Abstract
The completion of flower development in Impatiens balsamina requires continuous inductive (short-day) conditions. We have previously shown that a leaf-derived signal has a role in floral maintenance. The research described here analyzes the role of the leaf in flower development. Leaf removal treatments, in which plants were restricted to a specified number of leaves, resulted in flowers with increased petal number, up to double that of the undefoliated control. Similar petal number increases (as well as changes in bract number or morphology) were recorded when plants began their inductive treatment at a late developmental age or when plants of a nonreverting line (capable of floral maintenance in the absence of continuous short days) were transferred from short days to long days. Our data imply that the increased petal number was neither a response to stress effects associated with leaf removal nor a result of alterations in primordium initiation rates or substitutions of petals for stamens. Rather, the petal initiation phase was prolonged when the amounts of a leaf-derived signal were limiting. We conclude that a leaf-derived signal has a continuous and quantitative role in flower development and propose a temporal model for the action of organ identity genes in Impatiens. This work adds a new dimension to the prevailing ABC model of flower development and may provide an explanation for the wide variety and instabilities of floral form seen among certain species in nature.
INTRODUCTION
The number of organs (sepals, petals, stamens, and carpels) in a flower is often relatively consistent, to the extent that the floral formula can be used as an aid to plant identification. Nevertheless, both genetic and environmental factors can cause floral organ number to vary, offering the means to understand how this number is regulated. In Arabidopsis, genetically determined increases in organ numbers have been described in clavata1, floral organ number1 (superman), ettin, and perianthia mutants (Clark et al., 1993; Crone and Lord, 1993; Running and Meyerowitz, 1996; Huang and Ma, 1997, 1998; Sessions et al., 1997). All floral organ types are present in these mutants, but their numbers vary from that of the wild type. In various other species, including rose, carnation, Cannabis, and Silene, specific environmental conditions have been associated with floral organ number variation (Halevy and Zieslin, 1959; Heslop-Harrison and Woods, 1959; Garrod and Harris, 1974; Lyndon, 1979). An increase specifically in petal number produces horticulturally prized “double” flowers. The means by which this increase is achieved varies; it can involve modification of existing organs of the flower so that they become petal-like (e.g., petaloid stamens), replacement of organs by petals (homeotic transformation), or the initiation of extra petal whorls, which increases the total number of organs in the flower (Reynolds and Tampion, 1983).
The ABC model of flower development explains how the fates of primordia are specified and can provide a partial explanation of the molecular basis of double flowering (Coen and Meyerowitz, 1991). The model proposes that classes A, B, and C organ identity genes act in overlapping domains to specify the flower pattern. Expression of class A alone in whorl 1 specifies sepals; of classes A and B in whorl 2, petals; of classes B and C in whorl 3, stamens; and of class C alone in whorl 4, carpels. In double flowers, a class A gene, which usually is confined to whorls 1 and 2, may be ectopically expressed in whorl 3, leading to the specification of extra petals at the expense of stamens. This type of development is associated with class C gene mutations (Yanofsky et al., 1990; Bradley et al., 1993) and with genes acting by way of class C genes, such as POLYPETALA in Antirrhinum (McSteen et al., 1998). Class C genes establish meristem determinacy as well as specifying reproductive organs of the flower; as a result, both organ number and identity are usually affected in plants carrying mutations in these genes.
A, B, and C genes have been isolated from a wide range of species (reviewed in Irish and Kramer, 1998), but only recently has the regulation of these genes begun to be unraveled in model species (Ng and Yanofsky, 2000). In Arabidopsis, the floral meristem identity gene LEAFY has been identified as a key regulator, directly activating APETALA1 (class A) and AGAMOUS (class C) (Parcy et al., 1998; Busch et al., 1999; Wagner et al., 1999). UNUSUAL FLORAL ORGANS (UFO) has also been implicated as a potential mediator of the control of organ identity gene expression (Wilkinson and Haughn, 1995; Parcy et al., 1998).
Initiation and differentiation of organ primordia are usually considered as independently regulated events (Crone and Lord, 1994; Irish and Kramer, 1998), so for the specification of floral formulae to be consistent, some mechanism must link the timing and positioning of organ primordia initiation with the class A, B, and C genes controlling organ identity. The ability of environmental signals to influence timing, positioning, and identity of organs is highlighted by Impatiens balsamina. We have shown that a leaf-derived signal links flower induction with floral maintenance and the specification of floral organ identity domains throughout the process of flower development (Pouteau et al., 1997; Tooke et al., 1998). In the experiments reported here, we have found marked variations in petal number and bract number and morphology that can be explained as a quantitative response to this leaf-derived signal. Our observations indicate that there is a relationship between the quantity of this signal and organ type in the flower and that a model emphasizing this temporal control of gene expression is needed to explain flower development in Impatiens.
RESULTS
The Terminal Flower
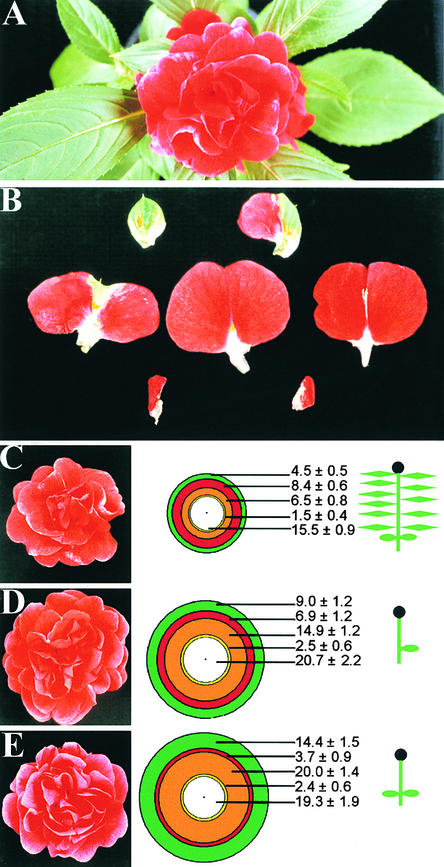
The terminal flower of I. balsamina cv Dwarf Bush Flowered (Figure 1A) is produced under inductive short-day (SD) conditions and is composed of a continuum of organ types (Pouteau et al., 1998a, 1998b). Figure 1B shows petal and petal-like organs of this continuum from a red-flowered plant. Partial petals have ⩾50% pigmentation; true petals have 100% pigmentation. Petals with white midveins have a central area of staminate tissue; in half petals, this area is very staminate in nature, and one lobe of the petal is missing or very much reduced. The number of each organ type in the terminal flower of Impatiens is variable, but in plants grown under SD conditions from day 0, it generally falls within a relatively small range (Figure 1C).
Figure 1.
The Terminal Flower of Impatiens: SD (Control) and Leaf Removal Treatments from Day 0.
(A) Terminal flower of red-flowered Impatiens grown in SD conditions.
(B) Petal and petal-like organs of the terminal flower of Impatiens. From left to right in the top row are a sepal-like bract and partial petal (bract/sepal/petal tissue). In the middle row are a partial petal, a true petal, and a petal with white midvein. In the bottom row, staminate petals with only one petal lobe are shown.
(C) to (E) Terminal flowers of plants from different treatments: (C) SD control; (D) SD-LR 1 cot treatment; and (E) SD-LR 2 cot treatment. Concentric circle diagrams to the right of each photograph show mean petal number and type and mean stamen number of flowers for each treatment. Distance between the circles is proportional to the number of petals of that type. The radius (from the center spot to the edge of the outermost circle) represents mean total number of petals and stamens for that treatment. Green denotes partial petals; red, true petals; orange, petals with white midveins; yellow, half petals with one petal lobe; and white, stamens. Means ±se are shown for each organ type. A central dot indicates carpels. Plant cartoons depict the experimental treatments (undefoliated, defoliated to one cotyledon, or defoliated to two cotyledons) giving rise to the terminal flower forms.
The effect of leaf number/leaf area on the form of the terminal flower of Impatiens was investigated by an experiment in which SD-grown plants were defoliated to a specified number of leaves, from either day 0 or day 8 (see Methods) of the experiment. In further experiments, the pattern of terminal flower development was assessed in treatments involving variable timings of the start of the inductive SD treatment and transferring from SD to long day (LD) those plants of a nonreverting purple-flowered line. This purple-flowered line is known to be able to continue flowering when transferred from SD to LD (Battey, 1985; Tooke et al., 1998), in contrast to the red-flowered line, which reverts, halting flowering and returning to leaf production, when so treated.
Day 0 Leaf Removal Treatments
The cotyledons of Impatiens remain on the plant throughout development and have been shown in earlier leaf-covering experiments to produce an inductive signal in SD conditions (Pouteau et al., 1997). In the first leaf-removal experiment, two leaf removal treatments were performed to restrict the number of leaves on the plant from day 0. Day 0 was designated 8 days after sowing, on the basis that the majority of the plants had produced a first true leaf 7 to 11 mm long. Plants within this class were selected to constitute a developmentally uniform population (described by Pouteau et al., 1998a). A control set of plants was left undefoliated for the duration of the experiment. In these SD control plants (no leaf removal), the terminal flower was composed of 2.3 (se = 0.2) bracts, 20.9 (se = 1.3) petals, and 15.5 (se = 0.9) stamens.
In the two leaf removal (LR) treatments, unfolding leaves (<12 mm long, lamina beginning to unfold) were removed from plants in SD conditions for 18 days (until the first bracts of the terminal flower were apparent), so that they were left with one cotyledon (SD-LR 1 cot treatment; the second cotyledon had been removed on day 0) or, in the second treatment, with two cotyledons (SD-LR 2 cot). Treatments are represented diagrammatically in Figures 1C to 1E. As a consequence of both leaf removal treatments, terminal flowers contained significantly more petals (P < 0.001) than terminal flowers of the SD control treatment. The mean petal number increase in SD-LR 1 cot plants over SD control plants was 59%, and in SD-LR 2 cot plants, the mean petal number of 40.5 (se = 1.6) petals was almost twice that in SD plants. Mean stamen number was similar in both leaf removal treatments and rather variable, with the result that it was not significantly different from that of SD controls.
Considering petal number in more detail revealed that SD-LR 1 cot plants had significantly more partial petals (P < 0.01) and petals with white midveins (P < 0.001) than did SD plants with no leaves removed (cf. Figures 1C and 1D). SD-LR 2 cot plants also developed terminal flowers in which the partial petal and petal with white midvein phases were significantly prolonged (P < 0.001), but the true petal phase was significantly truncated (P < 0.001) (cf. Figures 1C and 1E) and was in fact missing from two plants. As a result of these changes in duration, the first occurrence of each organ type was delayed in relation to SD control plants. For example, the SD control plants have five organs between the first pigmented organ and the first true petal, yet this development took an average of 12 organs in SD-LR 1 cot plants and 16.3 organs in SD-LR 2 cot plants. Similarly, ∼16.5 organs were initiated between the first true petal and the first stamen in plants with a full complement of leaves, but the number was 23 and 25.5 in SD-LR 1 cot and SD-LR 2 cot plants, respectively.
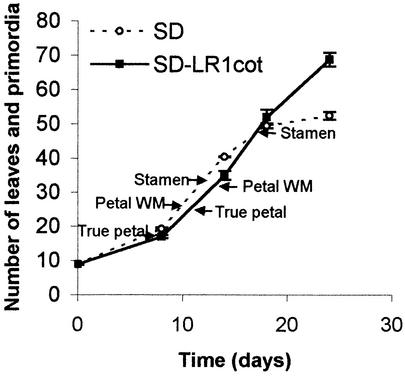
By dissecting samples of SD-LR 1 cot plants at the same time as SD controls, we were able to compare the primordium initiation rates in the two treatments (Figure 2). Although primordia were initiated at a similar rate in the two treatments over most of the period of flower initiation, the interpolation of transitions between floral organ types shows clear differences in the timing of developmental events. Variation in petal number was therefore not a consequence of altered initiation rate. The earlier decline in the rate of initiation in SD controls reflects the slowing associated with stamen/carpel initiation in Impatiens (Battey and Lyndon, 1984).
Figure 2.
Change in Mean Number of Leaves and Primordia over Time in SD and SD-LR 1 Cot Plants.
These data were derived from plant dissections performed on days 0, 8, 14, 18, and 24. The points of initiation of the first true petal, petal with white midvein (WM), and stamen (deduced from mature plant dissections) are marked.
The results from the leaf removal treatments performed from day 0 indicate that by reducing the leaf number or area on a plant, a limiting factor to the rate of progression of flower development is introduced, but this rate is not related to the rate of primordium initiation. With fewer leaves, the timing of expression of the genes required to determine primordium fate might be delayed and thereby prolong the petal phase of flower development. To assess directly the role of leaf area in flower development, leaf area was measured during the course of the experiment. On days 8, 14, 18, and 24, plants were sampled from SD and SD-LR 1 cot treatments, and the leaf areas were recorded. In the SD-LR 1 cot plants, the single cotyledon grew (mean 8.1 cm2 [se = 0.8] by day 24) as if to compensate for the lack of leaf area of the plant. In plants with no leaves removed, cotyledons were smaller (mean 4.3 cm2 [se = 0.2] by day 24), and their size remained relatively constant after day 8 (data not shown).
The timing of organ initiation patterns (in days) and the leaf area (cm2) in the two treatments were compared (Table 1) and demonstrated a delay in transitions between organ types in flowers from SD-LR 1 cot plants. Comparing the lengths of each phase, however, showed that the true petal phase was of a similar length in both SD and SD-LR 1 cot treatments (2.7 and 2.6 days, respectively). The petal with white midvein phase was ∼1.8 days longer, and the phase from first pigmentation to the first true petal was 1 day longer in SD-LR 1 cot plants than in SD plants. At the petal initiation rates noted (similar in both treatments), this roughly equates to an extra whorl (usually five to six organs). At each stage of organogenesis, SD control plants had at least 68% more leaf area than did SD-LR 1 cot plants. This suggests that the supply of a leaf-derived signal might be much more generous in control plants than in the SD-LR 1 cot plants, promoting the rapid and efficient floral development of the former.
Table 1.
Timing of Organ Initiation (First Node of Occurrence) and Leaf Area of SD and SD-LR 1 Cot Plants
| Day of Initiation
|
Leaf Area (cm2)
|
|||||
|---|---|---|---|---|---|---|
| SD | SD-LR 1 Cot | Difference (in days) | SD | SD-LR 1 Cot | Percentage of SD-LR/SDa | |
| Pigment | 3.7 | 5.8 | 2.1 | 11.5 | 3.7 | 32 |
| True petal | 7.6 | 10.7 | 3.1 | 20.0 | 5.2 | 26 |
| Petal white midvein | 10.3 | 13.3 | 3.0 | 34.0 | 6.1 | 18 |
| Stamen | 12.6 | 17.4 | 4.8 | 44.0 | 7.5 | 17 |
| Carpel | 21.0 | 24.7 | 3.7 | 135.0 | 8.1 | 6 |
The area of the cotyledon in the SD-LR 1 cot treatment as a percentage of the total leaf area of plants with no leaves removed (SD) at the time points given under “Day of Initiation”.
Day 8 Leaf Removal Treatments
A second set of leaf removal treatments was established on day 8. Plants that had experienced 8 SDs from day 0 (by which time the first true petals had been initiated) were selected into five different treatments of increasing leaf number: plants were defoliated to one cotyledon, two coty-ledons, or two cotyledons with one, two, or three leaves (represented diagrammatically in Figures 3A to 3E). Leaf removal was continued from day 8 to ensure that plants were maintained with these leaf numbers (see Methods). The aim of these treatments was to determine, first, whether the phases of flower development from day 8 onward would be increased in length through the reduction in leaf area and number and, second, whether the length of those phases would reflect the leaf area and number of the plants. We predicted that those with more leaves would have shorter phases of initiation of each organ and more efficient completion of the terminal flower than would those with fewer leaves.
Figure 3.
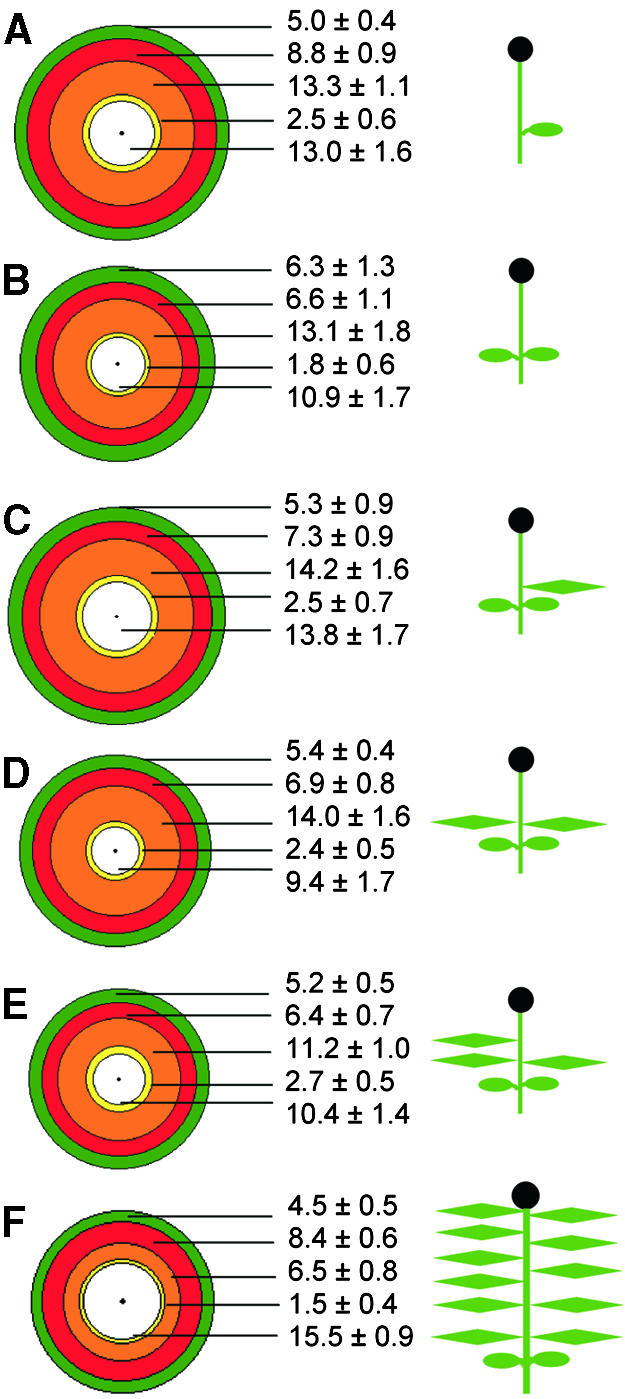
The Terminal Flower of Impatiens: Day 8 Leaf Removal Treatments.
The concentric circle diagrams show the mean petal number and type and mean stamen number for flowers of each day 8 treatment.
(A) Plants defoliated to one cotyledon.
(B) To two cotyledons.
(C) To two cotyledons and one leaf.
(D) To two cotyledons and two leaves.
(E) To two cotyledons and three leaves.
(F) The control treatment (induction in SD from day 0, no defoliation).
Distance between the circles is proportional to the number of petals of that type. The radius (from the center spot to the outermost circle) represents the mean total number of petals and stamens in the treatment. Green denotes partial petals; red, true petals; orange, petals with white midveins; yellow, half petals with one petal lobe; white, stamens. Means ±se are shown for each organ type. Plant cartoons depict the experimental treatments giving rise to the terminal flower forms.
In all day 8 treatments (Figures 3A to 3E), the partial petal phase was similar in length to that of the SD control plants (Figure 3F). This was expected because by day 8, plants had begun initiation of the phase of true petals. There were also no significant differences in the true petal phase of the day 8–treated and SD control plants, except for the plants with only two cotyledons and three leaves, in which this phase was significantly shorter than that in the control (P < 0.05).
The significantly higher (P < 0.01) total petal number of each day 8 treatment plant compared with the SD controls was the result of the significant increase in the number of petals with white midveins in these plants (P < 0.01). Whereas SD plants had on average 6.5 petals of this type (Figure 3F), the averages for the day 8 treatment plants were between 11.2 and 14.2 (Figures 3A to 3E). As with the SD-LR 1 cot and SD-LR 2 cot treatments (Figures 1D and 1E) imposed on day 0, the petal with white midvein phase was particularly susceptible to leaf removal treatments and was greatly prolonged.
In day 0 treatments, stamen number was not affected by leaf removal (no significant difference between SD, SD-LR 1 cot, and SD-LR 2 cot). However, in day 8 2 cot (P < 0.05), day 8 2 cot + 2 leaves (P < 0.01), and day 8 2 cot + 3 leaves (P < 0.05), stamens were significantly fewer than in SD controls.
Although petal initiation had commenced by day 8, defoliation treatments from this time resulted in prolonged initiation of petals compared with SD controls. However, no relationship between the leaf number of the plant and the efficiency of completion of flower development was found.
Timing of Inductive Treatment (Red-Flowered Plants)
To assess further the effect of leaf area and number on floral form, we grew plants in noninductive LD conditions for 14 days from day 0 before induction in SD (LD-SD treatment). On transfer to the inductive (SD) treatment, plants were vegetative and had 20.5 (se = 0.3) leaves and primordia and a leaf area of ∼40.5 cm2 (se = 1.7). Control plants induced in SD on day 0 had 8.9 (se = 0.2) leaves and primordia and a mean leaf area of 3.2 cm2 (se = 0.2). Total petal numbers were significantly greater in plants that received LD-SD treatment than in the SD controls (Table 2). This increase can be attributed exclusively to the petal with white midvein phase, because the earlier partial and true petal phases were directly comparable in length to those of the SD controls. Moreover, bract morphology was often altered under the LD-SD treatment. Sepaloid characteristics (organs reduced in size compared with bracts and with a pointed tip and yellow patches) were prominent in the transition from bracts to petals. The sepaloid phase was therefore much more evident in LD-SD than in SD control plants, which meant that the bracts could be clearly defined as leaflike (first bracts) or sepal-like (later bracts) in LD-SD plants but that they rarely displayed the patches of red pigmentation regularly observed in the bracts of controls induced in SD from day 0.
Table 2.
Mean Petal Numbers and Their Subtypes in Plants in SD from Day 0 and in SD after 14 LDs (LD-SD)
| Treatment | Total No. of Petalsa | Partial Petals | True Petals | Petals with White Midveins | Half Petals |
|---|---|---|---|---|---|
| SD | 20.9 ± 1.3 | 4.5 ± 0.5 | 8.4 ± 0.6 | 6.5 ± 0.8 | 1.5 ± 0.4 |
| LD-SD | 28.0 ± 2.1 | 4.9 ± 1.2 | 8.1 ± 1.2 | 13.2 ± 2.0 | 1.8 ± 0.6 |
The difference between total petal numbers of SD and LD-SD treatments is significant (P > 0.01).
Transfer from SD to LD (Purple-Flowered Plants)
Alterations in floral form were also apparent when plants of a nonreverting, purple-flowered line (Tooke et al., 1998) were transferred from inductive SD conditions to LD conditions. Plants were induced from day 0 for 5 SDs and then transferred to LD conditions. The flowers resulting from this treatment contained more bracts and many more petals with white midveins than did the SD controls, and their true petal phase was markedly shorter (Table 3). The implication of these results is that a leaf-derived signal is continuously involved in flower development and that the amount of the signal is altered when plants are taken out of strongly inductive conditions. This alteration is reflected in the form of the flower.
Table 3.
Mean Bract Numbers and Petal Numbers and Their Subtypes in Purple-Flowered Plants in Continuous SD and 5 SD + LD from Day 0a
| Treatment | Total No. of Bracts | Total No. of Petals | Partial Petals | True Petals | Petals with White Midveins | Half Petals |
|---|---|---|---|---|---|---|
| SD | 2.6 ± 0.2 | 29.9 ± 2.2, n = 19 | 6.4 ± 0.6 | 6.8 ± 0.5 | 13.4 ± 2.4, n = 19 | 3.1 ± 0.6, n = 19 |
| 5 SD + LD | 5.1 ± 0.5 | 52.7 ± 1.8 | 9.1 ± 0.7 | 2.1 ± 0.5 | 40.1 ± 1.8 | 1.4 ± 0.7 |
Combined data from three experiments. n = 21 unless otherwise stated.
DISCUSSION
In Impatiens, a Leaf-Derived Signal Is a Quantitative Determinant of Floral Form
In a leaf removal experiment, SD plants with only one or two cotyledons produced more petals in the terminal flower than did SD undefoliated plants. Similar petal number increases were found after leaf removal treatments that restricted plants to a specified leaf number from day 8 of the experiment. These results suggest that either the act of leaf removal or the limited number of leaves remaining on the plant affects floral development at the terminal meristem. Petal number increases were observed in separate experiments involving no leaf removal but including altered leaf number or conditions. When red-flowered plants were induced on day 14 rather than day 0 (LD-SD treatment), or when purple-flowered (nonreverting) plants were transferred from SD to LD conditions, petal numbers changed, indicating that the observed plasticity of floral development is driven by a leaf-derived signal and is not the result of the act of leaf removal itself. Situations in which a leaf-derived signal was deduced to be limiting led to prolonged phases of petal initiation. However, a simple linear relationship between leaf area and floral organ development was not clear, and although LD-SD–treated plants (SD conditions from day 14) had a greater leaf area at the start of the inductive treatment than did SD-treated plants (SD conditions from day 0), they had significantly more petals. This result suggests that at least in developmentally older plants, petal number is not controlled solely by leaf area/leaf number. The observation that the petal with white midvein phase was lengthened in these plants implies that a limiting factor was reached in the progression to stamen initiation, whereas the earlier petal phases were accomplished as efficiently as in plants grown in SD conditions from day 0.
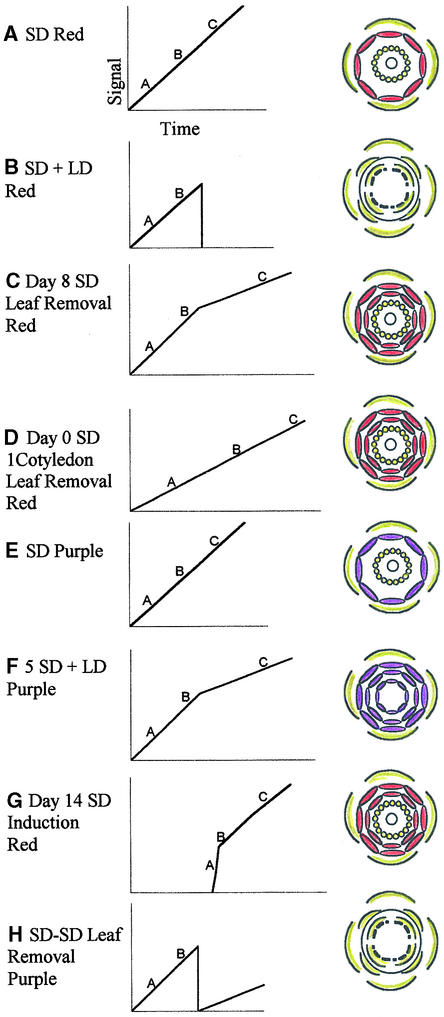
Although increases in the number of petals was the most striking alteration to the flower under all the treatments described here, bract number also increased in five SD + LD purple-flowered plants, and a strongly sepaloid phase, largely absent in day 0–induced plants, was revealed in red-flowered plants induced on day 14. This latter finding suggests that induction of older plants allows more complete suppression of vegetative features (prominent in bracts) and greater expression of floral features (sepals) than does induction at an early age. Our data suggest models for how production of a leaf-derived signal could give rise to the diverse patterns of floral development at the terminal meristem of red- and purple-flowered plants under different treatments (Figure 4). A floral signal could arrive from the leaf at a rate that is related to leaf area and could drive the process of flower development, which is summarized in the diagram as A, B, and C, representing the A, B, and C phases of meristem development.
Figure 4.
Various Forms of the Terminal Flower of Red- and Purple-Flowered Impatiens Produced in Response to SD + LD, LD − SD, Leaf Removal Treatments, or Combinations of These.
The diagrams show production of a hypothetical floral signal and its proposed quantitative relationship with organ identity under different treatments.
(A) Undefoliated red-flowered plant in SD treatment.
(B) Floral reversion of the red-flowered line, as described by Battey and Lyndon (1984) and Pouteau et al. (1997).
(C) Leaf removal in red-flowered plants to restricted leaf number from day 8, as described in text.
(D) Leaf removal in red-flowered plants to one cotyledon from day 0, as described in text. The effect of leaf removal on bract number is unclear, and this representation may therefore be an oversimplification.
(E) Undefoliated purple-flowered plant in SD conditions.
(F) Purple-flowered plants transferred to LD conditions after five SDs. Note that at the time of dissection, some plants in this treatment had produced stamens whereas others had not.
(G) Red-flowered plants transferred to SD conditions after 14 LDs (from day 0). LD-SD treatment is described in text.
(H) Reversion of the purple-flowered line as a result of leaf removal treatment, as described by Tooke et al. (1998). These plants are thought to be able to reflower after some time in LD conditions.
A, B, and C indicate phases of meristem development associated with action of class A, B, and C genes (see Coen and Meyerowitz, 1991). Flower colors represent the red- and purple-flowered lines used.
Reversion and “superflowering” of Impatiens may be considered extreme examples in this context. Reversion, a return to leaf production after a period of flower development, is shown in Figure 4B and occurs in reverting lines in response to transfer from SD to LD conditions (Krishnamoorthy and Nanda, 1968; Battey and Lyndon, 1984). The failure to complete flowering is proposed to result from the loss of a leaf-derived signal (Pouteau et al., 1997). In superflowering of the cultivar Buisson Fleuri, flower buds form on the placental column of the gynoecium axis in response to >16 SDs and a temperature of 25 to 28°C (Simon, 1973).
Temporal Model of Flower Development
The final form of a flower is defined by the number, position, size, orientation, and identity of the organs within it. The mechanism by which organ number is determined is not well understood but is thought to relate to organ positioning and to be established independent of organ identity (Meyerowitz, 1997; Chuang et al., 1999). Characterization of mutants with altered organ numbers in the Arabidopsis flower has highlighted several genes involved in this aspect of floral development. The PERIANTHIA and ETTIN genes are proposed to operate in organ patterning in the flower (Running and Meyerowitz, 1996; Sessions et al., 1997), whereas the CLAVATA and WIGGUM genes regulate meristem size—which is enlarged in the mutants, perhaps promoting excess organ initiation (Clark et al., 1993; Running et al., 1998). Mutations in the FLORAL ORGAN NUMBER1 gene allow the floral meristem to initiate organs that are longer than those in the wild type, resulting in proliferation late in floral development (Huang and Ma, 1997, 1998). The organ number increases in Impatiens, described here, appear to be unique among these cases. Although meristem size was not measured in these experiments, careful observations of dissected meristems provided no evidence for such a change; indeed, the Impatiens meristem is known not to change in size on flowering, reversion, or reflowering (Battey and Lyndon, 1984, 1986; Lyndon and Battey, 1985). There was also no obvious variation in organ patterning/positioning associated with the increases in organ numbers observed. Organ number variation in Impatiens apparently highlights a regulatory mechanism that controls both organ number and identity. The development of the terminal flower indicates that no spatial constraints affect floral meristem function in its capacity to initiate organ primordia or to specify their fate. This also suggests that continued organ initiation may be regulated, directly or indirectly, by controllers of organ identity.
The ABC model of flower development proposes that floral organ identity is conferred by class A, B, and C genes operating in overlapping domains A, B, and C demarcated on the meristem (Coen and Meyerowitz, 1991). Classes A and C are proposed to be antagonistic to one another and to act in the mutually exclusive domains A and C. In this spatial model, the pattern of floral development is largely predetermined (Meyerowitz, 1997), and alterations in floral form can be explained by shifts in domain boundaries such that the expansion in one domain may occur at the expense of another.
Little evidence, however, indicates that the development of the terminal flower of Impatiens is governed by a predetermined pattern mapped out early in the floral phase of the meristem. This lack of predetermination is underlined by the results of day 8 leaf removal treatments on plants, described here. Even though flowering was under way when leaves were removed, completion of the flower could be perturbed from the “normal” pattern of development such that extra petals were initiated. Experiments involving the undefoliated and SD-LR 1 cot plants indicate that variations in petal numbers were not related to changes in the rate at which primordia were produced, and in most cases, petal number increases were not at the expense of stamens—which were present in numbers not significantly different from those of the undefoliated SD control. The ultimate expression of the flexible nature of flower development in Impatiens is its ability to produce leaves from within the flower (i.e., revert), thus overriding mapped floral domains, if they were ever present.
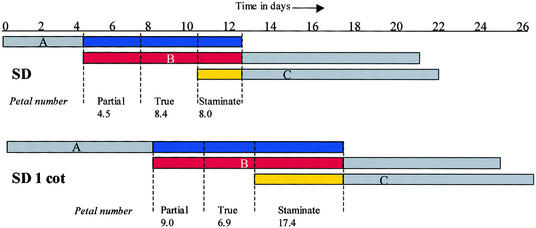
Our data favor a temporally based model (Lord et al., 1994) in which petal number is increased by initiation phases prolonged in time rather than extended in space. We suggest that the control of flower development in Impatiens may therefore be more similar to the control of phase change (Poethig, 1990), with class A, B, and C genes playing the role of phase regulators. In this model, the spatial patterning of organ whorls observed in the flower would arise as a result of temporal control. A model emphasizing this temporal aspect, and suggesting how class A, B, and C genes might act to produce the terminal flower in Impatiens induced to flower on day 0 (undefoliated and SD-LR 1 cot plants), is shown in Figure 5. Petal number increases described here were, most commonly, the result of prolonged initiation of petals with white midveins. Such organs, containing a staminate midvein, imply that action of class A, B, and C genes may occur within one organ, and the increase in their number in experiments described here implies that limited leaf-derived signal either represses the full action of a class C gene or prolongs the action of a class A gene.
Figure 5.
Temporal ABC Model of Flower Development in Impatiens.
The model shows deduced timing of A, B, and C action in the development of the terminal flower of I. balsamina grown in SD conditions with no leaf removal (SD) and with leaf removal to only one cotyledon (SD 1 cot). This model is based on the rate of primordium initiation (determined by primordia counts of both treatments throughout the experiment) correlated with results of mature plant dissections (SD, n = 8; SD 1 cot, n = 9). Colored areas denote petal development. Partial petals have ⩾50% pigmentation; true petals have 100% petal pigmentation. A, B, and C are thought to overlap to specify staminate petals (white midveins/half petals).
Impatiens as a General Model of Floral Form
The nature of the leaf-derived signal that controls flower development of Impatiens is not known, but evidence for a similar role for the leaf in flower development in other plants is provided by the id1 mutation of maize. In the absence of the leaf-expressed ID1 gene, floral maintenance of male flowers in the tassel is lost (Galinat and Naylor, 1951; Colasanti et al., 1998). Environmental conditions causing organ number variation in rose, Silene, Cannabis, carnation, and Gossypium (Halevy and Zieslin, 1959; Heslop-Harrison and Woods, 1959; Garrod and Harris, 1974; Lyndon, 1979; Wilson and Stapp, 1979) relate mainly to temperature and length of day—two factors that could influence the effectiveness of production or transport of a metabolite/hormone/signal. Reducing the leaf number of Impatiens might have a similar effect. Recently, the promoter of the LEAFY gene of Arabidopsis has been shown to integrate environmental signals in floral induction (Blázquez and Weigel, 2000). The role that the LEAFY gene plays in establishing floral meristem identity (Weigel et al., 1992) and its recognized abilities in homeotic gene activation (Busch et al., 1999; Wagner et al., 1999) imply that this gene may connect floral induction and floral development, processes often considered to be distinct. Furthermore, Okamuro et al. (1996) have shown that lfy-6 mutants grown in SD conditions display a type of reversion, returning to inflorescence development after initiation of a normal flower.
Evidence that organ number abnormalities occur at least to some extent when conditions for flowering as a whole are suboptimal comes from studies of natural populations. In work on Linanthus, a plant generally assumed to have a constant floral formula, Huether (1968)(1969) found that deviation from the normal pentamerous corolla occurred frequently and in relation to flowering time in the natural populations studied. Flowers developing at the start or end of the season had abnormal numbers of petals (ranging from one to 16). A similar investigation of floral formula constancy was performed on natural populations of Ipomopsis aggregata by Ellstrand (1983) and Ellstrand and Mitchell (1988); they also found the floral formula to vary over a season and cited the environmental conditions and age of the inflorescence as the factors potentially responsible. These studies imply that strongly inductive conditions may be a requirement for production of a “cofactor” in genetic determination of floral organ number and type and suggest that floral formulae may be more variable than commonly supposed.
METHODS
Seed sowing and plant growth were performed as described by Pouteau et al. (1997). Plants were grown in long-day (LD) conditions of 8 hr of light provided by tungsten and fluorescent bulbs at 271 to 309 μmol m−2 sec−1, followed by 16 hr of tungsten light at 4.2 to 5.2 μmol m−2 sec−1. Short-day (SD) conditions were 8 hr of tungsten and fluorescent light (as above) followed by 16 hr of darkness.
One or Two Cotyledons from Day 0
On day 0, 10 plants were selected at random from the population, and the number of leaves and primordia present on each was recorded. Thirty plants were kept in LD conditions, and 160 plants were transferred to SD conditions. From these 160 plants in SD conditions, 50 were selected at random for leaf removal (LR) to one coty-ledon (SD-LR 1 cot). One of the two cotyledons was removed from each plant by cutting the base of the petiole with a razor blade. Another 10 plants were selected as SD-LR 2 cot plants. Plants in these treatments were checked daily for 18 days (or until bracts were present), and any unfolding leaves were removed by hand. These two treatments meant plants were maintained with either one or two cotyledons. For SD-LR 1 cot plants, 11.9 (se = 0.5) leaves were removed; 11.9 (se = 0.3) leaves were removed from SD-LR 2 cot plants.
On days 8, 14, 18, and 24, 10 SD and 10 SD-LR 1 cot plants were dissected under a Wild M3 stereomicroscope (Leica, Heerbrugg, Switzerland) to determine the number of leaves and primordia present at each of these times in the two treatments. Ten SD, 10 SD-LR 1 cot, and 10 SD-LR 2 cot plants were grown to maturity.
Varying the Number of Leaves from Day 8
A second set of leaf removal treatments was established on day 8. From the SD plants, batches of 10 plants were selected for the following treatments: Leaves were removed on day 8 so that plants were left with one cotyledon, two cotyledons, two cotyledons and one leaf, two cotyledons and two leaves, or two cotyledons and three leaves. This required removing a mean of 5.0 (se = 0.25), 4.6 (se = 0.17), 3.8 (se = 0.37), 2.9 (se = 0.10), or 2.3 (se = 0.15) leaves in each treatment group, respectively. As with day 0 treatments, plants were checked daily for 18 days (or until bracts were present), and unfolding leaves were removed to maintain plants with the number of leaves designated for their treatment. The mean number of leaves removed from the plants after day 8 was 6.9 (se = 0.44), 5.8 (se = 0.34), 5.7 (se = 0.45), 6.2 (se = 0.21), or 6.6 (se = 0.40) in day 8 1 cot, day 8 2 cot, day 8 2 cot + 1 leaf, day 8 2 cot + 2 leaves, and day 2 cot + 3 leaves treatments, respectively. Ten SD plants were dissected on day 8 to establish the number of leaves and primordia present at the beginning of these treatments. All day 8 plants were grown to maturity. During the leaf removal period, axillary shoots were removed from plants, either as the leaf was removed or at a later stage, if they threatened apical dominance.
At the start of each treatment, the leaf area of sample plants was measured with an area meter (Delta T-devices; Burwell, Cambridge, UK). Leaf areas for SD and SD-LR 1 cot plants were also recorded by this method on sampling days.
Late Induction of Red-Flowered Plants
On day 14, 10 of the 30 plants kept in LD conditions on day 0 were dissected to establish the number of leaves and primordia present. Ten plants were transferred to SD conditions on day 14 (LD-SD treatment), and 10 plants were kept in LD conditions. Plants in all treatments were potted into M2 compost (Levington Horticulture, Ipswich, UK) on day 14 in 9-cm pots and on day 35 in 11-cm pots. At maturity (days 39 to 50), when the terminal flowers were open, plants were dissected, and their organ numbers and characteristics of their terminal flowers were recorded. Statistical analysis of petal and stamen number in different treatments was performed by t test.
Data shown in Table 3 for SD and 5 SD + LD treatments of the nonreverting purple-flowered line were collected in three separate experiments. The plants were grown as described for the red-flowered line by Pouteau et al. (1997)(1998a), initially under LD conditions of 8 hr of light provided by tungsten and fluorescent light at 260 to 290 μmol m−2 sec−1 followed by 16 hr of tungsten light at 3 to 4 μmol m−2 sec−1. On day 0, plants were selected as described above and transferred to SD conditions: 8 hr of tungsten and fluorescent light followed by 16 hr of darkness. After 5 SDs, plants were returned to LD conditions (5 SD + LD treatment) or remained in SD conditions for the duration of the experiment (SD treatment). Plants were potted into M2 compost, as described above.
Acknowledgments
This work was supported by grants from the State of Jersey and the Biotechnology and Biological Sciences Research Council. We thank Des Bradley (John Innes Centre, Norwich) for comments on the manuscript.
References
- Battey, N.H. (1985). Growth and Development at the Shoot Apex of Impatiens balsamina L. during Flowering and Reversion. PhD Dissertation (Edinburgh, UK: University of Edinburgh).
- Battey, N.H., and Lyndon, R.F. (1984). Changes in apical growth and phyllotaxis on flowering and reversion in Impatiens balsamina L. Ann. Bot. 54, 553–567. [Google Scholar]
- Battey, N.H., and Lyndon, R.F. (1986). Apical growth and modification of the development of primordia during re-flowering of reverted plants of Impatiens balsamina L. Ann. Bot. 58, 333–341. [Google Scholar]
- Blázquez, M.A., and Weigel, D. (2000). Integration of floral inductive signals in Arabidopsis. Nature 404, 889–892. [DOI] [PubMed] [Google Scholar]
- Bradley, D., Carpenter, R., Sommer, H., Hartley, N., and Coen, E. (1993). Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell 72, 85–95. [DOI] [PubMed] [Google Scholar]
- Busch, M.A., Bomblies, K., and Weigel, D. (1999). Activation of a floral homeotic gene in Arabidopsis. Science 285, 585–587. [DOI] [PubMed] [Google Scholar]
- Chuang, C.-F., Running, M.P., Williams, R.W., and Meyerowitz, E.M. (1999). The PERIANTHIA gene encodes a bZIP protein involved in the determination of floral organ number in Arabidopsis thaliana. Genes Dev. 13, 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1993). CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119, 397–418. [DOI] [PubMed] [Google Scholar]
- Coen, E.S., and Meyerowitz, E.M. (1991). The war of the whorls: Genetic interactions controlling flower development. Nature 353, 31–37. [DOI] [PubMed] [Google Scholar]
- Colasanti, J., Yuan, Z., and Sundaresan, V. (1998). The INDETERMINATE gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 93, 593–603. [DOI] [PubMed] [Google Scholar]
- Crone, W., and Lord, E.M. (1993). Flower development in the organ number mutant clavata1–1 of Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 80, 1419–1426. [Google Scholar]
- Crone, W., and Lord, E.M. (1994). Floral organ initiation and development in wild-type Arabidopsis thaliana (Brassicaceae) and in the organ identity mutants apetala-1 and agamous-1. Can. J. Bot. 72, 384–401. [Google Scholar]
- Ellstrand, N.C. (1983). Floral formula inconstancy within and among plants and populations of Ipomopsis aggregata (Polemoniaceae). Bot. Gaz. 144, 119–123. [Google Scholar]
- Ellstrand, N.C., and Mitchell, R.J. (1988). Spatial and temporal patterns of floral inconstancy in plants and populations of Ipomopsis aggregata (Polemoniaceae). Bot. Gaz. 149, 209–212. [Google Scholar]
- Galinat, W.C., and Naylor, A.W. (1951). Relation of photoperiod to inflorescence proliferation in Zea mays L. Am. J. Bot. 38, 38–47. [Google Scholar]
- Garrod, J.F., and Harris, G.P. (1974). Studies on the glasshouse carnation: Effects of temperature and growth substances on petal number. Ann. Bot. 38, 1025–1031. [Google Scholar]
- Halevy, A.H., and Zieslin, N. (1959). The development and causes of petal blackening and malformation of Baccara rose flowers. Acta Hortic. 14, 149–157. [Google Scholar]
- Heslop-Harrison, Y., and Woods, I. (1959). Temperature-induced meristic and other variation in Cannabis sativa. J. Linn. Soc. Lond. Bot. 56, 290–293. [Google Scholar]
- Huang, H., and Ma, H. (1997). FON1, an Arabidopsis gene that terminates floral meristem activity and controls flower organ number. Plant Cell 9, 115–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H., and Ma, H. (1998). The nature of the Arabidopsis fon-1 mutations. Plant Cell 10, 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huether, C.A. (1968). Exposure of natural genetic variability underlying the pentamerous corolla constancy in Linanthus androsaceus ssp. androsaceus. Genetics 60, 123–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huether, C.A. (1969). Constancy of the pentamerous corolla phenotype in natural populations of Linanthus. Evolution 23, 572–588. [DOI] [PubMed] [Google Scholar]
- Irish, V.F., and Kramer, E.M. (1998). Genetic and molecular analysis of angiosperm flower development. Adv. Bot. Res. 28, 197–230. [Google Scholar]
- Krishnamoorthy, H.N., and Nanda, K.K. (1968). Floral bud reversion in Impatiens balsamina under non-inductive photoperiods. Planta 80, 43–51. [Google Scholar]
- Lord, E.M., Crone, W., and Hill, J.P. (1994). Timing of events during flower organogenesis: Arabidopsis as a model system. Curr. Top. Dev. Biol. 29, 325–356. [DOI] [PubMed] [Google Scholar]
- Lyndon, R.F. (1979). Aberrations in flower development in Silene. Can. J. Bot. 57, 233–235. [Google Scholar]
- Lyndon, R.F., and Battey, N.H. (1985). The growth of the shoot apical meristem during flower initiation. Biol. Plant. 27, 339–349. [Google Scholar]
- McSteen, P.C.M., Vincent, C.A., Doyle, S., Carpenter, R., and Coen, E.S. (1998). Control of floral homeotic gene expression and organ morphogenesis in Antirrhinum. Development 125, 2359–2369. [DOI] [PubMed] [Google Scholar]
- Meyerowitz, E.M. (1997). Genetic control of cell division patterns in developing plants. Cell 88, 299–308. [DOI] [PubMed] [Google Scholar]
- Ng, M., and Yanofsky, M.F. (2000). Three ways to learn the ABCs. Curr. Opin. Plant Biol. 3, 47–52. [DOI] [PubMed] [Google Scholar]
- Okamuro, J.K., DenBoer, B.G.W., Lotys-Prass, C., Szeto, W., and Jofuku, K.D. (1996). Flowers into shoots: Photo and hormonal control of a meristem identity switch in Arabidopsis. Proc. Natl. Acad. Sci. USA 93, 13831–13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy, F., Nilsson, O., Busch, M.A., Lee, I., and Weigel, D. (1998). A genetic framework for floral patterning. Nature 395, 561–566. [DOI] [PubMed] [Google Scholar]
- Poethig, R.S. (1990). Phase change and the regulation of shoot morphogenesis in plants. Science 250, 923–930. [DOI] [PubMed] [Google Scholar]
- Pouteau, S., Nicholls, D., Tooke, F., Coen, E., and Battey, N. (1997). The induction and maintenance of flowering in Impatiens. Development 124, 3343–3351. [DOI] [PubMed] [Google Scholar]
- Pouteau, S., Nicholls, D., Tooke, F., Coen, E., and Battey, N. (1998. a). Transcription pattern of a FIM homologue in Impatiens during floral development and reversion. Plant J. 14, 235–246. [DOI] [PubMed] [Google Scholar]
- Pouteau, S., Tooke, F., and Battey, N. (1998. b). Quantitative control of inflorescence formation in Impatiens balsamina. Plant Physiol. 118, 1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, J., and Tampion, J. (1983). Double Flowers: A Scientific Study. (London: Polytechnic of Central London Press).
- Running, M.P., and Meyerowitz, E.M. (1996). Mutations in the PERIANTHIA gene of Arabidopsis specifically alter floral organ number and initiation pattern. Development 122, 1261–1269. [DOI] [PubMed] [Google Scholar]
- Running, M.P., Fletcher, J.C., and Meyerowitz, E.M. (1998). The WIGGUM gene is required for proper regulation of floral meristem size in Arabidopsis. Development 125, 2545–2553. [DOI] [PubMed] [Google Scholar]
- Sessions, A., Nemhauser, J.L., McColl, A., Roe, J.L., Feldmann, K.A., and Zambryski, P.C. (1997). ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124, 4481–4491. [DOI] [PubMed] [Google Scholar]
- Simon, L. (1973). L'Impatiens balsamina L. cultivar “Buisson Fleuri.” Morphogénèse et ontogénèse expérimentales. PhD Dissertation (Nantes, France: University of Nantes).
- Tooke, F., Pouteau, S., and Battey, N. (1998). Non-reversion of Impatiens in the absence of meristem commitment. J. Exp. Bot. 49, 1681–1688. [Google Scholar]
- Wagner, D., Sablowski, R.W.M., and Meyerowitz, E.M. (1999). Transcriptional activation of APETALA1 by LEAFY. Science 285, 582–584. [DOI] [PubMed] [Google Scholar]
- Weigel, D., Alvarez, J., Smyth, D.R., Yanofsky, M.F., and Meyerowitz, E.M. (1992). LEAFY controls floral meristem identity in Arabidopsis. Cell 69, 843–859. [DOI] [PubMed] [Google Scholar]
- Wilkinson, M.D., and Haughn, G.W. (1995). UNUSUAL FLORAL ORGANS controls meristem identity and organ primordia fate in Arabidopsis. Plant Cell 7, 1485–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, F.D., and Stapp, B.R. (1979). Flowers with abnormal numbers of involucral bracts in cotton. Crop Sci. 19, 204–208. [Google Scholar]
- Yanofsky, M.F., Ma, H., Bowman, J.L., Drews, G.N., Feldmann, K.A., and Meyerowitz, E.M. (1990). The protein encoded by the Arabidopsis homeotic gene AGAMOUS resembles transcription factors. Nature 346, 35–39. [DOI] [PubMed] [Google Scholar]