.A., Shipley, A.M., Rivers, B.A., Cresti, M., and Hepler, P.K. (1994). Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 6, 1815–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudaskoski, M., Åström, H., Perttila, K., Virtanen, I., and Louhelainen, J. (1987). Role of microtubule cytoskeleton in pollen tubes: An immunochemical and ultrastructural approach. Biol. Cell 61, 177–188. [Google Scholar]
- Rodionov, V.I., Gyoeva, F.K., Tanaka, E., Bershadsky, A.D., Vasiliev, J.M., and Gelfand, V.I. (1993). Microtubule-dependent control of cell shape and pseudopodial activity is inhibited by the antibody to kinesin motor domain. J. Cell Biol. 123, 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin, K.E., Mitchison, T.J., and Wordeman, L.G. (1992). Evidence for kinesin-related proteins in the mitotic apparatus using peptide antibodies. J. Cell Sci. 101, 303–313. [DOI] [PubMed] [Google Scholar]
- Saxton, W.M., Porter, M.E., Cohn, S.A., Scholey, J.M., Raff, E.C., and McIntosh, J.R. (1988). Drosophila kinesin: Characterization of microtubule motility and ATPase. Proc. Natl. Acad. Sci. USA 85, 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H., Golovkin, M., Reddy, A.S., and Endow, S.A. (1997). In vitro motility of AtKCBP, a calmodulin-binding kinesin protein of Arabidopsis. Proc. Natl. Acad. Sci. USA 94, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, G., and Schliwa, M. (1995). The Neurospora organelle motor: A distant relative of conventional kinesin with unconventional properties. Mol. Biol. Cell 6, 1605–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K., Nakatani, K., Mitsui, H., Ohashi, Y., and Takahashi, H. (1999). Characterization of katD, a kinesin-like protein gene specifically expressed in floral tissues of Arabidopsis thaliana. Gene 230, 23–32. [DOI] [PubMed] [Google Scholar]
- Thormahlen, M., Marx, A., Sack, S., and Mandelkow, E. (1998). The coiled-coil helix in the neck of kinesin. J. Struct. Biol. 122, 30–41. [DOI] [PubMed] [Google Scholar]
- Tiezzi, A., Moscatelli, A., Milanesi, C., Ciampolini, F., and Cresti, M. (1987). Taxol-induced structures derived from cytoskeletal elements of the Nicotiana pollen tube. J. Cell Sci. 88, 657–661. [Google Scholar]
- Tiezzi, A., Moscatelli, A., Cai, G., Bartalesi, A., and Cresti, M. (1992). An immunoreactive homolog of mammalian kinesin in Nicotiana tabacum pollen tubes. Cell Motil. Cytoskeleton 21, 132–137. [DOI] [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale, R.D., and Fletterick, R.J. (1997). The design plan of kinesin motors. Annu. Rev. Cell Dev. Biol. 13, 745–777. [DOI] [PubMed] [Google Scholar]
- Vale, R.D., Schnapp, B.J., Reese, T.S., and Sheetz, M.P. (1985). Organelle, bead, and microtubule translocations promoted by soluble factors from the squid giant axon. Cell 40, 559–569. [DOI] [PubMed] [Google Scholar]
- Wang, W., Takezawa, D., Narasimhulu, S.B., Reddy, A.S., and Poovaiah, B.W. (1996). A novel kinesin-like protein with a calmodulin-binding domain. Plant Mol. Biol. 31, 87–100. [DOI] [PubMed] [Google Scholar]
- Weiss, D.G. (1986). Visualization of the living cytoskeleton by video-enhanced microscopy and digital image processing. J. Cell Sci. 5 (suppl.), 1.–15. [DOI] [PubMed] [Google Scholar]
- Williams, R.C., Jr., and Lee, J.C. (1982). Preparation of tubulin from brain. Methods Enzymol. 85B, 376–385. [DOI] [PubMed] [Google Scholar]
- Williamson, R.E. (1993). Organelle movements. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 181–202. [Google Scholar]