Abstract
Cell wall formation in the syncytial endosperm of Arabidopsis was studied by using high-pressure-frozen/freeze-substituted developing seeds and immunocytochemical techniques. The endosperm cellularization process begins at the late globular embryo stage with the synchronous organization of small clusters of oppositely oriented microtubules (∼10 microtubules in each set) into phragmoplast-like structures termed mini-phragmoplasts between both sister and nonsister nuclei. These mini-phragmoplasts produce a novel kind of cell plate, the syncytial-type cell plate, from Golgi-derived vesicles ∼63 nm in diameter, which fuse by way of hourglass-shaped intermediates into wide (∼45 nm in diameter) tubules. These wide tubules quickly become coated and surrounded by a ribosome-excluding matrix; as they grow, they branch and fuse with each other to form wide tubular networks. The mini-phragmoplasts formed between a given pair of nuclei produce aligned tubular networks that grow centrifugally until they merge into a coherent wide tubular network with the mini-phragmoplasts positioned along the network margins. The individual wide tubular networks expand laterally until they meet and eventually fuse with each other at the sites of the future cell corners. Transformation of the wide tubular networks into noncoated, thin (∼27 nm in diameter) tubular networks begins at multiple sites and coincides with the appearance of clathrin-coated budding structures. After fusion with the syncytial cell wall, the thin tubular networks are converted into fenestrated sheets and cell walls. Immunolabeling experiments show that the cell plates and cell walls of the endosperm differ from those of the embryo and maternal tissue in two features: their xyloglucans lack terminal fucose residues on the side chain, and callose persists in the cell walls after the cell plates fuse with the parental plasma membrane. The lack of terminal fucose residues on xyloglucans suggests that these cell wall matrix molecules serve both structural and storage functions.
INTRODUCTION
In higher plants, new cell walls are formed during cytokinesis by fusion of Golgi-derived vesicles into cell plates. The structure that gives rise to the cell plate is the phragmoplast, a complex arrangement of microtubules, microfilaments, Golgi-derived vesicles, and endoplasmic reticulum that assembles during late anaphase and is dismantled once the new wall is complete (Staehelin and Hepler, 1996; Smith, 1999). The phragmoplast cytoskeleton consists of two oppositely oriented sets of microtubules with their plus ends in the plane of the cell plate and two corresponding sets of actin microfilaments that do not overlap or directly abut. The first phragmoplast microtubules appear to arise from remnants of the mitotic spindle, but all later ones are polymerized anew, forming a cylinder that consolidates by shortening in length and widening in girth. During cell plate expansion, the microtubules depolymerize in the center and repolymerize along the edge, transforming the phragmoplast into a barrel-like structure, which marks the growing margin of the cell plate (Staehelin and Hepler, 1996; Heese et al., 1998). The high density of microtubules in the phragmoplast establishes an organelle exclusion zone along the growing edges of the cell plate (Samuels et al., 1995).
In somatic plant cells, the development of a preprophase band signals the future site of fusion of the cell plate with the parental cell wall. A detailed model depicting the structural events associated with somatic cell plate formation in high-pressure-frozen/freeze-substituted tobacco root tips and BY-2 cells has been published by Samuels et al. (1995). Two central features of this model are the involvement of thin (20-nm-diameter) fusion tubes in the formation of a transient membrane network from Golgi-derived vesicles and the subsequent programmed series of maturation steps that convert the membrane network into a new cell wall.
In contrast, in syncytial systems such as the nuclear endosperm, meiocytes, and gametophytic cells, cytokinesis is uncoupled from mitosis, and no preprophase bands have been reported (Brown and Lemmon, 1991; Mineyuki, 1999). During endosperm development, the primary nucleus initially undergoes a series of divisions without cytokinesis, forming a syncytium. Shortly thereafter, the cellularization process is initiated by the formation of cell walls between sister and nonsister nuclei, which are perpendicular (i.e., anticlinal) to the syncytial cell wall. Although many studies have dealt with endosperm cellularization, the formation of the very first anticlinal walls between nonsister nuclei has remained controversial for more than 90 years (DeMason, 1997).
The most widely accepted theory of endosperm cellularization, developed from studies on cereals, postulates that the first anticlinal cell walls are deposited in the absence of mitosis and typical phragmoplasts (reviewed in Olsen et al., 1995; Heese et al., 1998; Berger, 1999). In this model, “free-growing” cell walls are postulated to arise from the syncytial cell wall like ingrowths in the absence of typical phragmoplast arrays and to then extend toward the central vacuole. This mechanism has been compared with the ingrowths that initiate cellularization of the blastoderm embryo in Drosophila (Heese et al., 1998). The sites at which these inward-growing cell walls form coincide with the interacting ends of opposing microtubule arrays that radiate from adjacent nuclei (Brown et al., 1996a; Olsen, 1998; Olsen et al., 1998). Furthermore, some have speculated that the points of initiation of the free-growing anticlinal walls are related to transfer ingrowths (Dute and Peterson, 1992; Chamberlin et al., 1994). Besides operating in cereals, this mechanism of cell wall formation has also been reported for the endosperm of Arabidopsis (Mansfield and Briarty, 1990; Brown et al., 1999), Euphorbia dulcis (Gori, 1987), Glycine max (Dute and Peterson, 1992; Chamberlin et al., 1994), Haemanthus katherinae (Newcomb, 1978), Helianthus annus (Newcomb, 1973), Papaver nudicaule (Olson, 1981), Phaseolus vulgaris (Yeung and Cavey, 1988), and Stellaria media (Newcomb and Fowke, 1973).
However, the findings of other studies seem to contradict this prevailing theory of endosperm cellularization. For example, cytokinesis involving conventional phragmoplasts and cell plate formation between two dividing nuclei have been described in the endosperm of wheat (Fineran et al., 1982) and Ranunculus sceleratus (XuHan and Van Lammeren, 1993). In addition, non-mitosis-related phragmoplasts and cell plates have been observed during endosperm cellularization of wheat (Van Lammeren, 1988) and P. vulgaris (XuHan and Van Lammeren, 1994).
In part, these contradictory reports, some even derived from observations on the same species, may be related to the difficulty of preserving endosperm cells by using chemical fixatives. To circumvent this problem, we have used high-pressure freezing/freeze-substitution techniques to preserve cellularizing endosperm cells of Arabidopsis. Samples so preserved demonstrate that the initial cellularization process in the syncytial endosperm includes the formation of novel and unique kinds of phragmoplasts and cell plates. We have named these structures mini-phragmoplasts and syncytial-type cell plates, respectively, to distinguish them from the more common somatic-type phragmoplasts and somatic-type cell plates. Mini-phragmoplasts contain very few microtubules, and multiple mini-phragmoplasts work in concert to produce a syncytial-type cell plate. Although this process is documented only for cell plate formation in the micropylar zone, the same structural intermediates have been observed during the initial stages of cell wall formation in the central and chalazal endosperm zones. The subsequent cell wall growth steps in these latter regions involve other intermediates, which will be reported elsewhere.
RESULTS
The Developing Endosperm
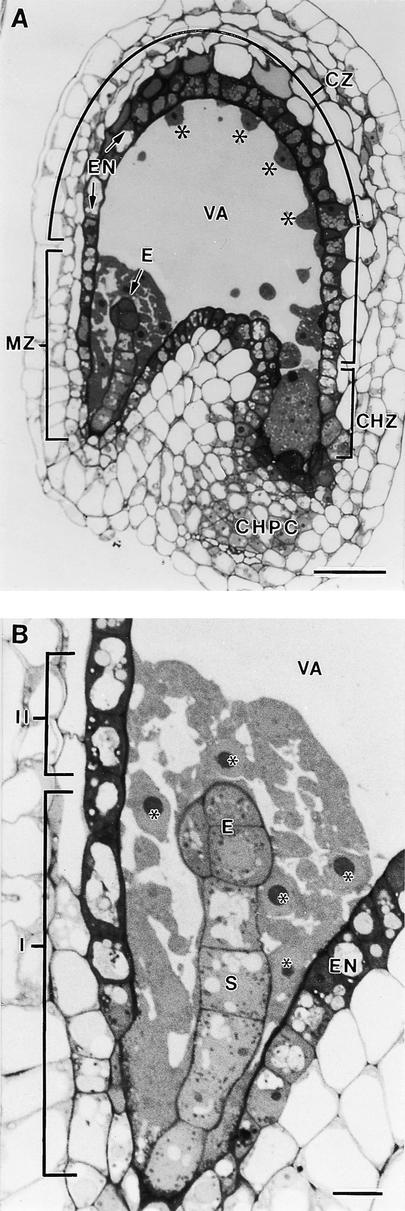
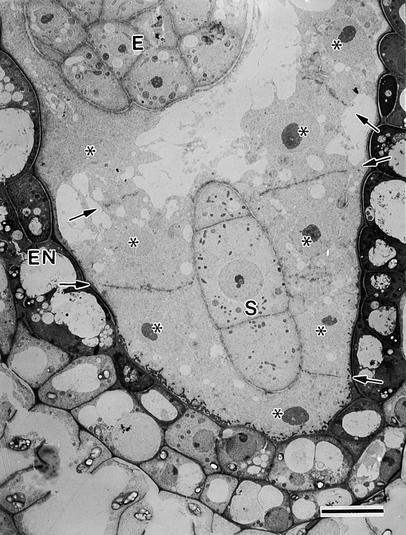
At 70 to 80 hr after anthesis, the syncytial endosperm consists of a layer of cytoplasm with free nuclei surrounding the large central vacuole (Figure 1A). At this stage, the free nuclear division cycles have been completed, and no mitotic spindle remains can be detected. The endosperm is surrounded by the endothelial cells, which typically exhibit a darkly staining cytoplasm (Figure 1A). Three distinct regions can be recognized in the highly asymmetric developing endosperm of Arabidopsis: the micropylar zone, the central zone, and the chalazal zone. The cytoplasm-rich micropylar zone completely envelops the developing embryo (Figures 1A and 1B). The adjacent central zone consists of a thin peripheral layer of cytoplasm with regularly spaced nuclei and a large central vacuole, whereas the chalazal zone displays a set of dispersed nuclei within a dense cytoplasm (Figure 1A). The latter zone is surrounded in part by the chalazal proliferating cells (Figure 1A).
Figure 1.
Light Microscopy of a Longitudinal Section of a Developing Arabidopsis Seed.
(A) Overview. Three distinct regions can be recognized in the developing endosperm: the micropylar zone (MZ) in which the embryo (E) is located, the central zone (CZ), and the chalazal zone (CHZ). The central zone consists of a thin peripheral layer of cytoplasm with regularly spaced nuclei (asterisks) and a large central vacuole (VA). The chalazal zone is partially enveloped by the chalazal proliferating cells (CHPC). The endosperm is surrounded by the endothelium (EN). 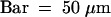 .
.
(B) Detail of the micropylar zone. Two different domains can be distinguished according to their relation with the central vacuole. One cytoplasmic domain (I) surrounds part of the embryo proper (E) and the suspensor (S) but does not abut the central vacuole (VA). The other domain (II) is bordered by the remaining part of the embryo proper and the central vacuole. Endosperm nuclei are indicated by asterisks. EN, endothelium. 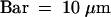 .
.
The micropylar zone can be divided into two different domains according to their relation to the central vacuole. As shown in Figure 1B, one cytoplasmic domain is sandwiched between the endothelial cells and the suspensor and part of the embryo proper but does not abut the central vacuole, whereas the other domain is bordered by the remaining part of the embryo proper and the central vacuole. Here, we document the formation of anticlinal cell walls that, when completed, form direct bridging structures between the syncytial cell walls adjacent to the endothelium and the embryo. We have also observed the same types of mini-phragmoplast and syncytial-type cell plate during the initial stages of cellularization of the central and chalazal zones. However, because the subsequent cell wall growth steps in these latter zones involve numerous other structural intermediates, a full report of the cellularization process in the central and chalazal zones will be presented elsewhere.
Near the suspensor, the syncytial cell wall facing the endothelium exhibits conspicuous transfer ingrowths surrounded by numerous mitochondria. The cytoplasm in the micropylar zone typically contains dilated endoplasmic reticulum cisternae, Golgi stacks, and plastids with grana (data not shown).
Structures Associated with Cell Plate Formation in the Micropylar Zone
The cellularization process in the micropylar zone of the endosperm begins at the late globular embryo stage with the synchronous assembly of small groups of oppositely oriented microtubules into phragmoplast-like structures and the formation of cell plates between both sister and nonsister nuclei (Figure 2). Because these “phragmoplasts” and cell plates differ in important respects from those produced by somatic cells, we refer to the structures reported here as mini-phragmoplasts and syncytial-type cell plates. Each mini-phragmoplast consists of two opposing sets of ∼10 microtubules each, and the cell plates are formed by multiple mini-phragmoplasts that act in concert between pairs of adjacent endosperm nuclei. These mini-phragmoplasts appear to deliver Golgi-derived vesicles ∼63 nm in diameter (sd ± 10, n = 20) to the equatorial plane between the nuclei, where their fusion initiates the process of cell plate formation (Figures 3A to 3F). Fusion of these vesicles involves hourglass-shaped intermediates (Figures 3B to 3D). Fusion tubes, the unique 20-nm-wide membrane tubes that mediate vesicle fusion in somatic cell plates (Samuels et al., 1995), were never observed.
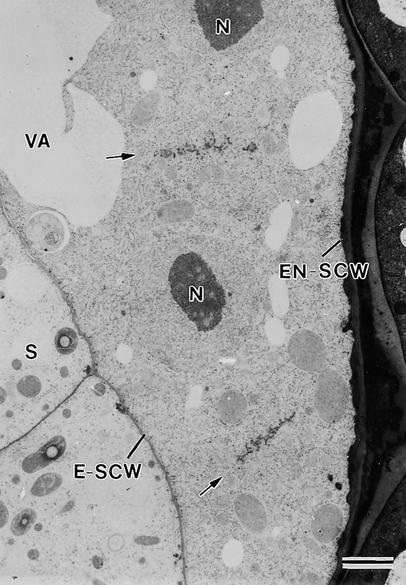
Figure 2.
Syncytial-Type Cell Plates Form between Nonsister Nuclei at the Onset of Cellularization in the Micropylar Zone.
Cell plates are aligned fairly perpendicular to the syncytial cell walls facing the embryo (E-SCW) and the endothelium (EN-SCW) (anticlinal orientation). Arrows indicate syncytial-type cell plates. N, nuclei; S, suspensor; VA, vacuole. 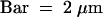 .
.
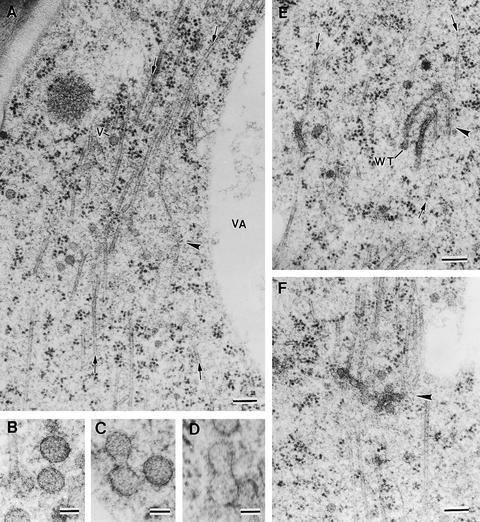
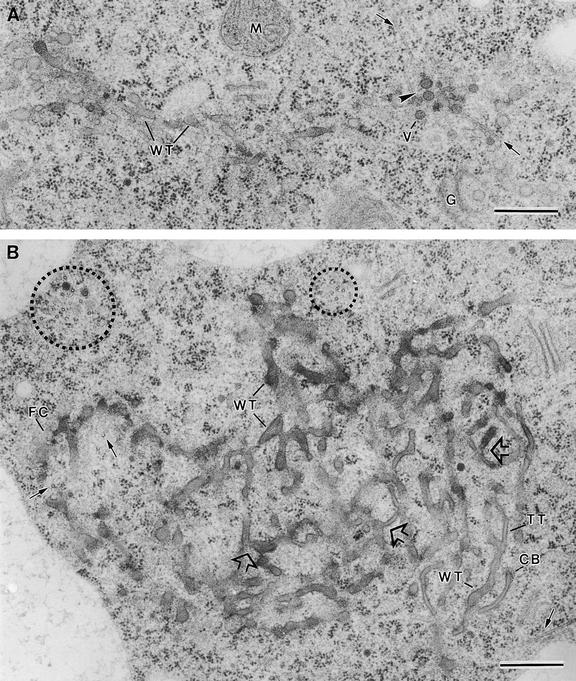
Figure 3.
Examples of Assembly of Mini-Phragmoplasts at the Onset of Endosperm Cellularization and Vesicle Fusion Steps.
(A) Mini-phragmoplast (arrowhead) consisting of two sets of opposed microtubules (arrows). Golgi-derived vesicles of ∼63 nm (V) are associated with the mini-phragmoplast microtubules. VA, vacuole.
(B) to (D) Vesicle fusion steps showing two Golgi-derived vesicles together (B), an hourglass-shaped intermediate resulting from the fusion of two vesicles (C), and a transient structure between an hourglass-shaped intermediate and a wide tubule (D).
(E) Wide tubules (WT) are aligned in a parallel orientation to the microtubules (arrows) in a mini-phragmoplast (arrowhead).
(F) Fused and branched wide tubules in a mini-phragmoplast (arrowhead).
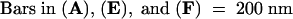 ;
; 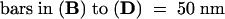 .
.
Fusion of the cell plate–forming vesicles results in the formation of wide membrane tubules with a diameter of 45 nm (sd ± 11, 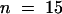 ) (Figures 3E and 4), which quickly become covered by a fuzzy coat of fine filamentous molecules (Figures 5A and 5B). As this filamentous mesh expands, it excludes ribosomes from the region at which the cell plates are forming. Often, the first cell plate–forming wide tubules appear aligned with the syncytial phragmoplast microtubules (Figure 3E). However, as they fuse with each other to form a branched wide tubule network, they assume a more equatorial orientation (Figures 3F, 4, 5A, and 5B).
) (Figures 3E and 4), which quickly become covered by a fuzzy coat of fine filamentous molecules (Figures 5A and 5B). As this filamentous mesh expands, it excludes ribosomes from the region at which the cell plates are forming. Often, the first cell plate–forming wide tubules appear aligned with the syncytial phragmoplast microtubules (Figure 3E). However, as they fuse with each other to form a branched wide tubule network, they assume a more equatorial orientation (Figures 3F, 4, 5A, and 5B).
Figure 4.
Two Wide Tubular Networks Formed in Two Adjacent Mini-Phragmoplasts.
WTN, wide tubular network. 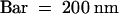 .
.
Figure 5.
Developmental Stages in Syncytial-Type Cell Plate Formation.
(A) Early stages in vesicle aggregation and wide tubule consolidation. Wide tubules are already covered by a fuzzy coat (FC).
(B) Wide tubular network covered by a dense fuzzy coat. Some microtubules (MT) and vesicles (V) are associated with the wide tubular network.
(C) Different membranous domains in a syncytial-type cell plate showing the transition from the wide tubular network to the thin tubular network (W-TTN) and some noncoated thin tubules corresponding to the thin tubular network (TTN). Note that the fuzzy coat is disassembled during the conversion of wide tubules into thin tubules.
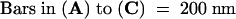 .
.
The next stage of syncytial cell plate maturation involves the conversion of the wide tubular network into a thin tubular network and the disassembly of the fuzzy coat (Figure 5C). During this process, the diameter of the tubules is reduced from ∼45 nm to ∼27 nm (sd ± 6, 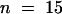 ). Coincident with the formation of noncoated thin tubules is the appearance of clathrin-coated buds and vesicles, mainly located at the ends of the thin tubules (Figure 6B).
). Coincident with the formation of noncoated thin tubules is the appearance of clathrin-coated buds and vesicles, mainly located at the ends of the thin tubules (Figure 6B).
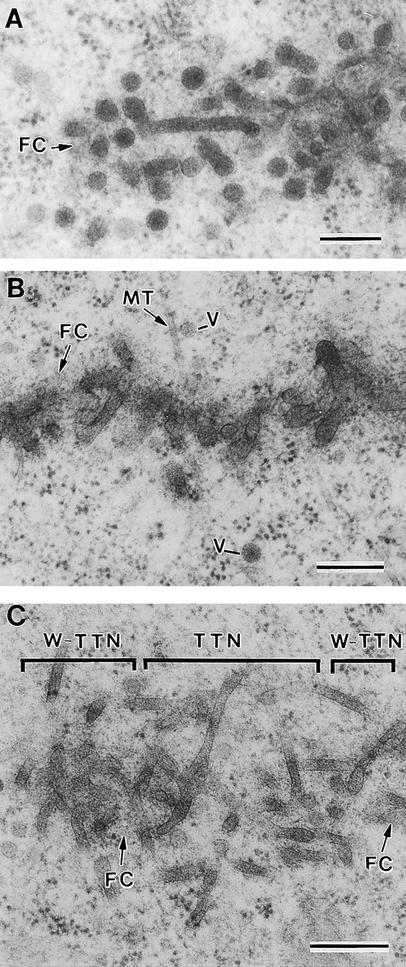
Figure 6.
Syncytial-Type Phragmoplast and Cell Plate.
(A) Transverse section of a syncytial-type cell plate. Wide tubules (WT) are seen in the tubular network. Golgi-derived vesicles (V) are associated with mini-phragmoplast microtubules (arrows) at the growing cell plate margin. Note that vesicles do not seem to fuse directly with the cell plate network but instead fuse first with each other (arrowhead). A mitochondrion (M) and a Golgi stack (G) seem close to the cell plate.
(B) Tangential section of a syncytial-type cell plate. Mini-phragmoplasts consisting of 4 to 12 microtubules (dotted circles) are located discontinuously along the margin of the growing cell plate. Some single microtubules (arrows) are interspersed among tubular elements. The nonuniform growth and maturation of these cell plates are evidenced by the presence of thin tubules (TT) between wide tubular domains (WT). Wide tubule to thin tubule transition sites are indicated by open arrows. Clathrin-coated membrane buds (CB) are associated with thin tubules. FC, fuzzy coat.
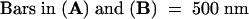 .
.
Although each mini-phragmoplast appears to act independently, those that form between a given pair of nuclei produce aligned cell plates that develop synchronously (Figure 4). The wide tubular networks formed by each mini-phragmoplast (Figure 4) grow centrifugally until they merge into a coherent cell plate network (Figures 6A and 6B). Because of the variable age of these different network domains, cell plate maturation occurs in a patchwork manner, as evidenced by the multiple sites involved in transition from wide tubules to thin tubules (Figure 6B, open arrows).
One of the most intriguing and defining features of the syncytial-type cell plate formation is the scarcity of microtubules involved in this process. These cell plates arise at sites defined by small sets of microtubules organized into mini-phragmoplasts (Figure 3). At later stages, the clusters of mini-phragmoplast microtubules (4 to 12 microtubules/mini-phragmoplast) are seen around the margins of the tubular cell plate networks (Figure 6B, dotted circles). In addition, a few single microtubules are seen between the network tubules (Figure 6B, arrows). The high frequency with which vesicles are observed adjacent to both clustered and individual microtubules (Figure 6A) suggests that their principal function is to deliver Golgi-derived vesicles to the forming cell plate.
Lateral growth of the syncytial-type cell plates appears to follow the same sequence of events described above for the initiation of the individual tubular networks (Figures 3B to 3D). First is the fusion of vesicles (Figure 6A) and the formation of wide tubules by way of hourglass intermediates; then comes the fusion of these tubules with elements of the wide tubular network at the growing edge of the cell plate. The incorporation of new vesicles to the cell plate by means of vesicle and tubule fusion processes occurs mostly near the groups of mini-phragmoplast-associated microtubules.
A consequence of the lack of a broad band of closely packed phragmoplast microtubules around the edge of syncytial-type cell plates is the absence of a structure for pushing larger organelles out of the way of the growing cell plate edge. Thus, it is not uncommon to see the narrow edge of an expanding syncytial-type cell plate colliding with a large organelle such as a plastid or a mitochondrion (data not shown). The small amount of buckling of the cell plate edge seen in instances where organelle denting is evident suggests that the cell plate margin is quite stiff.
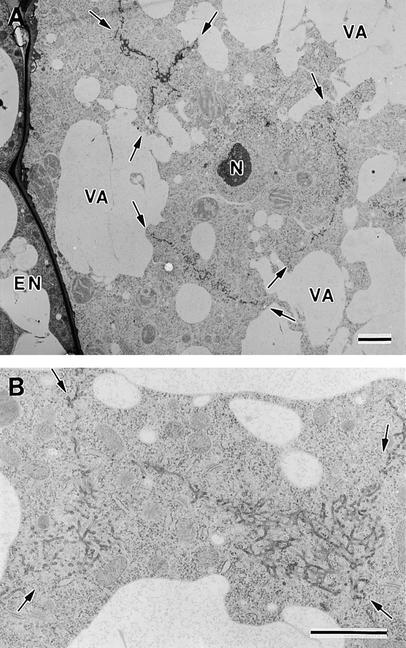
The cytoplasm of the micropylar zone of the endosperm contains a single layer of free nuclei (Figure 7, asterisks). Because this zone forms a cylindrical sheath around the suspensor, the nuclei in this sheath are organized in the form of a two-dimensional array parallel to the syncytial cell wall. Thus, the cellularization of this region of cytoplasm requires the formation of multiple cell plates around each nucleus to form a honeycomb-like organization of cell walls perpendicular to the syncytial cell walls (anticlinal cell walls). This process is illustrated in Figure 8, which depicts a tangential view through the endosperm cytoplasm in the micropylar zone. In particular, Figure 8A shows multiple cell plates around a single nucleus; moreover, these cell plates are in the process of linking with each other and with the other adjacent cell plates, forming Y- or T-shaped junctions. At higher magnifications (Figure 8B), this joining is seen to involve cell plates that are still in the tubular network stage of development. Maturation appears to be slow compared with network growth, as evidenced by the fact that the large cell plates retain mostly a tubular network architecture across their entire width, even as they fuse with each other (Figure 8). Once the adjoining cell plate networks have fused together, they link with the plasma membrane and the syncytial cell walls, while simultaneously maturing into fenestrated sheets (Figure 9). Clathrin-coated buds/vesicles are frequent during this stage (Figures 9A and 9B), and multivesicular bodies are common in the vicinity of the cell plate (Figure 9B). The fenestrated sheets finally mature into anticlinal cell walls, which contain numerous primary plasmodesmata (data not shown).
Figure 7.
Syncytial-Type Cell Plates Ready to Fuse with a Syncytial Cell Wall in the Cytoplasmic Domain That Does Not Abut the Central Vacuole.
See Figure 1 for an overview. Endosperm nuclei are indicated by asterisks. Syncytial-type cell plates are marked by arrows. E, embryo proper; EN, endothelium; S, suspensor. 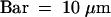 .
.
Figure 8.
Fusion of Syncytial-Type Cell Plates.
(A) Syncytial-type cell plates (arrows) forming simultaneously around an endosperm nucleus (N). All of the cell plates exhibit a tubular network architecture, and where they have joined, they give rise to Y- or T-shaped junctional domains. EN, endothelium; VA, vacuole.
(B) Detail of the network (arrows) resulting from the fusion of several syncytial-type cell plates.
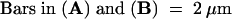 .
.
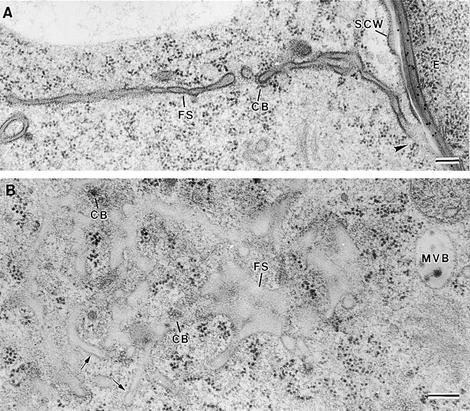
Figure 9.
Fusion of Syncytial-Type Cell Plates with the Syncytial Cell Wall.
(A) Transverse section of a flattened fenestrated sheet (FS) that has already fused (arrowhead) with the syncytial cell wall (SCW) facing the embryo (E).
(B) Tangential section of a syncytial-type cell plate at the fenestrated sheet stage. Some tubules (arrows) are still observed. Clathrin-coated structures associated with the cell plate and multivesicular bodies (MVB) are commonly observed at this stage.
CB, clathrin-coated bud; FS, fenestrated sheet. 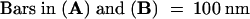 .
.
Composition of Syncytial-Type Cell Plates
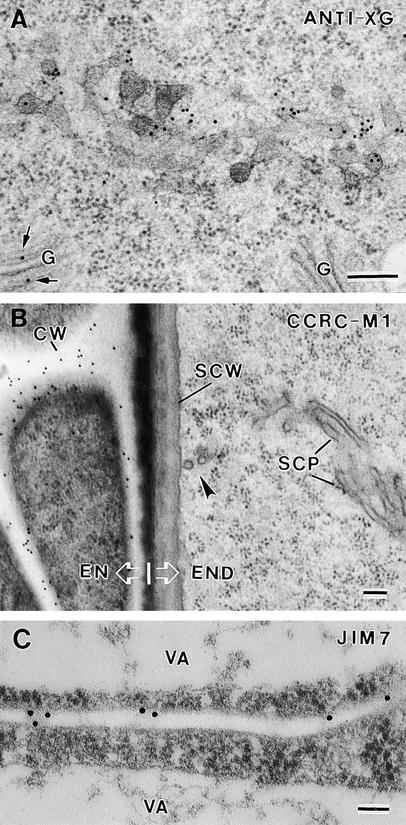
To determine whether syncytial-type cell plates differ in composition from somatic-type cell plates, we have labeled cryofixed/freeze-substituted and plastic-embedded specimens with a series of anti-polysaccharide antibodies and a cellulose binding probe (Figures 10 and 11). To assess the xyloglucan content of these cell plates, we used both a polyclonal anti-xyloglucan antibody, which recognizes the β-1,4–linked glucose backbone of xyloglucan (Lynch and Staehelin, 1992), and the monoclonal antibody CCRC-M1, which binds to the terminal fucosyl residue of the trimeric side chain of xyloglucan in the context of an extended xyloglucan conformation (Puhlman et al., 1991). The anti-xyloglucan antibody labeled the cell plates strongly, starting with the earliest stages of their assembly (Figure 10A). Labeling was also seen over Golgi stacks and vesicles. In contrast, the CCRC-M1 antibody failed to label any structure of the syncytial endosperm, including cell plates, Golgi stacks, or even cell walls (Figure 10B). However, CCRC-M1 labeling was observed over the cell walls of both the embryo and the integument and endothelial cells surrounding the endosperm (Figure 10B). Thus, the lack of CCRC-M1 labeling of the syncytial-type cell plates and the syncytial cell walls most likely reflects an absence of the xyloglucan–fucose epitope in these structures rather than an inability of the antibody to bind effectively to the epitopes in our sections.
Figure 10.
Labeling Cell Plates and Cell Walls with Anti-Xyloglucan and Anti-Pectic Polysaccharide Antibodies.
(A) Transverse section of a wide tubular network and Golgi stacks (G) labeled with anti-xyloglucan antibody (ANTI-XG). Arrows indicate labeled Golgi stack.
(B) Section labeled with CCRC-M1 (anti-xyloglucan fucose residues) antibodies. Note the lack of label on the syncytial-type cell plate (SCP) and the syncytial cell wall (SCW) and the strong labeling of the adjacent endothelium cell walls (CW). The arrowhead indicates the site of fusion between the syncytial-type cell plate and the syncytial cell wall. EN, endothelium; END, endosperm.
(C) Transverse section of a recently formed anticlinal cell wall labeled with JIM7. VA, vacuole.
 .
.
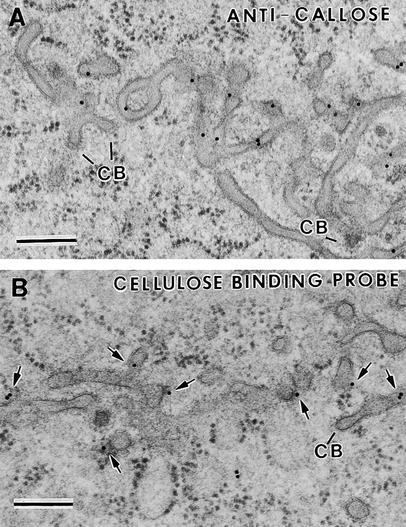
Figure 11.
Labeling Cell Plates and Cell Walls with Anti-Callose Antibodies and CBHI-Gold Probe.
(A) Tangential section of a thin tubular network and some wide tubules labeled with anti-callose antibodies. Two clathrin-coated membrane structures (CB) are seen budding off some tubules in the cell plate.
(B) Transverse section of a wide tubular network labeled with CBHI-gold (arrows).
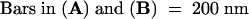 .
.
The presence of methyl-esterified pectins was tested by using the JIM7 monoclonal antibody (Knox et al., 1990). This antibody failed to bind esterified pectins on Epon-embedded (Ted Pella, Inc., Redding, CA) samples, but labeling was obtained on tissues embedded in London Resin White (Electron Microscopy Sciences, Fort Washington, PA). Although the preservation of syncytial-type cell plate with this latter resin was poor (data not shown), we were able to identify newly formed anticlinal cell walls in which JIM7 labeling was observed (Figure 10C).
Callose, which was first detected by means of anti-callose (anti–β-1,3-glucan) antibody (Meikle et al., 1991) during the wide tubule stage of syncytial-type cell plate formation, increases during cell plate maturation (Figure 11A). Strong labeling was also seen over recently completed cell walls in which all fenestrae had closed. On the other hand, no callose labeling was observed over Golgi stacks, vesicles, syncytial cell walls, or transfer ingrowths (data not shown).
The presence of low concentrations of cellulose in syncytial-type cell plates was suggested by the binding of the cellulose probe cellobiohydrolase I–gold (CBHI-gold) (Figure 11B). This labeling, however, was much less than that over the mature cell walls of the surrounding cells (data not shown). Cellulose could first be detected during the wide tubular network stage (Figure 11B) and increased through the fenestrated sheet stage. Because no labeling of Golgi stacks or of vesicles was observed, this labeling is highly likely to have resulted from binding to cellulose and not from binding of the probe to the backbone of xyloglucan molecules, which also consists of β-1,4–linked glucan residues.
DISCUSSION
The formation of the very first anticlinal walls between nonsister nuclei in the syncytial endosperm has remained controversial for more than 90 years (DeMason, 1997). Thus, despite general agreement about the role of nuclear-based radial microtubule systems for the positioning of anticlinal cell walls, the mechanism of assembly of these cell walls has remained an enigma. Briefly, three different theories have been put forward to explain this process: (1) extension of septal-like ingrowths of the syncytial walls in the absence of mitosis and typical phragmoplasts; (2) conventional phragmoplast-mediated cell plate formation after mitosis, and (3) non-mitosis-related phragmoplasts and cell plates. For Arabidopsis, two previous studies have reported that the first anticlinal cell walls in the endosperm arise from septal-like extensions of the syncytial cell wall (Mansfield and Briarty, 1990; Brown et al., 1999). In the present study, which is based on samples preserved by high-pressure freezing/freeze substitution rather than chemical fixation, we demonstrate that the initial anticlinal walls formed during endosperm cellularization do not arise from septal-like ingrowths but are produced instead by groups of mini-phragmoplasts, which give rise to unique cell plates, syncytial-type cell plates, that then mature into cell walls. The main stages of this process are summarized diagramatically in Figures 12 and 13.
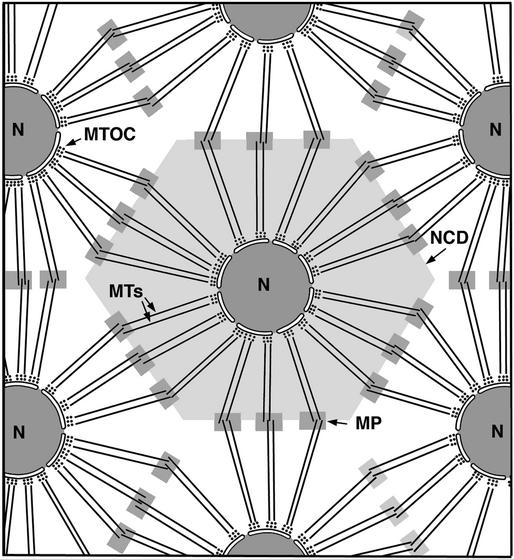
Figure 12.
Diagram Showing Microtubule Organization in the Syncytial Endosperm at the Onset of Cellularization and the Putative Origin of the Mini-Phragmoplasts from Opposing Overlapping Clusters of Microtubules.
Microtubules radiating from microtubule-organizing centers (MTOCs) in the nuclear envelope define the nuclear cytoplasmic domains (NCDs) and the sites of syncytial-type cell plate formation. MP, mini-phragmoplasts; N, nucleus.
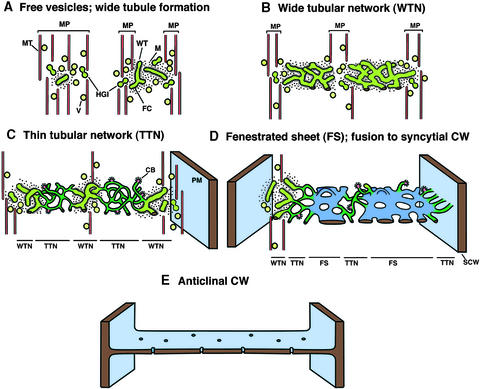
Figure 13.
Stages of Syncytial-Type Cell Plate and Cell Wall Formation.
The model depicts the main stages of syncytial-type cell plate and cell wall formation in Arabidopsis endosperm, with a newly formed anticlinal cell wall connecting the syncytial cell walls facing the endothelium and the embryo (E). CB, clathrin bud; CW, cell wall; FC, fuzzy coat; HGI, hourglass intermediate; M, ribosome-excluding matrix; MP, mini-phragmoplast; MT, microtubules; PM, plasma membrane; SCW, syncytial cell wall; V, vesicles; WT, wide tubules.
Small Clusters of Microtubules Become Organized into Mini-Phragmoplasts during Syncytial-Type Cell Plate Development
Cellularization of the endosperm in Arabidopsis starts with the formation of several mini-phragmoplasts between both sister and nonsister nuclei (Figures 3A, 3E, and 3F). Each of these mini-phragmoplasts comprises two opposing sets of ∼10 microtubules, which during later stages of cell plate development are seen to persist as distinct morphological units around the cell plate margins (Figure 6B). This particular type of phragmoplast organization has never been observed during plant cytokinesis in somatic cells (Baskin and Cande, 1990; Heese et al., 1998). The scarcity of microtubules associated with the mini-phragmoplasts during formation of syncytial-type cell plates probably reflects the fact that during the cellularization process, not enough tubulin is available to assemble simultaneously five or six conventional somatic cell-type phragmoplasts around each endosperm nucleus.
In somatic cells, the phragmoplast microtubules arise initially from remnants of the mitotic spindle. In developing Arabidopsis seeds, however, Brown et al. (1999) have observed that after completion of the cycles of free nuclear divisions, no mitotic spindle microtubules remain in the syncytial endosperm before assembly of the new cell walls. Instead, the syncytial endosperm becomes organized into nuclear cytoplasmic domains (Brown and Lemmon, 1992; Pickett-Heaps et al., 1999) that are defined by radial microtubule arrays produced by microtubule organizing centers associated with the external surface of the nuclear envelope (Lambert, 1995). This nuclear-based arrangement of microtubules has also been noticed in the syncytial endosperm of other species, such as rice, barley, wheat, and P. vulgaris (Van Lammeren, 1988; Brown et al., 1994; XuHan and Van Lammeren, 1994; Olsen et al., 1995; Brown et al., 1996a, 1996b) as well as in other types of syncytial systems such as developing microspores (Van Lammeren et al., 1985; Brown and Lemmon, 1991). The interaction of opposing microtubules emanating from adjacent nuclei has been suggested to play an important function in determining the sites of non-mitosis-related phragmoplast formation between nonsister nuclei (Van Lammeren, 1988; XuHan and Van Lammeren, 1994). Given the structural data presented here, we postulate that at the onset of cellularization in Arabidopsis endosperm, the mini-phragmoplast microtubules arise from the opposing overlapping clusters of microtubules radiating from neighbor nuclei (Figure 12). Because the same nuclear cytoplasmic domain organization has been observed in the micropylar, central, and chalazal zones of Arabidopsis endosperm (Brown et al., 1999), we think it likely that mini-phragmoplasts originate in the same manner in all of the endosperm zones.
Growth of Syncytial-Type Cell Plates Involves Fusion of Vesicles by Way of Hourglass Intermediates, Formation of Wide Tubules, and Fusion of Wide Tubules into Networks
One of the most striking differences between syncytial- and somatic-type cell plate formation pertains to the mechanism of fusion of the cell plate–forming vesicles. In dividing somatic cells, this process involves unique, 20-nm-diameter fusion tubes (Samuels et al., 1995; Verma and Gu, 1996), whereas in the syncytial endosperm, fusion seems to occur only between closely opposed vesicles, as evidenced by the hourglass-shaped fusion intermediates (Figures 3B to 3D).
At present, very little is known about the molecules involved in the fusion of cell plate–forming vesicles. The KNOLLE protein, a cytokinesis-specific syntaxin, plays a critical role in membrane fusion events associated with somatic-type cell plate formation, most likely by participating in the homotypic fusion of Golgi vesicles in somatic-type cell plate formation (Lukowitz et al., 1996; Lauber et al., 1997). Because syncytial-type cell plates are also derived from homotypic fusions of Golgi-derived vesicles, one would expect KNOLLE protein or a close homolog to be present in forming syncytial-type cell plates. This prediction has been verified in cellularizing Arabidopsis endosperm cells by means of anti-KNOLLE protein antibodies (Lauber et al., 1997). It will be interesting to determine if the knolle mutant displays the same ultrastructural alterations in syncytial- and somatic-type cell plates preserved by high-pressure freezing/freeze-substitution techniques.
The centrifugal growth of cell plates involves the incorporation of new Golgi-derived vesicles into the cell plate margin. In syncytial-type cell plates, the vesicles do not seem to fuse directly with the cell plate but instead fuse first with each other to form wide tubules. Only after vesicles fuse into tubules do they appear to gain the ability to fuse with elements of the wide tubular network in the growing cell plate margin. Thus, incorporation of new material into the growing cell plate seems to encompass two steps: the homotypic fusion between Golgi-derived vesicles and the homotypic fusion between wide tubules. Two other homotypic fusion steps occur during syncytial-type cell plate formation: fusion of the early tubular networks of adjacent mini-phragmoplasts, and fusion of adjoining cell plates at the sites of future cell corners to form the honeycomb-like wall configurations.
Conserved Structures Allow for the Identification of Corresponding Stages in Somatic- and Syncytial-Type Cell Plates
The assembly of both somatic- and syncytial-type cell plates involves transient membrane networks that undergo programmed changes during cell plate maturation, but as documented in this report, the geometries of these networks differ between the two systems. In view of the appearance of a characteristic, dense fuzzy coat on both the wide tubular network from syncytial-type cell plates (Figure 5B) and the tubulo-vesicular network from somatic-type cell plates (Samuels et al., 1995), we postulate that these two membrane networks represent corresponding stages in cell plate formation. The assembly of a very dense fibrous coat onto the surface of the initial membrane networks may serve to mechanically stabilize these delicate membrane systems until the cell wall–forming polysaccharides can take over this function (Staehelin and Hepler, 1996). The persistence of a dense fibrous coat on the wide tubules throughout the lengthy wide tubular network stage of syncytial-type cell plate formation (Figures 5B, 6A, and 6B) supports the argument of the membrane-strengthening function of the fibrous coat material. With the importance of this fuzzy coat material now firmly established for both types of cell plates, future studies need to identify the molecules involved in the formation of this coat.
The next stage in cell plate maturation, loss of the fuzzy coat and conversion of the associated membrane systems into a thin tubular network, is another feature in common in the two types of cell plates (Figures 5C and 6B; Samuels et al., 1995, Figures 4 and 5B). These changes are accompanied by two other developments in common: the appearance of clathrin-coated buds and vesicles, and the rapid buildup of callose deposits within the thin tubules. Whereas the former structures appear to be responsible for removing selected membrane proteins and excess membrane lipids from the cell plate networks, the initial accumulation of callose within the membrane tubes may provide the mechanical support that allows the safe dismantling of the fuzzy coat.
Syncytial-Type Cell Plates Mature Heterogeneously and More Slowly Than Do Somatic-Type Cell Plates
Syncytial-type cell plates mature in a patchwork manner, showing different domains at various developmental stages at any given time. This particular pattern of maturation is related to the fact that these cell plates are produced by multiple mini-phragmoplasts that act in concert but, because of the small number of microtubules, have limited capabilities for vesicle transport. Thus, initially, at the onset of cellularization, each mini-phragmoplast gives rise to its own tubular network, which then expands and fuses with other tubular networks to establish a large consolidated tubular cell plate network (Figures 6B and 8) that can be converted into a new cell wall. This multiple origin is one of the principal causes of the patchwork maturation of syncytial-type cell plates, but it is not the only one. A second cause is the mechanism of lateral cell plate enlargement, which continues to involve multiple independent mini-phragmoplasts located around the cell plate margins. Because transport of vesicles to the cell plate appears to occur along the microtubules of these mini-phragmoplasts, and because vesicle fusion and fusion of wide tubules with the growing edge also occur close to the mini-phragmoplasts, the enlargement of cell plates appears to be confined to localized domains around their margins.
This localized growth phenomenon leads to the question of how cell plate expansion is controlled. Given the discrete nature of the mini-phragmoplasts associated with syncytial-type cell plates, and considering the short life (t1/2) of the microtubules (Hepler and Hush, 1996), the even growth of the cell plate margins could involve either gradual, microtubule turnover–mediated shifts in mini-phragmoplast distribution or the complete breakdown of entire mini-phragmoplasts and the creation of new ones in different locations. Only observations on appropriately labeled living cells will enable researchers to distinguish between these alternatives.
Our micrographs also demonstrate that syncytial-type cell plates mature more slowly than somatic-type cell plates. This is shown by the extended duration of the wide tubular network stage of syncytial-type cell plate formation, even after fusion of adjacent syncytial-type cell plates (Figure 8). After all adjoining cell plates have linked to form a honeycomb-like configuration, they fuse with the parental plasma membrane and the syncytial cell wall. This fusion step coincides with conversion of the tubular network cell plate into a fenestrated sheet and, finally, into an anticlinal cell wall. A similar pattern of cell plate maturation after fusion with the parental cell wall has been demonstrated during the final phases of somatic-type cell plate assembly (Samuels et al., 1995) and has been related to the presence of maturational factors in the cortical division site (Mineyuki, 1999).
Xyloglucans in Syncytial-Type Cell Plates and Endosperm Cell Walls Are Devoid of Fuscose Residues
The strongest and most consistent labeling of all stages of syncytial-type cell plate formation as well as of Golgi stacks and Golgi-derived vesicles was obtained with the anti-xyloglucan backbone antibodies. This result is consistent with previous observations on somatic-type cell plates (Moore et al., 1986) and with the finding that xyloglucan is synthesized by Golgi-localized enzymes and delivered to the cell plate in vesicles (Moore et al., 1986; Zhang and Staehelin, 1992). In contrast, the same structures are not labeled with the anti-xyloglucan side chain antibody CCRC-M1, which recognizes the terminal fucose residues on the trisaccharide side chain of xyloglucan (Puhlman et al., 1991). Given that all of the walls of the cells surrounding the endosperm were positively labeled with this antibody, we can rule out the possibility that CCRC-M1 antibodies were unable to bind the xyloglucan–fucose epitope in our samples. Thus, we conclude that the xyloglucan molecules produced by Arabidopsis endosperm lack terminal fucose residues.
This finding is not completely unexpected, because the seed storage form of xyloglucans in nasturtium (Tropaeolum majus), legumes, and other plants is essentially devoid of terminal fucose residues (Reid, 1985). However, the nasturtium embryo produces two kinds of xyloglucans during development (Desveaux et al., 1998): a structural fucosylated xyloglucan that is delivered to the primary cell wall, and a storage nonfucosylated xyloglucan that accumulates in embryo cells in the periplasmic space between the plasma membrane and the primary cell wall. Our labeling data show that in contrast to nasturtium, in Arabidopsis endosperm neither the xyloglucans associated with the primary cell walls nor the xyloglucans in the cell plates become fucosylated. The absence of labeling with the CCRC-M1 antibodies on Golgi stacks, Golgi-derived vesicles, and cell plates in Arabidopsis endosperm supports the idea that neither transport nor deposition of xyloglucan in the extracellular matrix involves transient fucosylation of xyloglucan followed by a defucosylation step.
The lack of terminal fucose residues on the trisaccharide side chain of xyloglucan has several interesting functional implications. As shown by Levy et al. (1991)(1997), the presence of these terminal fucose residues leads to the stabilization of the straight, flat conformation of xyloglucan, which can bind to cellulose fibrils. In the absence of these fucose residues, the xyloglucan molecules tend to assume a twisted conformation that cannot bind to cellulose. The inability to bind to cellulose makes such fucose-less xyloglucan more accessible to hydrolytic enzymes and therefore more suitable as a storage form of polysaccharide. At the same time, the lack of stable xyloglucan binding to cellulose microfibrils will lead to cell walls that are less cross-linked and hence mechanically weaker, as evidenced by the decreased tensile strength of the cell walls of the mur1 cell wall mutant (Zablackis et al., 1996). Based on these considerations, the cell walls of Arabidopsis endosperm probably also lack tensile strength, but because the endosperm is surrounded and protected by mechanically strong tissues, this weakness seems to have no deleterious consequences. Indeed, the lack of strength and rigidity may be critical for endosperm function, given that the endosperm cell wall has to expand locally and give way as the embryo enlarges and impinges on the endosperm space.
Callose Serves Different Functions in Syncytial-Type Cell Plates and Endosperm Cell Walls
Callose is first detected during the wide tubular network stage of syncytial-type cell plate formation, increases during the formation of the thin tubular networks and fenestrated sheets, and persists in the mature endosperm cell wall. Whereas these findings parallel those reported for somatic-type cell plates, the presence of callose in the mature cell walls constitutes a unique property of endosperm cell walls (Stone and Clarke, 1992; Brown et al., 1997). The callose associated with somatic-type cell plates has been postulated to perform two functions (Samuels et al., 1995): (1) to mechanically stabilize the delicate membrane tubules as the fuzzy coat is dismantled and the cell plate–forming membranes are matured by the selective removal of membrane components by way of clathrin-coated vesicles, and (2) to provide the spreading force that widens the tubules and converts the network into a fenestrated sheet and a continuous cell wall. Support of these interpretations has come from studies with the drug caffeine, which prevents callose synthesis when somatic-type cell plates are forming and leads to their fragmentation, and from the demonstration that callose forms a membrane surface–adhering layer during the formation of fenestrated sheets (Samuels et al., 1995; Samuels and Staehelin, 1996). Given the similarity of the membrane structures with which callose is associated during syncytial-type cell plate formation and given the kinetics of callose deposition, we postulate that callose performs the same membrane stabilization and thin tubule expansion functions in both types of cell plates.
However, whereas callose disappears from somatic-type cell plates during their conversion into cell walls, the callose in syncytial-type cell plates not only persists throughout all stages of cell plate formation but also is maintained after cell walls have matured. The presence of callose in mature endosperm cell walls has also been reported for rice and other cereals (Stone and Clarke, 1992; Brown et al., 1997). One possible function of callose in endosperm cell walls is to serve, like the fucose-less xyloglucan, as a carbohydrate reserve for the growing embryo. In addition, because of its gel-like physical properties, callose could further increase the plasticity of the endosperm cell walls to allow for the remodeling of the endosperm tissue around the rapidly expanding embryo.
METHODS
Seeds of Arabidopsis thaliana (Landsberg erecta wild type) were planted in Metro-mix 200 growing medium (America Clay Works, Denver, CO) with Arabidopsis controlled-release fertilizer 17-6-12 + minor (Lehle Seeds, Round Rock, TX). Plants were grown in continuous fluorescent lighting, at a temperature of 27 ± 1°C and a relative humidity of 45%. Developing seeds were excised at various hours after anthesis.
High-Pressure Freezing/Freeze Substitution
Whole developing seeds were removed from the siliques. They were immediately loaded into sample hat holders that had been coated with a solution of 100 mg of lecithin per mL of chloroform. The holders were filled with a solution of 15% dextran or 0.1 M sucrose. The samples were frozen in a Baltec HPM 010 high-pressure freezer (Technotrade, Manchester, NH) and then transferred to liquid nitrogen for storage. Substitution was performed in 2% OsO4 in anhydrous acetone at −80°C for 72 hr, followed by slow warming to room temperature over 2 days. After several acetone rinses, some samples were teased from the holders and infiltrated in Epon or Spurr's resin (Ted Pella) according to the following schedule: 5% resin in acetone (4 hr), 10% resin (12 hr), 25% resin (12 hr), 50% (24 hr), 75% (24 hr), and 100% (24 hr). Polymerization was performed at 70°C for Epon and 60°C for Spurr's resin. Other samples were washed in ethanol after the acetone rinse and then embedded in London Resin White (Electron Microscopy Sciences) (25% in absolute ethanol, 50%, 75%, and 100%; 12 hr at each concentration); polymerization was performed at 60°C for 24 hr.
Immunolabeling and Cellobiohydrolase I–Gold Probe
Callose was localized by using a monoclonal antibody to β-1,3-glucan from Biosupplies Australia (Parkville, Victoria, Australia). Xyloglucans were localized by using the polyclonal antibody anti-xyloglucan (Moore et al., 1986) and the CCRC-M1 monoclonal antibody (Puhlman et al., 1991). Methyl-esterified pectins were localized by using the JIM7 monoclonal antibody (Knox et al., 1990).
Cellobiohydrolase I–gold was prepared as described by Krishnamurthy (1999). Cellobiohydrolase I was a gift of Dr. M. Shulein (Novo Nordisk, Danbury, CT) and was conjugated to 15-nm-diameter colloidal gold (BBInternational; Ted Pella).
Sections from Epon resin blocks (anti-callose, anti-xyloglucan, and CCRC-M1) or from LR White–embedded material (JIM7) were collected on formvar-coated nickel grids. Samples were blocked for 30 min with a 5% (w/v) solution of nonfat dried milk in PBST (Tween-20, 0.1%) (for anti-callose antibody and anti-xyloglucan antibody) or a 3% (w/v) solution of the same components (for CCRC-M1 and JIM7). Primary antibodies anti-callose (1:50 in PBST), anti-xyloglucan (1:10 in PBST), CCRC-M1 (1:10 in PBST), and JIM7 (1:1) were applied for 45 to 60 min at room temperature (anti-callose and anti-xyloglucan) or overnight at 4°C (CCRC-M1, JIM7). The sections were washed in a stream of PBST containing 0.5% Tween-20 and then transferred to the secondary antibodies (goat anti–mouse IgG for anti-callose and CCRC-M1, goat anti–rabbit IgG for anti-xyloglucan, and goat anti–rat IgG for JIM7) conjugated with 15-nm-diameter colloidal gold for 1 hr.
Controls in each case received the same treatments except that the primary antibodies were omitted.
In all cases, sections 70- to 90-nm thick were cut on a Leica Ultracut R and then stained with 2% uranyl acetate in 70% methanol for 10 min followed by Reynold's lead citrate (2.6% lead nitrate and 3.5% sodium citrate, pH 0.12) for 4 min. Samples were observed with a Philips (Hillsboro, OR) CM10 microscope. In some cases, serial sections were studied to try to elucidate the three-dimensional configuration of some cellular structures.
Acknowledgments
We thank Dr. Andreas Nebenfüehr for valuable comments on the manuscript. The donation of cellobiohydrolase I by Dr. M. Shulein (Novo Nordisk) is gratefully acknowledged. This work was supported by National Institutes of Health Grant No. 18639 and U.S. Department of Agriculture Grant No. 96-35304-3710 to L.A.S. and Fullbright/Antorchas Foundation and Comision Nacional Investigaciones Cientificas y Tecnicas, Argentina, Postdoctoral Fellowships to M.O.
References
- Baskin, T.I., and Cande, W.Z. (1990). The structure and function of the mitotic spindle in flowering plants. Annu. Rev. Plant Physiol. 41, 277–315. [Google Scholar]
- Berger, F. (1999). Endosperm development. Curr. Opin. Plant Biol. 2, 28–32. [DOI] [PubMed] [Google Scholar]
- Brown, R.C., and Lemmon, B.E. (1991). The cytokinesis apparatus in meiosis: Control of division plane in the absence of a preprophase band of microtubules. In The Cytoskeletal Basis of Plant Growth and Form, W.C. Lloyd, ed (London: Academic Press), pp. 259–273.
- Brown, R.C., and Lemmon, B.E. (1992). Cytoplasmic domain: A model for spatial control of cytokinesis in reproductive cells of plants. EMSA Bull. 22, 48–53. [Google Scholar]
- Brown, R.C., Lemmon, B.E., and Olsen, O.-A. (1994). Endosperm development in barley: Microtubule involvement in the morphogenetic pathway. Plant Cell 6, 1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, R.C., Lemmon, B.E., and Olsen, O.-A. (1996. a). Development of the endosperm in rice (Oryza sativa L.): Cellularization. J. Plant Res. 109, 301–313. [Google Scholar]
- Brown, R.C., Lemmon, B.E., and Olsen, O.-A. (1996. b). Polarization predicts the pattern of cellularization in cereal endosperm. Protoplasma 192, 168–177. [Google Scholar]
- Brown, R.C., Lemmon, B.E., Stone, B.A., and Olsen, A.-O. (1997). Cell wall (1–3)- and (1–3,1–4)—glucans during the early grain development in rice (Oryza sativa L.). Planta 202, 414–426. [DOI] [PubMed] [Google Scholar]
- Brown, R.C., Lemmon, B.E., Nguyen, H., and Olsen, O.-A. (1999). Development of endosperm in Arabidopsis thaliana. Sex. Plant Reprod. 12, 32–42. [Google Scholar]
- Chamberlin, M.A., Horner, H.T., and Palmer, R.G. (1994). Early endosperm, embryo, and ovule development in Glycine max (L.) Merr. Int. J. Plant Sci. 155, 421–436. [Google Scholar]
- DeMason, D.A. (1997). Endosperm structure and development. In Cellular and Molecular Biology of Plant Seed Development, B.A. Larkins and I.K. Vasil, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 73–115.
- Desveaux, D., Faik, A., and Maclachlan, G. (1998). Fucosyltransferase and the biosynthesis of storage and structural xyloglucans in developing nasturtium fruits. Plant Physiol. 118, 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dute, R.R., and Peterson, C.M. (1992). Early endosperm development in ovules of soybean, Glycine max (L.) Merr. (Fabaceae). Ann. Bot. 69, 263–271. [Google Scholar]
- Fineran, B.A., Wild, D.J., and Ingerfeld, M. (1982). Initial wall formation in the endosperm of wheat, Triticum aestivum: A reevaluation. Can. J. Bot. 60, 1776–1795. [Google Scholar]
- Gori, P. (1987). The fine structure of the developing Euphorbia dulcis endosperm. Ann. Bot. 60, 563–569. [Google Scholar]
- Heese, M., Ulrike, M., and Jürgens, G. (1998). Cytokinesis in flowering plants: Cellular process and developmental integration. Curr. Opin. Plant Biol. 1, 486–491. [DOI] [PubMed] [Google Scholar]
- Hepler, P.K., and Hush, J.M. (1996). Behavior of microtubules in living plant cells. Plant Physiol. 112, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox, J.P., Linstead, P.J., King, J., Cooper, C., and Roberts, K. (1990). Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181, 512–521. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy, K.V. (1999). Methods in Cell Wall Cytochemistry. (Boca Raton, FL: CRC Press).
- Lambert, A.-M. (1995). Microtubule-organizing centers in higher plants: Evolving concepts. Bot. Acta 108, 535–537. [Google Scholar]
- Lauber, M.H., Waizenegger, I., Steinmann, T., Schwarz, H., Mayer, U., Hwang, I., Lukowitz, W., and Jürgens, G. (1997). The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J. Cell Biol. 139, 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, S., York, W.S., Struike-Prill, R., Meyer, B., and Staehelin, L.A. (1991). Simulations of the static and dynamic molecular conformations of xyloglucan. The role of the fucosylated side chain in surface-specific side chain folding. Plant J. 1, 195–215. [PubMed] [Google Scholar]
- Levy, S., Maclachlan, G., and Staehelin, L.A. (1997). Xyloglucan side chains modulate binding to cellulose during in vitro binding assays as predicted by conformational dynamics simulations. Plant J. 11, 373–386. [DOI] [PubMed] [Google Scholar]
- Lukowitz, W., Mayer, U., and Jürgens, G. (1996). Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84, 61–71. [DOI] [PubMed] [Google Scholar]
- Lynch, M.A., and Staehelin, L.A. (1992). Domain-specific and cell type–specific localization of two types of cell wall matrix polysaccharides in the clover root tip. J. Cell Biol. 118, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1990). Endosperm cellularization in Arabidopsis thaliana L. Arabidopsis Inf. Serv. 27, 65–72. [Google Scholar]
- Meikle, P.J., Bönig, I., Hoogenraad, N.J., Clarke, A.E., and Stone, B.A. (1991). The location of (1-3)-glucans in the walls of pollen tubes of Nicotiana alata using a (1-3)-glucan–specific monoclonal antibody. Planta 185, 1–8. [DOI] [PubMed] [Google Scholar]
- Mineyuki, Y. (1999). The preprophase band of microtubules: Its function as a cytokinetic apparatus in higher plants. Int. Rev. Cytol. 187, 1–49. [Google Scholar]
- Moore, P.J., Darvill, A.G., Albersheim, P., and Staehelin, L.A. (1986). Immunogold localization of xyloglucan and rhamnogalacturonan I in the cell walls of suspension-cultured sycamore cells. Plant Physiol. 82, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb, W. (1973). The development of the embryo sac of sunflower Helianthus annus after fertilization. Can. J. Bot. 51, 879–890. [Google Scholar]
- Newcomb, W. (1978). The development of cells in the coenocytic endosperm of the African blood lily Haemanthus katherinae. Can. J. Bot. 56, 483–501. [Google Scholar]
- Newcomb, W., and Fowke, L.C. (1973). The fine structure of the change from the free-nuclear to cellular condition in the endosperm of chickweed Stellaria media. Bot. Gaz. 134, 236–241. [Google Scholar]
- Olsen, O.-A. (1998). Endosperm developments. Plant Cell 10, 485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, O.-A., Brown, R.C., and Lemmon, B.E. (1995). Pattern and process of wall formation in developing endosperm. BioEssays 17, 803–812. [Google Scholar]
- Olsen, O.-A., Lemmon, B.E., and Brown, R.C. (1998). A model for aleurone development. Trends Plant Sci. 3, 168–169. [Google Scholar]
- Olson, A.R. (1981). Embryo and endosperm development in ovules of Papaver nudicaule after in vitro placental pollination. Can. J. Bot. 59, 1738–1748. [Google Scholar]
- Pickett-Heaps, J.D., Gunning, B.E.S., Brown, R.C., Lemmon, B.E., and Cleary, A.L. (1999). The cytoplast concept in dividing plant cells: Cytoplasmic domains and the evolution of spatially organized cell division. Am. J. Bot. 86, 153–172. [PubMed] [Google Scholar]
- Puhlman, J., Dunning, N., Albersheim, P., Darvill, A., and Hahn, M.G. (1991). A monoclonal antibody that binds to sycamore maple xyloglucans recognizes a fucose-containing epitope. Proc. 3rd Int. Congr. Int. Soc. Plant Mol. Biol., 1028 (abstr.).
- Reid, J.S.G. (1985). Cell wall storage carbohydrates in seeds: Biochemistry of the seed “gums” and “hemicelluloses.” Adv. Bot. Res. 11, 125–155. [Google Scholar]
- Samuels, A.L., and Staehelin, L.A. (1996). Caffeine inhibits cell plate formation by disrupting membrane reorganization just after the vesicle fusion step. Protoplasma 195, 144–155. [Google Scholar]
- Samuels, A.L., Giddings, T.H., and Staehelin, L.A. (1995). Cytokinesis in tobacco BY-2 and root tip cells: A new model of cell plate formation in higher plants. J. Cell Biol. 130, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, L.G. (1999). Divide and conquer: Cytokinesis in plant cells. Curr. Opin. Plant Biol. 2, 447–453. [DOI] [PubMed] [Google Scholar]
- Staehelin, L.A., and Hepler, P.K. (1996). Cytokinesis in higher plants. Cell 84, 821–824. [DOI] [PubMed] [Google Scholar]
- Stone, B.A., and Clarke, A.E. (1992). Chemistry and Biology of (1-3)-Glucans. (Melbourne, Australia: La Trobe University Press).
- Van Lammeren, A.M.M. (1988). Structure and function of the microtubular cytoskeleton during endosperm development in wheat: An immunofluorescence study. Protoplasma 146, 18–27. [Google Scholar]
- Van Lammeren, A.A.M., Keijzer, C.J., Willemse, M.T.M., and Kieft, H. (1985). Structure and function of the microtubular cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval. Planta 165, 1–11. [DOI] [PubMed] [Google Scholar]
- Verma, D.P.S., and Gu, X. (1996). Vesicle dynamics during cell-plate formation. Trends Plant Sci. 5, 145–149. [Google Scholar]
- XuHan, X., and Van Lammeren, A.M. (1993). Microtubular configurations during the cellularization of coenocytic endosperm in Ranunculus scleratus L. Sex. Plant Reprod. 6, 127–132. [Google Scholar]
- XuHan, X., and Van Lammeren, A.A.M. (1994). Microtubular configurations during endosperm development in Phaseolus vulgaris. Can. J. Bot. 72, 1489–1495. [Google Scholar]
- Yeung, E.C., and Cavey, M.J. (1988). Cellular endosperm formation in Phaseolus vulgaris. I. Light and scanning electron microscopy. Can. J. Bot. 66, 1209–1216. [Google Scholar]
- Zablackis, E., York, W.S., Pauly, M., Hantus, S., Reiter, W.-D., Chapple, C.C.S., Albersheim, P., and Darvill, A. (1996). Substitution of l-fucose by l-galactose in cell walls of Arabidopsis mur1. Science 272, 1808–1810. [DOI] [PubMed] [Google Scholar]
- Zhang, G.F., and Staehelin, L.A. (1992). Functional compartmentalization of the Golgi apparatus of plant cells. An immunocytochemical analysis of high-pressure-frozen and freeze-substituted sycamore maple suspension culture cells. Plant Physiol. 99, 1070–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]