Abstract
The kinesin-like calmodulin (CaM) binding protein (KCBP), a minus end–directed microtubule motor protein unique to plants, has been implicated in cell division. KCBP is negatively regulated by Ca2+ and CaM, and antibodies raised against the CaM binding region inhibit CaM binding to KCBP in vitro; therefore, these antibodies can be used to activate KCBP constitutively. Injection of these antibodies into Tradescantia virginiana stamen hair cells during late prophase induces breakdown of the nuclear envelope within 2 to 10 min and leads the cell into prometaphase. However, mitosis is arrested, and the cell does not progress into anaphase. Injection of antibodies later during cell division has no effect on anaphase transition but causes aberrant phragmoplast formation and delays the completion of cytokinesis by ∼15 min. These effects are achieved without any apparent degradation of the microtubule cytoskeleton. We propose that during nuclear envelope breakdown and anaphase, activated KCBP promotes the formation of a converging bipolar spindle by sliding and bundling microtubules. During metaphase and telophase, we suggest that its activity is downregulated.
INTRODUCTION
The dynamics of the microtubule cytoskeleton during cell division in plant cells have been studied extensively (Hepler and Hush, 1996), but little is known about the roles of the motor proteins during mitosis and cytokinesis. Microtubule motor proteins have long been implicated in cell division in plants (Asada and Collings, 1997). For example, some kinesin proteins increase in concentration during mitosis, as shown for the kinesin-like proteins KatB and KatC in synchronized tobacco BY-2 cells (Mitsui et al., 1996), and several kinesins immunolocalize to the mitotic microtubule arrays but not to interphase arrays, as shown for KatAp in Arabidopsis and tobacco suspension cells (Liu et al., 1996). Chromosome pieces in Haemanthus endosperm cells that have been cut by laser beam move along microtubules in opposite directions (equator or pole) independent of kinetochore activity, depending on the division phase in vivo (Khodjakov et al., 1996). One of the most convincing functional analyses by Asada and co-workers has implicated the 125-kD tobacco kinesin-related polypeptide (TKRP125), a plus end–directed kinesin-like protein, in phragmoplast microtubule organization. Antibodies raised against the motor domain of this kinesin inhibit sliding of phragmoplast microtubules in permeabilized tobacco BY-2 cells (Asada and Shibaoka, 1994; Asada et al., 1997).
KCBP is a kinesin-like calmodulin (CaM) binding protein that has been identified in Arabidopsis, tobacco, and potato (Reddy et al., 1996a, 1996b; Wang et al., 1996). Arabidopsis KCBP (AtKCBP) consists of 1259 amino acids (140-kD protein) and forms dimers that have microtubule-stimulated ATPase activity (Reddy et al., 1996a), minus end–directed microtubule motor activity (Song et al., 1997), and microtubule-bundling activity within both the motor domain and the tail region (Kao et al., 2000). Other, not fully characterized binding domains possibly link the tail region to membranous cellular structures, such as the endoplasmic reticulum, or to vesicular cargo (Reddy and Reddy, 1999). Recently, the N-terminal tail region of KCBP has been found to interact with a plant-specific protein kinase (KIPK, or KCBP-interacting protein kinase) (Day et al., 2000). The concentration of KCBP is cell cycle–regulated, being high during mitosis and dropping to undetectable levels during interphase in synchronized tobacco BY-2 cultures (Bowser and Reddy, 1997). Immunolabeling studies with Arabidopsis and tobacco BY-2 cultured cells (Bowser and Reddy, 1997) have demonstrated that KCBP is localized to the preprophase band, the spindle apparatus, and the phragmoplast, but it is not colocalized with cortical microtubules during interphase. In Haemanthus endosperm cells, KCBP is localized to the perinuclear basket of microtubules during prophase, to the metaphase and anaphase spindle, and to the phragmoplast during telophase (Smirnova et al., 1998). Taken together, these results suggest a role for KCBP in cell division.
Recently, a CaM binding C-terminal kinesin (kinesin C) was cloned from sea urchin embryos that showed 56 and 35% sequence identity with the motor and CaM binding domains of plant KCBPs, respectively (Rogers et al., 1999). However, the tail regions of kinesin C and plant KCBPs have no sequence similarity. Given the CaM binding property of kinesin C, a Ca2+-regulated role for kinesin C in the early sea urchin development is suggested. As with KCBP, the CaM binding region of kinesin C is adjacent to the C-terminal motor domain (Reddy et al., 1996b). The CaM binding region of KCBP has been shown to bind three isoforms of Arabidopsis CaM in a Ca2+-dependent manner (Reddy et al., 1999). In the presence of Ca2+, CaM not only inhibits the KCBP and microtubule interaction but also is able to dissociate the two in microtubule sedimentation assays in vitro (Deavours et al., 1998; Narasimhulu and Reddy, 1998). Ca2+-CaM therefore negatively regulates the interaction of KCBP with microtubules.
Here, we have used live cells and specific antibodies to KCBP to determine the possible function and signal cascade of this protein. The affinity-purified polyclonal antibodies (hereafter designated as KCBP-Ab), which were raised against a synthetic 23–amino acid polypeptide containing the CaM binding domain of AtKCBP, were found to interfere with the Ca2+-CaM regulation but not with the microtubule binding activity of KCBP in vitro. In the presence of these antibodies, the interaction of KCBP with microtubules was not inhibited by activated CaM (Narasimhulu et al., 1997; Narasimhulu and Reddy, 1998). These antibodies are therefore thought to activate KCBP constitutively. To show that the antibody differentially affects cell division, we first determined that KCBP-Ab cross-reacts with endogenous KCBP from Tradescantia virginiana. We then microinjected living stamen hair cells of this plant with these antibodies and monitored the changes in morphology and mitotic transition times. The injections with KCBP-Ab differentially affected specific phases during cell division but not the cytoplasmic streaming or cell viability during interphase. We conclude that by driving the sliding and bundling of microtubules, KCBP plays a role in the formation of a converging bipolar spindle during nuclear envelope breakdown and anaphase. During metaphase and telophase, its activity is reduced to allow for microtubule dynamics during chromosome alignment and phragmoplast formation, respectively.
RESULTS
KCBP-Ab Recognizes a 140-kD Protein in T. virginiana
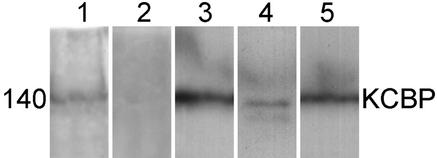
The polyclonal antibody directed against the unique CaM binding region of KCBP has previously been shown to cross-react with a single band in protein gel blots of total protein extracts from Arabidopsis seedlings and suspension cells and tobacco BY-2 suspension cells (Bowser and Reddy, 1997). To determine the effects of microinjecting these antibodies into T. virginiana stamen hair cells, we needed to ascertain whether the antibodies would also cross-react with endogenous KCBP. To detect endogenous KCBP, total protein extracts from T. virginiana inflorescences (Figure 1, lane 1) were compared with protein extracts from tobacco BY-2 culture cells (Figure 1, lane 3), lily anthers (Figure 1, lane 4), and Arabidopsis suspension cultures (Figure 1, lane 5) on protein gel blots. Equal amounts of protein (∼80 μg) were loaded in each lane. In all preparations, we detected a single band of ∼140 kD. The protein preparations of the tobacco and Arabidopsis cultured cells (Figure 1, lanes 3 and 5) indicate higher concentrations of KCBP than do the T. virginiana and lily preparations (Figure 1, lanes 1 and 4). This difference probably reflects the higher proportions of dividing cells in these cultures rather than suggesting differences between monocots and dicots, because Arabidopsis seedlings also showed lower concentrations of KCBP than did suspension cell cultures (results not shown). Incubation with preimmune serum did not result in any band (Figure 1, lane 2).
Figure 1.
Protein Gel Blot Analysis of KCBP.
Total protein preparations of T. virginiana inflorescences (lanes 1 and 2), tobacco BY-2 suspension cell culture (lane 3), Lidium longiflorum anthers (lane 4), and Arabidopsis suspension cell culture (lane 5) were separated on SDS–polyacrylamide gels, blotted, and incubated with 1:500 dilutions of affinity-purified KCBP-Ab or a 1:500 dilution of unpurified preimmune serum (lane 2). In lanes 1 and 3 to 5, a single band at 140 kD was detected, indicating the presence of endogenous KCBP.
KCBP-Ab Does Not Affect Viability and Cytoplasmic Streaming
To manipulate KCBP activity in vivo, cells were injected during all phases of cell division with affinity-purified KCBP-Ab, a probe thought to constitutively activate KCBP by binding to the CaM binding domain. To ascertain whether microinjection of these antibodies affects the cells in other ways, T. virginiana stamen hair cells were microinjected during interphase and observed for up to 1.5 hr (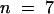 ). No effect on viability or cytoplasmic streaming was evident, as shown in Figure 2A. Cytoplasmic strands throughout the vacuoles remained intact, and organelle movement was not affected. Use of monoclonal antibodies raised against calmodulin (CaM-Ab) gave quite different results (Figure 2B): there was loss of transvacuolar strands and cytoplasmic streaming within 10 min, and organelles showed Brownian movement (
). No effect on viability or cytoplasmic streaming was evident, as shown in Figure 2A. Cytoplasmic strands throughout the vacuoles remained intact, and organelle movement was not affected. Use of monoclonal antibodies raised against calmodulin (CaM-Ab) gave quite different results (Figure 2B): there was loss of transvacuolar strands and cytoplasmic streaming within 10 min, and organelles showed Brownian movement (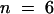 ). Most of the cytoplasm and the nucleus moved to one half of the cell and left the vacuole occupying the other half. After 30 min, streaming would sometimes recur and cytoplasmic strands would reappear (results not shown).
). Most of the cytoplasm and the nucleus moved to one half of the cell and left the vacuole occupying the other half. After 30 min, streaming would sometimes recur and cytoplasmic strands would reappear (results not shown).
Figure 2.
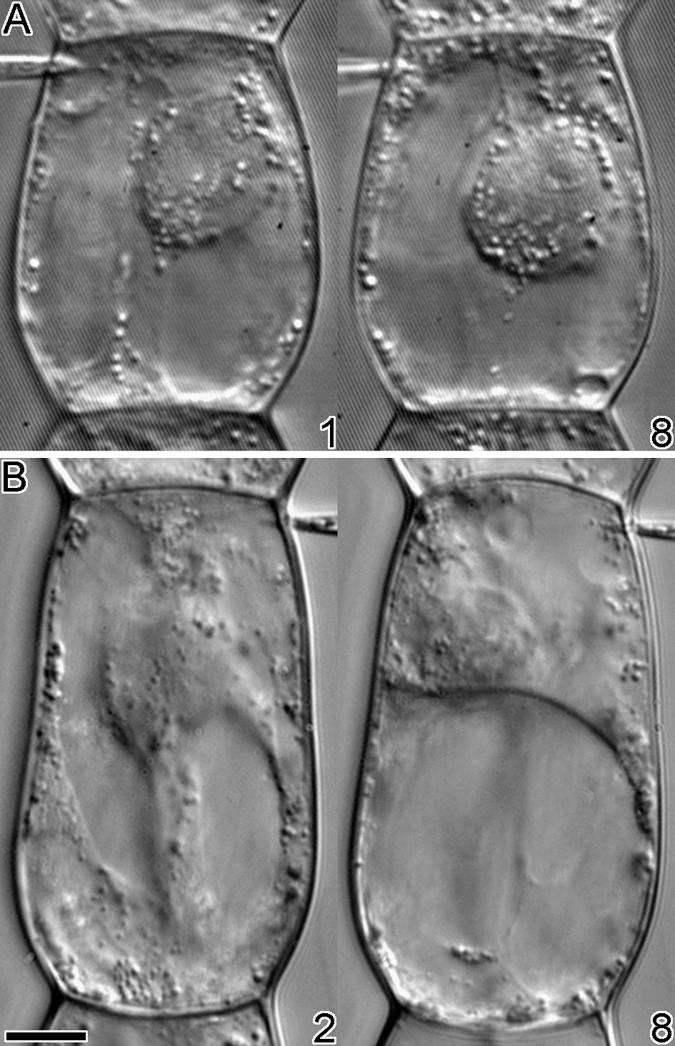
Injection of Antibodies into T. virginiana Stamen Hair Cells during Interphase.
(A) Eight minutes after microinjection with KCBP-Ab, no effects on cytoplasmic streaming or viability were visible.
(B) Within 8 min after injection with CaM-Ab, cytoplasmic streaming ceased, cytoplasmic strands collapsed, and most of the cytoplasm was localized to the lower part of the cell.
The time in minutes after injection is given at the bottom right of each photograph.  .
.
KCBP-Ab Induces Nuclear Envelope Breakdown
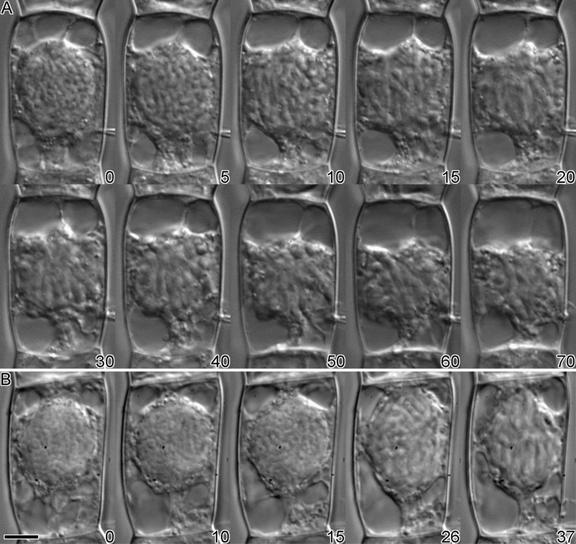
Microinjection of KCBP-Ab during late prophase, that is, when the chromatin was clearly condensed, resulted in early nuclear envelope breakdown, as shown in Figure 3A. Between 5 and 10 min after injection, the nuclear envelope disintegrated, and the chromosomes spread out and occupied the central area of the cell. All cells that were injected during late prophase showed nuclear envelope breakdown within 2 to 10 min after injection ( ), with a median time of 7.5 min (Figure 4A and Table 1). Three cells classified as being in early prophase did not show nuclear envelope breakdown at 15, 55, and 60 min after injection, respectively (Figure 4A). One of these cells actually reverted to interphase after 55 min (Figure 4A), which is a relatively common observation for stamen hair cells of T. virginiana. None of the cells that showed accelerated nuclear envelope breakdown reverted to interphase.
), with a median time of 7.5 min (Figure 4A and Table 1). Three cells classified as being in early prophase did not show nuclear envelope breakdown at 15, 55, and 60 min after injection, respectively (Figure 4A). One of these cells actually reverted to interphase after 55 min (Figure 4A), which is a relatively common observation for stamen hair cells of T. virginiana. None of the cells that showed accelerated nuclear envelope breakdown reverted to interphase.
Figure 3.
Injection of Antibodies during Late Prophase.
(A) In a KCBP-Ab–injected cell, the nuclear envelope broke down between 5 and 10 min after injection. Initially, the chromosomes lined up at the metaphase plate, but at ∼40 min after injection they started losing their orientation. After an hour, the cell was still arrested in prometaphase or metaphase.
(B) As the control, a late-prophase cell was injected with affinity-purified preimmune serum. Nuclear envelope breakdown did not occur until ∼20 min after injection, after which the cell completed cell division normally (data not shown).
The time in minutes after injection is shown at the bottom right of each photograph.  .
.
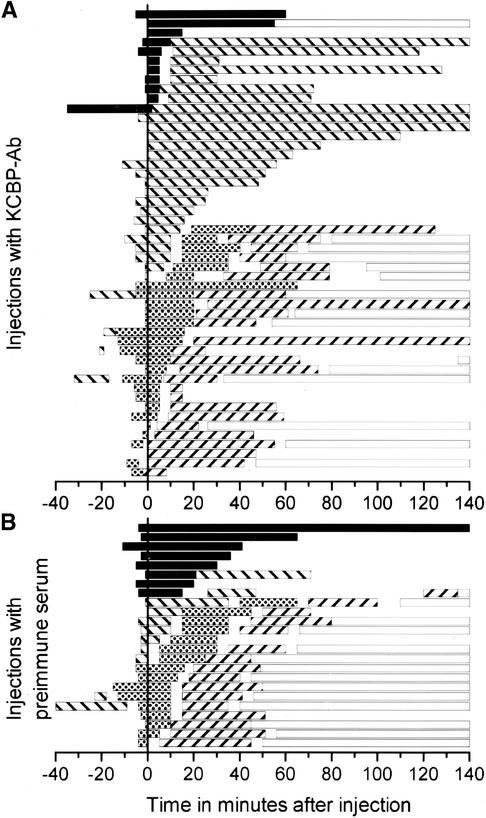
Figure 4.
Mitosis Transition Times and Observation Times.
(A) Dividing cells injected with KCBP-Ab.
(B) Dividing cells injected with preimmune serum.
In both (A) and (B), the microinjections are ordered according to the length of time spent in a mitotic phase after injection at time 0. Observations >140 min were cut off. Each bar represents the observation time (not necessarily the beginning and end of a mitotic phase), except for the bars representing interphase, which were continued to show the observed completion of cell division. Clearly visible are the differences in the lengths of prophase and metaphase and the variation in the length of telophase between KCBP-Ab–injected cells and their controls. Prophase is indicated with black bars; metaphase with striped (\) bars; anaphase with stippled bars; telophase with striped (//) bars; and interphase with open bars.
Table 1.
Mitosis Transition and Observation Times after Injection with KCBP-Ab and Preimmune Serum
| Transition | Injections with KCBP-Aba |
Injections with Preimmune Seruma |
|---|---|---|
| Time to nuclear envelope breakdown |
7.5 (2–10) (n = 8)b |
33 (20–172) (n = 8)b |
| Metaphase | 88.3 (22.5–1377) (n = 8)b63 (25–120.5) (n = 19)c |
32 (15–50) (n = 4)c |
| Anaphase | 25.5 (20–30) (n = 9)d |
26.3 (12.5–32.5) (n = 8)d |
| Telophase | 45 (30.5–60.5) (n = 22)e |
31 (25–38.5) (n = 16)e |
Median transition or observation times and 95% confidence limits in minutes in parentheses.
Cells injected during late prophase.
Cells injected during late prophase or prometaphase.
Cells injected before the onset of anaphase and followed to telophase.
Cells injected before or during telophase and observed in telophase for at least 10 min.
Microinjection of preimmune serum that had been treated according to the affinity purification method used with KCBP-Ab did not accelerate nuclear envelope breakdown. In Figure 3B, a cell that was injected with preimmune serum did not show nuclear envelope breakdown until ∼20 min after injection. The cell then progressed into prometaphase (at 26 and 37 min after injection; Figure 3B) and completed cell division (data not shown). Cells were observed in prophase for a median time of 33 min (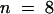 ) (Figure 4B and Table 1); this time differed significantly from that for KCBP-Ab–injected cells (5% level of significance; Mann–Whitney U test).
) (Figure 4B and Table 1); this time differed significantly from that for KCBP-Ab–injected cells (5% level of significance; Mann–Whitney U test).
KCBP-Ab Induces Metaphase Arrest
After nuclear envelope breakdown, all cells that were injected with KCBP-Ab during late prophase entered prometaphase, but they did not progress into anaphase. The cell in Figure 3A entered prometaphase between 5 and 10 min after injection and was arrested for 128 min, after which observation was discontinued. During the first half hour of prometaphase, many of the chromosomes lined up at the metaphase plate with their arms arranged parallel to the spindle axis. However, the cell did not progress into anaphase. Instead, chromosomes disoriented again throughout the center of the cell, although they remained clearly visible and refractile during the observation time. The median time during which cells were observed in metaphase, after injection during late prophase, was 88.3 min (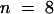 ) (Figure 4A and Table 1). Similar results were observed when cells were injected during prometaphase (Figure 4A). However, cells that were injected later during metaphase, that is, when all the chromosomes had lined up at the metaphase plate, did not arrest but progressed into anaphase. Taken together, cells that were injected during late prophase or prometaphase—excluding those that progressed into anaphase—remained in this prometaphase or metaphase state for a median time of 63 min (
) (Figure 4A and Table 1). Similar results were observed when cells were injected during prometaphase (Figure 4A). However, cells that were injected later during metaphase, that is, when all the chromosomes had lined up at the metaphase plate, did not arrest but progressed into anaphase. Taken together, cells that were injected during late prophase or prometaphase—excluding those that progressed into anaphase—remained in this prometaphase or metaphase state for a median time of 63 min (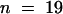 ) (Table 1).
) (Table 1).
Cells that were injected with preimmune serum were not arrested in prometaphase or metaphase (Figure 4B). The median time during which cells were observed in prometaphase or metaphase after injection in late prophase or prometaphase was 32 min (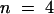 ) (Table 1). The number of observations for control cells was too few to underpin statistically the difference between cells injected with KCBP-Ab and preimmune serum (
) (Table 1). The number of observations for control cells was too few to underpin statistically the difference between cells injected with KCBP-Ab and preimmune serum (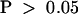 ; Mann–Whitney U test). The average metaphase transition time, that is, from nuclear envelope breakdown to the onset of anaphase, for noninjected cells is 32.5 ±3.9 min (
; Mann–Whitney U test). The average metaphase transition time, that is, from nuclear envelope breakdown to the onset of anaphase, for noninjected cells is 32.5 ±3.9 min (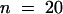 ) (Hepler, 1985).
) (Hepler, 1985).
KCBP-Ab Does Not Affect Anaphase
Microinjection of KCBP-Ab during late metaphase or anaphase did not affect the anaphase cell morphology and transition time. The anaphase chromosome arrangements of the cell in Figure 5A (from 0 to 10 min; Figure 5A) were comparable with those of cells injected with preimmune serum (0 and 10 min; Figure 5B) and with those of cells that were not injected (results not shown). For KCBP-Ab–injected cells, the median anaphase transition time—the time measured from the onset to the cessation of chromatid separation or to the first signs of vesicle aggregation at the center of the phragmoplast—was 25.5 min (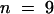 ) (Figure 4A and Table 1). For preimmune serum–injected cells, the median anaphase transition time was 26.3 min (
) (Figure 4A and Table 1). For preimmune serum–injected cells, the median anaphase transition time was 26.3 min (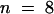 ) (Figure 4B and Table 1), which is not statistically different (
) (Figure 4B and Table 1), which is not statistically different (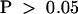 ; Mann–Whitney U test).
; Mann–Whitney U test).
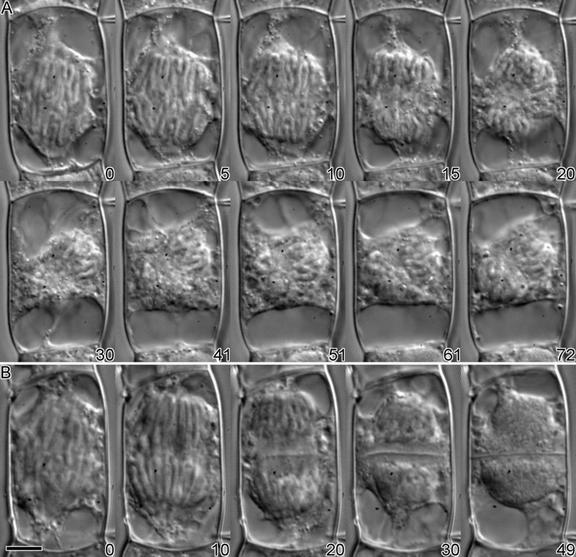
Figure 5.
Injection of Antibody during Anaphase.
(A) Injection with KCBP-Ab did not affect anaphase; the chromatids separated normally (0 to 15 min after injection). At the end of anaphase, no clear zone or initiation of the cell plate was visible (20 min). The whole spindle apparatus tilted 45° (30 min), and the cell was arrested in telophase for >2 hr.
(B) The cell was injected with preimmune serum and progressed through anaphase and telophase normally. At the end of anaphase (20 min), a phragmoplast formed and vesicles coalesced to form a cell plate. The new cell wall was formed in ∼30 min (49 min after injection).
The time in minutes after injection is shown at the bottom right of each photograph.  .
.
KCBP-Ab Causes Aberrant Phragmoplast Formation
Normally, at the end of anaphase, a clear zone arises between the two sets of daughter chromosomes (Staehelin and Hepler, 1996). However, after KCBP-Ab injection during midanaphase, in severe cases no phragmoplast formed, and trailing chromosome arms and larger organelles were not excluded from the central area of the cell (Figure 5A). In addition, there was no sign of vesicle accumulation between the two half spindles. Later, the whole spindle apparatus, including the two sets of chromosomes, tilted at the division plane, possibly because there was no phragmoplast to establish a connection with the parental cell membrane (30 to 72 min; Figure 5A). The cell did not recover and had not completed cytokinesis after 2 hr. In general, we found a large variation in morphological effects during telophase after KCBP-Ab injection. The most common effects for cells that were observed for at least 10 min during telophase were wavy cell plates (nine of 22 cells), misaligned cell plates (five of 22 cells), and unclear or completely absent phragmoplasts and cell plates (four of 22 cells; Figure 5A). Sometimes medium-sized organelles were observed to move into the area of the phragmoplast (four of 22 cells; Figure 5A). In addition, the length of time in which the above-mentioned cells were in telophase varied substantially. Fourteen cells completed cytokinesis and were followed from the first vesicles aggregating at the cell plate to the disintegration of the phragmoplast; seven others were arrested in telophase. The median observation or telophase transit time was 45 min (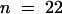 ) (Figure 4A and Table 1). We were unable to determine a correlation between the time of injection and the severity of the effect.
) (Figure 4A and Table 1). We were unable to determine a correlation between the time of injection and the severity of the effect.
Injection of preimmune serum that had been treated according to the affinity purification method for KCBP-Ab did not affect cytokinesis (Figure 5B). Vesicles aggregated at the center of the young phragmoplast within 20 min after injection during midanaphase. Within 30 min after the onset of cytokinesis, the distinctly refractile cell plate connected to the parental cell membrane, and the phragmoplast broke down (20 to 49 min; Figure 5B). The median telophase transition time of control cells was 31 min (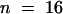 ) (Figure 4B and Table 1), statistically different from that of KCBP-Ab–injected cells (
) (Figure 4B and Table 1), statistically different from that of KCBP-Ab–injected cells (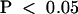 ; Mann–Whitney U test).
; Mann–Whitney U test).
Microtubule Cytoskeleton Is Not Destroyed after KCBP-Ab Injection
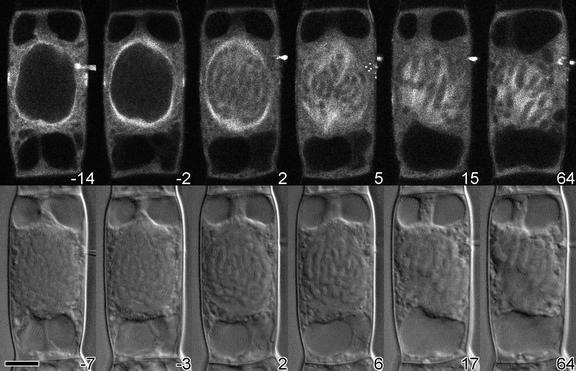
To determine the effects of changes in the KCBP activity on the microtubule cytoskeleton, we injected cells with rhodamine-conjugated bovine tubulin, allowed the tubulin to incorporate into the endogenous microtubule arrays, and injected the cells a second time with KCBP-Ab. Confocal and differential interference contrast images were taken alternately. The double-injection procedure is very complex, and only a few attempts were successful. The cell shown in Figure 6 was injected with tubulin 32 min before injection with KCBP-Ab. During that time, the bovine tubulin incorporated into the preprophase band and the perinuclear spindle. The preprophase band narrowed slightly (cf. images at 14 and 2 min before injection with KCBP-Ab; Figure 6) as the cell progressed through prophase. After the needle was withdrawn and the cell was in late prophase, KCBP-Ab was injected. Two minutes after injection, the nuclear envelope had broken down and the preprophase band had disappeared. The microtubules of the perinuclear spindle then moved into the nuclear area and formed a bipolar spindle with distinct kinet-ochore microtubule bundles. Although the cell was arrested in prometaphase or metaphase for >1 hr, the morphology and orientation of the microtubule cytoskeleton were not destroyed (cf. images at 15 and 64 min after injection of KCBP-Ab; Figure 6).
Figure 6.
Microtubule Distribution after Injection with KCBP-Ab.
A cell in mid-prophase was injected with rhodamine-labeled bovine tubulin; after 32 min, the same cell was injected with KCBP-Ab. The bovine tubulin was incorporated into the endogenous microtubule arrays of the preprophase band and the perinuclear basket. Within 2 min after injection of KCBP-Ab, the nuclear envelope broke down, the microtubules entered the nuclear area, and a bipolar spindle started to form. Although chromosomes lined up at the metaphase plate and kinetochore microtubule bundles were visible (15 and 17 min after injection), after 1 hr the cell was still arrested.
(Top) Tubulin fluorescence.
(Bottom) Differential interference contrast images of the same cell.
The time in minutes after KCBP-Ab injection is shown at the bottom right of each photograph. 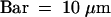 .
.
Two cells that were successfully injected with tubulin and KCBP-Ab had normal anaphase microtubule arrays. One of the cells progressed normally through telophase with no delay, but the other had a phragmoplast that was misaligned and a cell plate that did not connect to the parental cell membrane on one side (results not shown).
DISCUSSION
KCBP Is Involved in Cell Division
Using an antibody that putatively activates KCBP, we have shown that this minus-end kinesin is differentially active during the various phases of cell division in stamen hair cells of T. virginiana. Thus, injection of cells with KCBP-Ab results in the premature breakdown of the nuclear envelope and early onset of prometaphase (Figures 3 and 6). However, these same cells subsequently arrest in late prometaphase or metaphase and do not enter anaphase. Injection later during cell division causes aberrant formation of the phragmoplast and cell plate, and completion of cytokinesis is delayed or inhibited (Figure 5). Cells during anaphase (Figure 5) and interphase (Figure 2) possibly are not affected because either KCBP is naturally activated, in which case it does not matter whether we activate it, or KCBP does not play a role during these phases. Given the immunolocalization to spindle microtubules during anaphase and the greatly reduced amounts of KCBP during interphase (Bowser and Reddy, 1997; Smirnova et al., 1998), we hypothesize that the first explanation holds true during anaphase and that the second one is relevant during interphase. The combination of strong effects on cytoplasmic streaming after CaM-Ab injection and the lack thereof after KCBP-Ab injection (Figure 2) provides us with an internal control and suggests that antibody injections do not necessarily disrupt cellular chemistry.
The outcome of the KCBP-Ab injections appears to depend on the timing of the injection (Figure 4). Whereas injections during late prophase induced breakdown of the nuclear envelope, earlier injections did not; similarly, cells that were injected in late metaphase did not arrest. This suggests the involvement of key checkpoints, for example, one in late prophase that had not yet been reached and another in late prometaphase that had already been passed. After the prophase checkpoint, the activation of KCBP hastened nuclear envelope breakdown, whereas after the late prometaphase checkpoint, the activation of KCBP no longer arrested cells in metaphase. It is also possible that the antibody was degraded. However, the duration of the metaphase arrest and the morphological effects found during telophase 30 min after injection with KCBP-Ab suggest that the antibodies were active for longer periods.
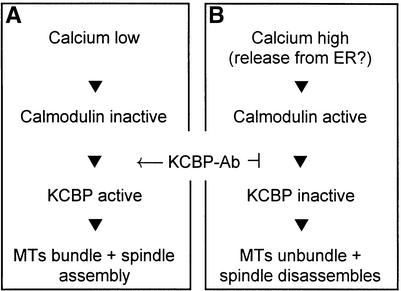
Model for KCBP Function and Regulation
Accurate and dependable chromosome separation may require a convergent bipolar spindle (Smirnova and Bajer, 1998), and spindle organization may depend, in part, on microtubule motors that exhibit both sliding and microtubule-bundling properties (Bajer and Molè-Bajer, 1986; Mitchison, 1992; Walczak et al., 1998). Our results suggest that KCBP is a candidate for one of the microtubule motors involved in plant cell division. In our simplified model, we suggest that microinjection of KCBP-Ab may induce early nuclear envelope breakdown by precociously activating KCBP to bundle and pull on the perinuclear microtubules (Song et al., 1997; Kao et al., 2000) (Figure 7A). Metaphase arrays of microtubules are formed after KCBP-Ab injection, as shown in Figure 6, but the induction of microtubule bundling and sliding, through the activation of KCBP, leads to metaphase arrest. Therefore, it is plausible that normally during metaphase, the Ca2+ concentration within the spindle is elevated, thereby activating CaM and inactivating KCBP (Figure 7B). Although CaM does not specifically localize within the spindle during the various phases of cell division (Vos and Hepler, 1998), it is nevertheless present and would be available to respond to a Ca2+ amplitude signal.
Figure 7.
Simplified Functional Model of KCBP during Cell Division.
(A) Diagram of a cell at nuclear envelope breakdown or anaphase.
(B) Representation of a cell in prometaphase, metaphase, or telophase. KCBP-Ab inhibits the deactivation of KCBP and bypasses regulation by Ca2+-CaM. ER, endoplasmic reticulum; MTs, microtubules.
Anaphase is characterized by the lack of KCBP-Ab effects on cell morphology and transition timing. We favor the idea that KCBP is already active, and consequently the Ca2+ concentration within the spindle poles is low (Figure 7A). Immunolocalization studies suggest that between early and late anaphase, the localization of KCBP is shifted toward the spindle pole; these studies also confirm the hypothesis of a role for KCBP in the formation of a converging bipolar spindle during anaphase (Smirnova et al., 1998).
During telophase, this converging spindle needs to transform into a bipolar but planer phragmoplast (Staehelin and Hepler, 1996). Possibly KCBP activity is reduced so that a plus end–directed microtubule motor such as TKRP125 (Asada et al., 1997) might facilitate the formation and growth of the phragmoplast. KCBP-Ab injection interferes with this dynamic microtubule process, causing abnormalities in the phragmoplast structure and delays in the telophase transition time (Figure 7B). Constitutive activation of KCBP may disrupt the delicate balance between plus end– and minus end–directed microtubule motors during spindle and phragmoplast assembly (Saunders et al., 1997; Cottingham et al., 1999).
Other possible mechanisms by which the antibody injections could differentially affect cell division are through steric hindrance or through cross-linking KCBP molecules by the antibody. Both mechanisms would act through the inactivation of KCBP rather than its activation, which would imply that the CaM activity and Ca2+ concentration in the spindle are opposite of that described above. However, these models disagree with the results of blot overlay and high-speed sedimentation assays (Narasimhulu et al., 1997; Narasimhulu and Reddy, 1998), which suggests that the antibody prevents the dissociation of KCBP from microtubules by Ca2+-CaM. The models also disagree with the zwichel (zwi) mutants (Oppenheimer et al., 1997; Krishnakumar and Oppenheimer, 1999) in which the motor domain is inactivated; nevertheless, they grow normally.
The role of calcium during cell division has been the subject of a large body of research. In animal cells, breakdown of the nuclear envelope, onset of anaphase, and formation of the cleavage furrow have been correlated with Ca2+ spikes or waves (Steinhardt and Alderton, 1988; Tombes et al., 1992; Stricker, 1995; Silver, 1996). However, relatively little direct evidence correlates increases in cytoplasmic free Ca2+ with specific cell division processes in T. virginiana stamen hair cells (Hepler, 1994; P.K. Hepler, unpublished results). Nonetheless, considerable indirect data point to a regulatory role for Ca2+, based on examinations with Ca2+ chelators and transport blockers (Hepler, 1985; Larsen et al., 1989; Wolniak, 1991), Ca2+ injections (Zhang et al., 1992), various 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′,- tetraacetic acid buffers (Jürgens et al., 1994), and caffeine (Valster and Hepler, 1997). Perhaps the closely associated endoplasmic reticulum membrane system within the spindle and phragmoplast is able to regulate the release and sequestration of Ca2+ in microdomains without affecting the overall cytoplasmic concentration of the ion (Hepler, 1994). Ca2+, represented as local and temporal gradients, is likely to be a central regulatory component of cell division, and furthermore, its encrypted message is probably transduced in part by CaM. However, the spatiotemporal pattern of CaM activation during mitosis differs from the pattern of Ca2+ signals in sea urchin embryos (Török et al., 1998), which suggests that increases in cytoplasmic free Ca2+ do not always reflect the activation of CaM but are a consequence of local interactions between CaM and its targets (Török et al., 1998; Zielinski, 1998). Currently, the hypothesized differential activation of KCBP does not contradict the indirectly measured calcium activity in dividing T. virginiana stamen hair cells.
KCBP and Trichomes
KCBP was also discovered independently by Oppenheimer et al. (1997) as the ZWI gene product during an analysis of Arabidopsis trichome mutants. Although the ZWI gene is expressed in the tissue of developing leaves as well as in most other plant tissue, the zwi mutants had alterations only in their trichome morphology. Their data suggest that KCBP is required for elongation of the stalk or the correct placement of branches, probably through reorganization of the cortical microtubule cytoskeleton (Oppenheimer, 1998). Although studies indicate that KCBP is specifically not colocalized with interphase microtubules (Bowser and Reddy, 1997; Smirnova et al., 1998), our results do not exclude a role for KCBP in the differentiation of nondividing cells. The approach used in the study of Oppenheimer et al. (1997) differs greatly from that applied in our study. For example, all of their mutants had truncated, nonfunctional KCBP or downregulated amounts of KCBP, whereas our experiments were based on artificially activating the protein during particular phases of cell division. Perhaps in the Arabidopsis mutants, other kinesins assume the role of KCBP in nontrichome cells, or the truncated versions of KCBP still perform some of the functions (Oppenheimer et al., 1997). We believe that instantaneous activation of KCBP with antibodies does not allow the cell to redirect cell division functions. It will be interesting to determine whether KCBP mutants with a deleted CaM binding domain have defects in cell division, trichome branching, or both.
Analogy among KCBP, Xenopus C-Terminal Kinesin 2, and Drosophila Nonclaret Disjunctional
Related to KCBP, both in sequence homology and function, are two other minus end–directed kinesins, Xenopus C-terminal kinesin 2 (XCTK2) (Walczak et al., 1997) and Drosophila Nonclaret disjunctional (Ncd) (Endow et al., 1990). In an elegant study using antibodies to inhibit spindle formation around DNA-coated beads in Xenopus egg extracts, Walczak et al. (1998) proved that in the absence of centrosomes, XCTK2 plays a role in focusing the spindle poles. A similar function has been proposed for Ncd (Matthies et al., 1996; Moore and Endow, 1996). Drosophila ncd mutants are characterized by high numbers of mistakes in chromosome separation during meiosis and in the first mitotic divisions thereafter (Endow et al., 1994; Endow and Komma, 1997). Live female oocytes in ncd null mutants have a high index of unstable, “frayed” multipolar or apolar spindles and do not properly arrest during late metaphase I (Matthies et al., 1996).
We propose that KCBP has a function similar to XCTK2 and Ncd. Like XCTK2 and Ncd, KCBP is a member of the C-terminal kinesin subfamily (for a phylogenetic tree of kinesins, see the kinesin home page http://www.blocks.fhcrc.org/~kinesin/index.html or Hirokawa [1998]), and like them, KCBP has been proposed to participate in the formation of convergent bipolar spindles through sliding and bundling microtubules (Matthies et al., 1996; Smirnova et al., 1998; Walczak et al., 1998). All three kinesins are localized toward the poles in mitotic spindles but are not associated with free cytoplasmic microtubules during interphase (Matthies et al., 1996; Bowser and Reddy, 1997; Walczak et al., 1997; Smirnova et al., 1998). Finally, we note that like Xenopus and Drosophila oocytes, dividing plant cells lack centrosomes. In the oocyte, the karyosome plays a central role in nucleating the spindle (Matthies et al., 1996); in the plant cell, this role is initially performed by the nuclear envelope (Lambert et al., 1991).
Concluding Remarks
There are many different kinesins in plants. The Arabidopsis genome database, in which ∼88% of the genome is sequenced, has >20 kinesin-like proteins (Reddy, 2000). Undoubtedly, several of these kinesin-like proteins play roles that overlap with that of KCBP or play roles during other steps of spindle assembly and cytokinesis, as does TKRP125. Further functional characterization of KCBP, for example, by specifically inhibiting its function, has recently been initiated. Also, we are testing the cellular localizations of various green fluorescent protein–KCBP fusions and the subcellular localization of KCBP in an immunogold electron microscopy study on cryofixed Gibasis sp stamen hair cells. These and functional analyses of other motor proteins involved in mitosis and cytokinesis will expand our understanding of this fundamental process.
METHODS
Plant Material
Plants (Tradescantia virginiana) were grown in growth chambers under long-day conditions (18 hr of light and 6 hr of dark) at 20 to 25°C. Young parts of inflorescences were used for total protein preparations, and stamen hairs were isolated from young flower buds for microinjection. Tobacco BY-2 suspension cultures (originating from the Nicotiana tabacum cv Bright Yellow; kindly provided by S. Gelvin, Purdue University, West Lafayette, IN) were grown in Murashige and Skoog medium (Sigma) supplemented with vitamins, 3% sucrose, 0.2 mg/L 2,4-D, 1 mg/L thiamine HCl, and 370 mg/L KH2PO4, as described previously (Bowser and Reddy, 1997). Cultures at log phase were used for protein extraction. Plants (Lilium longiflorum) were grown in pots from bulbs at 15 to 20°C in a greenhouse. Young anthers with pollen mother cells were dissected and used for protein extraction. Arabidopsis thaliana suspension cultures (kindly provided by B. Palevitz, University of Georgia, Athens) were grown in B5 medium (Sigma) supplemented with B-5 vitamin mixture, 3% sucrose, and 0.6 mg/L 2,4-D in the dark at 22°C, with shaking as described previously (Bowser and Reddy, 1997). Cultures at log phase were used for protein extraction.
SDS-PAGE and Protein Gel Blotting
To detect endogenous kinesin-like calmodulin (CaM) binding protein (KCBP), protein extracts from young inflorescences of T. virginiana, tobacco BY-2 culture cells, lily anthers, and Arabidopsis suspension-grown cells were prepared as described previously (Bowser and Reddy, 1997). In brief, material was ground in liquid nitrogen and extracted in 50 mM Tris, pH 7.4, 250 mM sucrose, 5 mM EDTA, 5 mM DTT, 10 μg/mL Nα-p-tosyl-l-arginine methyl ester (Sigma), and Complete protease inhibitor cocktail (Boehringer Mannheim). After centrifugation at 100,000g for 20 min, the supernatant was collected and used for electrophoresis. Proteins were separated on standard 7.5% SDS-PAGE gels and transferred overnight onto polyvinylidene difluoride membranes. Unbound sites on the membranes were blocked with 0.2% casein, 0.5% gelatin, and 0.1% Tween 20 in PBS for 1 hr; the membrane was then incubated with affinity-purified antibodies raised against the CaM binding region of KCBP (KCBP-Ab) (Narasimhulu et al., 1997) at 1:500 dilution for 1 hr in blocking solution without gelatin to detect KCBP. Further processing of membranes and KCBP detection was according to the Western-Light chemiluminescent detection system (Tropix, Bedford, MA), or KCBP was detected colorimetrically with nitroblue tetrazolium and 5-bromo- 4-chloro-3-indolyl phosphate. Duplicate blots were processed as given above, except that they were incubated in preimmune serum (1:500 dilution) instead of KCBP-Ab.
Microinjection
T. virginiana stamen hair cells were prepared and microinjected as described previously (Vos et al., 1999). Cells were immobilized on cover slips in a thin layer of 1% agarose and 0.025% Triton X-100 in culture medium (5 mM Hepes, pH 7.0, 1 mM KCl, 1 mM MgCl2, and 0.1 mM CaCl2) and received pressure microinjections with affinity-purified KCBP-Ab (needle concentrations of 0.2 to 1.7 mg/mL) in 5 mM Hepes, pH 7.0, 100 mM KCl, and as much as 0.5 mM carboxyfluorescein. As a control, the above-mentioned Hepes buffer or preimmune serum was injected into other cells. Preimmune serum was treated according to the same affinity purification procedure as was used for KCBP-Ab. As a positive control, interphase cells were injected with an antibody raised against CaM (clone 6D4; Sigma) (CaM-Ab) at a needle concentration of 3.2 to 32 mg/mL in 5 mM Hepes, pH 7.0, and 100 mM KCl. Differential interference contrast images of injected cells were captured every 5 min with a Zeiss inverted microscope (Zeiss, Thornwood, NY) and a video camera (Dage-MTI, Michigan City, IN) using Image 1 software (Universal Imaging, West Chester, PA).
Injections with rhodamine-labeled bovine tubulin (Cytoskeleton, Denver, CO) at a needle concentration of 0.25 mg/mL in 20 mM l-glutamate, 0.5 mM MgSO4, and 1.0 mM EGTA, pH 7.0, were performed and imaged on a Nikon inverted microscope (Nikon, Melville, NY) equipped with an MRC 600 confocal krypton/argon laser and standard T1/GR2 filter combination (Bio-Rad, Hercules, CA). Images were processed by using Photoshop 5.0 (Adobe, San Jose, CA).
Statistics and Graphics
Observation and transition times of various mitotic phases after injection of KCBP-Ab and preimmune serum are nonlinearly distributed and were therefore expressed as medians with 95% confidence limits. Differences between the two data sets were analyzed with the Mann–Whitney U test at the 5% level of significance (Campbell, 1974). Graphic representations of mitotic transition and observation times were produced with Origin 5.0 (Microcal, Northampton, MA).
Acknowledgments
We thank Drs. John Nambu, Patricia Wadsworth, and Alice Cheung at the University of Massachusetts for helpful discussion; Dr. Michael Sutherland at the Statistical Consulting Center (University of Massachusetts) for advice; and Drs. Stan Gelvin (Purdue University) and Barry Palevitz (University of Georgia) for plant material. This research was supported by Grant No. 94-37304-1180 from the U.S. Department of Agriculture to P.K.H. and Grant No. MCB-9630782 from the National Science Foundation to A.S.N.R. The Central Microscopy Facility at the University of Massachusetts is supported by a grant from the National Science Foundation (No. BBS 8714235).
References
- Asada, T., and Collings, D. (1997). Molecular motors in higher plants. Trends Plant Sci. 2, 29–37. [Google Scholar]
- Asada, T., and Shibaoka, H. (1994). Isolation of polypeptides with microtubule-translocating activity from phragmoplasts of tobacco BY-2 cells. J. Cell Sci. 107, 2249–2257. [DOI] [PubMed] [Google Scholar]
- Asada, T., Kuriyama, R., and Shibaoka, H. (1997). TKRP125, a kinesin-related protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J. Cell Sci. 110, 179–189. [DOI] [PubMed] [Google Scholar]
- Bajer, A.S., and Molè-Bajer, J. (1986). Reorganization of microtubules in endosperm cells and cell fragments of the higher plant Haemanthus in vivo. J. Cell Biol. 102, 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser, J., and Reddy, A.S.N. (1997). Localization of a kinesin-like calmodulin-binding protein in dividing cells of Arabidopsis and tobacco. Plant J. 12, 1429–1437. [DOI] [PubMed] [Google Scholar]
- Campbell, R.C. (1974). Statistics for Biologists. (London: Cambridge University Press).
- Cottingham, F.R., Gheber, L., Miller, D.L., and Hoyt, M.A. (1999). Novel roles for Saccharomyces cerevisiae mitotic spindle motors. J. Cell Biol. 147, 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, I.S., Miller, C., Golovkin, M., and Reddy, A.S.N. (2000). Interaction of a kinesin-like calmodulin-binding protein with a protein kinase. J. Biol. Chem. 275, 13737–13745. [DOI] [PubMed] [Google Scholar]
- Deavours, B.E., Reddy, A.S.N., and Walker, R.A. (1998). Ca2+/calmodulin regulation of the Arabidopsis kinesin-like calmodulin-binding protein. Cell Motil. Cytoskeleton 40, 408–416. [DOI] [PubMed] [Google Scholar]
- Endow, S.A., and Komma, D.J. (1997). Spindle dynamics during meiosis in Drosophila oocytes. J. Cell Biol. 137, 1321–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow, S.A., Henikoff, S., and Soler-Niedziela, L. (1990). Mediation of meiotic and early mitotic chromosome segregation in Drosophila by a protein related to kinesin. Nature 345, 81–83. [DOI] [PubMed] [Google Scholar]
- Endow, S.A., Chandra, R., Komma, D.J., Yamamoto, A.H., and Salmon, E.D. (1994). Mutants of the Drosophila ncd microtubule motor protein cause centrosomal and spindle pole defects in mitosis. J. Cell Sci. 107, 859–867. [DOI] [PubMed] [Google Scholar]
- Hepler, P.K. (1985). Calcium restriction prolongs metaphase in dividing Tradescantia stamen hair cells. J. Cell Biol. 100, 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler, P.K. (1994). The role of calcium in cell division. Cell Calcium 16, 322–330. [DOI] [PubMed] [Google Scholar]
- Hepler, P.K., and Hush, J.M. (1996). Behavior of microtubules in living plant cells. Plant Physiol. 112, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa, N. (1998). Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279, 519–526. [DOI] [PubMed] [Google Scholar]
- Jürgens, M., Hepler, L.H., Rivers, B.A., and Hepler, P.K. (1994). BAPTA-calcium buffers modulate cell plate formation in stamen hairs of Tradescantia: Evidence for calcium gradients. Protoplasma 183, 86–99. [Google Scholar]
- Kao, Y.-L., Deavours, B.E., Phelps, K.K., Walker, R.A., and Reddy, A.S.N. (2000). Binding of microtubules by motor and tail domains of a kinesin-like calmodulin-binding protein from Arabidopsis: Regulation by Ca2+/calmodulin. Biochem. Biophys. Res. Commun. 267, 201–207. [DOI] [PubMed] [Google Scholar]
- Khodjakov, A., Cole, R.W., Bajer, A.S., and Rieder, C.L. (1996). The force for poleward chromosome motion in Haemanthus cells acts along the length of the chromosome during metaphase but only at the kinetochore during anaphase. J. Cell Biol. 132, 1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumar, S., and Oppenheimer, D.G. (1999). Extragenic suppressors of the Arabidopsis zwi-3 mutation identify new genes that function in trichome branch formation and pollen growth. Development 126, 3079–3088. [DOI] [PubMed] [Google Scholar]
- Lambert, A.-M., Vantard, M., Schmit, A.-C., and Stoeckel, H. (1991). Mitosis in plants. In The Cytoskeletal Basis of Plant Growth and Form, C.W. Lloyd, ed (London: Academic Press), pp. 199–208.
- Larsen, P.M., Chen, T.L., and Wolniak, S.M. (1989). Quin2-induced metaphase arrest in stamen hair cells can be reversed by 1,2-dioctanoylglycerol but not by 1,3-dioctanoylglycerol. Eur. J. Cell Biol. 48, 212–219. [PubMed] [Google Scholar]
- Liu, B., Cyr, R.J., and Palevitz, B.A. (1996). A kinesin-like protein, KatAp, in the cells of Arabidopsis and other plants. Plant Cell 8, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies, H.J.G., McDonald, H.B., Goldstein, L.S.B., and Theurkauf, W.E. (1996). Anastral meiotic spindle morphogenesis: Role of the Non-claret disjunctional kinesin-like protein. J. Cell Biol. 134, 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison, T.J. (1992). Self-organization of polymer-motor systems in the cytoskeleton. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 336, 99–106. [DOI] [PubMed] [Google Scholar]
- Mitsui, H., Hasezawa, S., Nagata, T., and Takahashi, H. (1996). Cell cycle–dependent accumulation of a kinesis-like protein, KatB/C, in synchronized tobacco BY-2 cells. Plant Mol. Biol. 30, 177–181. [DOI] [PubMed] [Google Scholar]
- Moore, J.D., and Endow, S.A. (1996). Kinesin proteins: A phylum of motors for microtubule-based motility. BioEssays 18, 207–219. [DOI] [PubMed] [Google Scholar]
- Narasimhulu, S.B., and Reddy, A.S.N. (1998). Characterization of microtubule binding domains in the Arabidopsis kinesin-like calmodulin binding protein. Plant Cell 10, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhulu, S.B., Kao, Y.L., and Reddy, A.S.N. (1997). Interaction of Arabidopsis kinesin-like calmodulin-binding protein with tubulin subunits: Modulation by Ca2+-calmodulin. Plant J. 12, 1139–1149. [DOI] [PubMed] [Google Scholar]
- Oppenheimer, D.G. (1998). Genetics of plant cell shape. Curr. Opin. Plant Biol. 1, 520–524. [DOI] [PubMed] [Google Scholar]
- Oppenheimer, D.G., Pollock, M.A., Vacik, J., Szymanski, D.B., Ericson, B., Feldmann, K., and Marks, M.D. (1997). Essential role of a kinesin-like protein in Arabidopsis trichome morphogenesis. Proc. Natl. Acad. Sci. USA 94, 6261–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, A.S.N. (2000). Molecular motors and their functions in plants. Int. Rev. Cytol., in press. [DOI] [PubMed]
- Reddy, A.S.N., Narasimhulu, S.B., Safadi, F., and Golovkin, M. (1996. a). A plant kinesin heavy chain–like protein is a calmodulin-binding protein. Plant J. 10, 9–21. [DOI] [PubMed] [Google Scholar]
- Reddy, A.S.N., Safadi, F., Narasimhulu, S.B., Golovkin, M., and Hu, X. (1996. b). A novel plant calmodulin-binding protein with a kinesin heavy chain motor domain. J. Biol. Chem. 271, 7052–7060. [DOI] [PubMed] [Google Scholar]
- Reddy, V.S., and Reddy, A.S.N. (1999). A plant calmodulin-binding motor is part kinesin and part myosin. Bioinformatics 15, 1055–1057. [DOI] [PubMed] [Google Scholar]
- Reddy, V.S., Safadi, F., Zielinski, R.E., and Reddy, A.S.N. (1999). Interaction of a kinesin-like protein with calmodulin isoforms from Arabidopsis. J. Biol. Chem. 274, 31727–31733. [DOI] [PubMed] [Google Scholar]
- Rogers, G.C., Hart, C.L., Wedaman, K.P., and Scholey, J.M. (1999). Identification of kinesin-C, a calmodulin-binding carboxy-terminal kinesin in animal (Strongylocentrotus purpuratus) cells. J. Mol. Biol. 294, 1–8. [DOI] [PubMed] [Google Scholar]
- Saunders, W., Lengyel, V., and Hoyt, M.A. (1997). Mitotic spindle function in Saccharomyces cerevisiae requires a balance between different types of kinesin-related motors. Mol. Biol. Cell. 8, 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, R.B. (1996). Calcium, BOBs, QEDs, microdomains and a cellular decision: Control of mitotic cell division in sand dollar blastomeres. Cell Calcium 20, 161–179. [DOI] [PubMed] [Google Scholar]
- Smirnova, E.A., and Bajer, A.S. (1998). Early stages of spindle formation and independence of chromosome and microtubule cycles in Haemanthus endosperm. Cell Motil. Cytoskeleton 40, 22–37. [DOI] [PubMed] [Google Scholar]
- Smirnova, E.A., Reddy, A.S.N., Bowser, J., and Bajer, A.S. (1998). Minus end–directed kinesin-like motor protein, KCBP, localizes to anaphase spindle poles in Haemanthus endosperm. Cell Motil. Cytoskeleton 41, 271–280. [DOI] [PubMed] [Google Scholar]
- Song, H., Golovkin, M., Reddy, A.S.N., and Endow, S.A. (1997). In vitro motility of AtKCBP, a calmodulin-binding kinesin protein of Arabidopsis. Proc. Natl. Acad. Sci. USA 94, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin, L.A., and Hepler, P.K. (1996). Cytokinesis in higher plants. Cell 84, 821–824. [DOI] [PubMed] [Google Scholar]
- Steinhardt, R.A., and Alderton, J. (1988). Intracellular free calcium rise triggers nuclear envelope breakdown in the sea urchin embryo. Nature 332, 364–365. [DOI] [PubMed] [Google Scholar]
- Stricker, S.A. (1995). Time-lapse confocal imaging of calcium dynamics in starfish embryos. Dev. Biol. 170, 496–518. [DOI] [PubMed] [Google Scholar]
- Tombes, R.M., Simerly, C., Borisy, G.G., and Schatten, G. (1992). Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J. Cell Biol. 117, 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török, K., Wilding, M., Groigno, L., Patel, R., and Whitaker, M. (1998). Imaging the spatial dynamics of calmodulin activation during mitosis. Curr. Biol. 8, 692–699. [DOI] [PubMed] [Google Scholar]
- Valster, A.H., and Hepler, P.K. (1997). Caffeine inhibition of cytokinesis: Effect on the phragmoplast cytoskeleton in living Tradescantia stamen hair cells. Protoplasma 196, 155–166. [Google Scholar]
- Vos, J.W., and Hepler, P.K. (1998). Calmodulin is uniformly distributed during cell division in living stamen hair cells of Tradescantia virginiana. Protoplasma 201, 158–171. [Google Scholar]
- Vos, J.W., Valster, A.H., and Hepler, P.K. (1999). Methods for studying cell division in higher plants. In Methods in Cell Biology, Vol. 61, C.L. Rieder, ed (San Diego, CA: Academic Press), pp. 413–437. [DOI] [PubMed]
- Walczak, C.E., Verma, S., and Mitchison, T.J. (1997). XCTK2: A kinesin-related protein that promotes mitotic spindle assembly in Xenopus laevis egg extracts. J. Cell Biol. 136, 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C.E., Vernos, I., Mitchison, T.J., Karsenti, E., and Heald, R. (1998). A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 8, 903–913. [DOI] [PubMed] [Google Scholar]
- Wang, W., Takezawa, D., Narasimhulu, S.B., Reddy, A.S.N., and Poovaiah, B.W. (1996). A novel kinesin-like protein with a calmodulin-binding domain. Plant Mol. Biol. 31, 87–100. [DOI] [PubMed] [Google Scholar]
- Wolniak, S.M. (1991). Patterns of regulation during mitosis. In The Cytoskeletal Basis of Plant Growth and Form, C.W. Lloyd, ed (London: Academic Press), pp. 209–226.
- Zhang, D.H., Wadsworth, P., and Hepler, P.K. (1992). Modulation of anaphase spindle microtubule structure in stamen hair cells of Tradescantia by calcium and related agents. J. Cell Sci. 102, 79–89. [Google Scholar]
- Zielinski, R.E. (1998). Calmodulin and calmodulin-binding proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 697–725. [DOI] [PubMed] [Google Scholar]