Abstract
Islet cell autoantigen (ICA) 512 is a receptor-tyrosine phosphatase-like protein associated with the secretory granules of neuroendocrine cells, including pancreatic β-cells. Binding of its cytoplasmic tail to β2-syntrophin suggests that ICA512 connects secretory granules to the utrophin complex and the actin cytoskeleton. Here we show that stimulation of insulin secretion from INS-1 cells triggers the biosynthesis of pro-ICA512 and the degradation of its mature form. Inhibition of calpain, which is activated upon stimulation of insulin secretion, prevents the Ca2+-dependent proteolysis of ICA512. In vitro µ-calpain cleaves ICA512 between a putative PEST domain and the β2-syntrophin binding site, whereas binding of ICA512 to β2-syntrophin protects the former from cleavage. β2-syntrophin and its F-actin-binding protein utrophin are enriched in subcellular fractions containing secretory granules. ICA512 preferentially binds phospho-β2-syntrophin and stimulation of insulin secretion induces the Ca2+-dependent, okadaic acid-sensitive dephosphorylation of β2-syntrophin. Similarly to calpeptin, okadaic acid inhibits ICA512 proteolysis and insulin secretion. Thus, stimulation of insulin secretion might promote the mobilization of secretory granules by inducing the dissociation of ICA512 from β2-syntrophin–utrophin complexes and the cleavage of the ICA512 cytoplasmic tail by µ-calpain.
Keywords: actin/diabetes/IA-2/secretory granule/tyrosine phosphatase
Introduction
The exocytosis of neurosecretory granules is associated with a focal and transient reorganization of the cortical actin cytoskeleton. Actin may control secretion in a variety of ways. For instance, evidence that depolymerization of actin microfilaments increases stimulated secretion (Aunis and Bader, 1988; Trifaro, 1990) led to the suggestion that the actin web acts as a barrier that prevents granules from approaching sites of exocytosis (Sontag et al., 1988; Vitale et al., 1991; Nakata and Hirokawa, 1992). The reorganized actin filaments can also directly support the movement of granules across the subcortical region (Lang et al., 2000). Actin bundles and filamentous proteins, in addition, may tether secretory granules to each other and to the plasma membrane, thereby restraining vesicle mobility (Senda et al., 1988, 1989; Lang et al., 2000). Thus, the dynamic relationship of secretory granules with the actin network is likely to account, at least in part, for the existence of different pools of secretory granules, which differ in respect to their readiness to undergo exocytosis upon stimulation of secretion (Trifaro, 1990; Klenchin and Martin, 2000). The identity of the molecule(s) that mediates the interaction between secretory granules and the actin cortex, however, is still unclear.
Recent studies from our laboratory have pointed to islet cell autoantigen (ICA) 512, also known as IA-2, as a potential link between secretory granules and the actin cytoskeleton in insulin-secreting cells (β-cells) of the pancreatic islets. ICA512 is an atypical member of the receptor protein tyrosine phosphatase (RPTP) family with a single protein tyrosine phosphatase (PTP) domain in its cytoplasmic domain (Rabin et al., 1994; Lan et al., 1994). The presence of an alanine and an aspartate at critical positions within the PTP domain prevents ICA512 from dephosphorylating typical PTP substrates (Magistrelli et al., 1996). ICA512 is mostly expressed in neurons and peptide-secreting endocrine cells, including pancreatic β-cells (Solimena et al., 1996). In neuroendocrine cells, ICA512 is enriched in the membrane of secretory granules and its appearance at the cell surface is increased upon stimulation of regulated secretion. The cytoplasmic domain of ICA512 interacts with βIV spectrin (Berghs et al., 2000), a novel protein of the spectrin family, as well as with the PDZ domain of the adapter protein β2-syntrophin (Ort et al., 2000). β2-syntrophin, in turn, binds the F-actin-binding protein utrophin. Confocal microscopy on rat insulinoma INS-1 cells indicated that ICA512 and β2-syntrophin are co-localized in the cell cortex on intracellular organelles, presumably secretory granules. We have suggested, therefore, that interaction of ICA512 with the β2-syntrophin–utrophin complex may anchor secretory granules to the actin cytoskeleton.
Here we report that stimulation of insulin secretion in INS-1 cells results in the dephosphorylation of β2-syntrophin, which, in turn, leads to a weakening of the interaction between ICA512 and β2-syntrophin, followed by the cleavage of ICA512 by µ-calpain. Inhibition of β2-syntrophin dephosphorylation and calpain activity both reduce insulin secretion. Thus, we propose that modulation of the association between ICA512 and β2-syntrophin, and cleavage of ICA512 by µ-calpain, are among the mechanisms regulating the exocytosis of secretory granules.
Results
Biosynthesis of pro-ICA512 and degradation of ICA512 transmembrane fragment upon insulin secretion
Newly synthesized pro-ICA512 migrates as an N-glycoprotein of 110 kDa by SDS–PAGE (Hermel et al., 1999). Along the secretory pathway, pro-ICA512 is first converted into a protein of 130 kDa and then is cleaved at a consensus site for furin-like convertases between residues 448 and 449 in the ectodomain (Solimena et al., 1996). The resulting transmembrane fragment (ICA512 TMF) includes the N-glycosylated portion of the ectodomain (amino acids 449–575), the transmembrane region (amino acids 576–600) and the cytoplasmic tail (amino acids 601–979). ICA512 TMF is enriched in the membrane of secretory granules and usually appears on western blotting as a protein doublet that migrates between 60 and 70 kDa. N-terminal microsequencing indicated that both proteins correspond to ICA512 TMFs generated by cleavage at residue 449 (Hermel et al., 1999). Thus, these multiple ICA512 TMFs may differ because of alternative splicing or post-translation modifications (Hermel et al., 1999; Diez et al., 2001). To determine possible changes in ICA512 associated with insulin release, INS-1 cells were stimulated for 1.5 h with high glucose (25 mM) and high KCl (55 mM) concentrations (later referred to as HGHK stimulation). Stimulation of insulin secretion with both secretagogues was accompanied by a significant increase in the levels of 110 kDa pro-ICA512 and by a ∼42% decrease in the amount of ICA512 TMF (Figure 1A). Notably, similar results were observed regardless of whether the western blottings were performed on total cell lysates from resting and stimulated INS-1 cells or the corresponding Triton X-100-soluble extracts. Thus, the diminished levels of ICA512 TMF upon stimulation could be not explained by a possible redistribution of ICA512 TMF between Triton X-100-soluble and -insoluble fractions, but rather are accounted for by the balance between the synthesis of pro-ICA512 and the degradation of mature ICA512 TMF. Stimulation with 55 mM KCl alone for the same time reduced the levels of ICA512 TMF by ∼37%, but did not induce the synthesis of pro-ICA512 (Figure 1B). Stimulation with 25 mM glucose, on the other hand, promoted the synthesis of pro-ICA512, but only slightly affected the levels of ICA512 TMF (Figure 1B). These data are consistent with, and further extend previous studies indicating that ICA512 mRNA levels are increased in INS-1 cells treated with high glucose (Seissler et al., 2000). The apparent dissociation between the biosynthesis of ICA512 and the proteolysis of ICA512 TMF upon stimulation in various conditions can be explained by considering the different properties of glucose and KCl as secretagogues. In INS-1 cells, 25 mM glucose was a weak secretagogue that only stimulated insulin secretion ∼2.5-fold over basal release, compared with an ∼10-fold increase by 55 mM KCl (Figure 1C). Glucose is the primary physiological insulin secretagogue. Its entry into β-cells initiates a metabolic signaling cascade that activates the transcription of many secretory genes, including insulin and ICA512 (Shushan et al., 1999; Seissler et al., 2000), and triggers insulin secretion by promoting the closure of ATP-dependent K+ channels. High K+, instead, is a potent non-physiological secretagogue that directly depolarizes membranes by reversing the concentration gradient of K+ between the intra- and extra-cellular compartments. Thus, our findings suggest that degradation of ICA512 TMF depends on the mode by which regulated secretion of INS-1 cells is activated.
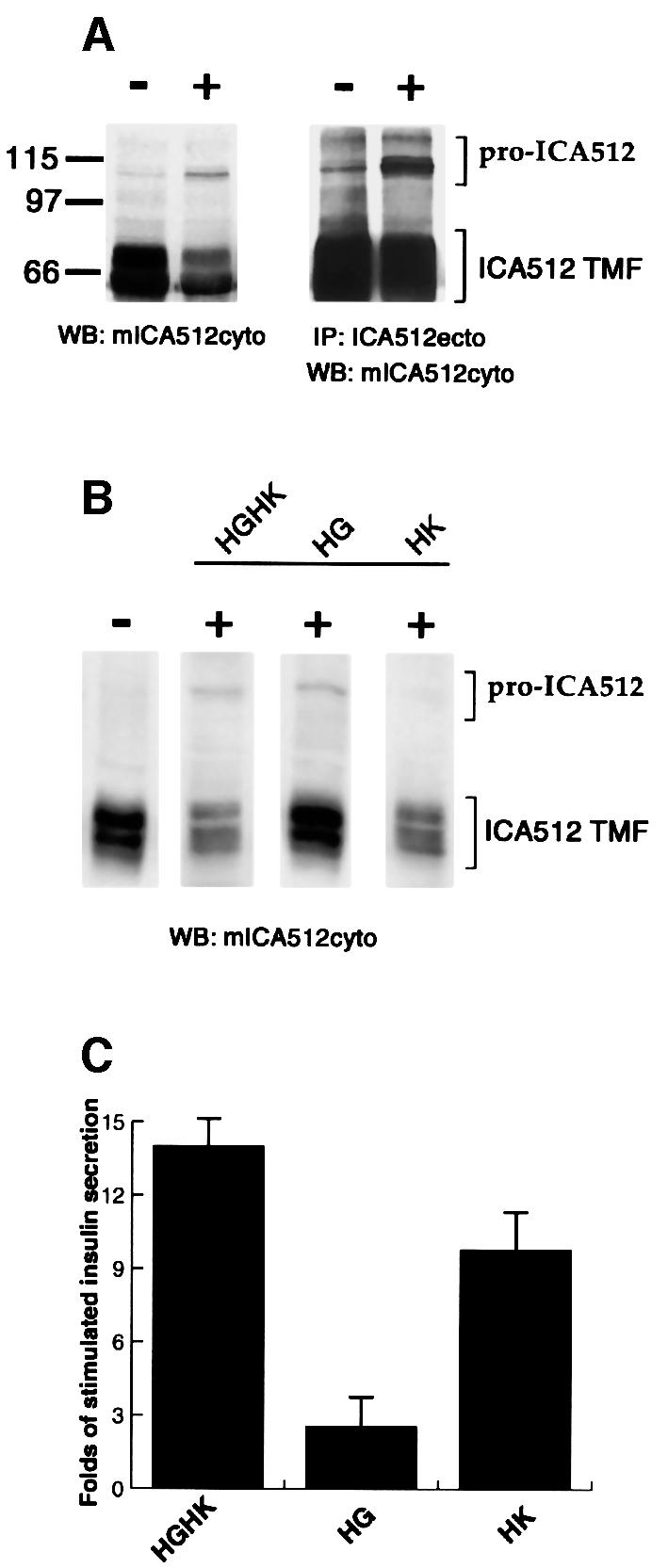
Fig. 1. Expression of ICA512 in resting and stimulated INS-1 cells. (A) Western blotting with mICA512cyto on Triton X-100-soluble extracts (left panel) or immunoprecipitates with ICA512ecto antibody (right panel) from INS-1 cells kept in resting conditions (–) or stimulated (+) for 1.5 h with high glucose and high potassium (HGHK). (B) Western blotting with mICA512cyto on Triton X-100-soluble extracts from INS-1 cells kept in resting conditions (–) or stimulated (+) for 1.5 h with 25 mM glucose (HG), 55 mM potassium (HK) or both (HGHK). (C) Fold insulin secretion above basal level from INS-1 cells stimulated with HGHK, HG or HK. The values presented are the means of three independent experiments performed in triplicate.
Calpain-dependent proteolysis of ICA512 TMF
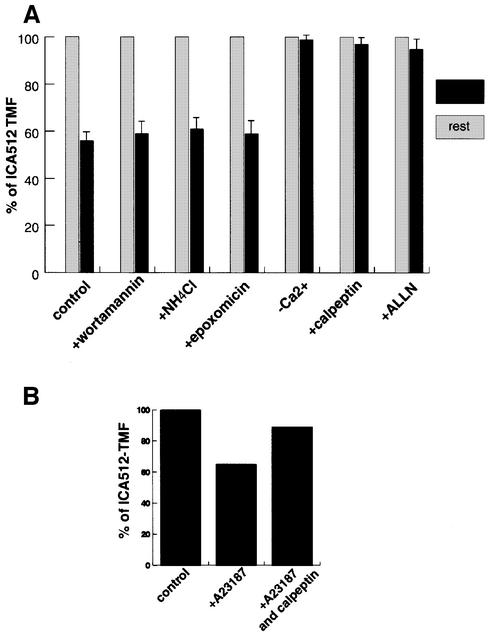
To investigate the mechanisms responsible for ICA512 degradation, INS-1 cells were stimulated with HGHK in the presence of substances that directly or indirectly affect various proteolytic pathways. Two hundred nanomolar wortmannin, which inhibits PI3 kinase and prevents the transport of endocytic proteins to the endosolysosomal compartment (Davidson, 1995), had no stabilizing effect on ICA512 TMF (Figure 2A). Fifty millimolar NH4Cl, a weak base that interferes with the activity of lysosomal enzymes by neutralizing the pH in the lysosomes (Galloway et al., 1983), and 1 µM epoxomicin, a selective proteosome inhibitor (Sin et al., 1999), also failed to prevent the degradation of ICA512 TMF. Conversely, the absence of extracellular Ca2+ completely protected ICA512 TMF from proteolysis, while it did not affect the biosynthesis of pro-ICA512. The search for a potential degradation signal in ICA512 TMF led to the identification of a single putative PEST domain between amino acids 644 and 653 (score: +8.53, high significance: >+5.0 values) in the cytoplasmic tail of ICA512 (Figure 2C). Proteins with PEST sequences are preferential targets for degradation by the proteosome and by the Ca2+-dependent protease calpain (Rechsteiner, 1990; Mykles, 1998). Since proteolysis of ICA512 TMF appeared to be Ca2+ sensitive and proteosome insensitive, we tested whether calpain could be involved in this process. Consistent with this hypothesis, 1 µM calpeptin and 10 µM N-acetyl-Leu-Leu-Nle-CHO (ALLN), two distinct calpain inhibitors, stabilized ICA512 TMF (Figure 2A). Likewise, calpeptin could largely inhibit the proteolyis of ICA512 TMF induced by incubation of INS-1 cells with the Ca2+ ionophore A23187 (Figure 2B).
Fig. 2. Effect of different pharmacological treatments on secretion-induced ICA512 TMF degradation in INS-1 cells. (A) Graphic representation of the amount of ICA512 TMF detected by western blotting with mICA512cyto and [125I]protein G in INS-1 cells stimulated with high glucose and high potassium for 1.5 h in the absence (control) or presence of the different drugs. The amount of ICA512 TMF detected in resting cells represents 100%. Drug concentrations were as follows: 200 nM wortmannin, 50 mM NH4Cl, 1 µM epoxomicin, 1µM calpeptin, 1 µM ALLN. The values presented are the means of three independent experiments. (B) Quantification of ICA512 TMF in control INS-1 cells (100%) or INS-1 cells treated with calcium ionophore A23187 in the absence or presence of 1 µM calpeptin. (C) Primary amino acid sequence of the cytoplasmic region of ICA512. The residues within the PEST motif are underlined with plus signs.
Activation of µ-calpain in INS-1 cells upon stimulation of insulin secretion
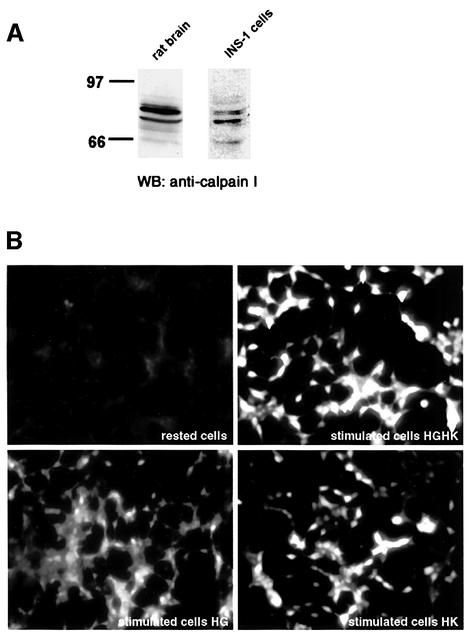
Two ubiquitous Ca2+-dependent calpain isoforms are known: µ-calpain and m-calpain. Activation of µ-calpain in vitro requires 5–50 µM Ca2+, while m-calpain requires 0.2–1.0 mM Ca2+ (Johnson et al., 1997; Sorimachi et al., 1997; Carafoli and Molinari, 1998). Since stimulation of regulated secretion is associated with the rise in cytosolic Ca2+ concentration in the micromolar range and ALLN is a specific inhibitor of µ-calpain, it was assumed that µ-calpain could be responsible for ICA512 TMF degradation. Western blotting with an anti-µ-calpain antibody revealed the expression of two proteins of ∼80 and 77 kDa in INS-1 cells (Figure 3A). Two proteins of identical size were detected in brain, which was used as a positive control tissue. The 80 and 77 kDa proteins corresponds to the pro- and activated forms of µ-calpain, respectively (Carafoli and Molinari, 1998).
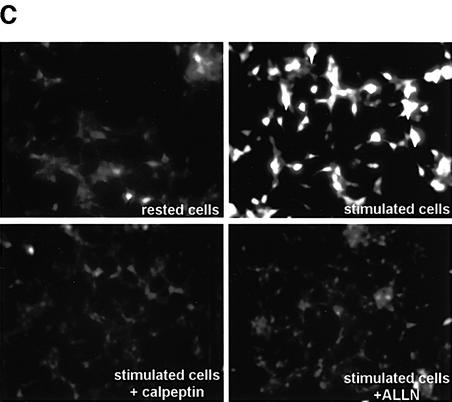
Fig. 3. Expression and activation of µ-calpain in INS-1 cells. (A) Western blotting for µ-calpain I on total protein extracts from rat brain and INS-1 cells. (B) Fluorescence microscopy in live INS-1 cells pre-loaded with the calpain substrate Boc-LM-CMAC and kept in resting conditions or stimulated with 25 mM glucose (HG), 55 mM KCl (HK) or both (HGHK) for 1.5 h. (C) Fluorescence microscopy in live INS-1 cells pre-loaded with the calpain substrate Boc-LM-CMAC and kept in resting conditions or stimulated with HGHK for 1.5 h in the absence or presence of the calpain inhibitors calpeptin and ALLN.
The rise in intracellular Ca2+ concentration that triggers the exocytosis of secretory granules should conceivably activate µ-calpain. To test this possibility, INS-1 cells were incubated with t-butoxycarbonyl-Leu-Met-chloromethylaminocoumarin (Boc-LM-CMAC). Cleavage of Boc-LM-CMAC, an equally good substrate for m- and µ-calpains, generates a blue fluorescent signal that can be detected by microscopy. INS-1 cells stimulated either with 25 mM glucose, 55 mM KCl, or both, were significantly more fluorescent than resting cells (Figure 3B). Maximal activation of calpain was observed in cells stimulated with both high glucose and high potassium, i.e. in the conditions that are the most effective in promoting the cleavage of ICA512 TMF (Figure 1B) and insulin secretion (Figure 1C). The fluorescence in cells stimulated with high glucose and high KCl in the presence of calpeptin or ALLN, on the other hand, was not increased above basal levels (Figure 3C). These results indicate that stimulation of exocytosis is accompanied by the activation of µ-calpain, which, in turn, may act on substrates, including ICA512.
ICA512 proteolysis by µ-calpain in vitro
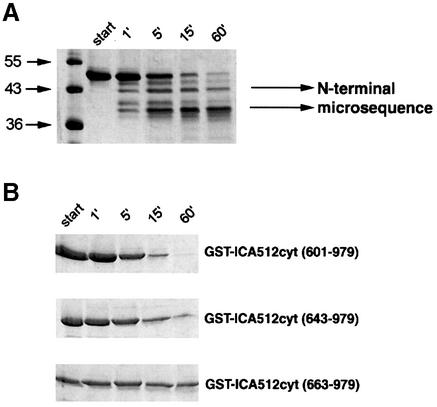
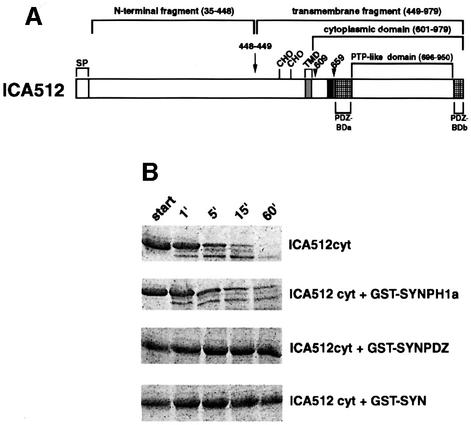
To test whether µ-calpain recognizes ICA512 as a sub strate, the recombinant cytoplasmic domain of ICA512 (ICA512cyt) was incubated in vitro with purified µ-calpain for different periods of time. The peptide profile of each reaction was then examined by SDS–PAGE and Coomassie Blue staining. Figure 4A shows that cleavage of ICA512cyt was almost complete after a 15 min incubation with 15 nM µ-calpain, and resulted in the generation of multiple proteolytic fragments, the smallest and most stable of which was an ∼39–40 kDa polypeptide. To establish the cleavage sites of µ-calpain, the two most prominent proteolytic fragments (Figure 4A, arrows) were analyzed by N-terminal microsequencing. The cleavage site of the largest fragment was located between residues 608 and 609, near the transmembrane domain, while the final cleavage product, which lacked the PEST domain, started at residue 659 (Figure 5A). Removal of residues 601–643 did not affect cleavage of ICA512cyt by µ-calpain, whereas further deletion of residues 644–662, which includes the PEST domain, prevented ICA512cyt degradation (Figure 4B). These data strongly suggest that the PEST domain is necessary for the cleavage of ICA512cyt by µ-calpain.
Fig. 4. In vitro cleavage of recombinant ICA512cyt by µ-calpain. (A) Time course digestion of recombinant HisTagICA512cyt after 0, 1, 15 and 60 min incubation with µ-calpain. Arrows indicate the proteolytic products that were submitted to N-terminal microsequencing. (B) Time course digestion of various GST–ICA512cyt fusion proteins.
Fig. 5. Domain structure and PEST domain of ICA512. (A) Schematic representation of the domain structure of ICA512. The primary amino acid sequence of ICA512 includes a putative signal peptide (SP), an extracellular domain with two potential N-glycosylation sites (CHO), a transmembrane domain (TMD) and a cytoplasmic domain with a single protein tyrosine phosphatase (PTP)-like domain. The arrow indicates the processing site in the extracellular domain. This cleavage generates a transmembrane fragment of ICA512 (ICA512 TMF). The cytoplasmic domain contains a PEST motif (black box), and two non-contiguous domains that are both required for binding the PDZ domain of β2-syntrophin. Arrowheads point to the localization of µ-calpain cleavage sites in vitro. (B) Time course digestion by µ-calpain of recombinant HisTagICA512cyt pre-incubated with or without the indicated recombinant GST–β2-syntrophin fusion fragments.
Stabilization of ICA512 upon binding of β2-syntrophin
The more distal cleavage site of µ-calpain is adjacent to a region of ICA512 that is required for binding the PDZ domain of β2-syntrophin (Figure 5A). We therefore tested whether binding of β2-syntrophin could shield ICA512 from cleavage by µ-calpain. Incubation of ICA512cyt with recombinant full-length β2-syntrophin or β2-syntrophin PDZ domain almost completely inhibited the degradation of ICA512 by µ-calpain (Figure 5B). Conversely, β2-syntrophin PH1a domain, which does not bind ICA512, did not prevent µ-calpain from cleaving ICA512cyt. These data suggest that the association with β2-syntrophin may regulate the cleavage of ICA512 by µ-calpain.
Enrichment of β2-syntrophin and utrophin on secretory granules
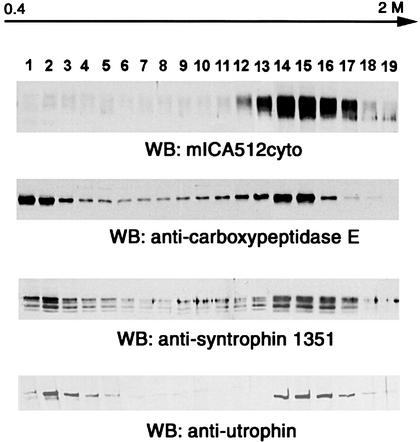
Syntrophins are molecular adapter proteins of ∼58 kDa that in muscle cells mediate the interaction between the actin-binding proteins of the dystrophin family and signaling molecules such as voltage-gated Na2+ channels and neuronal nitric oxide synthase (nNOS) (Brenman et al., 1996; Froehner et al., 1997; Gee et al., 1998; Schultz et al., 1998). β2-syntrophin, in particular, is a syntrophin isoform with a wide tissue distribution that binds the ubiquitous dystrophin isoform utrophin (Peters et al., 1994, 1997). It is also the prevalent syntrophin expressed in rat pancreatic islets and INS-1 cells, where it binds both utrophin and ICA512 (Ort et al., 2000). Thus, β2-syntrophin accounts for virtually all of the reactivity detected in INS-1 cells with the monoclonal pan-syntrophin 1351 antibody. To test whether β2-syntrophin and utrophin are associated with secretory granules, isotonic homogenates of INS-1 cells were separated on continuous sucrose density gradients and the resulting fractions analyzed by western blotting. Both β2-syntrophin and utrophin were largely enriched in dense fractions (∼1.4–1.7 M sucrose) (Figure 6, two bottom panels). Their peaks coincided with the peaks of the secretory granule markers ICA512 and carboxypeptidase E (Figure 6, two top panels). Unlike ICA512, β2-syntrophin and utrophin were also recovered in light fractions of the gradient, as expected for soluble cytosolic proteins. Carboxypeptidase E is a secretory granule protein that is only partially associated with the luminal leaflet of the secretory granule membrane. Thus, the pool of carboxypeptidase E found in light sucrose fractions most likely corresponds to the portion of the enzyme that was released upon destruction of secretory granules during the homogenization procedure. These results corroborate the hypothesis that β2-syntrophin–utrophin complexes are associated with secretory granules, presumably through their interaction with ICA512.
Fig. 6. Subcellular distribution of syntrophin and utrophin in INS-1 cells. Post-nuclear supernatants of INS-1 cells were fractionated on continuous sucrose density gradients (0.4–2 M). Aliquots of each gradient fraction were immunoblotted with antibodies directed against ICA512 (mICA512cyto), carboxypeptidase E, syntrophin (1351) and utrophin.
Dephosphorylation of β2-syntrophin upon stimulation of insulin secretion
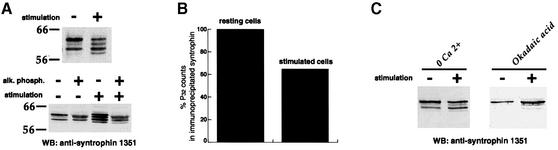
During the course of our studies we observed that β2-syntrophin appeared as a ladder of either two or four ∼55–58 kDa proteins by immunoblotting on extracts from resting or stimulated INS-1 cells, respectively (Figure 7A, top panel). Incubation of extracts from resting and stimulated INS-1 cells with alkaline phosphatase led to the disappearance in both cases of the slowest migrating protein and the corresponding accumulation of the second slowest migrating band (Figure 7A, lower panel). These results suggest that the top band is a phosphorylated species of β2-syntrophin. Notably, this species was less abundant in stimulated versus resting cells (Figure 7A, top panel), suggesting that β2-syntrophin is partially dephosphorylated upon stimulation of insulin secretion. This possibility was confirmed by immunoprecipitation of β2-syntrophin from 32P-labeled INS-1 cells. [32P]β2-syntrophin recovered from stimulated cells was indeed only ∼65% of [32P]β2-syntrophin recovered from resting cells (Figure 7B). Dephosphorylation of β2-syntrophin was Ca2+ dependent (Figure 7C, left panel) and sensitive to okadaic acid (Figure 7C, right panel), an inhibitor of serine/threonine protein phosphatases 1 and 2A. Specific ally, the slowest migrating isoform of β2-syntrophin was virtually the only species detected both in resting and stimulated conditions upon treatment with okadaic acid. This result implies that all the isoforms detected in resting or stimulated INS-1 cells (Figure 7A) represent species of β2-syntrophin that differ with respect to their degree of phosphorylation. It also suggests that β2-syntrophin cycles between different phosphorylation states even in resting cells. The persistence of multiple species after alkaline phosphatase treatment, on the other hand, indicates that some phosphorylation sites of β2-syntrophin are not accessible to this phosphatase in vitro.
Fig. 7. Phosphorylation of syntrophin in INS-1 cells. (A) Western blotting on Triton X-100-soluble extracts from resting (–) or HGHK-stimulated (+) INS-1 cells with anti-syntrophin 1351 antibody (top panel). The lower panel shows the western blotting on extracts from resting (left lanes) or stimulated INS-1 cells (right lanes), untreated or treated with alkaline phosphatase. (B) Levels of 32P-labeled syntrophin immunoprecipitated from resting or HGHK-stimulated INS-1 cells with the anti-syntrophin antibody 1351. (C) Western blotting with the anti-syntrophin antibody 1351 on Triton X-100-soluble extracts from resting (–) or HGHK stimulated (+) INS-1 cells incubated with no extracellular calcium or 1 µM okadaic acid.
ICA512 preferentially interacts with phosphorylated β2-syntrophin
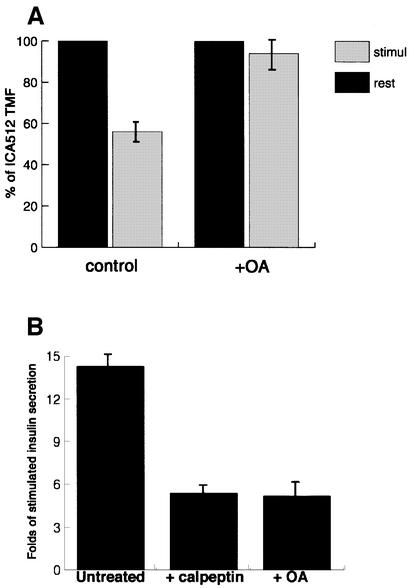
We next investigated whether phosphorylation of β2-syntrophin affects its binding to ICA512. The extracts from stimulated INS-1 cells were used as the source of β2-syntrophin for pull-down assays with glutathione S-transferase (GST)–ICA512cyt and for immunoprecipitation with ICA512ecto antibody. Figure 8A shows that ICA512cyt preferentially bound the top phosphorylated species of β2-syntrophin. Similarly, the same top phosphorylated form is the only species of β2-syntrophin that was co-immunoprecipitated with ICA512ecto antibody (Figure 8B). These data, and the finding that β2-syntrophin protects ICA512 from µ-calpain cleavage, point to phosphorylation/dephosphorylation of β2-syntrophin as a potential mechanism for regulating both the association of ICA512 with β2-syntrophin–utrophin complexes and the stimulus-induced proteolysis of ICA512 TMF by µ-calpain. According to this scenario, treatment of INS-1 cells with okadaic acid, which traps β2-syntrophin in its maximally phosphorylated state, should promote the binding of β2-syntrophin to ICA512 and thus protect the latter from µ-calpain-mediated proteolysis. Indeed, the levels of ICA512 TMF after stimulation of INS-1 cells in the presence of okadaic acid were unaffected (Figure 9A).
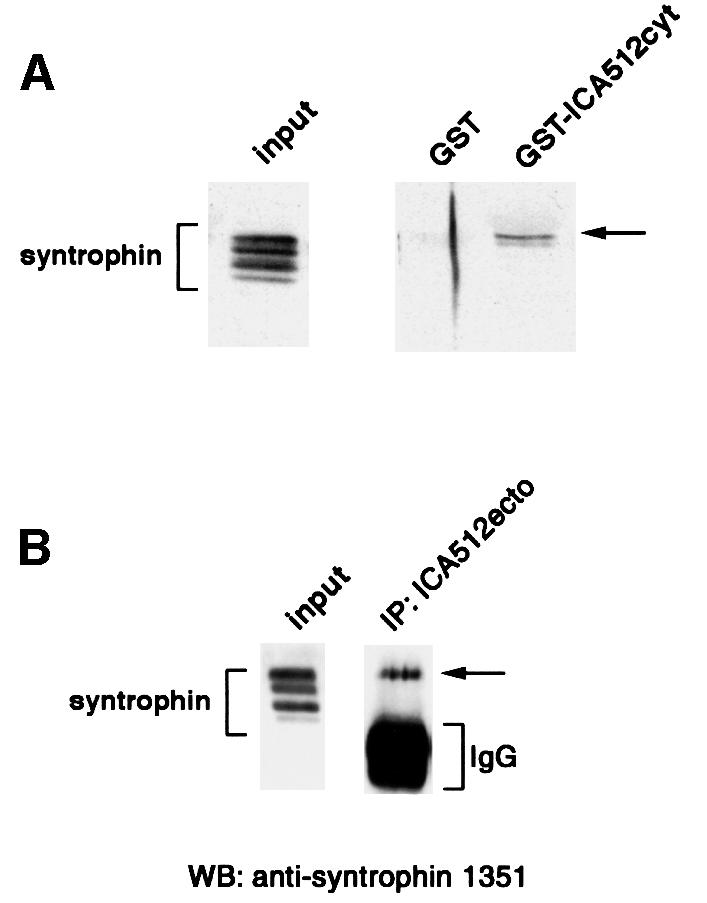
Fig. 8. Binding of ICA512 to phosphorylated syntrophin. (A) Triton X-100-soluble extracts from stimulated INS-1 cells were incubated with glutathione beads coupled to GST or GST–ICA512cyt. Aliquots of the starting and bound material were immunoblotted with the anti-syntrophin 1351 antibody. (B) Western blotting with the anti-syntrophin 1351 antibody on starting material from stimulated INS-1 cells and on the immunoprecipitate with ICA512ecto antibody from the corresponding Triton X-100 soluble extract.
Fig. 9. Degradation of ICA512 and insulin secretion in INS-1 cells treated with okadaic acid. (A) Quantification of the amount of ICA512 TMF in stimulated INS-1 cells untreated (control) or treated with 1 µM okadaic acid. The amount of ICA512 TMF detected in resting cells represents 100%. The values presented are the means of three independent experiments. (B) Fold insulin secretion above basal level from INS-1 cells stimulated with HGHK, untreated or treated with calpeptin or okadaic acid. The values presented are the means of three independent experiments performed in triplicate.
Inhibition of insulin secretion by okadaic acid and calpeptin
Binding of ICA512 to β2-syntrophin–utrophin complexes may anchor secretory granules to the actin cytoskeleton. Conversely, dissociation of ICA512 from β2-syntrophin followed by µ-calpain-mediated proteolysis of ICA512 may allow the mobilization of secretory granules and facilitate insulin secretion. Consistent with this scenario, insulin secretion was similarly reduced by ∼61% in INS-1 cells stimulated in the presence of either okadaic acid or calpeptin (Figure 9B).
Discussion
A large body of evidence indicates that exocytosis of β-cell secretory granules is hindered by cortical actin microfilaments (Orci et al., 1972; Li et al., 1994; Cable et al., 1995; Bruun et al., 2000; Wilson et al., 2001). To our knowledge, there are no data concerning a direct association between actin and insulin-containing secretory granules. Actin and its binding proteins spectrin and α-actinin can associate with the secretory granules in other model systems of regulated secretion, such as the adrenal chromaffin cells and the pancreatic acinar cells, (Bader and Aunis, 1983; Aunis and Perrin, 1984; Valentijn et al., 1999, 2000). Actin and spectrin may also interact with synaptic vesicles, the other regulated secretory vesicles of neuroendocrine cells (Ceccaldi et al., 1995; Zimmer et al., 2000). The nature of the molecular association between neurosecretory granules and microfilaments, however, is still not understood.
Our previous studies indicated that, in INS-1 cells, ICA512 TMF is associated with syntrophin–utrophin complexes (Ort et al., 2000). This interaction is likely to account for the enrichment of β2-syntrophin and utrophin in the sucrose density fractions containing insulin-releasing secretory granules. Syntrophin and utrophin are generally regarded as scaffold proteins at the plasma membrane. Recently, the association of syntrophin with post-Golgi vesicles that transport acetylcholine receptors to the cell surface has been documented by immunoelectron microscopy in the Torpedo electrocyte (Marchand et al., 2001). In general, there is increasing evidence that interactions involving PDZ domains, like the one between syntrophin and ICA512, play a role in regulating membrane trafficking (Butz et al., 1998; Cao et al., 1999; Thomas et al., 2000). For example, the association of the PDZ domain of TACIP18 to transforming growth factor-α (TGF-α) is necessary for the trafficking of the latter to the cell surface (Fernandez-Larrea et al., 1999). We propose that the ICA512–syntrophin–utrophin complex links secretory granules to actin microfilaments. The N-terminus of utrophin contains two calponin homology domains that are known to bind actin (Amann et al., 1999; Keep et al., 1999; Moores et al., 2000). Unlike its related protein, dystrophin, utrophin does not contain additional actin-binding sites in the spectrin-like repeats (Rybakaova et al., 1996). Thus, actin filaments may bind utrophin less tightly than dystrophin. This mode of weaker binding could facilitate the rapid release of secretory granules from the actin-based cytoskeleton.
Our data indicate that, in INS-1 cells, β2-syntrophin is phosphorylated at multiple sites and ICA512 preferentially interacts with phospho-β2-syntrophin. To our knowledge, this is the first demonstration that phosphorylation of a PDZ domain-containing protein regulates its interaction with a ligand. Phosphorylation within the syntrophin family has previously been reported only in the case of α-syntrophin, which can be a substrate of Ca2+/calmodulin kinase II (Madhavan and Jarrett, 1999), and stress-activated protein kinase 3 (M.Hasegawa et al., 1999). Evidence that recombinant β2-syntrophin can also bind ICA512 implies that phosphorylation of β2-syntrophin is not an absolute requirement for this association to occur, but may affect the affinity of the interaction. Stimulation of insulin secretion results in the Ca2+-dependent dephosphorylation of β2-syntrophin, while inhibition of β2-syntrophin dephosphorylation with okadaic acid correlates with a significant reduction of insulin secretion. Okadaic acid appears to have opposite effects on insulin secretion. A stimulatory effect on insulin release, which only lasted a few minutes, was observed in Rin5mF cells treated with okadaic acid (Haby et al., 1994). Similar to our findings, however, this drug was found to inhibit the cumulative insulin secretion from freshly isolated and cultured perifused mouse islets upon stimulation for 1 h (Sato et al., 1998). These results may be reconciled by assuming that okadaic acid potentiates the first phase of insulin secretion, while inhibiting its second phase. Interestingly, Henquin’s group already proposed that okadaic acid might reduce insulin secretion by interfering with the reorganization of the cytoskeleton (Sato et al., 1998).
Stimulation of insulin secretion causes a significant reduction in the levels of ICA512 TMF. The degradation of ICA512 TMF has been found to be Ca2+ dependent and sensitive to calpain inhibitors, including one (ALLN) that is specific for µ-calpain. Accordingly, we have shown that stimulation of INS-1 cells activates µ-calpain and that this protease generates multiple ICA512cyt fragments in vitro. The largest of these fragments analyzed by N-terminal microsequencing starts at residue 609, just a few residues downstream of the ICA512 transmembrane domain. The smallest and more stable in vitro fragment begins at residue 659, adjacent to the region required for the association with β2-syntrophin. By using deleted constructs of ICA512cyt, we have further narrowed the region required for recognition of ICA512 by µ-calpain to residues 643–659. This is the region that contains the PEST domain. PEST-containing proteins are among the preferential substrates of cytosolic proteases, including calpains. Soluble and receptor tyrosine phosphatases often have one or more PEST domains, the significance of which has been investigated only in few cases (Gu and Majerus, 1996; Bult et al., 1997; K.Hasegawa et al., 1999; Nguyen et al., 1999). The mechanisms that target PEST-containing proteins to proteolysis are largely unclear. In the case of IκBα, the PEST domain is required for binding and cleavage by µ-calpain (Shumway et al., 1999). In the case of ICA512, however, we could not demonstrate a direct interaction with µ-calpain (our unpublished observation).
The detection of ICA512 fragments generated in vivo by µ-calpain was hampered by the epitope specificity of the mICA512cyto antibody. Since this antibody recognizes an epitope located between residues 643 and 663 of ICA512, it cannot react with the stable proteolytic fragment encompassing amino acids 659–979. It recognizes instead several of the larger ICA512cyt fragments produced by µ-calpain in vitro. However, no fragments of corresponding sizes were detected with this antibody by western blotting on extracts from stimulated INS-1 cells. Given their short half-life in vitro, it is possible that one or more of these fragments are produced in vivo and yet have escaped our detection. Interestingly, the cytoplasmic domain of ICA512 contains a putative tyrosine-based internalization motif (YQDL) (Trowbridge et al., 1993) between residues 628 and 631. Cleavage of ICA512 downstream of this motif would generate a transmembrane fragment that could still be effectively internalized from the plasma membrane following the exocytosis of secretory granules.
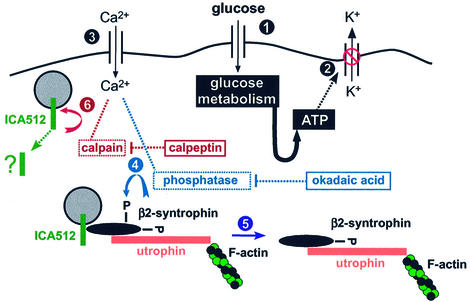
As mentioned previously, the PEST domain and the µ-calpain cleavage site between residues 658 and 659 are adjacent to a region (663–700) that is necessary for the binding of ICA512 to the β2-syntrophin PDZ domain (Ort et al., 2000). Our in vitro studies show that such binding is sufficient to shield ICA512cyt from degradation. In view of the preferential binding of ICA512cyt to phospho-β2-syntrophin, it is conceivable that phosphorylation of β2-syntrophin controls the accessibility of ICA512 to µ-calpain. This possibility is corroborated by the fact that okadaic acid, which inhibits the dephosphorylation of β2-syntrophin, blocks the degradation of ICA512 TMF upon stimulation of INS-1 cells. Inhibition of ICA512 TMF degradation as well as insulin secretion was also observed upon treatment of INS-1 cells with calpeptin. Based on these data, we propose that the increased levels of intracellular Ca2+ evoked by stimulation of INS-1 cells result in the dephosphorylation of β2-syntrophin, thus weakening its association with ICA512 TMF (Figure 10). This, in turn, may allow the cleavage of ICA512 TMF by activated µ-calpain, leading to the mobilization of secretory granules previously anchored to actin microfilaments via the ICA512–β2-syntrophin–utrophin complex. This process, i.e. the recruitment of granules from the large resting pool, is likely to contribute to the second phase of insulin secretion. Obviously, the effects on insulin secretion of okadaic acid and calpeptin may be accounted for, at least in part, by mechanisms different from the one proposed here.
Fig. 10. ICA512 and the trafficking of secretory granules. Entry (step 1) and metabolism of glucose in β-cells led to increased levels of intracellular ATP, closure of ATP-sensitive K+ channels (step 2), membrane depolarization and Ca2+ influx (step 3). The raised levels of intracellular Ca2+ stimulate the dephosphorylation of β2-syntrophin (step 4), and the dissociation of β2-syntrophin–utrophin–F-actin complexes from ICA512 and secretory granules (step 5). Upon dissociation of β2-syntrophin, the cytoplasmic domain of ICA512 TMF becomes available for cleavage by µ-calpain (step 6), which is also activated by Ca2+. This process would result in the mobilization of secretory granules from the cytomatrix and facilitate their exocytosis. Okadaic and calpeptin inhibit steps 5 and 6, respectively, and thus insulin release.
Recently, an alternative spliced variant of ICA512 has been identified that lacks exon 14 (ICA512Δ14; Diez et al., 2001), which encodes the PDZ-BDa domain. It would be interesting to establish whether expression of ICA512Δ14, which should not bind β2-syntrophin, has any effect on insulin secretion. Similar considerations may be raised about β2-syntrophin28 (Ort et al., 2000). This alternative spliced variant of β2-syntrophin, which is expressed in pancreatic islets, contains the PDZ domain, but lacks the C-terminal half of β2-syntrophin responsible for the association with utrophin. Binding of ICA512 TMF to β2-syntrophin28 could prevent its cleavage, but also preclude its indirect association with utrophin and actin microfilaments. These examples illustrate how regulation of the expression of ICA512 and β2-syntrophin isoforms could allow the generation of various subsets of secretory vesicles that differ in regard to their ability to interact with the cytoskeleton and undergo exocytosis.
The finding that ICA512 is a calpain substrate could have relevant clinical implications. Recently, calpain-10 has been identified as the first susceptibility gene in type 2 diabetes (Baier et al., 2000; Horikawa et al., 2000). Preliminary studies suggest that calpain-10 is expressed in pancreatic islets, but no physiological substrate for it has yet been identified (Altshuler et al., 2000; Horikawa et al., 2000). Unlike µ-calpain, calpain-10 may not be Ca2+ regulated. Stimuli such as acetylcholine and amino acids, on the other hand, enhance insulin secretion via the activation of signaling pathways distinct from those elicited by glucose. Thus, it would be important to investigate whether activation of these pathways promotes the proteolysis of ICA512 and the mobilization of secretory granules by stimulating calpain-10.
Materials and methods
The following antibodies were described previously: the affinity-purified rabbit antibody ICA512ecto directed against the extracellular domain (amino acids 389–575) of human ICA512 (Solimena et al., 1996); the mouse monoclonal antibody (mAb) mICA512cyto directed against the cytoplasmic domain (amino acids 601–979) of human ICA512 (Hermel et al., 1999); the mouse mAb 1351 directed against the PDZ domain of α1-, β1- and β2-syntrophin (Froehner et al., 1987; Peters et al., 1997). The following antibodies were purchased from commercial suppliers: the mouse mAb NCL-DRP2 directed against the N-terminus of utrophin (Novocastra, Newcastle upon Tyne, UK); the mouse monoclonal anti-calpain I antibody (Chemicon International, Inc., Temecula, CA); the mouse mAb directed against carboxypeptidase E (Transduction Laboratories, San Diego, CA); peroxidase-conjugated goat anti-rabbit (Sigma, St Louis, MO) and anti-mouse (Bio-Rad, Hercules, CA) IgGs. Calpeptin, ALLN, wortmannin and purified µ-calpain from erythrocytes were obtained from Calbiochem (La Jolla, CA). Calcium ionophore A23187 and okadaic acid were obtained from Sigma. The proteosome inhibitor epoxomicin was a kind gift of Dr C.M.Crew (Yale University, New Haven, CT) (Sin et al., 1999).
Culture and stimulation of INS-1 cells
Rat insulinoma INS-1 cells were cultured in RPMI 1640 medium (Gibco-BRL) supplemented with 10 mM HEPES, 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 1 mM sodium pyruvate and 50 µM 2-mercaptoethanol as described previously (Asfari et al., 1992). For stimulation experiments, 1 × 106 cells were plated 3 days before treatment in six-well plates. On the fourth day, cells were washed and pre-incubated for 1 h in resting buffer at 37°C (5 mM KCl, 120 mM NaCl, 24 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, 1 mg/ml ovalbumin, 15 mM HEPES pH 7.4). The cells were then incubated for an additional 1.5 h in the resting buffer or in the stimulating buffer (55 mM KCl, 25 mM glucose, 70 mM NaCl, 24 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, 1 mg/ml ovalbumin, 15 mM HEPES pH 7.4). Inhibitors were added during the last 20 min of the pre-incubation and were present throughout the stimulation period. After incubation, the cells were briefly washed with ice-cold phosphate-buffered saline (PBS) and then immediately extracted with PBS including 1 mM phenylmethylsulfonyl flouride (PMSF), 10 mM benzamidine, 1 µg/ml aprotinin, 1 µg/ml pepstatin A, 1 µg/ml antipain, 5 mM EDTA, 1 mM sodium orthovanadate and 1% Triton X-100. After centrifugation at 14 000 g for 30 min, 3× SDS sample buffer was added. Equivalent amounts of protein were loaded in each lane and separated by SDS–PAGE for western blotting. The protein concentration in cell extracts was determined using the BCA assay (Pierce, Rockford, IL).
Western blotting
After transfer onto nitrocellulose, proteins were immunoblotted with the one of the following antibodies: 1351 (1:5000), mICA512cyto (1:10), NCL-DRP2 (1:100), µ-calpain antibody (1:100) or carboxypeptidase E antibody (1:2000). After three 10-min washes in 20 mM Tris–HCl pH 7.8, 150 mM NaCl, 0.1% Tween-20, the blots were incubated with horseradish-conjugated goat anti-mouse or anti-rabbit IgG (1:5000). Signal was detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). To quantify the amount of ICA512 TMF in the cell extracts, the blots were incubated with the antibody mICA512cyto, washed, and then incubated with [125I]protein G (Amersham Pharmacia Biotech). After extensive washing, the blots were exposed for autoradiography with X-OMAT-AR film (Kodak, Rochester, NY) and the signals were quantified using 1D Image Analysis software (Kodak). The value given is the mean from at least three independent experiments.
Phospholabeling of INS-1 cells
INS-1 cells grown to ∼70% confluence in 10 mm plates were pre-incubated at 37°C for 15 min in labeling media containing phosphate-free RPMI 1640 (Gibco-BRL) and 10% dialyzed fetal calf serum. Cells were then equilibrated in the labeling media containing 32Pi (200 µCi/dish; Amersham Pharmacia Biotech) for 1 h, followed by incubation in resting or stimulating buffers as described above. Cell extracts were prepared as indicated above and pre-cleaned by incubation with protein G–Sepharose. Syntrophin was immunoprecipitated with the antibody 1351 bound to protein G–Sepharose. Immunoprecipitates were eluted in 1× SDS sample buffer and separated by SDS–PAGE. PhosphorImaging analysis was performed with a PhosphorImager SI (Molecular Dynamics, Sunnyvale, CA).
Alkaline phosphatase treatments
Extracts from rested and stimulated cells (500 µg) were incubated with or without 50 U of calf intestinal alkaline phosphatase (Gibco-BRL) for 30 min at 30°C in 50 mM Tris buffer pH 8.0 containing 1 mM MgCl2. The samples were then analyzed by SDS–PAGE, followed by western blotting as described above.
Recombinant proteins
The following cDNA constructs were amplified by PCR and subcloned into pGEX4T-1 (Amersham Pharmacia Biotech) for expression of GST fusion recombinant proteins in Escherichia coli: GST–SYN (full-length human β2-syntrophin58, amino acids 1–521), GST–SYN PH1a (PH1a domain of human β2-syntrophin58, amino acids 1–115) and GST–SYN PDZ (PDZ domain of human β2-syntrophin, amino acids 116–195). The constructs for ICA152 cytoplasmic domain (ICA512cyt, amino acids 601–979 of human ICA512) in pET28a (Clontech) and pGEX4T-1 were described previously (Ort et al., 2000). The construct for truncated form of ICA512cyt (amino acids 643–979, 663–979) was also described previously (Ort et al., 2000). All constructs were verified by automated sequence analysis. Recombinant proteins were purified on a glutathione 4B–Sepharose affinity matrix (Amersham Pharmacia Biotech) or on a Ni-charged NTA–agarose matrix (Novagen, Madison, WI).
Pull-down assays and immunoprecipitation
Stimulated Triton X-100-soluble extracts from INS-1 cells were pre-cleaned by incubation for 1 h with 200 µl (50% slurry) of protein A–Sepharose beads (Amersham Pharmacia Biotech) coupled with 10 µg of rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Pre-cleaned lysates were then incubated for 2 h with 10 µl of the ICA512ecto antibodies. Immune complexes were captured by the addition of 100 µl (50% slurry) of protein A–Sepharose beads. After 1 h incubation, and extensive washing in PBS buffer plus 1% Triton X-100, immunoprecipitated proteins were eluted in 2% SDS sample buffer, and then analyzed by western blotting with 1351 anti-syntrophin antibody. For pull-down assays, 5 mg of protein from stimulated Triton X-100-soluble extracts of INS-1 cells were incubated with glutathione– Sepharose beads coupled to GST or GST fused with the cytoplasmic domain of ICA512 (GST–ICA512cyt). The beads were washed with PBS containing 1% Triton X-100. The bound proteins were eluted in 1× SDS sample buffer, and analyzed by western blotting with the pan-syntrophin antibody 1351.
Bio-assay for calpain activity
Calpain activity in INS-1 cells was assessed by fluorescence microscopy using the artificial calpain substrate Boc-LM-CMAC (Molecular Probes, Eugene, OR) (Glading et al., 2000). Boc-LM-CMAC is retained within cells by reacting with intracellular protein thiols through the chloromethylaminocoumarin portion of the molecule, while calpain cleavage results in a blue fluorescent glutathione conjugate. Briefly, cells on glass coverslips were incubated at 37°C for 20 min in the presence of 50 µM Boc-LM-CMAC and then kept in resting conditions or stimulated as described above in the presence or absence of calpain inhibitors. To facilitate the dispersion of the hydrophobic Boc-LM-CMAC, an equal volume of 25% (w/w) pluronic F-127 (Sigma) solution in DMSO was added to the stock solution of Boc-LM-CMAC. The coverslips were then wet mounted on glass slides and observed for chloromethylaminocoumarin fluorescence using a Zeiss Axiophot fluorescent microscope (Germany) with Quantix Photometrics camera. The image exposure settings were identical within each experiment.
In vitro digestion of ICA512 by µ-calpain
Recombinant HisTagICA512cyt and GST–ICA512cyt fusion constructs (∼5–10 µg per reaction) were incubated for different periods of time in 20 mM Tris–HCl pH 7.5, 5 µM CaCl2, 1 mM l-cysteine (Sigma) with ∼0.1 µg of µ-calpain (Calbiochem) at room temperature. To determine the influence of β2-syntrophin binding to ICA512 on µ-calpain cleavage, the reactions were performed with HisTagICA512cyt either in the absence or presence of a molar excess (1:3) of recombinant GST–β2-syntrophin fusion fragments. The reactions were stopped by the addition of an equal volume of 2× SDS sample buffer. Following SDS–PAGE, proteins were either stained with Coomassie Blue R-250 (Bio-Rad) or transferred onto polyvinylidene difluoride (PVDF) membranes for N-terminal microsequencing. N-terminal microsequencing of the proteolytic fragments of ICA512 was performed by the Protein Chemistry Facility at Yale University School of Medicine.
Fractionation of INS-1 cells by sucrose density gradients
Subcellular fractionation of INS-1 cells on continuous sucrose density gradients was performed as described previously (Iezzi et al., 1999). Briefly, ∼1 × 108 cells were homogenized with a ball-bearing cell cracker in 0.32 M sucrose, 4 mM HEPES pH 7.4, 2 mg/ml pepstatin and 0.1 mM PMSF. The postnuclear supernatant was loaded onto a 10 ml continuous sucrose density gradient ranging from 0.4 to 2.0 M sucrose. After centrifugation at 110 000 g for 18 h at 4°C, 0.5 ml fractions were collected from the top of the gradients. The sucrose concentration in the fractions was determined by measuring the refractive index of the solution. Proteins in each fraction were precipitated with a 1.5 vol of ethanol at –20°C overnight, pelleted by centrifugation at 14 000 g for 30 min and then dissolved in 1× SDS sample buffer for separation by PAGE. The distribution of ICA512, syntrophin, utrophin and carboxypeptidase E throughout the gradients was assessed by western blotting.
Insulin secretion assay
INS-1 cells were grown in six-well plates for 3 days. On the fourth day, cells were incubated in resting or stimulating buffers as described above. After 1.5 h, the medium was collected while cells were briefly washed twice with PBS and then incubated overnight with acid ethanol at 0°C for the extraction of intracellular insulin. Supernatants were obtained by centrifuging the harvested cells at 14 000 g for 15 min at 4°C. Insulin content in the media and in the supernatants was measured by radioimmunoassay as described previously (Albano et al., 1972).
Others
The presence of PEST regions in ICA512 was assessed by using the PESTfind software (http://www.at.embnet.org/embnet/tools/bio/PESTfind; Rogers et al., 1986).
Acknowledgments
Acknowledgements
We would like to thank Drs A.Wells and A.Glading for advice on the in vivo calpain activation assay, Dr C.Wollheim for providing us with INS-1 cells, Dr C.Crew for the gift of epoxomicin, Drs D.U.Rabin, J.A.Shapiro, D.Accili and P.Hanson for helpful discussion, and Dr P.De Camilli for his constant encouragement and critical reading of the manuscript. This study was supported by grants from the NIH (DK 54913, DK 45735, DK 53015 and DK 41230) and the American Diabetes Association to M.S. T.O. is the recipient of a Hudson Brown-Coxe Postdoctoral Fellowship.
References
- Albano J.D., Ekins,R.P., Maritz,G. and Turner,R.C. (1972) A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol., 70, 487–509. [DOI] [PubMed] [Google Scholar]
- Altshuler D., Daly,M. and Kruglyak,L. (2000) Guilt by association. Nature Genet., 26, 135–137. [DOI] [PubMed] [Google Scholar]
- Amann K.J., Guo,A.W.-X. and Ervasti,J.M. (1999) Utrophin lacks the rod domain actin binding activity of dystrophin. J. Biol. Chem., 274, 35375–35380. [DOI] [PubMed] [Google Scholar]
- Asfari M., Janjic,D., Meda,P., Li,G., Halban,P.A. and Wollheim,C.B. (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology, 130, 167–178. [DOI] [PubMed] [Google Scholar]
- Aunis D. and Bader,M.F. (1988) The cytoskeleton as a barrier to exocytosis in secretory cells. J. Exp. Biol., 139, 253–266. [DOI] [PubMed] [Google Scholar]
- Aunis D. and Perrin,D. (1984) Chromaffin granule membrane–F-actin interactions and spectrin-like protein of subcellular organelles: a possible relationship. J. Neurochem., 42, 1558–1569. [DOI] [PubMed] [Google Scholar]
- Bader M.F. and Aunis,D. (1983) The 97-kD α-actinin-like protein in chromaffin granule membranes from adrenal medulla: evidence for localization on the cytoplasmic surface and for binding to actin filaments. Neuroscience, 8, 165–181. [DOI] [PubMed] [Google Scholar]
- Baier L.J. et al. (2000) A calpain-10 gene polymorphism is associated with reduced muscle mRNA levels and insulin resistance. J. Clin. Invest., 106, R69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghs S. et al. (2000) βIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J. Cell Biol., 151, 985–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman J.E. et al. (1996) Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell, 84, 757–767. [DOI] [PubMed] [Google Scholar]
- Bruun T.Z., Hoy,M. and Gromada,J. (2000) Scinderin-derived actin-binding peptides inhibit Ca2+- and GTPγS-dependent exocytosis in mouse pancreatic β-cells. Eur. J. Pharmacol., 403, 221–224. [DOI] [PubMed] [Google Scholar]
- Bult A., Zhao,F., Dirkx,R.,Jr, Raghunathan,A., Solimena,M. and Lombroso,P.J. (1997) STEP: a family of brain-enriched PTPs. Alternative splicing produces transmembrane, cytosolic and truncated isoforms. Eur. J. Cell Biol., 72, 337–344. [PubMed] [Google Scholar]
- Butz S., Okamoto,M. and Sudhof,T.C. (1998) A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell, 94, 773–782. [DOI] [PubMed] [Google Scholar]
- Cable H.C., el-Mansoury,A. and Morgan,N.G. (1995) Activation of α-2-adrenoceptors results in an increase in F-actin formation in HIT-T15 pancreatic B-cells. Biochem. J., 307, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T.T., Deacon,H.W., Reczek,D., Bretscher,A. and von Zastrow,M. (1999) A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature, 401, 286–290. [DOI] [PubMed] [Google Scholar]
- Carafoli E. and Molinari,M. (1998) Calpain: a protease in search of a function? Biochem. Biophys. Res. Commun., 247, 193–203. [DOI] [PubMed] [Google Scholar]
- Ceccaldi P.E., Grohovaz,F., Benfenati,F., Chieregatti,E., Greengard,P. and Valtorta,F. (1995) Dephosphorylated synapsin I anchors synaptic vesicles to actin cytoskeleton: an analysis by videomicroscopy. J. Cell Biol., 128, 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H.W. (1995) Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J. Cell Biol., 130, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez J., Park,Y., Zeller,M., Brown,D., Garza,D., Ricordi,C., Hutton,J., Eisenbarth,G.S. and Pugliese,A. (2001) Differential splicing of the IA-2 mRNA in pancreas and lymphoid organs as a permissive genetic mechanism for autoimmunity against the IA-2 type 1 diabetes autoantigen. Diabetes, 50, 895–900. [DOI] [PubMed] [Google Scholar]
- Fernandez-Larrea J., Merlos-Suarez,A., Urena,J.M., Baselga,J. and Arribas,A. (1999) A role for a PDZ protein in the early secretory pathway for the targeting of proTGF-α to the cell surface. Mol. Cell, 3, 423–433. [DOI] [PubMed] [Google Scholar]
- Froehner S.C., Murnane,A.A., Tobler,M., Peng,H.B. and Sealock,R. (1987) A postsynaptic Mr 58,000 (58K) protein concentrated at acetylcholine receptor-rich sites in Torpedo electroplaques and skeletal muscle. J. Cell Biol., 104, 1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S.C., Adams,M.E., Peters,M.F. and Gee,S.H. (1997) Syntrophins: modular adapter proteins at the neuromuscular junction and the sarcolemma. Soc. Gen. Physiol. Ser., 52, 197–207. [PubMed] [Google Scholar]
- Galloway C.J., Dean,G.E., Marsh,M., Rudnick,G. and Mellman,I. (1983) Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc. Natl Acad. Sci. USA, 80, 3334–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee S.H., Madhavan,R., Levinson,S.R., Caldwell,J.H., Sealock,R. and Froehner,S.C. (1998) Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J. Neurosci., 18, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A., Chang,P., Lauffenburger,D.A. and Wells,A. (2000) Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J. Biol. Chem., 275, 2390–2398. [DOI] [PubMed] [Google Scholar]
- Gu M. and Majerus,P.W. (1996) The properties of the protein tyrosine phosphatase PTPMEG. J. Biol. Chem., 271, 27751–27759. [DOI] [PubMed] [Google Scholar]
- Haby C., Larsson,O., Islam,M.S., Aunis,D., Berggren,P.O. and Zwiller,J. (1994) Inhibition of serine/threonine protein phosphatases promotes opening of voltage-activated L-type Ca2+ channels in insulin-secreting cells. Biochem. J., 298, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Yajima,H., Katagiri,T., Ogimoto,M., Arimura,Y., Mitomo,K., Mashima,K., Mizuno,K. and Yakura,H. (1999) Requirement of PEST domain tyrosine phosphatase PEP in B cell antigen receptor-induced growth arrest and apoptosis. Eur. J. Immunol., 29, 887–896. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Cuenda,A., Spillantini,M.G., Thomas,G.M., Buee-Scherrer,V., Cohen,P. and Goedert,M. (1999) Stress-activated protein kinase-3 interacts with the PDZ domain of α1-syntrophin. A mechanism for specific substrate recognition. J. Biol. Chem., 274, 12626–12631. [DOI] [PubMed] [Google Scholar]
- Hermel J.M., Dirkx,R.,Jr and Solimena,M. (1999) Post-translational modifications of ICA512, a receptor tyrosine phosphatase-like protein of secretory granules. Eur. J. Neurosci., 11, 2609–2620. [DOI] [PubMed] [Google Scholar]
- Horikawa Y. et al. (2000) Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nature Genet., 26, 163–175. [DOI] [PubMed] [Google Scholar]
- Iezzi M., Escher,G., Meda,P., Charollais,A., Baldini,G., Darchen,F., Wollheim,C.B. and Regazzi,R. (1999) Subcellular distribution and function of Rab3A, B, C, and D isoforms in insulin-secreting cells. Mol. Endocrinol., 13, 202–212. [DOI] [PubMed] [Google Scholar]
- Johnson G.V. and Guttmann,R.P. (1997) Calpains: intact and active? BioEssays, 19, 1011–1018. [DOI] [PubMed] [Google Scholar]
- Keep N.H., Norwood,F.L.M., Moores,C.A., Winder,S.J. and Kendrick-Jones,S. (1999) The 2.0 Å structure of the second calponin homology domain from the actin-binding region of the dystrophin homologue, utrophin. J. Mol. Biol., 285, 1257–1264. [DOI] [PubMed] [Google Scholar]
- Klenchin V.A. and Martin,T.F. (2000) Priming in exocytosis: attaining fusion-competence after vesicle docking. Biochimie, 82, 399–407. [DOI] [PubMed] [Google Scholar]
- Lan M.S., Lu,J., Goto,Y. and Notkins,A.L. (1994) Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol., 13, 505–514. [DOI] [PubMed] [Google Scholar]
- Lang T., Wacker,I., Wunderlich,I., Rohrbach,A., Giese,G., Soldati,T. and Almers W. (2000) Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys. J., 78, 2863–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Rungger-Brandle,E., Just,I., Jonas,J.C., Aktories,K. and Wollheim,C.B. (1994) Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Mol. Biol. Cell, 5, 1199–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan R. and Jarrett,H.W. (1999) Phosphorylation of dystrophin and α-syntrophin by Ca2+-calmodulin dependent protein kinase II. Biochim. Biophys. Acta, 1434, 260–274. [DOI] [PubMed] [Google Scholar]
- Magistrelli G., Toma,S. and Isacchi,A. (1996) Substitution of two variant residues in the protein tyrosine phosphatase-like PTP35/IA-2 sequence reconstitutes catalytic activity. Biochem. Biophys. Res. Commun., 227, 581–588. [DOI] [PubMed] [Google Scholar]
- Marchand S., Stetzkowski-Marden,F. and Cartaud,J. (2001) Differential targeting of components of the dystrophin complex to the postsynaptic membrane. Eur. J. Neurosci., 13, 221–229. [PubMed] [Google Scholar]
- Moores C.A. and Kendrick-Jones,J. (2000) Biochemical characterisation of the actin-binding properties of utrophin. Cell Motil. Cytoskeleton, 46, 116–128. [DOI] [PubMed] [Google Scholar]
- Mykles D.L. (1998) Intracellular proteinases of invertebrates: calcium-dependent and proteasome/ubiquitin-dependent systems. Int. Rev. Cytol., 184, 157–289. [DOI] [PubMed] [Google Scholar]
- Nakata T. and Hirokawa,N. (1992) Organization of cortical cytoskeleton of cultured chromaffin cells and involvement in secretion as revealed by quick-freeze, deep-etching, and double-label immmunoelectron microscopy. J. Neurosci., 12, 2186–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.H., Paul,S., Xu,Y., Gurd,J.W. and Lombroso,P,J. (1999) Calcium-dependent cleavage of striatal enriched tyrosine phosphatase (STEP). J. Neurochem., 73, 1995–2001. [PubMed] [Google Scholar]
- Orci L., Gabbay,K.H. and Malaisse,W.J. (1972) Pancreatic β-cell web: its possible role in insulin secretion. Science, 175, 1128–1130. [DOI] [PubMed] [Google Scholar]
- Ort T., Maksimova,E., Dirkx,R., Kachinsky,A., Berghs,S., Froehner,S. and Solimena,M. (2000). The receptor tyrosine phosphatase like-protein ICA512 binds the PDZ domains of β2-syntrophin and nNOS in pancreatic β-cells. Eur. J. Cell Biol., 79, 621–630. [DOI] [PubMed] [Google Scholar]
- Peters M.F., Kramarcy,N.R., Sealock,R. and Froehner,S.C. (1994) β2-syntrophin: localization at the neuromuscular junction in skeletal muscle. Neuroreport, 5, 1577–1580. [PubMed] [Google Scholar]
- Peters M.F., Adams,M.E. and Froehner,S.C. (1997) Differential association of syntrophin pairs with the dystrophin complex. J. Cell Biol., 138, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin D.U., Pleasic,S.M., Shapiro,J.A., Yoo-Warren,H., Oles,J., Hicks,J.M., Goldstein,D.E. and Rae,P.M. (1994) Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J. Immunol., 152, 3183–3188. [PubMed] [Google Scholar]
- Rechsteiner M. (1990) PEST sequences are signals for rapid intracellular proteolysis. Semin. Cell Biol., 1, 433–440. [PubMed] [Google Scholar]
- Rogers S., Wells,R. and Rechsteiner,M. (1986) Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science, 234, 364–368. [DOI] [PubMed] [Google Scholar]
- Rybakaova I.N., Amann,K.J. and Ervasti,J.M. (1996) A new model for the interaction of dystrophin with F-actin. J. Cell Biol., 135, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Mariot,P., Detimary,P., Gilon,P. and Henquin,J.C. (1998) Okadaic acid-induced decrease in the magnitude and efficacy of the Ca2+ signal in pancreatic β cells and inhibition of insulin secretion. Br. J. Pharmacol., 123, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J., Hoffmuller,U., Krause,G., Ashurst,J., Macias,M.J., Schmieder,P., Schneider-Mergener,J. and Oschkinat,H. (1998) Specific interactions between the syntrophin PDZ domain and voltage-gated sodium channels. Nature Struct. Biol., 5, 19–24. [DOI] [PubMed] [Google Scholar]
- Seissler J., Nguyen,T.B., Aust,G., Steinbrenner,H. and Scherbaum,W.A. (2000) Regulation of the diabetes-associated autoantigen IA-2 in INS-1 pancreatic β-cells. Diabetes, 49, 1137–1141. [DOI] [PubMed] [Google Scholar]
- Senda T., Ban,T. and Fujita,H. (1988) Scanning electron microscopy of cytoplasmic filaments in rat anterior pituitary cells. Arch. Histol. Cytol., 51, 371–388. [DOI] [PubMed] [Google Scholar]
- Senda T., Fujita,H., Ban,T., Zhong,C., Ishimura,K., Kanda,K. and Sobue,K. (1989) Ultrastructural and immunocytochemical studies on the cytoskeleton in the anterior pituitary of rats, with special regard to the relationship between actin filaments and secretory granules. Cell Tissue Res., 258, 25–30. [DOI] [PubMed] [Google Scholar]
- Shumway S.D., Maki,M. and Miyamoto,S. (1999) The PEST domain of IκBα is necessary and sufficient for in vitro degradation by µ-calpain. J. Biol. Chem., 274, 30874–30881. [DOI] [PubMed] [Google Scholar]
- Shushan E.B., Cerasi,E. and Melloul,D. (1999) Regulation of the insulin gene by glucose: stimulation of trans-activation potency of human PDX-1 N-terminal domain. DNA Cell Biol., 18, 471–479. [DOI] [PubMed] [Google Scholar]
- Sin N., Kim,K.B., Elofsson,M., Meng,L., Auth,H., Kwok,B.H. and Crews,C.M. (1999) Total synthesis of the potent proteasome inhibitor epoxomicin: a useful tool for understanding proteasome biology. Bioorg. Med. Chem. Lett., 9, 2283–2288. [DOI] [PubMed] [Google Scholar]
- Solimena M., Dirkx,R.,Jr, Hermel,J.M., Pleasic-Williams,S., Shapiro,J.A., Caron,L. and Rabin,D.U. (1996) ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J., 15, 2102–2114. [PMC free article] [PubMed] [Google Scholar]
- Sontag J.M., Aunis,D. and Bader,M.F. (1988) Peripheral actin filaments control calcium-mediated catecholamine release from streptolysin-O-permeabilized chromaffin cells. Eur. J. Cell Biol., 46, 316–326. [PubMed] [Google Scholar]
- Sorimachi H., Ishiura,S. and Suzuki,K. (1997) Structure and physiological function of calpains. Biochem. J., 328, 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U., Ebitsch,S., Gorczyca,M., Koh,Y.H., Hough,C.D., Woods,D., Gundelfinger,E.D. and Budnik,V. (2000) Synaptic targeting and localization of discs-large is a stepwise process controlled by different domains of the protein. Curr. Biol., 10, 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaro J.M. (1990) The 1989 Upjohn Award lecture. Cellular and molecular mechanisms in hormone and neurotransmitter secretion. Can. J. Physiol. Pharmacol., 68, 1–16. [DOI] [PubMed] [Google Scholar]
- Trowbridge I.S., Collawn,J.F. and Hopkins,C.R. (1993) Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol., 9, 129–161. [DOI] [PubMed] [Google Scholar]
- Valentijn K., Valentijn,J.A. and Jamieson,J.D. (1999) Role of actin in regulated exocytosis and compensatory membrane retrieval: insights from an old acquaintance. Biochem. Biophys. Res. Commun., 266, 652–661. [DOI] [PubMed] [Google Scholar]
- Valentijn J.A., Valentijn,K., Pastore,L.M. and Jamieson,J.D. (2000) Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc. Natl Acad. Sci. USA, 97, 1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale M.L., Rodriguez Del Castillo,A., Tchakarov,L. and Trifaro,J.M. (1991) Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J. Cell Biol., 113, 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.R., Ludowyke,R.I. and Biden,T.J. (2001) A redistribution of actin and myosin IIA accompanies Ca2+-dependent insulin secretion. FEBS Lett., 492, 101–106. [DOI] [PubMed] [Google Scholar]
- Zimmer W.E., Zhao,Y., Sikorski,A.F., Critz,S.D., Sangerman.J., Elferink,L.A., Xu,X.S. and Goodman,S.R. (2000) The domain of brain β-spectrin responsible for synaptic vesicle association is essential for synaptic transmission. Brain Res., 881, 18–27. [DOI] [PubMed] [Google Scholar]