Abstract
In tobacco cultivars resistant to tobacco mosaic virus (TMV), infection results in the death of the infected cells accompanying the formation of necrotic lesions. To identify the genes involved in this hypersensitive reaction, we isolated the cDNA of tobacco DS9, the transcript of which decreases before the appearance of necrotic lesions. The DS9 gene encodes a chloroplastic homolog of bacterial FtsH protein, which serves to maintain quality control of some cytoplasmic and membrane proteins. A large quantity of DS9 protein was found in healthy leaves, whereas the quantity of DS9 protein in infected leaves decreased before the lesions appeared. In transgenic tobacco plants containing less and more DS9 protein than wild-type plants, the necrotic lesions induced by TMV were smaller and larger, respectively, than those on wild-type plants. These results suggest that a decrease in the level of DS9 protein in TMV-infected cells, resulting in a subsequent loss of function of the chloroplasts, accelerates the hypersensitive reaction.
INTRODUCTION
The hypersensitive reaction (HR) is a plant defense system against pathogen attack. HR is defined as the rapid death of infected cells accompanying formation of necrotic lesions in which pathogens are thought to be enclosed (Goodman and Novacky, 1994). The HR of tobacco to tobacco mosaic virus (TMV) occurs in cultivars carrying the resistance gene N (NN tobacco) but not in cultivars lacking the N gene (nn tobacco) (Holmes, 1938; Whitham et al., 1994). Induction of the N gene–mediated HR requires the temperature to be below 22 to 26°C (Kassanis, 1952; Shimomura, 1971; Weststeijn, 1981), suggesting that the gene can function at temperatures below this range.
We previously observed that when TMV-inoculated leaves were treated with heat shock or actinomycin D, necrotic lesions were induced in not only NN but also in nn tobacco, even at 30°C, a nonpermissive temperature for the N gene (Shimomura and Ohashi, 1971; Ohashi and Shimomura, 1972). In the actinomycin D–treated leaves in which necrotic lesions were induced, the synthesis of nucleic acid in the host plant was greatly suppressed, whereas the synthesis of TMV RNA was suppressed only slightly (Shimomura and Ohashi, 1980). In contrast to treatment with actinomycin D, treatment with other chemicals, including rifampicin, 2-thiouracil, and cycloheximide, which suppress RNA or protein synthesis in both the TMV and the host plant, did not induce necrotic lesions to form (Ohashi and Shimomura, 1972). Given that actinomycin D forms intercalation complexes with DNA, thereby blocking transcription (Waring, 1981), and that heat shock suppresses transcription of housekeeping genes (Lindquist, 1986; Nover et al., 1989), we hypothesized that suppressing the transcription of certain housekeeping genes in NN tobacco plants after infection with TMV could induce the HR.
To isolate such candidate genes, we used a synchronous HR-inducing system based on a temperature shift. Incubation at 30°C of TMV-infected NN tobacco leaves results in multiplication and systemic spread of the virus without lesion formation (Weststeijn, 1981). Shifting TMV-infected leaves from 30 to 20°C induces synchronous lesion formation in the infected region. We found that necrotic lesions were induced in TMV-infected NN tobacco leaves even at 30°C by transient incubation at 20°C for >4 hr during the 30°C incubation period (Ohtsubo et al., 1999). These phenomena suggest that the period of 20°C treatment required to induce the HR, in other words, “the point of no return” for N gene expression and subsequent induction of HR, is 4 hr in our system. Therefore, by using differential screening (Seo et al., 1995), we isolated the cDNA (DS clones) of genes for which transcripts specifically increased or decreased 3 hr after the temperature shift from 30 to 20°C. Of the transcripts of the six unique cDNA clones obtained, only the DS9 transcripts were found to decrease during the formation of necrotic lesions. This observation allowed us to study the behavior of the DS9 protein in the TMV-induced HR in NN tobacco. Here, we present evidence that a decreased amount of DS9 protein in TMV-infected NN tobacco leaves accelerates the HR.
RESULTS
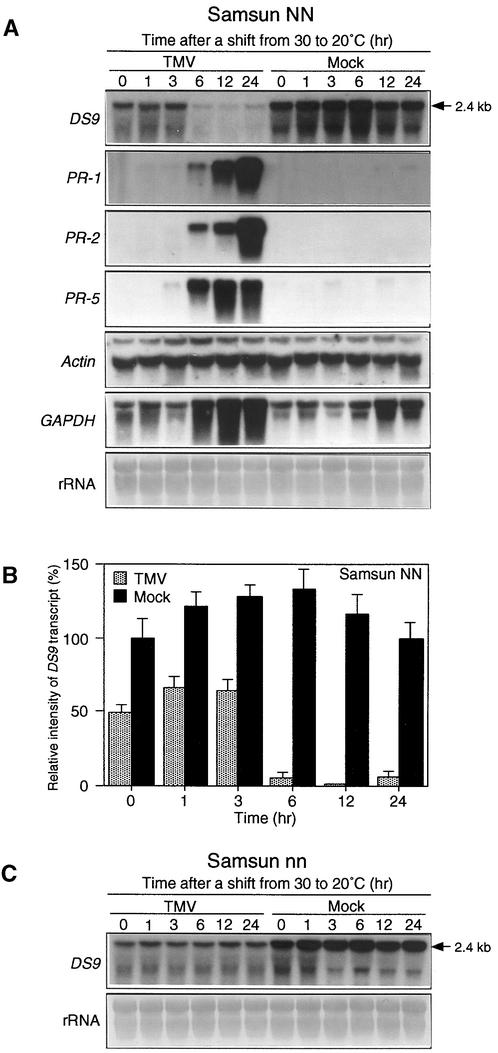
The Amount of DS9 Transcripts Decreases during the N Gene–Mediated HR
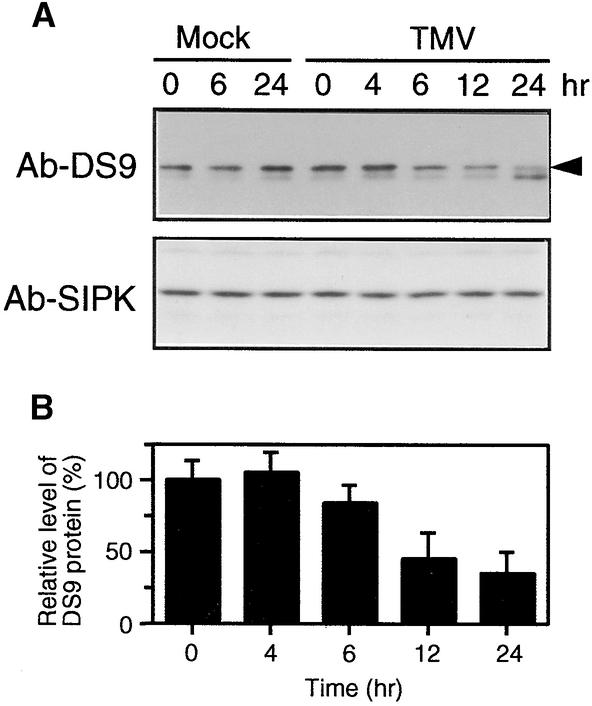
We examined changes in the amounts of DS9 transcripts during the formation of necrotic lesions in TMV-infected NN tobacco. Leaves of Samsun NN tobacco, a tobacco cultivar carrying the N gene, were inoculated with TMV, incubated for 40 hr at 30°C to allow TMV to infect as many cells as possible, and then shifted to 20°C. Under these conditions, necrotic lesions appeared 8 to 10 hr after the shift to 20°C. Using this synchronous system, we found that in TMV-infected NN tobacco leaves, the DS9 transcripts began to decrease as early as 6 hr after the shift to 20°C (Figures 1A and 1B). Although the DS9 cDNA was originally isolated as a cDNA clone for which transcript levels decreased 3 hr after the shift from 30 to 20°C (see Introduction), no decrease in the amount of DS9 transcripts was observed at that time point. These contradictory results can be explained by the difference in hybridization conditions used between differential screening and RNA gel blot analysis.
Figure 1.
RNA Gel Blot Analysis of the DS9, PR-1, PR-2, PR-5, Actin, and GAPDH Genes in TMV- and Mock-Inoculated Tobacco Leaves after Temperature Shift.
Detached healthy leaves of 50-day-old Samsun NN and 70-day-old Samsun nn tobacco plants were inoculated with TMV (10 μg/mL) or buffer only (Mock), incubated at 30°C for 40 hr, and then shifted to 20°C at time 0.
(A) Leaves from Samsun NN tobacco plants were harvested at the indicated times after the temperature shift and used for total RNA extraction. Aliquots of 20 μg of total RNA per lane were fractionated by gel electrophoresis, transferred to nylon membranes, and successively subjected to hybridization with the indicated radioactively labeled probes after stripping the former probe. To standardize RNA loading, the blot was stained with methylene blue (rRNA). The 2.4-kb RNA represents the length of the DS9 gene.
(B) Relative intensity of DS9 transcripts as shown in (A). The amounts of DS9 transcript are expressed as a ratio with that present in the control sample (0 hr after the temperature shift of mock-inoculated leaves), which was set equal to 100%. Values are the mean ±sd from four independent experiments.
(C) Leaves from Samsun nn tobacco plants were harvested at the indicated times after the temperature shift and used for total RNA extraction. RNA gel blot hybridization was performed as described in (A), using the DS9 cDNA probe. The experiment was repeated twice with similar results. The 2.4-kb RNA represents the length of the DS9 gene.
In TMV-inoculated leaves of nn tobacco cultivars, such as Samsun nn, SR1, and Xanthi, the shift to 20°C did not cause a marked decrease in DS9 transcripts (Figure 1C). In mock-inoculated leaves of both NN and nn tobacco cultivars, no substantial decrease in DS9 transcripts was seen after the temperature shift. Even before the shift to 20°C, the amount of DS9 transcripts in TMV-inoculated NN tobacco leaves was ∼50% of that in mock-inoculated ones (Figure 1B). Interestingly, a similar TMV infection–dependent decrease in the DS9 transcripts was observed in nn tobacco (Figure 1C). Together, these results suggest that during the HR in TMV-infected NN tobacco leaves, the DS9 transcripts are decreased by the multiplication of TMV, and DS9 transcripts are also lower in tissue in which the N gene is expressed.
Induction of pathogenesis-related (PR) protein genes is known to be associated with the HR in TMV-infected NN tobacco (Bol et al., 1990). In our synchronous system, the transcripts of PR-1, PR-2, and PR-5 genes indeed began to accumulate 6 hr after the shift to 20°C (Figure 1A), indicating that during the N gene–mediated HR, the pattern of expression of the gene encoding DS9 is in striking contrast with that of the PR-1, PR-2, and PR-5 genes.
Healthy leaves contained high amounts of DS9 transcripts, comparable with that observed in mock-inoculated leaves (see time 0 in mock-inoculated leaves in Figure 1A), suggesting that the DS9 gene is constitutively expressed as a housekeeping gene. This allowed us to examine whether the response after infection with TMV varies between the DS9 gene and other housekeeping genes, such as those encoding actin and the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH). In TMV-inoculated NN tobacco leaves, the amount of actin transcripts remained constant at least between 0 and 24 hr after the shift to 20°C, whereas the GAPDH transcripts rapidly increased 6 hr after the shift (Figure 1A). Thus, so-called housekeeping genes seem to exhibit different expression patterns during the N gene–mediated HR.
Sequence Analysis of DS9 cDNA
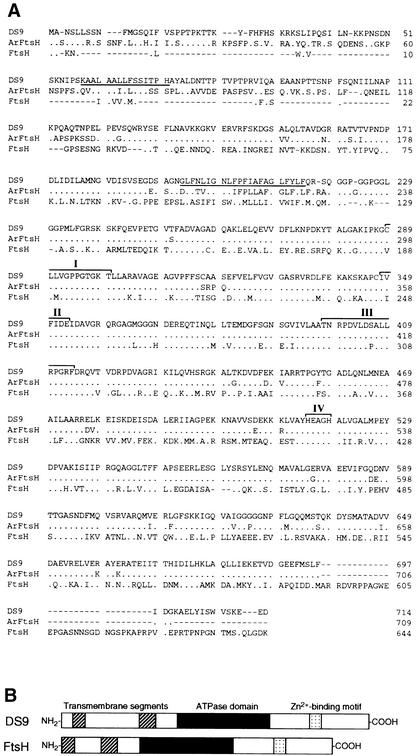
The cDNA sequence of DS9 contains a 2447-bp open reading frame encoding a protein of 714 amino acids (Figure 2A). The putative amino acid sequence shows 41 and 81% identity with Escherichia coli FtsH metalloprotease and the Arabidopsis FtsH homolog, respectively (Figure 2A). FtsH, which was originally identified in E. coli (Tomoyasu et al., 1993a), is a member of the AAA (for ATPase associated with diverse cellular activities) superfamily (Confalonieri and Duguet, 1995). The central region of DS9 contains the ATPase domain, which contains two ATP binding sites, and the SRH (for second region of homology), which is found in all members of the AAA family (Figure 2A). Furthermore, two distinctive features of the FtsH protein are present in the DS9 protein: the N terminus of DS9 contains two putative transmembrane domains that are rich in hydrophobic amino acids (Tomoyasu et al., 1993a, 1993b), and the C terminus contains a putative zinc binding motif that is responsible for metalloprotease activity (Tomoyasu et al., 1995). Recently, Summer and Cline (1999) isolated a cDNA encoding a FtsH-like protein from tobacco. The putative amino acid sequence shows 47% identity with DS9 (data not shown), indicating that tobacco has at least two FtsH-related genes. A diagrammatic alignment of the predicted DS9 protein sequence and the E. coli FtsH protein sequence is shown in Figure 2B.
Figure 2.
Comparison of the Deduced Amino Acid Sequences of DS9, Arabidopsis FtsH Homolog, and E. coli FtsH.
(A) Lines at top, center, and below represent the amino acid sequence of DS9 (GenBank accession number AB017480), an Arabidopsis FtsH homolog (Lindahl et al., 1996), and E. coli FtsH (Tomoyasu et al., 1993a), respectively. The underlined amino acids represent putative membrane-spanning regions. Dots represent identical amino acid residues, and dashes indicate gaps introduced to maximize alignment. The roman numerals indicate two regions of ATP binding motif (I and II), a second region of homology(III), and a Zn2+ binding motif (IV).
(B) Diagrammatic alignment of the primary structures of DS9 and E. coli FtsH. Hatched, black, and stippled boxes represent two putative transmembrane segments, the ATPase domain, and the putative Zn2+ binding motif, respectively. The ATPase domain contains an ATP binding motif and a second region of homology (Confalonieri and Duguet, 1995). The putative Zn2+ binding motif is HEXXH, where X indicates nonconserved amino acid residues.
Production of Recombinant DS9 Protein and Its Biochemical Characterization
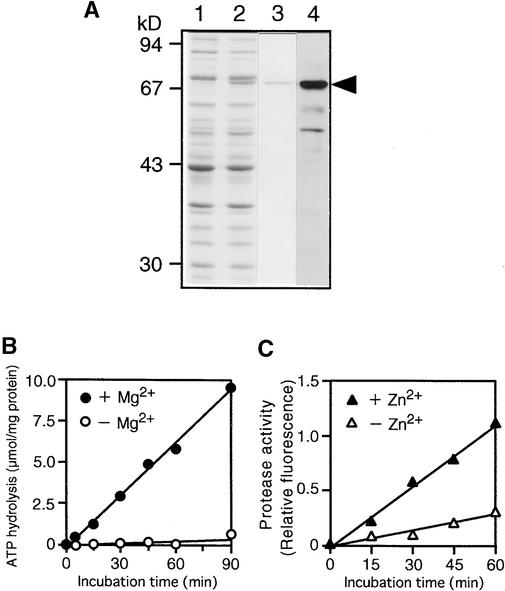
Protein degradation by FtsH metalloprotease in E. coli requires both Mg2+-dependent ATP hydrolysis and Zn2+ (Tomoyasu et al., 1995). To examine the biochemical profile of the DS9 protein, we first produced a recombinant DS9 protein as a glutathione S-transferase (GST) fusion protein with the truncated DS9 protein lacking 131 N-terminal residues (GST–DS9ΔN). DS9ΔN separated from GST by digesting GST–DS9ΔN with thrombin was found to have Mg2+-dependent ATPase activity (data not shown). However, DS9ΔN did not exhibit protease activity when casein, a general substrate for proteases, was used as the substrate. This failure to exhibit protease activity was apparently the result of the lack of the 131 N-terminal amino acid residues.
Therefore, the protein encoded by the full-length coding region of DS9 cDNA was overexpressed by isopropyl β-d-thiogalactopyranoside (IPTG) induction in E. coli cells as a protein with a six-histidine tag by using the pET-15b expression vector (Figure 3A, lane 2). The histidine-tagged protein (HisDS9) was partially purified with a nickel-chelate matrix column (Figure 3A, lane 3), and the purified protein was used to measure ATPase and protease activities. The HisDS9 protein exhibited not only Mg2+-dependent ATP-hydrolyzing activity (Figure 3B) but also Zn2+-stimulated casein-hydrolyzing activity (Figure 3C), both of which increased in a time-dependent manner. These results, together with the results presented in Figure 2, demonstrate that the protein encoded by the DS9 gene is an FtsH homolog.
Figure 3.
Expression and Enzymatic Activity of the Recombinant DS9 Protein.
(A) Expression and purification of the recombinant HisDS9 protein from E. coli cells. Total cellular extracts prepared from cells not treated with IPTG (lane 1) and IPTG-treated cells (lane 2) from E. coli were separated by electrophoresis on a 10% SDS–polyacrylamide gel; the gel was stained with Coomassie Brilliant Blue R 250. The recombinant HisDS9 protein (arrowhead) was purified by affinity chromatography (lane 3) and subjected to protein gel blot analysis (lane 4) with antibodies raised against DS9ΔN. Size markers are indicated at left in kilodaltons.
(B) Mg2+-dependent ATP-hydrolyzing activity in the recombinant HisDS9 protein. Reactions were performed in the presence (closed circles) or absence (open circles) of 5 mM MgCl2.
(C) Zn2+-stimulated casein-hydrolyzing activity in the recombinant HisDS9 protein. Fluorescein isothiocyanate–labeled casein was used as the substrate. Reactions were performed in the presence (closed triangles) or absence (open triangles) of 12.5 μM zinc acetate.
Antisera against the recombinant DS9ΔN protein were generated. The antisera recognized HisDS9 (Figure 3A, lane 4), and this antibody (anti-DS9 antibody) was used for immunological detection of the DS9 protein in tobacco plants.
The DS9 Protein Is Expressed Constitutively in Healthy Tobacco Leaves
In extracts of healthy tobacco leaves, the anti-DS9 antibody reacted strongly with an abundant 78-kD protein and with a less-abundant protein of lower molecular mass (∼72 kD; Figure 4, lane1). The 78-kD band probably corresponds to a mature form of the DS9 protein because the DS9 gene product with the N terminus containing a putative chloroplast-targeting peptide, including a few acidic residues and several serine and alanine residues (von Heijne et al., 1989; see Figure 2A), could be translocated in chloroplasts and processed to its mature size. The size of the principal immunoreactive component is comparable with the 78-kD molecular mass of the mature form of Arabidopsis FtsH protein reported by Lindahl et al. (1996). The 72-kD band may be a degraded product of the DS9 protein.
Figure 4.
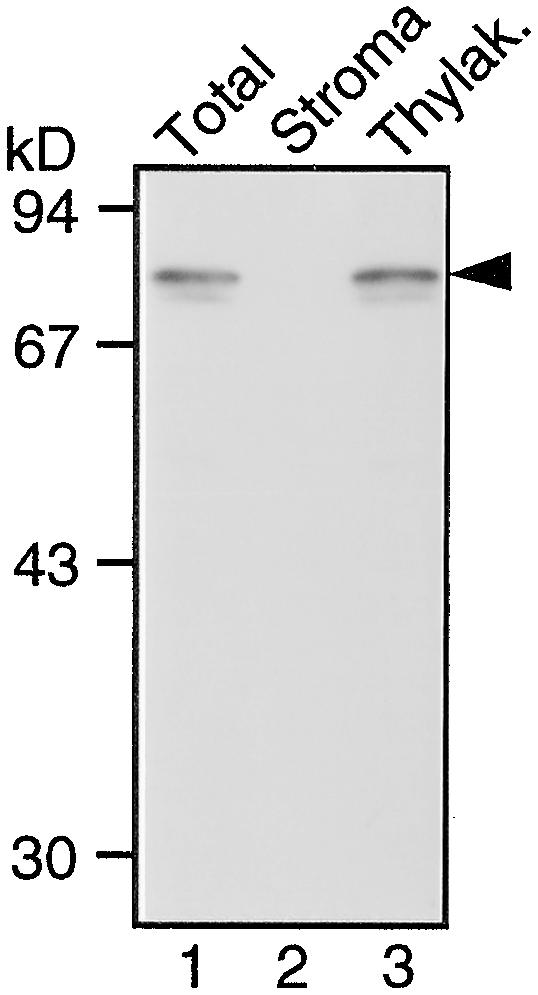
Protein Gel Blot Analysis of the DS9 Protein in Tobacco Leaf Tissue.
Total protein extracts (lane 1), a stroma fraction (lane 2), and a thylakoid (Thylak.) fraction (lane 3) prepared from healthy leaves of Samsun NN tobacco plants were subjected to protein gel blot analysis with antibodies raised against DS9ΔN. Lanes 1, 2, and 3 contain total protein extracts containing 20 μg of total protein, the stroma fraction equivalent to the volume of thylakoid membrane fraction used, and the thylakoid membrane fraction containing 5 μg of chlorophyll, respectively. The arrowhead indicates the relative molecular masses of ∼78 kD. Size markers are indicated at left in kilodaltons.
In eukaryotes, FtsH homologs associate with mitochondria and chloroplasts (Andersson and Aro, 1997; Gottesman et al., 1997), although no mitochondrial FtsH homolog has been found in plants. Localization of the DS9 protein in chloroplasts was studied with stroma and thylakoid membrane fractions. The 78-kD band was detected in the thylakoid membrane fraction (Figure 4, lane 3), whereas no band was detected in the stroma fraction (Figure 4, lane 2). This result is in agreement with the results published by Lindahl et al. (1996).
Localization of the DS9 Protein in Chloroplasts
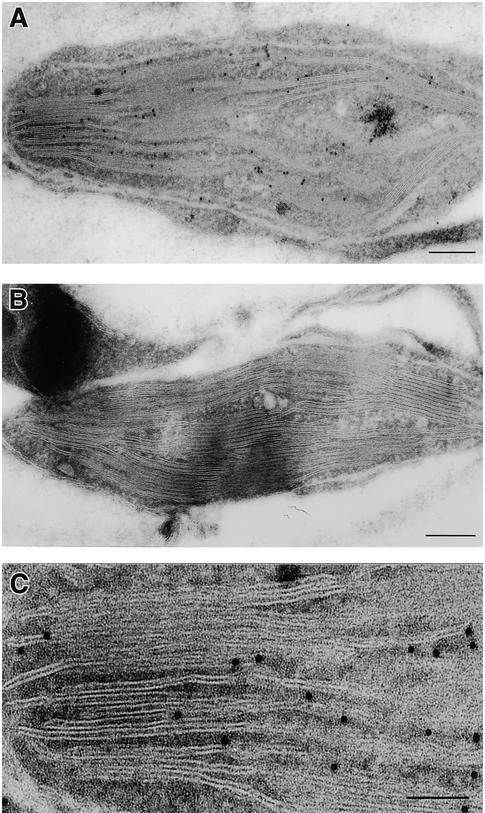
The localization of the DS9 protein in chloroplasts was further confirmed by immunoelectron microscopic analysis. When an ultrathin section of healthy leaf tissue was treated with the anti-DS9 antibody and anti–rabbit IgG conjugated with 10-nm (in diameter) particles of gold, immunogold particles specific for the DS9 protein were observed in several chloroplasts of mesophyll cells (Figure 5A). Treatment of an ultrathin section with preimmune serum produced very little labeling (Figure 5B). Immunogold particles specific for the DS9 protein were frequently associated with the stroma side of the thylakoidal membrane in chloroplasts (Figure 5C).
Figure 5.
Immunoelectron Microscopy in Frozen Sections of Leaf Tissue.
(A) Immunogold localization of the DS9 protein in a tobacco mesophyll cell treated with antibodies raised against DS9ΔN and 10-nm gold-conjugated anti–rabbit IgG. 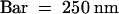 .
.
(B) A tobacco mesophyll cell treated with preimmune serum and 10-nm gold-conjugated anti–rabbit IgG. 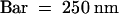 .
.
(C) Greater magnification of (A). 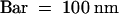 .
.
When tissue sections treated with the anti-DS9 antibody and conjugated with 10-nm gold particles were examined, the chloroplasts were found to contain most of the gold particles: 90.8, 4.9, and 4.3% of the total number of gold particles were found in the chloroplast, mitochondrion, and nucleus, respectively (Table 1).
Table 1.
Number of Gold Particles in the Chloroplasts, Mitochondrion, and Nucleus When the DS9 Protein Was Immunogold-Labeled
| Number of Gold Particles (μm−2)
|
||
|---|---|---|
| Cell Organelle | Treatmenta(nc = 22) | Controlb(n = 10) |
| Chloroplast | 12.337 ± 1.690d | 0.053 ± 0.009 |
| Mitochondrion | 0.661 ± 0.285 | 0.299 ± 0.199 |
| Nucleus | 0.585 ± 0.078 | 0.098 ± 0.020 |
Ultrathin sections of healthy leaf tissue embedded in London Resin White in the treatment group were treated with antibodies raised against DS9ΔN and 10-nm gold-conjugated anti–rabbit IgG.
Ultrathin sections of healthy leaf tissue in the control group were treated with preimmune serum and 10-nm gold-conjugated anti–rabbit IgG.
Number of cells in which the number of gold particles in the indicated cell organelle was enumerated.
Each value represents the mean ±se.
The Amount of DS9 Protein Decreases during the N Gene–Mediated HR
Changes in the amount of DS9 protein during the N gene–mediated HR were determined by protein gel blot analysis. When TMV-inoculated NN tobacco leaves were shifted from 30 to 20°C, the 78-kD protein began to decrease 6 hr after the shift, reaching a minimal level 24 hr later (Figure 6A). In contrast, in mock-inoculated leaves, no substantial decrease in the amount of the 78-kD protein was observed after the shift to 20°C. Even before the shift to 20°C, the amount of the 78-kD protein in TMV-inoculated leaves was almost equal to that in mock-inoculated ones (Figure 6B), suggesting that TMV multiplication had little effect on the decrease in DS9 protein. In TMV-inoculated leaves, the 72-kD protein increased 24 hr after the shift to 20°C. To confirm whether equal amounts of proteins had been loaded onto the gel, the blots were reprobed with an antibody raised against salicylic acid–induced protein kinase (SIPK), a protein reported to maintain constant amounts during the N gene–mediated HR (Zhang and Klessig, 1998). As shown in Figure 6A, there were no major differences in SIPK signals during the time course.
Figure 6.
Protein Gel Blot Analysis of the DS9 Protein in TMV- and Mock-Inoculated NN Tobacco Leaves after the Temperature Shift.
(A) Detached healthy leaves of Samsun NN tobacco plants were inoculated with TMV (10 μg/mL) or buffer only (Mock) and shifted from 30 to 20°C at time 0. Leaves were harvested at the indicated times after the temperature shift and used for protein extraction. Twenty micrograms of total protein was immunoblotted with antibodies raised against DS9ΔN (Ab-DS9). The arrowhead indicates the relative molecular masses of ∼78 kD. The blots were reprobed with antibodies (Ab-SIPK) against a synthetic peptide corresponding to the N-terminal 24–amino acid sequence of SIPK as a loading control.
(B) Relative amount of the DS9 protein in TMV-inoculated leaves as shown in (A), expressed as a ratio with that present in the control sample (0 hr after the temperature shift), which was set equal to 100%. Values are the mean ±sd from three independent experiments.
Actinomycin D and Heat Shock Individually Induce an HR-like Response in Leaves Systemically Infected with TMV
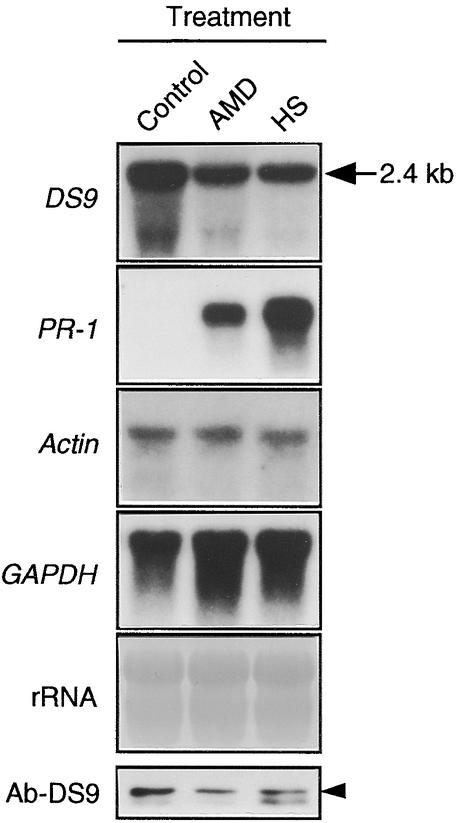
As described above, treatment with either actinomycin D or heat shock induced necrotic lesions on NN tobacco leaves systemically infected with TMV at 30°C. After treatment, we were able to examine whether the formation of lesions induced by each of these treatments is accompanied by decreases in both the DS9 transcripts and the DS9 protein. TMV-inoculated leaves of Samsun NN tobacco plants were incubated for 40 hr at 30°C and then treated with actinomycin D or subjected to heat shock, reincubated at 30°C for 18 hr, and subjected to RNA and protein gel blot analyses. The amounts of DS9 transcript and DS9 protein in actinomycin D–treated leaves were 55 and 50%, respectively, of those in untreated TMV-inoculated leaves (Figure 7). For the heat shock–treated samples, 50 and 48%, respectively, were detected. Moreover, in both the actinomycin D– and the heat shock–treated leaves, an accumulation of PR-1 transcripts was found, whereas no notable decreases in the actin and GAPDH transcripts were seen. These observations indicate that treatment of TMV-inoculated leaves with both actinomycin D and heat shock mimic the situation found after the temperature shift from 30 to 20°C.
Figure 7.
RNA Gel Blot Analysis of the DS9, PR-1, Actin, and GAPDH Genes and Protein Gel Blot Analysis of the DS9 Protein in TMV-Inoculated Leaves Treated with Actinomycin D or Heat Shock.
Detached leaves of Samsun NN tobacco plants were inoculated with TMV (10 μg/mL), incubated at 30°C for 40 hr, treated with actinomycin D (AMD; 200 μg per gram [fresh weight] of leaf tissues) or subjected to heat shock (HS) at 50°C for 2 min, reincubated at 30°C for 18 hr, and then used for total RNA extraction and protein extraction. Aliquots of RNA (20 μg per lane) were subjected to RNA gel blot analysis with the indicated cDNA probes. The 2.4-kb RNA (arrow) represents the length of the DS9 gene. Twenty micrograms of total protein was subjected to protein gel blot analysis with antibodies raised against DS9ΔN (Ab-DS9). TMV-inoculated leaves that were not treated with actinomycin D or subjected to heat shock were used as the control. The arrowhead indicates the relative molecular masses of ∼78 kD.
Generation of Transgenic NN Tobacco Plants Containing Various Amounts of DS9 Protein
To examine the role of the DS9 protein in TMV-induced HR, we attempted to generate transgenic NN tobacco plants that would express various amounts of DS9 protein. The DS9 cDNA was introduced into Samsun NN tobacco plants by the Agrobacterium infection method. The cDNA was under the control of the constitutive 35S cauliflower mosaic virus promoter and was inserted in the sense and antisense orientations. Kanamycin-resistant shoots were then generated under a light intensity of 120 μmol of photons m−2 sec−1. When the sense gene was introduced, all 20 shoots rooted in a kanamycin-containing medium. In contrast, no shoots were generated in the tobacco plants introduced with the antisense gene, despite the same transformation scale. From similar results in repeated experiments, we noticed that the light intensity may in fact affect the transformation efficiency. Therefore, all transformation procedures were performed under a weak light intensity of 10 μmol of photons m−2 sec−1. Under these improved conditions, 15 shoots were generated when the antisense gene was introduced, but only two shoots actually rooted.
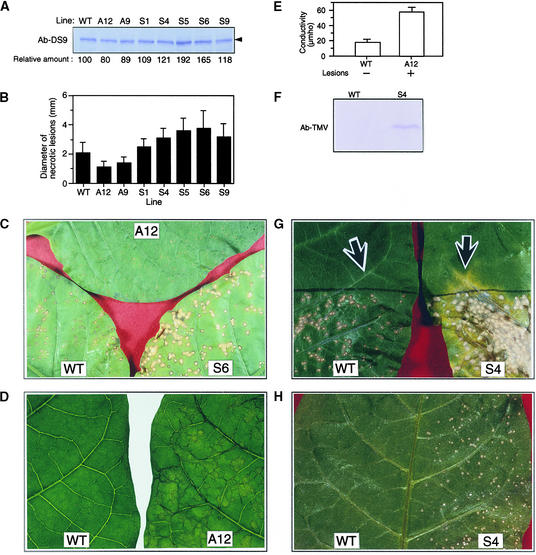
The amount of the 78-kD protein in healthy leaves of five sense DS9 lines (S1, S4, S5, S6, and S9) ranged between 109 and 192% of that in healthy leaves of untransformed, wild-type plants (Figure 8A). Conversely, the 78-kD protein in two antisense lines, A9 and A12, was 89 and 80%, respectively, of that in the untransformed plants. The phenotypes of these sense and antisense plants were apparently normal; neither chlorosis nor necrosis was observed in the healthy leaves.
Figure 8.
Analysis of Transgenic Tobacco Plants Containing Various Amounts of the DS9 Protein.
The upper, fully expanded healthy leaves of 3-month-old wild-type Samsun NN (WT) and transgenic tobacco plants (S4, S6, and A12) were used for each experiment.
(A) Total protein was extracted from the wild-type and transgenic tobacco plants carrying antisense (lines A12 and A9) and sense (lines S1, S4, S5, S6, and S9) constructs. Twenty-five micrograms of total protein from each line was subjected to protein gel blot analysis with antibodies raised against DS9ΔN (Ab-DS9). The numbers below the gel indicate the amount of DS9 protein expressed as a ratio of that present in the wild type, which was set equal to 100%. The arrowhead indicates the relative molecular masses of ∼78 kD.
(B) Leaves were inoculated with TMV (2 μg/mL) and incubated at 20°C under 120 μmol of photons m−2 sec−1 fluorescence illumination. In each line, the diameter of 60 local lesions 5 days after inoculation was measured with a stereoscopic microscope. Each bar represents the mean ±sd.
(C) Necrotic lesions that had formed on the leaves of a wild-type plant and the S6 and A12 lines, as described in (B).
(D) Leaves of line A12 that had been inoculated with TMV (10 μg/mL) and incubated for 40 hr at 30°C were shifted to 20°C, where they were incubated for 3.5 hr and returned to 30°C. As a control, leaves of wild-type plants were used. A photograph was taken 24 hr after returning the leaves to 30°C. The experiment was repeated twice with similar results.
(E) After photographing the leaves as shown in (D), five leaf discs were punched from each leaf and measured for electrolyte leakage. Values are the mean ±sd from three independent experiments. +, lesions appeared; −, no lesions appeared.
(F) Protein gel blot analysis of TMV coat protein. The experiment was repeated twice with similar results. Ab-TMV, anti-TMV antibody.
(G) The bottom half of leaves of wild-type and S4 plants were inoculated with TMV (2 μg/mL) and incubated at 20°C. Seven days after inoculation, the leaves were photographed. Two discs (0.7 cm in diameter each) from each leaf were punched out from the sites indicated by the arrows,
which were 1.5 cm away from the black borderline that indicates the edge of the inoculated region. One of the two discs from each leaf was used for protein gel blot analysis with the anti-TMV antibody (F), and the other was used for the TMV bioassay (H).
(H) TMV bioassay. For the TMV bioassay, crude leaf extracts from wild-type plants and the S4 line were inoculated onto the left and right half, respectively, of Samsun NN tobacco leaves. Five days after inoculation, the leaf was photographed. The experiment was repeated twice with similar results.
Acceleration of HR in Transgenic Tobacco Plants Containing Less DS9 Protein Than the Wild-Type Plants
Leaves of wild-type and transgenic plants were inoculated with TMV and incubated at 20°C to allow necrotic lesions to form. Five days after inoculation, the diameter of localized necrotic lesions in the A9 and A12 lines was 70 and 60%, respectively, of that in the wild-type plants (Figures 8B and 8C). In contrast, the diameter of localized necrotic lesions in all five sense plants was 120 to 180% of that in the wild-type plants.
Because lesion area is proportional to the viral content, the relatively smaller lesions of the A9 and A12 lines are indicative of increased resistance of these lines to TMV infection. We assumed that the formation of such small lesions was due to acceleration of the HR. If accelerated HR occurs, then <4 hr of 20°C treatment would be sufficient for formation of necrotic lesions (see Introduction). When TMV-inoculated leaves of an antisense DS9 plant (the A12 line) were incubated at 30°C for 40 hr, transiently held at 20°C for 3.5 hr, and returned to 30°C, visible necrotic lesions were induced (Figure 8D, right). However, in the leaves of wild-type plants subjected to the same experimental conditions, no visible lesions were found (Figure 8D, left). Because electrolyte leakage is known to increase during the formation of necrotic lesions in TMV-infected NN tobacco (Ohashi and Shimomura, 1976; Mittler et al., 1995), we compared the amounts of electrolyte leakage between the A12 line and wild-type plants. Electrolyte leakage from the lesion-forming leaves of the A12 line was approximately three times greater than that from the non-lesion-forming leaves of wild-type plants (Figure 8E). Similar enhanced electrolyte leakage was observed in the A9 line (data not shown).
Delayed HR in Transgenic Tobacco Plants Containing More DS9 Protein Than the Wild-Type Plants
Lesions in the sense DS9 plants were larger than those in wild-type plants (Figures 8B and 8C). This observation suggested that in the sense DS9 plants infected with TMV, the development of the HR was slow; consequently, the pathogen could multiply and spread to uninfected cells, resulting in the formation of large lesions. In such a delayed HR, TMV-multiplying cells would be present in the uninfected area around lesions. To test this assumption, we inoculated the bottom half of leaves of a sense DS9 plant (the S4 line) with TMV and incubated them at 20°C. Seven days after inoculation, the upper, uninoculated region adjacent to the bottom half where lesions had been induced became yellow along the veins (Figure 8G). In contrast, no yellow regions were visible in the uninoculated top half of leaves of wild-type plants. Leaf discs from both the yellow region and the corresponding position of wild-type plants were subjected to protein gel blot analysis to detect TMV; they were also bioassayed to confirm TMV multiplication. The TMV coat protein was detectable in the yellow region from the S4 line but not in the leaf discs from wild-type plants (Figure 8F). A TMV bioassay with NN tobacco leaves revealed that TMV had multiplied in the yellow region of the S4 line and also confirmed that the virus had not spread and multiplied in the corresponding region of wild-type plants (Figure 8H). Such a delayed HR observed in the S4 line was also observed in the S6 line (data not shown). Thus, in transgenic tobacco plants containing high quantities of DS9 protein, TMV was not restricted to the necrotic lesions but rather spread to uninoculated regions.
Photosynthetic Electron Transport Is Inhibited during the N Gene–Mediated HR
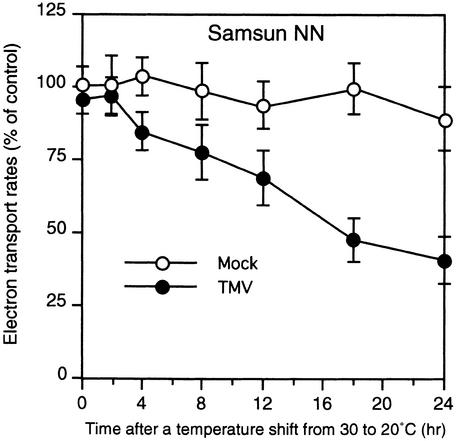
Because the DS9 protein is a thylakoid-bound protein, we assumed that a decreased amount of DS9 protein in TMV-infected cells would affect photosynthetic events in the thylakoid membranes in the chloroplasts. Therefore, we examined the temporal relationship between a decrease in DS9 protein and changes in the rate of photosynthetic electron transport, which is used to monitor a photosynthetic event, during the N gene–mediated HR. Thylakoid membranes were isolated from intact chloroplasts prepared from TMV- and mock-inoculated leaves after the shift from 30 to 20°C and were used to measure whole-chain electron transport with methyl viologen as an electron acceptor. In TMV-inoculated leaves, the rate of whole-chain electron transport began to decrease 4 hr after the temperature shift (Figure 9). The decrease continued until at least 24 hr after the shift. In TMV-inoculated leaves, the timing of the initial decrease in the rate of the photosynthetic electron transport was 2 hr earlier than the initial decrease in DS9 protein (Figure 6B), suggesting that the first trigger for the inhibition of transport might not be the protein decrease. In mock-inoculated leaves, there was no marked decrease in the rate of whole-chain electron transport after the temperature shift.
Figure 9.
Changes in the Rate of Photosynthetic Electron Transport after the Temperature Shift.
Detached healthy leaves of Samsun NN tobacco plants were inoculated with TMV (10 μg/mL; closed circles) or buffer only (Mock; open circles) and shifted from 30 to 20°C at time 0. Thylakoid membranes were isolated from leaves harvested at the indicated times after the temperature shift and were used to measure the whole-chain electron transport. Activity was expressed as the percentage of the control sample (0 hr after the temperature shift of mock-inoculated leaves). Values are the mean ±sd of three independent measurements.
DISCUSSION
Here, we have demonstrated that decreases in the amount of DS9 protein, a chloroplastic homolog of FtsH metalloprotease, in TMV-infected NN tobacco leaves accelerate the HR.
TMV-induced HR in NN tobacco always accompanied a decrease in DS9 protein: the quantity of DS9 protein in TMV-inoculated leaves decreased not only after the shift to 20°C (Figure 6) but also after treatment with actinomycin D and with heat shock (Figure 7). In transgenic tobacco plants containing low and high amounts of DS9 protein compared with wild-type plants, TMV-induced HR was accelerated and delayed, respectively (Figure 8). This demonstrates a close correlation between the amounts of DS9 protein and the degree of the HR. Together, these results indicate that a decrease in DS9 protein in NN tobacco leaves after infection with TMV is crucial to the development of the HR.
No decrease in DS9 protein was observed in TMV-inoculated leaves just before the shift to 20°C or in mock-inoculated leaves before and after the shift. These observations clearly indicate that both multiplication of TMV and expression of the N gene are necessary to reduce the quantity of DS9 protein in infected cells at 20°C. The simplest possible mechanism involved in such a protein decrease is the direct reflection of a greatly decreased content of DS9 transcripts. After the shift of temperature for TMV-infected NN tobacco leaves from 30 to 20°C, the timing of the initial decrease in DS9 transcripts was almost the same as that for the decrease in DS9 protein. The amount of DS9 transcripts in TMV-inoculated NN tobacco leaves at 6 hr after the shift was <5% of that in mock-inoculated leaves immediately after the shift. Such a small amount of the DS9 transcripts could lead to a reduced production of DS9 protein. On the other hand, we observed that TMV multiplication decreased the amount of DS9 transcripts but not that of DS9 protein—which may imply that a TMV infection–dependent decrease in DS9 transcripts has little effect on the change in the amount of DS9 protein present. It remains to be elucidated why the DS9 transcripts are decreased when a plant is infected with TMV.
Actinomycin D and heat shock reduce the quantity of the DS9 protein, probably by mimicking the possible function of the N gene in decreasing the DS9 transcripts. Neither actinomycin D nor heat shock treatments decreased the actin and GAPDH transcripts (Figure 7), however, which suggests that the DS9 gene is more sensitive to these treatments than are the actin and GAPDH genes. Actually, a low sensitivity of actin and GAPDH genes to actinomycin D or heat shock has been reported (Brodl and Ho, 1991; Blattner et al., 1999). Interestingly, actinomycin D is an inducer of apoptosis, a genetically determined cell suicide program, in animals (Devitt et al., 1999; Li et al., 1999). Transcriptional suppression is a major event for apoptosis induction by actinomycin D (Muscarella et al., 1998). In addition, heat shock induces cell death in yeast (Davidson et al., 1996). It is thus not surprising that actinomycin D and heat shock treatments were able to induce an HR, which is a form of programmed cell death in plants (Dangl et al., 1996; Mittler and Lam, 1996), in leaves systemically infected with TMV. Because the response to actinomycin D (Muscarella et al., 1998) or heat shock (Lindquist, 1986) varies between cell lines or cell types, TMV-infected cells are probably more sensitive to actinomycin D or heat shock treatment than are uninfected cells. If TMV-infected NN tobacco leaves are treated with actinomycin D or subjected to heat shock, then transcription of specific housekeeping genes, including the DS9 gene, would be suppressed to a greater extent in infected cells than in uninfected cells. As a consequence, the HR-like response in the infected cells would be accelerated.
A shift from 30 to 20°C, similar to treatments with actinomycin D and heat shock, selectively decreased the amount of DS9 transcripts in TMV-inoculated NN tobacco leaves, suggesting that suppression of transcription of specific housekeeping genes contributes to the TMV-induced HR. Another candidate (housekeeping gene) for being suppressed is the ubiquitin genes, the products of which function in a protein degradation system (von Kampen et al., 1996). Suppressed expression of a ubiquitin gene, which may cause a disturbance of cellular homeostasis, has been reported to be involved in the TMV-induced HR in tobacco (Becker et al., 1993; Karrer et al., 1998).
In the transformation experiment under high-intensity light, no shoots were generated in the tobacco plants carrying the antisense DS9 gene. Under low-intensity light, however, two antisense transgenic plants with an apparently normal phenotype were generated—although healthy leaves of these plants from the A9 and A12 lines contained only 89 and 80%, respectively, of the wild-type level of the DS9 protein. These findings suggest a threshold value for DS9 protein such that a minimum amount of the protein may be necessary for cell survival under certain circumstances, for example, light stress or infection with TMV. Conversely, under such circumstances, if the quantity of DS9 protein in tobacco cells drops below that threshold, the cells do not survive. Although no visible spontaneous lesions were found in healthy leaves of the antisense A12 line, infection of its leaves with TMV resulted in an accelerated HR. This observation may suggest that in TMV-infected leaves of wild-type, NN tobacco plants, the HR is accelerated when the DS9 protein decreases to <80% of its basal value. Thus, a slight decrease in the DS9 protein seems to be critical in the acceleration of the HR in response to TMV.
FtsH protein of E. coli is involved in degradation of both soluble regulatory proteins, such as the heat shock transcription factor δ32 (Herman et al., 1995; Tomoyasu et al., 1995) and the λ lysis-lysogeny determination protein cIII (Herman et al., 1997), and membrane proteins, such as the secretory machinery subunit SecY (Akiyama et al., 1996b; Kihara et al., 1996) and the subunit of the F1F0 ATPase complex (Akiyama et al., 1996a). In addition to its function as a protease, the FtsH protein functions as a chaperone involved in protein assembly and folding (Akiyama et al., 1994). Notably, depletion of the FtsH protein inhibits cell growth and protein export in E. coli (Akiyama et al., 1994). Furthermore, disruption of the ftsH gene leads to a loss of viability in Helicobacter pylori (Ge and Taylor, 1996). In eukaryotes, mitochondrial homologs of FtsH are involved in both protein degradation (Nakai et al., 1995) and protein assembly (Rep et al., 1996), similar to bacterial FtsH. Interestingly, the mutations at ftsH-related genes in yeast cause a loss of function of mitochondria, leading to an increase in DNA leakage from the mitochondria (Thorsness et al., 1993) and to a decrease in activity for respiratory chain enzymes (Nakai et al., 1995), thus indicating that FtsH-related proteins are essential for normal mitochondrial function. These findings support our suggestion that a decrease in DS9 protein affects cell viability.
Proteases in chloroplasts are thought to be essential for the degradation of unassembled, mistargeted, denatured, and unfolded chloroplastic proteins to maintain homeostasis (Adam, 1996; Andersson and Aro, 1997). The chloroplast FtsH is the only known ATP-dependent metalloprotease in thylakoid membranes (Lindahl et al., 1996). This protease was shown to be involved in the degradation of the unassembled Rieske FeS protein (Ostersetzer and Adam, 1997). The identity of the target substrates of the DS9 protein in vivo remains to be determined.
Although the precise mechanism for the acceleration of HR by a decrease in DS9 protein is not known, both chloroplasts and light are likely to be important factors for the HR, because of the localization of the DS9 protein in chloroplasts and a reduction in the transformation efficiency of tobacco plants containing the antisense DS9 gene under high-intensity light. Indeed, a light-stimulated HR has been found in several plant species (Peever and Higgins, 1989; Genoud et al., 1998; Allen et al., 1999). In addition, photosynthesis is inhibited during the G-protein activator mastoparan-induced HR in Asparagus sprengeri mesophyll cells (Allen et al., 1999). Exposing chloroplasts to excess light results in a loss of their photosynthetic capacity (Powles, 1984). The primary target in this process is photosystem II (PSII), leading to inhibition of photosynthetic electron transport. This so-called photoinhibition generates toxic singlet oxygen (1O2). The molecule generated then damages the D1 protein of the PSII reaction center, and the damaged D1 protein is degraded (Barber and Andersson, 1992; Aro et al., 1993). The degradation of the damaged D1 protein during photoinhibition leads to de novo synthesis of D1 protein; consequently, the newly synthesized protein replaces the damaged one, contributing to recovery of PSII activity. Spetea et al. (1999) proposed that chloroplast FtsH is involved in clean-up proteolysis of a fragment resulting from the primary degradation of the photodamaged D1 protein. We found that photosynthetic electron transport was inhibited during TMV-induced HR (Figure 9).
It is possible that the inhibition of photosynthetic electron transport in the chloroplasts in the infected cells of NN tobacco leaves infected with TMV at 20°C leads to the generation of 1O2, which damages the D1 protein. Assuming that the DS9 protein is involved in D1 protein degradation, the damaged D1 protein cannot be cleaned up because of a decrease in the amount of DS9 protein; as a result, PSII activity in the chloroplasts cannot be recovered. We speculate that the inhibition of photosynthesis through the loss of PSII activity in infected cells disturbs the cellular homeostasis necessary for survival of the cells, which in turn accelerates the HR, leading to necrotic lesion formation. Obviously, further work is required to elucidate whether a decrease in the DS9 protein in chloroplasts results in a loss of photosynthesis.
In this report, we suggest that a chloroplastic protease participates in a virus-induced HR. Our results will provide insights into the mechanisms involved in the HR in plants.
METHODS
Plant Materials
Plants (Nicotiana tabacum cv Samsun NN, Samsun nn, SR-1, and Xanthi) were grown in a temperature-controlled greenhouse at 25°C under sunlight. Unless otherwise stated, the upper, fully expanded healthy leaves of 50-day-old plants were used.
Tobacco Mosaic Virus Inoculation and a Temperature Shift Experiment
For general inoculation, the detached leaves of plants were dusted with carborundum (Mesh 600; Kishida Chemical Co., Osaka, Japan) and mechanically inoculated with tobacco mosaic virus (TMV) suspended in 10 mM phosphate buffer, pH 7.0. All of the inoculated leaves were set on wet filter paper in transparent plastic boxes and incubated in incubators previously maintained at 30 or 20°C. The temperature shift was performed by transferring the leaves themselves to transparent plastic boxes containing wet filter paper that had previously been maintained in an incubator at 30 or 20°C. All incubations of inoculated leaves were performed under continuous illumination at an intensity of 120 μmol of photons m−2 sec−1.
DNA Sequencing
The DS9 cDNA was subcloned into the pBluescript SK− (Stratagene, La Jolla, CA) phagemid vector and sequenced by the dideoxy chain termination method with a model 373A DNA sequencer (Applied Biosystems Inc., Foster City, CA). Nucleotide and amino acid sequences were analyzed with the Genetyx-Mac (Software Development Co., Tokyo, Japan) software system.
RNA Gel Blot Analysis
Total RNA extraction, blotting, and hybridization were performed as detailed previously (Seo et al., 1999). cDNA probes of the tobacco pathogenesis-related (PR) genes PR-1, PR-2, and PR-5 were synthesized by polymerase chain reaction using synthetic primers to yield fragments of 231 bp (corresponding to positions 507 to 737; Matsuoka et al., 1987), 225 bp (corresponding to positions 1504 to 1728; Linthorst et al., 1990), and 187 bp (corresponding to positions 1781 to 1967; van Kan et al., 1989), respectively. A cDNA probe for the tobacco glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was described previously (Seo et al., 1995). The actin cDNA probe was kindly supplied by H. Sano (Nara Institute of Science and Technology, Nara, Japan). The blots were analyzed with a BioImage analyzer (PhosphorImager; Molecular Dynamics Inc., Sunnyvale, CA) to quantify relative transcript amounts and by autoradiography.
Production of Recombinant DS9 Proteins
To construct a plasmid for a glutathione S-transferase (GST) protein fusion with DS9 (GST–DS9ΔN), we subcloned the coding region of DS9 cDNA (corresponding to positions 412 to 2241) to the BamHI and EcoRI sites of pGEX-2T (Pharmacia), which had been fused to the C terminus of the GST protein. The resulting construct, pGEX–DS9ΔN, was expressed in Escherichia coli JM109 by incubation in 0.4 mM isopropyl β-d-thiogalactopyranoside (IPTG) for 2 hr at 37°C. The cells were collected by centrifugation at 6000g for 10 min at 4°C, washed, and suspended in buffer A (20 mM Tris-HCl, pH 8.0, 30 mM NaCl, 10 mM EDTA, and 2 mM phenylmethylsulfonyl fluoride). After addition of a one-tenth volume of lysozyme (20 mg/mL in buffer A), the suspension was incubated for 1 hr on ice and disrupted with an ultrasonic disrupter (model UR-200P; Tomy Seiko Co., Tokyo, Japan) at a middle setting (level 7) for 1 min on ice.
The insoluble fraction was collected by centrifugation at 6000g for 10 min at 4°C, washed with buffer B (20 mM Tris-HCl, pH 7.5, and 30 mM NaCl) three times, centrifuged at 8000g for 10 min at 4°C, and then resuspended in 10 mM EDTA, pH 8.0. After the addition of 8 M guanidine-HCl, pH 8.3, to a final molar concentration of 6.22 M, the lysate was subjected to centrifugation at 12,000g for 30 min at 4°C. The supernatant was dialyzed first against buffer C (2 M guanidine-HCl, 0.2 mM EDTA, pH 8.0, and 5 mM 2-mercaptoethanol) for 2 hr at 4°C and then against buffer D (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.5 mM EDTA, and 5 mM 2-mercaptoethanol) for >4 hr at 4°C. After collection by ultracentrifugation at 100,000g for 30 min at 4°C, the supernatant was further purified with glutathione–agarose beads. The protein concentration was determined by a protein assay kit (Bio-Rad) with BSA as the standard.
To construct a plasmid for a recombinant DS9 protein with a six-histidine tag (HisDS9), we subcloned the full-length coding region of DS9 cDNA (corresponding to positions 22 to 2163) into the BamHI site of pET-15b (Novagen, Madison, WI) in frame. The resulting construct, pET-DS9, was expressed in E. coli BL21(DE3) by incubation in 0.4 mM IPTG for 2.5 hr at 37°C. The cells were collected by centrifugation at 5000g for 6 min at 4°C, suspended in buffer E (10 mM Tris-HCl, pH 8.0, 20 mM imidazole, 10 mM 2-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride), and treated with lysozyme. After the addition of Nonidet P-40, glycerol, and NaCl to final concentrations of 0.5% (v/v), 10% (v/v), and 0.3 M, respectively, the suspension was incubated for 15 min on ice and sonicated as described above. After the removal of insoluble materials by centrifugation, the supernatant was loaded onto a nickel-chelating column (Pharmacia). The column was washed with buffer E containing 0.5% Nonidet P-40, 10% glycerol, and 0.3 M NaCl and was eluted with a 20 to 500 mM linear gradient of imidazole in the same buffer. The peak fractions of HisDS9 were dialyzed extensively against buffer F (10 mM Tris-HCl, pH 8.0, 10% glycerol, 0.5% Nonidet P-40, and 1 mM DTT). The protein concentration was determined as described above.
Production of Antibodies
The anti-DS9 antibody was produced against the DS9 protein separated from GST–DS9ΔN. The salicylic acid–induced protein kinase (SIPK)–specific antibody was produced against synthetic peptides encoding the N terminus of SIPK (MDGSGQQTDTMMSDAGAEQPPTAP), as reported by Zhang et al. (1998). The anti-TMV antibody was produced against the TMV particles.
Assay of ATPase Activity
ATPase activity was assayed according to the method of Chan et al. (1986). The reaction mixture (60 μL) contained 50 mM Tris-HCl, pH 7.5, 1 mM DTT, 2.5 mM Tris-acetate, 10 μM zinc acetate, 1 mM ATP, and 500 ng of the recombinant HisDS9 protein. Reactions were performed in the presence or absence of 5 mM MgCl2 at 37°C for the appropriate periods. The reaction was stopped by the addition of 240 μL of a color reagent (malachite green–polyvinyl alcohol–ammonium molybdate–H2O, 1:1:2:2 [v/v]) and 30 μL of 34% sodium citrate. After incubation for 20 min at 25°C, the absorbance of the color complex was measured at 660 nm, as described previously (Akiyama et al., 1996b). The amount of inorganic phosphate in the assay was determined by using known concentrations of potassium dihydrogen phosphate as standards.
Assay of Protease Activity
Protease assays using fluorescein isothiocyanate–labeled casein were conducted basically according to the method of Twining (1984). The reaction mixture (60 μL) contained 50 mM Tris-HCl, pH 7.5, 1 mM DTT, 5 mM Tris-acetate, 5 mM MgCl2, 4 mM ATP, 2 μg of fluorescein isothiocyanate–labeled casein (Molecular Probes, Inc., Eugene, OR), and 200 ng of the recombinant HisDS9 protein. The samples were reacted in the presence or absence of 12.5 μM zinc acetate at 37°C for various periods. The reaction was stopped by adding 6 μL of 10 mg/mL BSA and 60 μL of 10% trichloroacetic acid, and the mixtures were incubated for 10 min on ice and centrifuged for 10 min at 15,000g. An aliquot (60 μL) of the supernatant was diluted to 2 mL with 0.5 M Tris-HCl, pH 8.5. Fluorescence of the diluted samples was measured with excitation at 490 nm and emission at 525 nm. The background fluorescence intensity of a control sample (0 min of incubation) was subtracted for each sample.
Chloroplast Isolation and Fractionation
All isolation and fractionation procedures were performed at 4°C. Intact chloroplasts were isolated by Percoll gradient centrifugation (Price et al., 1987) and lysed by osmotic shock in 5 mM Tricine-KOH and 5 mM MgCl2, pH 7.9. The lysate was fractionated into stroma and thylakoid fractions by centrifugation at 3000g for 5 min. The chlorophyll concentration was determined according to the method of Arnon (1949).
Protein Gel Blot Analysis
Protein samples were separated by electrophoresis on a 10% SDS–polyacrylamide gel and transferred to polyvinylidene difluoride or nitrocellulose membranes. After transfer, the polyvinylidene difluoride membranes (Figure 8A) were stained with Ponceau S (Sigma) to verify transfer efficiency, according to Ausubel et al. (1987). The Ponceau stain of the membranes revealed no differences in the intensity of the band corresponding to the large subunit of Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase, the most abundant protein in leaf tissue) in each lane, demonstrating that equal amounts of proteins had been loaded onto the gel. After the removal of Ponceau S, the membranes were used for immunological detection of the DS9 protein, as described below. The dilution of the primary antibodies used was 1:4000 for the anti-DS9 antibody and 1:2000 for the anti-SIPK antibody. For the polyvinylidene difluoride membranes, alkaline phosphatase–conjugated goat anti–rabbit IgG (Organon Teknika Corp., Durham, NC) was used as a secondary antibody at a dilution of 1:3000, and the immune complexes were visualized by hydrolysis of tetrazolium-5-bromo-4-chloro-3-indolyl phosphate as the substrate (Figure 8A). For the nitrocellulose membranes (Figures 3A, 4, 6, and 7), horseradish peroxidase–conjugated anti–rabbit IgG was used as a secondary antibody at a dilution of 1:5000, and the immune complexes were visualized by using an enhanced chemiluminescence system (Amersham). Quantitation of the relative content of DS9 protein was determined with the National Institutes of Health (Bethesda, MD) Image 1.61 software program.
For protein gel blot analysis of the TMV coat protein, a leaf disc was ground in an ice-cold mortar with liquid nitrogen and extracted with 400 μL of 0.1 M sodium phosphate buffer, pH 7.0, containing 1 mM EDTA, 2 mM DTT, and one tablet of complete protease inhibitors per 10 mL (complete, Mini; Boehringer Mannheim). After the addition of 200 μL of 2 × SDS gel loading buffer (1 × SDS gel loading buffer is 65 mM Tris-HCl, pH 6.8, 2.95% SDS, 720 mM 2-mercaptoethanol, 0.01% bromophenol blue, and 10% glycerol), the extraction solution was transferred to a 1.5-mL microtube and centrifuged at 18,000g for 15 min. Aliquots (15 μL) of the supernatant were loaded on a 15% SDS–polyacrylamide gel, electrophoresed, and transferred to a polyvinylidene difluoride membrane. The membrane was probed with the anti-TMV antibody at a dilution of 1:2000. The immune complexes were visualized by observing the color reaction of alkaline phosphatase, as described above.
Immunoelectron Microscopy
Immunoelectron microscopy was performed essentially as described by Suzuki and Kataoka (1992) and Tomoyasu et al. (1993b), except that the leaves were cut into 1 × 1-mm pieces and fixed with 0.1% glutaraldehyde and 4% paraformaldehyde in sodium cacodylate, pH 7.4, under vacuum conditions. The tissues were embedded in London Resin White (London Resin Co., London, UK) at −20°C. The sections, cut with an ultramicrotome, were incubated with the anti-DS9 antibody (diluted 1:250) and then reacted with anti–rabbit IgG conjugated with 10-nm (in diameter) particles of gold (diluted 1:100; Biocell Research Laboratories, Cardiff, UK) for 30 min at 37°C. After immunolabeling, the sections were stained with uranyl acetate. As the cytochemical control, specimens were incubated with nonimmune rabbit IgG or the primary antibody was omitted from the labeling process. For immunoelectron microscopy of the ultrathin frozen sections, some pieces of fixed tissue were infused with a mixture of 20% polyvinylpyrrolidone (molecular weight 10,000; Sigma) and 1.6 M sucrose, frozen in liquid propane, and cryosectioned as described by Tokuyasu (1989). The section was immunogold-labeled by the same procedure used for the samples embedded in London Resin White, after which it was embedded in a thin layer of polyvinyl alcohol (molecular weight 10,000; Sigma) containing uranyl acetate. Samples were observed under a model H-7100 transmission electron microscope (Hitachi, Tokyo, Japan).
Imaging of the Gold Particles
Negatives of the electron microphotographs were digitized with a model GT-9000 flat bed scanner (1800 DPI; Seiko Epson, Nagano, Japan) and stored in a TIFF format. The digitized images were normalized to enhance the contrast (Fukui, 1986) by using Adobe Photoshop version 3.0 (Adobe Systems, Inc., Mountain View, CA). The boundaries of the nuclei, chloroplasts, mitochondria, and microsomes were traced on each electron microphotograph, and the area of each organelle was digitally measured. The number of gold particles in each organelle was then visually counted.
Actinomycin D and Heat Shock Treatments
Actinomycin D (Wako Pure Chemicals, Osaka, Japan) at 10 mg/mL in methanol was the stock solution and was subsequently diluted with distilled water to various concentrations. For application to the plants, the detached leaves were fed through the petiole with the agent to a final concentration of 200 μg per gram fresh weight of leaf tissues. This application was performed in an incubator maintained at 30°C and took 1 hr. For the heat shock treatment, whole leaves were completely immersed in a water bath maintained at 50°C for 2 min and then incubated again at 30°C.
Construction of Antisense and Sense DS9 Transgenes and Tobacco Transformation
The coding region of the DS9 cDNA (corresponding to positions 22 to 2166) was amplified by polymerase chain reaction with the following primers: primer A, 5′-ATGGCCAATTCTCTCCTCTC-3′; and primer B, 5′-CTAATCCTCCTCCTTAGAGA-3′. For the sense construct, the BamHI and SacI sites were linked at the 5′ end of primers A and B, respectively. For the antisense construct, the SacI and BamHI sites were linked at the 5′ end of primers A and B, respectively. The polymerase chain reaction products were digested with BamHI and SacI and then ligated, in the sense and antisense orientation relative to a promoter, to pBI121 (Clontech, Palo Alto, CA), which had previously been digested with BamHI and SacI. The sense and antisense DS9 expression constructs were introduced into Agrobacterium tumefaciens LBA4404 by electroporation.
Transformation of Samsun NN tobacco was performed by the leaf disc cocultivation method (Horsch et al., 1985). Leaf discs were immersed for 5 min in a Luria-Bertani solution (0.1% Bacto tryptone, 0.05% Bacto yeast extract, and 0.1% NaCl) containing Agrobacterium cells, placed on an incubation medium (basal Murashige–Skoog medium [Nihon Seiyaku Co., Tokyo, Japan] containing 3% sucrose and B-5 vitamins) containing naphthaleneacetic acid (100 μg/L) and benzyl amino purine (1 mg/L) for 2 days at 25°C under the continuous illumination of a white fluorescence lamp at an intensity of 10 μmol of photons m−2 sec−1, and transferred to the same medium but with 250 μg/mL cefotaxime (Chugai Pharmaceutical Co., Tokyo, Japan) added. After 2 days, the leaf discs were transferred to selection medium (incubation medium containing 250 μg/mL cefotaxime and 100 μg/mL kanamycin). Plates containing leaf discs on selection medium were incubated at 25°C under a cycle of 16 hr of light and 8 hr of dark at an intensity of 10 μmol of photons m−2 sec−1. The leaf discs were transferred to new selection medium every 10 days until green shoots were generated. The shoots were transferred to hormone-free selection medium to allow rooting. Plantlets were transplanted to pots containing vermiculite and grown in an isolated greenhouse maintained at 25°C under sunlight.
Electrolyte Leakage Measurement
Leaf discs (1 cm in diameter) were washed with distilled water and incubated for 30 min in 5 mL of distilled water in a 50-mL beaker on a reciprocal shaker that was operated at 70 strokes per min. After incubation, the conductivity of the solution was measured with a model CDD-6A conductivity detector (Shimadzu, Kyoto, Japan).
TMV Bioassay
For the TMV bioassay, a leaf disc (7 mm in diameter) was ground with a fine glass rod in a 1.5-mL microtube containing 200 μL of 10 mM sodium phosphate buffer, pH 7.0. Aliquots (50 μL) of the extract were applied to leaves. The inoculated leaves were incubated at 20°C.
Photosynthetic Electron Transport Measurement
The rate of the whole-chain electron transport in thylakoid membranes was estimated as oxygen uptake resulting from the reoxidation of methyl viologen. The assay medium contained 300 mM sucrose, 40 mM Tricine-KOH, pH 7.5, 10 mM NaCl, 2 mM MgCl2, 1 mM ADP, 5 mM disodium hydrogen phosphate, thylakoid fractions containing 20 μg of chlorophyll per milliliter, and 100 μM methyl viologen as an electron acceptor. Oxygen uptake was monitored at 25°C with a Clark-type liquid-phase oxygen electrode (Hansatech Instruments, Norfolk, UK).
Acknowledgments
We thank Ichiro Mitsuhara and Norihiro Ohtsubo for helpful discussions; Tomohiko Kuwabara and Mitsue Miyao-Tokutomi for valuable discussions; Hiroshi Sano for providing an actin cDNA probe; and Yoko Gotoh, Hisako Ochiai, and Yumi Naitoh for maintenance of the plant materials. This work was supported by the Enhancement of Center of Excellence (COE), Special Coordination Funds for Promoting Science and Technology, the Science and Technology Agency, Japan.
References
- Adam, Z. (1996). Protein stability and degradation in chloroplasts. Plant Mol. Biol. 32, 773–783. [DOI] [PubMed] [Google Scholar]
- Akiyama, Y., Shirai, Y., and Ito, K. (1994). Involvement of FtsH in protein assembly into and through the membrane. II. Dominant mutations affecting FtsH functions. J. Biol. Chem. 269, 5225–5229. [PubMed] [Google Scholar]
- Akiyama, Y., Kihara, A., and Ito, K. (1996. a). Subunit a of proton ATPase F0 sector is a substrate of the FtsH protease in Escherichia coli. FEBS Lett. 399, 26–28. [DOI] [PubMed] [Google Scholar]
- Akiyama, Y., Kihara, A., Tokuda, H., and Ito, K. (1996. b). FtsH (HflB) is an ATP-dependent protease selectively acting on SecY and some other membrane proteins. J. Biol. Chem. 271, 31196–31201. [DOI] [PubMed] [Google Scholar]
- Allen, L.J., MacGregor, K.B., Koop, R.S., Bruce, D.H., Karner, J., and Bown, A.W. (1999). The relationship between photosynthesis and a mastoparan-induced hypersensitive response in isolated mesophyll cells. Plant Physiol. 119, 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, B., and Aro, E.-M. (1997). Proteolytic activities and proteases of plant chloroplasts. Physiol. Plant. 100, 780–793. [Google Scholar]
- Arnon, D.I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro, E.-M., Virgin, I., and Andersson, B. (1993). Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143, 113–134. [DOI] [PubMed] [Google Scholar]
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K., eds (1987). Current Protocols in Molecular Biology. (New York: Wiley Interscience).
- Barber, J., and Andersson, B. (1992). Too much of a good thing: Light can be bad for photosynthesis. Trends Biochem. Sci. 17, 61–66. [DOI] [PubMed] [Google Scholar]
- Becker, F., Buschfeld, E., Schell, J., and Bachmair, A. (1993). Altered response to viral infection by tobacco plants perturbed in ubiquitin system. Plant J. 3, 875–881. [Google Scholar]
- Blattner, C., Sparks, A., and Lane, D. (1999). Transcription factor E2F–1 is upregulated in response to DNA damage in a manner analogous to that of p53. Mol. Cell. Biol. 19, 3704–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol, J.F., Linthorst, H.J.M., and Cornelissen, B.J.C. (1990). Plant pathogenesis-related proteins induced by virus infection. Annu. Rev. Phytopathol. 28, 113–138. [Google Scholar]
- Brodl, M.R., and Ho, T.-H.D. (1991). Heat shock causes selective destabilization of secretory protein mRNAs in barley aleurone cells. Plant Physiol. 96, 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, K.-M., Delfert, D., and Junger, K.-D. (1986). A direct colorimeric assay for Ca2+-stimulated ATPase activity. Anal. Biochem. 157, 375–380. [DOI] [PubMed] [Google Scholar]
- Confalonieri, F., and Duguet, M. (1995). A 200-amino acid ATPase module in search of a basic function. Bioessays 17, 639–650. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L., Dietrich, R.A., and Richberg, M.H. (1996). Death don't have no mercy: Cell death programs in plant–microbe interactions. Plant Cell 8, 1793–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, J.F., Whyte, B., Bissinger, P.H., and Schiestl, R.H. (1996). Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93, 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt, G.P., Creagh, E.M., and Cotter, T.G. (1999). The antioxidant 4b,5,9b,10-tetrahydroindeno[1,2-b]indole inhibits apoptosis by preventing caspase activation following mitochondrial depolarization. Biochem. Biophys. Res. Commun. 264, 622–629. [DOI] [PubMed] [Google Scholar]
- Fukui, K. (1986). Standardization of karyotyping plant chromosomes by a newly developed chromosome image analyzing system (CHIAS). Theor. Appl. Genet. 72, 27–32. [DOI] [PubMed] [Google Scholar]
- Ge, Z., and Taylor, D.E. (1996). Sequencing, expression, and genetic characterization of the Helicobacter pylori ftsH gene encoding a protein homologous to members of a novel putative ATPase family. J. Bacteriol. 178, 6151–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud, T., Millar, A.J., Nishizawa, N., Kay, S.A., Schafer, E., Nagatani, A., and Chua, N.-H. (1998). An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell 10, 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, R.N., and Novacky, A.J. (1994). The Hypersensitive Reaction in Plants to Pathogens: A Resistance Phenomenon. (St. Paul, MN: American Phytopathological Society Press).
- Gottesman, S., Wickner, S., and Maurizi, M.R. (1997). Protein quality control: Triage by chaperones and proteases. Genes Dev. 11, 815–823. [DOI] [PubMed] [Google Scholar]
- Herman, C., Thévenet, D., D'Ari, R., and Bouloc, P. (1995). Degradation of δ32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc. Natl. Acad. Sci. USA 92, 3516–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, C., Thévenet, D., D'Ari, R., and Bouloc, P. (1997). The HflB protease of Escherichia coli degrades its inhibitor λ cIII. J. Bacteriol. 179, 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, F.O. (1938). Inheritance of resistance to tobacco-mosaic disease in tobacco. Phytopathology 28, 553–561. [Google Scholar]
- Horsch, R.B., Fry, J.E., Hoffmann, N.L., Eichholtz, D., Rogers, S.G., and Fraley, R.T. (1985). A simple and general method for transferring genes into plants. Science 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Karrer, E.E., Beachy, R.N., and Holt, C.A. (1998). Cloning of tobacco genes that elicit the hypersensitive response. Plant Mol. Biol. 36, 681–690. [DOI] [PubMed] [Google Scholar]
- Kassanis, B. (1952). Some effects of high temperature on the susceptibility of plants to infection with viruses. Ann. Appl. Biol. 39, 358–369. [Google Scholar]
- Kihara, A., Akiyama, Y., and Ito, K. (1996). A protease complex in the Escherichia coli plasma membrane: HflKC (HflA) forms a complex with FtsH (HflB), regulating its proteolytic activity against SecY. EMBO J. 15, 6122–6131. [PMC free article] [PubMed] [Google Scholar]
- Li, J., Bombeck, C.A., Yang, S., Kim, Y.-M., and Billiar, T.R. (1999). Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J. Biol. Chem. 274, 17325–17333. [DOI] [PubMed] [Google Scholar]
- Lindahl, M., Tabak, S., Cseke, L., Pichersky, E., Andersson, B., and Adam, Z. (1996). Identification, characterization, and molecular cloning of a homologue of the bacterial FtsH protease in chloroplasts of higher plants. J. Biol. Chem. 271, 29329–29334. [DOI] [PubMed] [Google Scholar]
- Lindquist, S. (1986). The heat-shock response. Annu. Rev. Biochem. 55, 1151–1191. [DOI] [PubMed] [Google Scholar]
- Linthorst, H.J.M., Melchers, L.S., Mayer, A., van Roekel, J.S.C., Cornelissen, B.J.C., and Bol, J.F. (1990). Analysis of gene families encoding acidic and basic β-1,3-glucanases of tobacco. Proc. Natl. Acad. Sci. USA 87, 8756–8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, M., Yamamoto, N., Kano-Murakami, Y., Tanaka, Y., Ozeki, Y., Hirano, H., Kagawa, H., Oshima, M., and Ohashi, Y. (1987). Classification and structural comparison of full-length cDNAs for pathogenesis-related proteins. Plant Physiol. 85, 942–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler, R., and Lam, E. (1996). Sacrifice in the face of foes: Pathogen-induced programmed cell death in plants. Trends Microbiol. 4, 10–15. [DOI] [PubMed] [Google Scholar]
- Mittler, R., Shulaev, V., and Lam, E. (1995). Coordinated activation of programmed cell death and defense mechanisms in transgenic tobacco plants expressing a bacterial proton pump. Plant Cell 7, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscarella, D.E., Rachlinski, M.K., Sotiriadis, J., and Bloom, S.E. (1998). Contribution of gene-specific lesions, DNA-replication-associated damage, and subsequent transcriptional inhibition in topoisomerase inhibitor–mediated apoptosis in lymphoma cells. Exp. Cell. Res. 238, 155–167. [DOI] [PubMed] [Google Scholar]
- Nakai, T., Yasuhara, T., Fujiki, Y., and Ohashi, A. (1995). Multiple genes, including a member of the AAA family, are essential for degradation of unassembled subunit 2 of cytochrome c oxidase in yeast mitochondria. Mol. Cell. Biol. 15, 4441–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover, L., Neumann, D., and Scharf, K.-D. (1989). Heat shock and other stress response systems of plants. In Results and Problems in Cell Differentiation, Vol. 16, L. Nover, D. Neumann, and K.D. Scharf, eds (Berlin: Springer-Verlag), pp. 43–59. [PubMed]
- Ohashi, Y., and Shimomura, T. (1972). Induction of localized necrotic lesions by actinomycin D on leaves systemically infected with tobacco mosaic virus. Virology 48, 601–603. [DOI] [PubMed] [Google Scholar]
- Ohashi, Y., and Shimomura, T. (1976). Leakage of cell constituents associated with local lesion formation on Nicotiana glutinosa leaf infected with tobacco mosaic virus. Ann. Phytopathol. Soc. Jpn. 42, 436–441. [Google Scholar]
- Ohtsubo, N., Mitsuhara, I., Koga, M., Seo, S., and Ohashi, Y. (1999). Ethylene promotes the necrotic lesion formation and basic PR gene expression in TMV-infected tobacco. Plant Cell. Physiol. 40, 808–817. [Google Scholar]
- Ostersetzer, O., and Adam, Z. (1997). Light-stimulated degradation of an unassembled Rieske FeS protein by a thylakoid-bound protease: The possible role of the FtsH protease. Plant Cell 9, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever, T.L., and Higgins, V.J.. (1989). Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol. 90, 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles, S.B. (1984). Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 35, 15–44. [Google Scholar]
- Price, C.A., Cushman, J.C., Mendiola-Morgenthaler, L.R., and Reardon, E.M. (1987). Isolation of plastids in density gradients of Percoll and other silica sols. Methods Enzymol. 148, 157–179. [Google Scholar]
- Rep, M., van Dijl, J.M., Suda, K., Schatz, G., Grivell, L.A., and Suzuki, C.K. (1996). Promotion of mitochondrial membrane complex assembly by a proteolytically inactive yeast Lon. Science 274, 103–106. [DOI] [PubMed] [Google Scholar]
- Seo, S., Okamoto, M., Seto, H., Ishizuka, K., Sano, H., and Ohashi, Y. (1995). Tobacco MAP kinase: A possible mediator in wound signal transduction pathways. Science 270, 1988–1992. [DOI] [PubMed] [Google Scholar]
- Seo, S., Sano, H., and Ohashi, Y. (1999). Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura, T. (1971). Necrosis and localization of infection in local lesion hosts. Phytopathol. Z. 10, 185–196. [Google Scholar]
- Shimomura, T., and Ohashi, Y. (1971). Conditioning of local lesion formation by a brief heat or cold treatment of leaves systemically infected with TMV. Virology 43, 531–532. [DOI] [PubMed] [Google Scholar]
- Shimomura, T., and Ohashi, Y. (1980). Necrotic lesion formation and localization of infection in virus-infected plants. Rev. Plant Prot. Res. 13, 74–96. [Google Scholar]
- Spetea, C., Hundal, T., Lohmann, F., and Andersson, B. (1999). GTP bound to chloroplast thylakoid membranes is required for light-induced, multienzyme degradation of the photosystem II D1 protein. Proc. Natl. Acad. Sci. USA 96, 6547–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer, E.J., and Cline, K. (1999). The nucleotide sequence of a tobacco homolog of Pftf. Evidence for two plastid-localized AAA-family proteins in higher plants (accession no. AF117339) (PGR 99–029). Plant Physiol. 119, 1147. [Google Scholar]
- Suzuki, E., and Kataoka, K. (1992). Lectin cytochemistry in the gastrointestinal tract with special reference to glycosylation in the Golgi apparatus of Brunner's gland cells. J. Histochem. 40, 379–385. [DOI] [PubMed] [Google Scholar]
- Thorsness, P.E., White, K.H., and Fox, T.D. (1993). Inactivation of YME1, a member of the ftsH-SEC18–PAS1-CDC48 family of putative ATPase-encoding genes, causes increased escape of DNA from mitochondria in Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 5418–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu, K. (1989). Use of poly(vinylpyrrolidone) and poly(vinylalcohol) for cryoultramicrotomy. Histochem. J. 21, 163–171. [DOI] [PubMed] [Google Scholar]
- Tomoyasu, T., Yuki, T., Morimura, S., Mori, H., Yamanaka, K., Niki, H., Hiraga, S., and Ogura, T. (1993. a). The Escherichia coli FtsH protein is a prokaryotic member of a protein family of putative ATPases involved in membrane functions, cell cycle control, and gene expression. J. Bacteriol. 175, 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu, T., Yamanaka, K., Murata, K., Suzaki, T., Bouloc, P., Kato, A., Niki, H., Hiraga, S., and Ogura, T. (1993. b). Topology and subcellular localization of FtsH protein in Escherichia coli. J. Bacteriol. 175, 1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu, T., Gamer, J., Bukau, B., Kanemori, M., Mori, H., Rutman, A.J., Oppenheim, A.B., Yura, T., Yamanaka, K., Niki, H., Hiraga, S., and Ogura, T. (1995). Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor δ32. EMBO J. 14, 2551–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining, S.S. (1984). Fluorescein isothiocyanate–labeled casein assay for proteolytic enzymes. Anal. Biochem. 143, 30–34. [DOI] [PubMed] [Google Scholar]
- van Kan, J.A.L., van de Rhee, M.D., Zuidema, D., Cornelissen, B.J.C., and Bol, J.F. (1989). Structure of tobacco genes encoding thaumatin-like proteins. Plant Mol. Biol. 12, 153–155. [DOI] [PubMed] [Google Scholar]
- von Heijne, G., Steppuhn, J., and Herrmann, R.G. (1989). Domain structure of mitochondrial and chloroplast targeting peptides. Eur. J. Biochem. 180, 535–545. [DOI] [PubMed] [Google Scholar]
- von Kampen, J., Wettern, M., and Schulz, M. (1996). The ubiquitin system in plants. Physiol. Plant. 97, 618–624. [Google Scholar]
- Waring, M.J. (1981). DNA modification and cancer. Annu. Rev. Biochem. 50, 159–192. [DOI] [PubMed] [Google Scholar]
- Weststeijn, E.A. (1981). Lesion growth and virus localization in leaves of Nicotiana tabacum cv. Xanthi nc. after inoculation with tobacco mosaic virus and incubation alternately at 22°C and 32°C. Physiol. Plant Pathol. 18, 357–368. [Google Scholar]
- Whitham, S., Dinesh-Kumar, S.P., Choi, D., Hehl, R., Corr, C., and Baker, B. (1994). The product of the tobacco mosaic virus resistance gene N: Similarity to Toll and the interleukin-1 receptor. Cell 78, 1101–1115. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998). Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 95, 7433–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Du, H., and Klessig, D.F. (1998). Activation of the tobacco SIP kinase by both a cell wall–derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell 10, 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]