Abstract
Members of the innexin protein family are structural components of invertebrate gap junctions and are analogous to vertebrate connexins. Here we investigate two Drosophila innexin genes, Dm-inx2 and Dm-inx3 and show that they are expressed in overlapping domains throughout embryogenesis, most notably in epidermal cells bordering each segment. We also explore the gap-junction–forming capabilities of the encoded proteins. In paired Xenopus oocytes, the injection of Dm-inx2 mRNA results in the formation of voltage-sensitive channels in only ∼ 40% of cell pairs. In contrast, Dm-Inx3 never forms channels. Crucially, when both mRNAs are coexpressed, functional channels are formed reliably, and the electrophysiological properties of these channels distinguish them from those formed by Dm-Inx2 alone. We relate these in vitro data to in vivo studies. Ectopic expression of Dm-inx2 in vivo has limited effects on the viability of Drosophila, and animals ectopically expressing Dm-inx3 are unaffected. However, ectopic expression of both transcripts together severely reduces viability, presumably because of the formation of inappropriate gap junctions. We conclude that Dm-Inx2 and Dm-Inx3, which are expressed in overlapping domains during embryogenesis, can form oligomeric gap-junction channels.
INTRODUCTION
Gap-junction channels allow small molecules and ions to pass between cells, thus mediating processes such as electrical coupling, maintenance of homeostasis, and cell–cell signaling (reviewed in Bruzzone et al., 1996). In vertebrates, these channels are composed of proteins called connexins. Six connexins associate to form a hexameric ring structure (connexon) in the plasma membrane that intercellularly docks with a corresponding connexon in an adjacent cell to form a continuous channel linking the cytoplasms (Yeager and Nicholson, 1996; Unger et al., 1999). Despite the fact that gap junctions are also found throughout invertebrate tissues, no connexins have been identified in the Caenorhabditis elegans or Drosophila genomes, for which near complete sequence data are available (Wilson, 1999; Flybase website: http://fly.ebi.ac.uk:7081/).
It has recently been shown that invertebrate gap-junction channels are composed of proteins now named innexins (Phelan et al., 1998a,b; Landesman et al., 1999; reviewed in Phelan, 2000). These bear no sequence homology to the connexins but possess an identical predicted topology of four transmembrane domains and intracellular N- and C-termini (Crompton et al., 1995; Starich et al., 1996).
Innexin genes have been identified in several invertebrates. C. elegans has at least 24 innexins (Barnes and Hekimi, 1997), but few of these genes have been investigated in detail. Mutations in the unc-7 and unc-9 genes result in uncoordinated phenotypes (Starich et al., 1993, 1996; Barnes and Hekimi, 1997), and in eat-5 mutants electrical and dye coupling are abolished between some pharyngeal muscle cells, leading to feeding defects (Avery, 1993; Starich et al., 1996). In Drosophila, five innexin gene loci have been described, and products of two of these loci, shaking-B(lethal), shaking-B(neural) (previously known as passover [Krishnan et al., 1993]), shaking-B(N2)-(N4) (Zhang et al., 1999), and ogre, have been characterized. shaking-B(lethal) mutations result in the animal's death after an extended first larval instar (Crompton et al., 1995). Mutations such as shak-B2 that disrupt the other products of the locus result in the loss of electrical synapses (essentially gap junctions, Bennett, 1997) in the giant fiber (Phelan et al., 1996; Blagburn et al., 1999) and haltere neural systems (Trimarchi and Murphey, 1997) and the abolition of dye coupling between some muscles during embryogenesis (Todman et al., 1999). A mutation in ogre leads to a reduced number of neurons in the optic lobes and an abnormal electroretinogram (Lipshitz and Kankel, 1985; Watanabe and Kankel, 1990). Three additional Drosophila innexin genes have been identified recently (Curtin et al., 1999) but mutations are not yet available.
These mutant phenotypes are consistent with the involvement of innexin genes in gap-junction function. However, the most compelling evidence that innexins are structural gap-junction proteins, and not merely accessory factors, is that both Shaking-B(lethal) (Phelan et al., 1998a) and one of the C. elegans innexins, Ce-Inx-3 (Landesman et al., 1999) can form functional channels in paired Xenopus oocytes, a heterologous system commonly used to model connexin function.
Shaking-B(neural) (Phelan et al., 1998a), which is partially identical to Shaking-B(lethal), and Eat-5 (Landesman et al., 1999) fail to form homotypic channels (composed of just one innexin type) in the Xenopus oocyte system. This raises the possibility that innexins, like connexins, form mixed junctions with a different type of hemi-channel in each membrane (heterotypic channels) (Swenson et al., 1989; Werner et al., 1989; Barrio et al., 1991; reviewed in Bruzzone et al., 1996) or with more than one type of innexin in each hemi-channel (heteromeric channels) (Stauffer, 1995; Jiang and Goodenough, 1996; Lee and Rhee, 1998; Ebihara et al., 1999; He et al., 1999). In view of the large number of innexins identified and the overlapping expression patterns of those that have been investigated so far (Crompton et al., 1995; Curtin et al., 1999), the occurrence of mixed channels seems likely. Here we show that the innexin, Dm-inx2 (prp33, Curtin et al., 1999), and the newly identified family member, Dm-inx3, exhibit overlapping expression domains throughout Drosophila embryogenesis. We provide electrophysiological evidence that the encoded proteins interact to form functional channels in paired Xenopus oocytes and support this with in vivo data from ectopic expression studies in Drosophila.
MATERIALS AND METHODS
cDNA and Genomic Clone Characterization
I.M.A.G.E. Consortium (Lawrence Livermore National Laboratory, Livermore, CA) cDNA clones, LD11362 and LD17559 (Lennon et al., 1996), were identified in the Berkeley Drosophila Genome Project (BDGP)/Howard Hughes Medical Institute EST project databases and obtained from Genome Systems (St. Louis, MO). We have named the corresponding genes Dm-inx2 and Dm-inx3, respectively (hereafter referred to as inx2 and inx3). To obtain genomic sequence, gridded genomic P1 clone filters (Genome Systems) and gridded genomic cosmid clone filters (Human Genome Mapping Project Resources Centre, Hinxton, Cambridge, UK) were screened using standard techniques. A cosmid clone, Dros17F19 (HGMP Resource Centre), containing the inx2 gene and two P1 clones, DS03216 (Hartl et al., 1994) and DS04968 (BDGP), containing inx3 were identified. Sequencing was performed directly on these genomic clones and on fragments subcloned into pBluescript II KS+ (Stratagene, La Jolla, CA) using primers designed to the corresponding cDNAs and to pBluescript. DNA was prepared using QIAprep spin columns (Qiagen, Crawley, West Sussex, UK) and sequencing was either performed on site using an Applied Biosystems 370A DNA sequencer or off site by MWG-Biotech UK (Milton Keynes, UK) using the LI-COR 4200 system. Sequence analysis was performed using LaserGene software (DNAstar, Madison, WI), and the multiple sequence alignment was assembled using CLUSTAL X (Thompson et al., 1997) and decorated using SeqVu 1.1 (Garvan Institute of Medical Research, Sydney, Australia).
Chromosome In Situ Hybridization
In situ hybridization to salivary gland polytene chromosomes was performed according to the method of Laverty et al. (BDGP, detailed at: http://www.fruitfly.org/methods/cytogenetics.html) using digoxigenin-labeled DNA probes and a horseradish peroxidase–conjugated antidigoxigenin antibody for probe detection (Roche Diagnostics, Lewes, East Sussex, UK).
mRNA In Situ Hybridization to Embryos
In situ hybridization, using digoxigenin-labeled RNA probes (Roche Diagnostics), was carried out as described by Lehmann and Tautz (1994) except that Proteinase K treatment was with 25 μg/ml Proteinase K for 3 min. In the case of LD11362 (inx2), downstream AT-rich regions were removed before probe synthesis to reduce nonspecific background staining. A 1.5-kb EcoRI fragment of LD11362, containing the coding sequence for Inx2 and part of the noncoding upstream and downstream regions, was subcloned into the EcoRI site of pBluescript II KS+ (Stratagene). LD17559 (a pBluescript II SK+ clone), which encodes Inx3, was used for probe synthesis without modification.
Transcription of mRNAs
inx2 and inx3 coding regions were cloned into the SPJC2L vector (gifted by H. Woodland, Warwick, UK) between upstream and downstream Xenopus β globin gene sequences to give inx2-SPJC2L and inx3-SPJC2L. These plasmids were linearized using XhoI and NotI, respectively, and transcribed in the presence of m7G(5′)ppp(5′)G (Roche Diagnostics) from the SP6 promoter. The resulting capped mRNAs were stored in aliquots at −20°C and thawed only once before use.
Translation of inx2 and inx3 in Xenopus Oocytes
Xenopus oocytes were injected with inx2 and/or inx3 mRNAs (18.4 nl of 0.5 ng/nl) and l-methionine [35S] (0.23 μCi, ICN) using a Drummond Nanoject (Laser Laboratory Systems, Southampton, UK). The cells were incubated for 24 h at 20°C, and membrane extracts were prepared using the “sucrose cushion” method (Colman, 1984). Protein samples were solubilized in SDS gel-loading buffer (2.5× stock: 312.5 mM Trizma base pH 6.8, 5% SDS, 25% glycerol, 12.5% β-mercaptoethanol, 2.5 mM dithiothreitol, 0.1% bromophenol blue) at room temperature for 1 h and then at 80°C for 10 min before separating on a 12.5% SDS-polyacrylamide gel alongside a prestained, broad range protein marker (New England Biolabs, Beverly, MA). After washing in 30% methanol/3% glycerol for 30 min, the gels were heat-dried under vacuum and exposed to Super RX medical x-ray film (Fuji, Tokyo, Japan) for up to a week. Densitometry readings were obtained from scanned autoradiographs imported as TIFF files into ImageMaster 1.10 (Pharmacia, Uppsala, Sweden).
Expression in the Paired Xenopus Oocyte System
Methods for oocyte isolation, injection and pairing were essentially as previously described (Swenson et al., 1989; Phelan et al., 1998a). Cells were preinjected with 20 ng Cx38 DNA antisense oligonucleotides (5′-CTGACTGCTCGTCTGTCCACACAG-3′), 24 h before the injection of 2–20 ng innexin mRNA in 18.4 nl H2O or H2O only (Barrio et al., 1991). After pairing the oocytes and incubating in Barth's saline at 20°C for 24–48 h, each oocyte of a pair was impaled with two 1–5 mΩ borosilicate glass microelectrodes (filled with 3 M KCl, 10 mM HEPES, 10 mM EGTA; pH 7.5) and recorded using a double voltage-clamp procedure (Spray et al., 1981). Junctional conductance (gj) and channel sensitivity to transjunctional voltage (Vj) and inside-outside voltage (Vi-o) were determined using methods described previously (Verselis et al., 1991; Phelan et al., 1998a). Data were analyzed and exponentials fitted using Axograph 4 software.
Drosophila Transformation
Flies were raised on standard Drosophila medium at 25°C. To prepare constructs for transformation, a 1.5-kb EcoRI fragment of LD11362 (inx2) and the complete LD17559 cDNA (inx3) were cloned into pUAST (Brand and Perrimon, 1993) giving UAS-inx2 and UAS-inx3 constructs, respectively. Each was purified twice over a CsCl gradient before coinjection with pπ25.7wc (a transposase source; Karess and Rubin, 1984) into yellow white (y w) embryos at concentrations of 400 and 100 μg/ml, respectively. Standard methods for P-element–mediated transformation were used (Spradling and Rubin, 1982). Multiple lines were obtained in a y w background, and the chromosomal positions of the insertions were mapped by standard genetic methods.
Ectopic Expression Studies
The UAS/GAL4 system (Brand and Perrimon, 1993) was used to ectopically express innexins in Drosophila, and the hatch and eclosion rates of these animals were determined. 24B-GAL4 was obtained from A. Brand (Cambridge, UK) and armadillo-GAL4 from the Bloomington Stock Center (stock number 1561; donated to the Center by J.-P. Vincent, National Institute for Medical Research, London, UK). Stocks homozygous for both UAS-inx2 and UAS-inx3 were constructed using standard genetic techniques. Males from these stocks (UAS-inx2;UAS-inx3) and the original UAS-inx2 and UAS-inx3 lines were each crossed to 24B-GAL4 or arm-GAL4 virgin females, and the embryos were collected in batches of 50, gridded onto media, and transferred to vials. These embryos were kept in humidified conditions at 25°C. Hatch rates were assessed after 2 days, and the numbers of adults that eclosed also were counted.
Innexin Nomenclature
cDNA clones LD11362 and LD17559 were identified in the BDGP EST database on the basis of their homology to other innexins. The corresponding genes were named Dm-inx2 and Dm-inx3, respectively, (shortened to inx2 and inx3 for convenience in this article). The two-letter prefix identifies the organism, and inx denotes innexin.
Ganfornina et al. (1999) have recently presented a Schistocerca americana protein, Sa-Inx(1), which is an orthologue of Drosophila Ogre. In light of this work, Dm-Inx1 must now be considered as an alternative nomenclature for Ogre. Additionally, Ganfornina et al. (1999) have identified another grasshopper innexin, Sa-Inx(2). Subsequently, Curtin et al. (1999) isolated a gene encoding its Drosophila orthologue (prp33). However, a partial sequence corresponding to this Drosophila gene had already been deposited in the BDGP EST database, and it was from here that we obtained the cDNA clone for inx2 described in this article. inx3, which was also identified in the BDGP database, has not been reported previously and, as yet, has no known orthologues in other organisms.
RESULTS
Molecular Characterization of inx2 and inx3
Clones LD11362 (inx2) and LD17559 (inx3) were sequenced, and the chromosomal positions of the corresponding genes were mapped to 6E4–5 and 98E4–6, respectively, by in situ hybridization to polytene chromosomes. P1 clones containing inx3, DS03216 and DS04968, have also been mapped to 98E3–6 and 98E4–6, respectively, by the BDGP. We have confirmed that the predicted polypeptide encoded by inx2 is 367 amino acids in length (Prp33, Curtin et al., 1999) and 42.49 kDa in mass with an isoelectric point (pI) of 6.096. inx3, a newly identified innexin, is predicted to encode a polypeptide of 395 amino acids with a mass of 45.36 kDa and a pI of 8.4.
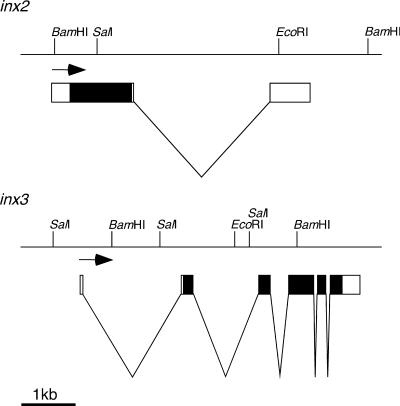
To obtain data on both the sequence and genomic organization of the inx2 and inx3 genes, gridded genomic library filters were screened using the cDNAs as probes. A cosmid clone covering inx2 and two P1 clones covering the inx3 region were identified and partially mapped (Figure 1). inx2 includes one intron that lies downstream of the coding region. It is likely that there are no other splice forms resulting in additional proteins from the inx2 locus because the intron is outside the coding region. However, the cDNA isolated by Curtin et al. (1999) that corresponds to inx2 is unspliced, and the sequence of this transcript continues into the intron for 240 bases before terminating. The polypeptides encoded by both cDNAs are identical, so this differential splicing could only have an effect on the regulation or localization of the transcripts. inx3 possesses five introns, four of which interrupt the open reading frame. No other splice forms of inx3 were detected in 12- to 24-h embryonic cDNA libraries (N. Brown, Wellcome/CRC Institute, Cambridge, UK) using PCR and primers to either end of the existing cDNA clone (Todman, unpublished data).
Figure 1.
The genomic organizations of the inx2 and inx3 genes. inx2 and inx3 map to chromosome positions 6E4–5 on the X chromosome and 98E4–6 on chromosome 3, respectively. EcoRI, BamHI, and SalI restriction sites are shown, and the direction of transcription is given by the arrows. Genomic DNA is indicated by horizontal lines, coding regions by filled boxes, noncoding sequences by open boxes, and intronic regions by a “v.”
The polypeptide sequences for Inx2 and Inx3 are 42% identical and are homologous to other innexin sequences in Drosophila melanogaster (Shaking-B, Ogre), Caenorhabditis elegans (Eat 5, Unc 7, Unc 9), Schistocerca americana (Sa-Inx(1), Sa-Inx(2)), and Bombyx mori (Bm-Inx2). Strongest homology is seen in the transmembrane domains and around the conserved cysteine residues in the extracellular loops (Figure 2). Inx2 is 76% identical to Sa-Inx(2) (Ganfornina et al., 1999). A third orthologue is found in the silk moth, Bombyx mori (Bm-Inx2), for which only a partial N-terminal sequence is available in the GenBank nucleotide sequence database. Inx2 is 84% identical to Bm-Inx2 and 81% identical to Sa-Inx(2) over this N-terminal region, and Sa-Inx(2) and Bm-Inx2 show 86% identity over the same region.
Figure 2.
Multiple polypeptide sequence alignment of some innexins. From top to bottom; Drosophila Innexin2 (Dm-Inx2), Schistocerca americana Innexin2 (Sa-Inx(2)), Bombyx mori Innexin2 (Bm-Inx2, only a partial sequence is available), Drosophila Innexin3 (Dm-Inx3) and Shaking-B(lethal). Predicted transmembrane regions and conserved cysteines are boxed. Amino acids that are identical to those in Dm-Inx2 are shaded. These sequence data are available from GenBank under the following accession numbers: Dm-Inx2, AF172257; Sa-Inx(2), AF115854; Bm-Inx2, AU003649; Dm-Inx3, AF172258; Shaking-B(lethal), S78495.
Embryonic Expression of inx2 and inx3
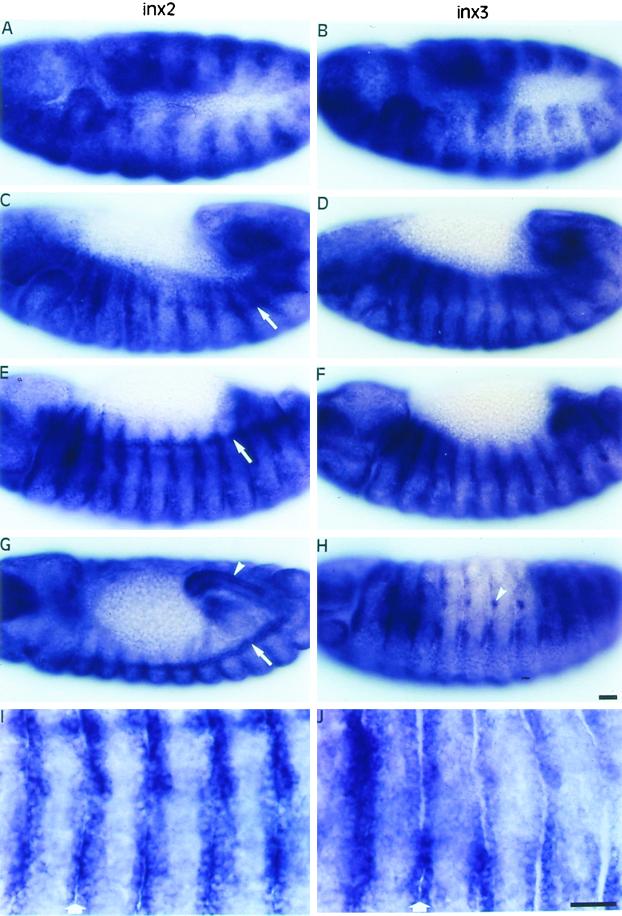
In general, inx2 and inx3 exhibit very similar expression patterns in the embryo, although there are some differences. In the blastoderm, inx3 mRNA is asymmetrically localized to anterior and ventral regions, whereas inx2 transcripts are evenly distributed (our unpublished results). Expression of both transcripts was detected throughout the germ band during early gastrulation until stage 10, when some modulation in the pattern began to be detectable. A segmentally reiterated pattern of expression emerges during germ band extension (stage 11, Figure 3, A and B). The pattern is refined further as the germ band retracts (stage 12, Figure 3, C and D) until only one or two rows of cells at each side of the segment borders express both transcripts strongly (stage 13, Figure 3, E, F, I, and J). Likewise, inx2 and inx3 epidermal expression in the head and terminal regions becomes restricted to the segment borders from stage 12 onward as the germ band retracts.
Figure 3.
Distribution of inx2 (A, C, E, G, I) and inx3 (B, D, F, H, J) mRNAs during embryogenesis. Anterior is to the left and dorsal is up unless otherwise stated. In germ band extended embryos (stage 11), inx2 (A) and inx3 (B) are expressed in broad segmentally repeated bands. As the germ band retracts (stage 12), expression becomes localized to the segment borders (inx2, C; inx3, D), where it is maintained through stage 13 (inx2, E; inx3, F). In stage 14 embryos, both transcripts were detected in the foregut (out of focus) and hindgut (arrowhead, only shown for inx2, dorsal view, G) and in lateral cell clusters around the spiracular openings (arrowhead, only shown for inx3, H). Unlike inx3, inx2 is expressed in the tracheal system dorsal trunk and the cell placodes that give rise to this structure (arrows, C, E, G). (I) and (J) show regions of epidermis, comprising 5 segments, at higher magnification in stage 13 embryos. The expression of inx2 (I) and inx3 (J) is clearly highest around the segment borders (arrows). Bars, 20 μm.
Both transcripts were also detected in the hindgut and possibly the foregut with little or no expression in the midgut. This expression is strong from stage 11 onward and is particularly apparent in the hindgut of stage 14 embryos (Figure 3G, arrowhead). Expression was also detected in a few segmentally repeated cells around the spiracular openings to the immature tracheal system (stage 14, Figure 3H, arrowhead).
The most noticeable difference between inx2 and inx3 expression was that only inx2 was detected in the dorsal trunk (the main anterior-posterior tracheal branch) and in the precursor cells of this structure from stage 11 onward (Figure 3, C, E, and G, arrows). inx2, but not inx3, is expressed strongly in the segmentally repeated tracheal placodes once they become internalized (stage 11). Tracheal expression was most clearly seen as cells of the placodes in adjacent segments migrate to meet at stage 13 and form a continuous tube (stage 14, Figure 3G, arrow).
Translation of Inx2 and Inx3 in Xenopus Oocytes
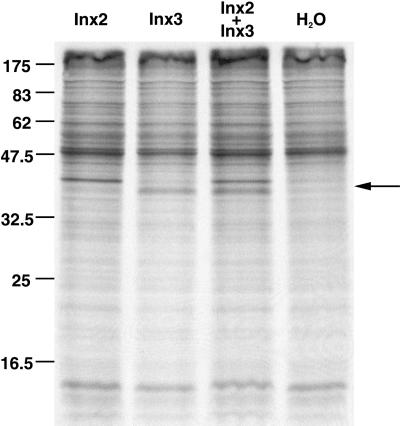
Membrane extracts prepared from Xenopus oocytes injected with inx2 and/or inx3 mRNA(s) and a radiolabeled methionine source were separated on SDS-polyacrylamide gels. Protein bands with apparent sizes of ∼ 41 and 39 kDa can be seen in the Inx2 and Inx3 lanes, respectively (Figure 4, Inx2 and Inx3 lanes), both protein bands are present in the Inx2+Inx3 membrane extract, (Figure 4, Inx2+Inx3), and neither are detected in the membrane preparations from oocytes injected with water (Figure 4, H2O). The apparent protein sizes are smaller than expected, considering the predicted sizes of the Inx2 (42.49 kDa) and Inx3 (45.36 kDa) polypeptides (deduced from sequence data). Additionally, Inx3, which was predicted to be larger than Inx2, gives an apparent band size that is slightly below that of Inx2. The difference, however, is unlikely to be significant because other innexins run anomalously on SDS-polyacrylamide gels (Phelan et al., 1998a).
Figure 4.
Translation of Inx2 and Inx3 in Xenopus oocytes. Ten nanograms inx2 mRNA, 10 ng inx3 mRNA, or 5 ng each of inx2 and inx3 mRNAs were translated in Xenopus oocytes. Membrane preparations (one oocyte equivalent loaded) were separated on SDS-polyacrylamide gels. Lanes were loaded as follows from left to right: Inx2; Inx3; Inx2+Inx3; H2O (no mRNA added). The positions of the protein size marker bands are given on the left, and protein bands unique to the Inx2, Inx3 and Inx2+Inx3 lanes are indicated by the arrow on the right.
When densitometry measurements were taken and standardized to take loading into account, Inx2 bands had optical densities ∼ 1.5 times greater than those for Inx3 bands, both when the proteins were expressed singly (Figure 4, Inx2 and Inx3 lanes) and when expressed together (Figure 4, Inx2+Inx3). Because there are half the number of methionines in Inx2 than in Inx3 (8 and 16, respectively), the translation and/or membrane insertion of Inx3 is less efficient by a factor of three.
Expression in Paired Xenopus Oocytes
We expressed the proteins encoded by inx2 and inx3 mRNAs in paired Xenopus oocytes to determine whether they are sufficient to form intercellular channels. Although in this series of experiments we never detected coupling in water-injected oocyte pairs, as a precaution, the oocytes were routinely pretreated with Cx38 antisense oligonucleotides to deplete any endogenous channels (Barrio et al., 1991; Phelan et al., 1998a).
inx3 mRNA, at amounts up to 20 ng, never induced channel formation in oocytes; the average junctional conductance (gj) in inx3-injected cell pairs (0.02 ± 0.02 μS, for 24 cell pairs), was not significantly different from gj in H2O-injected control pairs (0.00 ± 0.05 μS, for 19 cell pairs, H2O; Table 1). By contrast, inx2 mRNA induced measurable conductances in some cell pairs. However, even at amounts of 10 ng mRNA, only 44% were coupled.
Table 1.
Junctional conductances in oocyte pairs injected with inx2 or inx2+inx3 mRNAs
| RNA injected | Number coupled/totala | Conductance (μS)b |
|---|---|---|
| H2O (—) | 0 /19 (0) | 0.00 ± 0.05 (19) |
| inx2 (10 ng) | 17 /39 (44) | 4.62 ± 1.05 (17) |
| inx2+inx3 (2+2 ng) and (5+5 ng) | 76 /78 (97) | 17.99 ± 1.58 (76) |
mRNAs were injected at the amounts indicated (ng); cells were paired and recorded electrophysiologically to determine intercellular coupling. In the inx2+inx3 experiments, both mRNAs were injected into each cell. Junctional conductance (gj) was calculated from double voltage-clamp recordings as described in Figure 5. Values are the maximum gj, recorded at transjunctional voltage steps of 10–20 mV. Note that the mean gj value for inx2 and inx2+inx3 experiments does not include noncoupled cell pairs.
Values in parentheses are percentages.
Values are means ± SEM for n cell pairs, in parentheses.
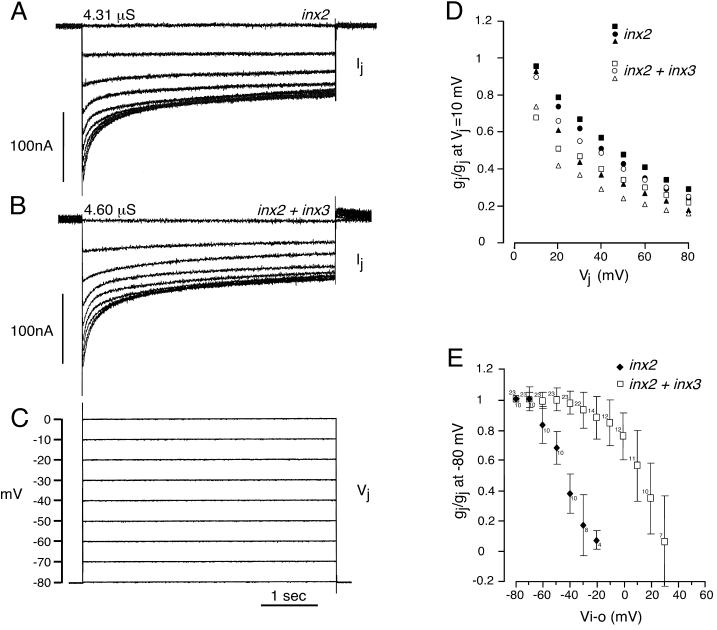
Because the expression domains of inx2 and inx3 were found to be partially overlapping in the Drosophila embryo (Figure 3), we were interested to determine whether the encoded proteins might interact to form functional channels in the oocyte system. To investigate the possibility that they form heterotypic channels, we paired cells expressing inx2 with cells expressing inx3; such pairs did not develop conductances (inx2/inx3, mean gj = 0.01 ± 0.02 μS, for 16 cell pairs). In contrast, coexpression of inx2 and inx3 in both oocytes of pairs at amounts that failed to induce (inx3), or unreliably induced (inx2), conductances when expressed alone, resulted in the formation of intercellular channels (inx2+inx3, Figure 5, A, B, and D and Table 1). When 2 ng of each mRNA was injected, channels were formed in essentially all cell pairs (97.4% of cell pairs electrically coupled) and the magnitude of gj (17.57 ± 2.12 μS) increased significantly when compared with cells expressing inx2 alone (mean gj = 4.62 ± 1.05 μS; Table 1). Increasing the amounts of each mRNA from 2 to 5 ng did not significantly increase the magnitude of gj, possibly because the translational machinery was saturated (inx2+inx3, 5 ng of each, mean gj = 18.54 ± 2.40 μS). These data suggest that Inx3 in some way either promotes channel formation by Inx2 or directly interacts with Inx2 in the hemi-channel to assemble heteromeric junctions. In an attempt to distinguish between these possibilities we compared the electrical properties of the homotypic (Inx2) and presumptive heteromeric (Inx2+Inx3) channels.
Figure 5.
Electrical properties of Inx2 and Inx2+Inx3 channels. The intercellular channels formed when inx2 was expressed alone, and when inx2 was coexpressed with inx3 were differentially sensitive to applied voltage. Cells were individually microinjected with the mRNAs alone or in combination, paired, and recorded 1 day later using the double voltage-clamp technique. (A–C) Recordings from cell pairs injected with inx2 mRNA only (10 ng, A) and inx2+inx3 mRNAs (2 ng each, B). Both oocytes of a pair were initially clamped to a holding potential of −80 mV. Transjunctional voltages (Vj) were generated by depolarizing one cell of the pair in 10-mV steps (C) and the current (Ij) required to maintain its paired neighbor at the holding potential was simultaneously recorded (A, B). For the pairs shown, the maximum junctional conductances (Ij/Vj, measured at the beginning of the 10-mV step) were 4.31 μS (Inx2) and 4.60 μS (Inx2+Inx3). For the inx2+inx3-injected cell pair, this was slightly greater than the steady-state gj, because the channels were strongly voltage-dependent and began to close at Vj steps of 10 mV. Vj-dependent closure was very obvious for the second (20 mV) and subsequent, larger voltage steps for both inx2- and inx2+inx3-injected cell pairs (A, B). (D) gj/Vj relation for the inx2 and inx2+inx3 pairs shown in (A) and (B) (inx2+inx3, □; inx2, ▪) and additional cell pairs with conductances up to 6.04 μS. Data were normalized to the maximum instantaneous gj at a Vj of 10 mV. Cell pairs injected with inx2 and inx3 tended to show slightly more voltage sensitivity than pairs injected with inx2 only, particularly for smaller Vj steps. (E) gj/Vi-o relation. Both oocytes of a pair were stepped equally and simultaneously over a range of negative and positive membrane potentials (Vi-o). At each potential, a 10-mV depolarizing pulse was delivered to one cell to measure gj; values (±SD for numbers of pairs indicated) were normalized to gj at −80 mV. Data shown are from pairs with conductances in the range 0.5–20 μS. Restricting the analysis to pairs with gjs of 5 μS or below did not change these curves significantly. Intercellular conductances in oocyte pairs expressing inx2 only (filled symbols) were significantly more sensitive to Vi-o than gjs in pairs expressing both innexins (open symbols).
The sensitivity of the intercellular conductance to transjunctional voltage (Vj, the voltage difference between the two cells) and transmembrane voltage (Vi-o, the voltage difference between the cytoplasm and the extracellular space) was examined in cell pairs injected with inx2 only or with inx2+inx3 mRNAs. We measured Vj by depolarizing one cell of the pair from a holding potential of −80 mV, and therefore the observed Vj sensitivity may have included a component of Vi-o sensitivity. Both inx2 and inx2+inx3–injected cell pairs showed similar sensitivity to Vj when depolarizing voltage steps were applied (Figure 5, A and B, shows typical recordings from inx2 and inx2+inx3 cell pairs). In both cases, the near steady-state gj (measured at the end of the step change) decreased as Vj increased from 10 to 80 mV (Figure 5, A and B). When larger Vj steps were imposed, the curves of declining Ij with Vj (Figure 5, A and B) did not follow single exponentials, suggesting that the transition is not simply from one state to another but involves an intermediate state(s). At Vj steps larger that 30 mV, time constants for the fitted bi- and tri-exponentials at each Vj were very similar for both channels. However, at 10- and 20-mV voltage steps, Inx2+Inx3 channels tended to be slightly more voltage sensitive than Inx2 channels, and the fitted curves differed. When gj (normalized to maximum instantaneous gj at 10 mV) was plotted against Vj (Figure 5D), only slight differences were apparent in the overall sensitivity of these channels to voltage. inx2+inx3 cell pairs tended to be marginally more voltage dependent than inx2 cell pairs at smaller Vj steps (Figure 5, A–D). This difference was unlikely to be due to access resistance effects because channels with conductances >6.04 μS were excluded from these data (Wilders and Jongsma, 1992). We were unable to examine the symmetry of the gj/Vj relationship around 0 mV because we could not maintain the holding potential of the passive cell when the stepped cell of the pair was hyperpolarized. Asymmetry of the gj/Vj plot would have indicated channel sensitivity to Vi-o.
However, we were able to measure Vi-o directly by stepping cell pairs over a range of negative and positive membrane potentials. The junctional conductances in cell pairs injected with inx2 mRNA only (Figure 5E, filled symbols) were significantly more sensitive to Vi-o than conductances in cell pairs in which inx3 was also injected (Figure 5E, open symbols). The majority of the channels in inx2 cell pairs were closed at Vi-o values of −20 to −10 mV. In contrast, there was little significant reduction in the gj of inx2+inx3 cell pairs at negative Vi-o, and half maximal gj was evident only at approximately +10 mV (Figure 5E). Because the imposition of Vj steps necessarily alters Vi-o, one might have expected this clear difference in Vi-o sensitivity to be more obvious in the gj/Vj relation (Figure 5, A–D). One possibility is that Inx2+Inx3 channels are more sensitive to transjunctional voltage than Inx2 channels, partially compensating for the greater Vi-o sensitivity of Inx2 channels and resulting in the very similar traces seen in Figure 5, A and B. An alternative explanation could be that Vi-o sensitivity develops relatively slowly and was not resolved during the time course (4.5 s) of the Vj steps used in our recordings. In some insect preparations, full resolution of Vi-o requires a longer time course (Bukauskas et al., 1992; Churchill and Caveney, 1993).
These physiological data show that coexpression of inx2 and inx3 causes channels to form between oocyte pairs that are distinct, in terms of probability of formation, conductance, and voltage sensitivity, from those formed when inx2 is expressed alone. The simplest interpretation of these results is that Inx2 and Inx3 proteins do collaborate to form heteromeric channels.
Ectopic Expression in Drosophila
Inx2 and Inx3 clearly cooperate to form gap-junction channels between Xenopus oocytes. To determine whether these proteins might also cooperate to form gap-junction channels in vivo, we ectopically expressed inx2 and inx3 in Drosophila embryos and assessed the survival rates of these animals (Figure 6). Using the UAS/GAL4 system (Brand and Perrimon, 1993), expression was driven in embryonic muscles (24B-GAL4, A. Brand) and, more ubiquitously, using armadillo-GAL4 (FlyBase website: http://fly.ebi.ac.uk:7081/). UAS-shaking-B(lethal) was crossed to each of these GAL4 lines as a positive control; this line was used because we had previously noted that in vivo ectopic expression of this innexin (which forms fully functional gap junctions in the Xenopus oocyte system) results in a lethal phenotype. As a negative control, each of these driver lines was crossed to the yellow white (y w) injection stock.
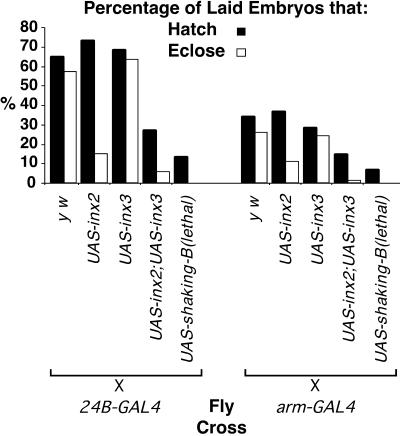
Figure 6.
Fly crosses were performed as shown using two different GAL4 driver lines (all UAS lines are in a y w background). Hatch (black bars) and eclosion (white bars) rates are expressed as a percentage of the total number of embryos examined. For 24B-GAL4 crosses n = 400 and for arm-GAL4 crosses, n = 600. Note that none of the embryos in which shaking-B(lethal) was ectopically expressed reached adulthood.
Progeny of the UAS-inx3 × 24B-GAL4 cross exhibited hatch and eclosion rates very similar to those of y w × 24B-GAL4 progeny. Ectopic expression of inx2 using the 24B-GAL4 driver line resulted in a hatch rate similar to that of 24B-GAL4 × y w progeny, but only 15% of the total number of embryos survived to adulthood. When both inx2 and inx3 were coexpressed, viability was significantly lower than that seen in UAS-inx2 × 24B-GAL4 experiments; only 27% of embryos hatched and only 5.5% reached adulthood. Survival rates of flies resulting from the UAS-inx2 × arm-GAL4 and the UAS-inx2/UAS-inx3 × arm-GAL4 crosses were also significantly reduced compared with the y w × arm-GAL4 progeny (Figure 6). Experiments using independently isolated lines of UAS-inx2 and UAS-inx3 confirmed that the observed results were not due to position effects. Additionally, mRNA in situ hybridization using inx2 and inx3 as probes was performed on all the progeny, and we found no obvious difference between the expression levels of inx2 or inx3 when ectopically expressed singly or in combination (our unpublished results). We conclude that the simultaneous ectopic expression of Inx2 and Inx3 proteins significantly reduces the viability of Drosophila.
DISCUSSION
We have characterized two new members of the Drosophila innexin gene family, inx2 and inx3. Both are expressed at high levels throughout embryogenesis; the transcripts become localized to epidermal cells bordering each embryonic segment at stage 12, and inx2 exhibits additional expression in parts of the tracheal system. In Xenopus oocytes Inx2 alone, but not Inx3, formed channels in ∼ 40% of oocyte pairs. As might be expected of a channel forming protein, Inx2 reduced viability when ectopically expressed in Drosophila embryos. In contrast, when both innexin proteins were coexpressed in the same cells, channels that were clearly distinct from Inx2 channels were reliably formed in oocyte pairs, and there was a much more profound effect on Drosophila viability. We conclude that Inx2 and Inx3 are likely to interact to form heteromeric gap-junction channels.
Additional Members of the Innexin Family
cDNAs corresponding to inx2 and inx3 were identified in the BDGP EST databases on the basis of their homology to known innexins. inx3 is a newly identified gene; inx2 has been independently isolated by PCR methods (prp33; Curtin et al., 1999). This increases the number of published Drosophila innexin proteins to eight.
Innexins have also been found in other insects. Three orthologues of inx2 have now been sequenced, from Drosophila, Bombyx mori (partial sequence, Mita, Morimyo, Shimada, Okano, and Maeda, unpublished results) and Schistocerca americana (Ganfornina et al., 1999). Sa-Inx(2) and Bm-Inx2 are more similar to each other (86% identity) than to Dm-Inx2 (81 and 84% identity, respectively) over the N-terminal region available for Bm-Inx2. Their degree of identity to each other is much higher than identity between innexin family members within Drosophila, which ranges from 29 to 47% (Curtin et al., 1999) (excluding the high identity between Shaking-B{N + 16} [Zhang et al., 1999], Shaking-B(neural) and Shaking-B(lethal)). Sequence comparison between orthologues in different species allows some examination of functional conservation. For example, the C-terminal regions of Sa-Inx(2) and Dm-Inx2 are divergent, whereas the N-terminal cytoplasmic tails of all three orthologues are highly conserved, suggesting that this region may be crucial to the functioning of this specific innexin. Shaking-B(neural) and Shaking-B(lethal) differ only in their N-terminal regions and they behave very differently both in vivo and in the Xenopus oocyte system. Additionally, the N-terminal regions of some connexins have been implicated in specifying which connexins can associate to form hetero-oligomeric hemi-channels (Falk et al., 1997).
inx2 and inx3 Are Expressed in Embryonic Tissues that are Known to Possess Gap Junctions
inx2 and inx3 expression was examined in the embryonic epidermis, hindgut, foregut, and tracheal system, all of which are ectodermal in origin and possess gap junctions during embryogenesis (Tepass and Hartenstein, 1994). In the insect epidermis, all cells, regardless of their position with respect to the segment border, are electrically coupled. However, although larger ions such as Lucifer Yellow transfer freely from cell to cell within a segment, movement across the segmental border is restricted in both larval and adult epidermis (Warner and Lawrence, 1982; Blennerhassett and Caveney, 1984; Ruangvoravat and Lo, 1992). This suggests that gap junctions at segmental borders may have permeability properties different from those within a segment. Because inx2 and inx3 are expressed most strongly around the borders of each segment, they may contribute to these differences. Both inx2 and inx3 transcripts were detected in the hindgut and foregut, which are ectodermally derived, but not in the midgut, which is mainly endodermal in origin. The type and number of cell junctions in the midgut is known to differ from those in the hindgut and foregut (Tepass and Hartenstein, 1994). Although gap junctions are present throughout the gut, our data suggest that the constituents of the gap-junction channels in these endodermally and ectodermally derived regions differ. We have also demonstrated that inx2, but not inx3, is expressed in the dorsal trunk, a multicellular primary branch of the tracheal system, and in precursors of this structure. During germ band retraction, segmentally repeated clusters of cells, the tracheal placodes, reorganize to form the initial outgrowths of the tracheal branches (Manning and Krasnow, 1993; Samakovlis et al., 1996). Some of these cells then migrate to link adjacent segments and form the dorsal trunk. Gap junctions are known to be present between the cells of this structure (Tepass and Hartenstein, 1994), and they may be involved in coordinating the migration and reorganization of dorsal trunk cells from adjacent placodes. Given that inx2 mRNA is expressed in these cells, Inx2 protein is likely to be a constituent of at least some of these tracheal gap-junction channels.
Inx2 Forms Homomeric Channels and Cooperates with Inx3 to Form Heteromeric Channels in Paired Xenopus Oocytes
Paired Xenopus oocytes have been used extensively for functional expression of proteins of the connexin family and, with the exception of Cx31.1, Cx32.7 and Cx33, all connexins so far characterized form homotypic channels in this system (Hennemann et al., 1992; Bruzzone et al., 1995; Chang et al., 1996; reviewed in Bruzzone et al., 1996). In a previous study, we expressed the two partially identical innexins, Shaking-B(neural) and Shaking-B(lethal), in oocyte pairs and found that only the latter forms homotypic channels (Phelan et al., 1998a). In the present study, we found that a second innexin, Inx2, also forms voltage-sensitive channels in the oocyte system. However, although Inx2 was clearly competent to form homotypic channels, it did so much less readily than Shaking-B(lethal). Injecting only 0.5 ng of shaking-B(lethal) mRNA gives rise to junctional conductances (mean gj = 15.87 ± 1.45 μS, Phelan et al., 1998a) in essentially all cell pairs whereas at 10 ng inx2 mRNA, only 44% of cell pairs developed measurable conductances (mean gj = 4.62 ± 1.05 μS). This could be accounted for if essential assembly/regulatory molecules are missing from the oocyte or if, ordinarily, Inx2 is a component of hetero-oligomeric channels. Although we cannot rule out a requirement for additional cofactors or channel subunits, we have presented evidence in this article that Inx3, the distribution of which overlaps that of Inx2 in some Drosophila tissues, may partner Inx2 in heteromeric channels.
Inx3, like Shaking-B(neural) (Phelan et al., 1998a), did not form homotypic channels in paired oocytes. However, when Inx3 was present in cells expressing Inx2, channels were formed that had voltage properties distinct from the Inx2 homotypic channels (which clearly also might have assembled in these cell pairs). Notably, the two channel subtypes were differentially sensitive to transmembrane voltage. The conductance of Inx2 channels dropped dramatically upon depolarization, to negligible levels at membrane potentials of −10 mV; the channels in inx2+inx3 cell pairs, although also influenced by Vi-o, showed no significant reduction in gj at negative potentials. Considering the results of ectopic expression in selected Drosophila tissues (see below), we interpret our oocyte expression data to imply that Inx2 and Inx3 form heteromeric channels. Similarly, electrical properties (Ebihara et al., 1999; He et al., 1999) and also pH sensitivity (Bevans and Harris, 1999) have been shown to distinguish connexin heteromeric channels from homotypic channels formed by the same proteins. Alternative approaches such as coimmunoprecipitation (Stauffer, 1995; Jiang and Goodenough, 1996) would be required to directly demonstrate protein–protein interactions within a channel but as yet suitable probes are not available for Inx2 and Inx3.
In terms of their sensitivity to Vi-o, the innexin channels described here differ from both Shaking-B(lethal) and Ce-Inx-3 channels, which are Vi-o insensitive (Phelan et al., 1998a; Landesman et al., 1999). However, the Inx2+Inx3 channels, in particular, are reminiscent of intercellular channels (of unknown molecular composition) characterized in many invertebrate tissues. Gap junctions in Drosophila embryonic muscle cells (Gho, 1994), salivary gland cells from Drosophila and Chironomus (Obaid et al., 1983; Verselis et al., 1991), and several insect cell lines (Bukauskas et al., 1992, 1997) are gated by Vi-o, such that the conductances decline as the membrane potential becomes more positive.
The Activity of Inx2 and Inx2+Inx3 Channels In Vivo Reflects Their Activity in Paired Xenopus Oocytes
Because inx2 and inx3 expression domains partially overlap and the encoded proteins appeared to interact in the paired oocyte system, we were prompted to investigate whether they might also cooperate in vivo. The rationale behind these experiments was based on unpublished observations (M.G.T.) that ectopic expression of Shaking-B(neural) has no effect on viability (our unpublished results) and that this protein fails to form functional junctions in the oocyte system (Phelan et al., 1998a). In contrast, ectopic expression of Shaking-B(lethal) in vivo results in death (Figure 6), and this protein consistently forms intercellular channels in oocyte pairs (Phelan et al., 1998a).
Similarly, when inx2 and inx3 were ectopically expressed together, only very few animals survived to adulthood, mirroring the efficacy of presumptive Inx2+Inx3 oligomeric channels in Xenopus oocyte pairs. Ectopic expression of inx2 alone was less harmful to the organism, in keeping with its reduced ability to form functional gap junctions in oocytes. These data could, if taken in isolation, be accounted for by a variety of interactions between inx2, inx3 and/or their products, or by non–gap-junction–related effects resulting from the misexpression of membrane proteins. However, because we have presented evidence that heteromeric channels are formed in Xenopus oocytes when both proteins are present, the most parsimonious explanation is that heteromeric channels are also formed in vivo. The most direct way to confirm this would be to look for differences in intercellular dye-coupling between epidermal cells in wild-type, inx2-, inx3-, and inx2/inx3-deficient embryos. These studies await the generation of appropriate mutant stocks.
Conclusions
Of the four Drosophila innexins so far expressed in paired oocytes, two are unable to form functional homotypic channels. Similarly, some C. elegans innexins also appear not to form homotypic channels (Landesman et al., 1999; reported as a personal communication in Curtin et al., 1999). So what additional factors are required for these innexins to form channels? This article has provided strong evidence that two innexins form heteromeric channels, raising the possibility that hetero-oligomerization is a common feature of invertebrate gap-junction channels. Some data from mutational studies in C. elegans support this; mutations in two innexin genes, unc-7 and unc-9, exhibit almost identical phenotypes, possibly because their encoded proteins are components of the same gap-junction channel (Barnes and Hekimi, 1997). As more family members are functionally expressed, some general rules of innexin compatibility should emerge.
ACKNOWLEDGMENTS
We thank Elizabeth Brint for maintaining and crossing fly stocks, Richard Baines and Kirsten Jacobs for advice and discussion, and Chris Ford for help with the initial stages of this project. This work was supported by the Biotechnology and Biological Sciences Research Council, UK, to J.P.B. and J.A.D., and in part by a Wellcome Trust grant to P.P.
REFERENCES
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TM, Hekimi S. The Caenorhabditis elegans avermectin resistance and anesthetic response gene unc-9 encodes a member of a protein family implicated in electrical coupling of excitable cells. J Neurochem. 1997;69:2251–2260. doi: 10.1046/j.1471-4159.1997.69062251.x. [DOI] [PubMed] [Google Scholar]
- Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, Bennett MVL, Nicholson BJ. Gap junctions formed by Connexin 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci USA. 1991;88:8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MVL. Gap junctions as electrical synapses. J Neurocytol. 1997;26:349–366. doi: 10.1023/a:1018560803261. [DOI] [PubMed] [Google Scholar]
- Bevans CG, Harris AL. Regulation of connexin channels by pH—Direct action of the protonated form of taurine and other aminosulfonates. J Biol Chem. 1999;274:3711–3719. doi: 10.1074/jbc.274.6.3711. [DOI] [PubMed] [Google Scholar]
- Blagburn JM, Alexopoulos H, Davies JA, Bacon JP. Null mutation in shaking-B eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: a structural study. J Comp Neurol. 1999;404:449–458. [PubMed] [Google Scholar]
- Blennerhassett MG, Caveney S. Separation of developmental compartments by a cell type with reduced junctional permeability. Nature. 1984;309:361–364. [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Yoshizaki G, Patino R, Paul DL. Intercellular channels in teleosts: functional characterization of two connexins from Atlantic croaker. FEBS Lett. 1995;358:301–304. doi: 10.1016/0014-5793(94)01457-c. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Kempf C, Weingart R. Electrical coupling between cells of the insect Ades albopictus. J Physiol. 1992;448:321–337. doi: 10.1113/jphysiol.1992.sp019044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Vogel R, Weingart R. Biophysical properties of heterotypic gap junctions newly formed between two types of insect cells. J Physiol. 1997;499:701–713. doi: 10.1113/jphysiol.1997.sp021962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Werner R, Dahl G. A role for an inhibitory connexin in testis? Dev Biol. 1996;175:50–56. doi: 10.1006/dbio.1996.0094. [DOI] [PubMed] [Google Scholar]
- Churchill D, Caveney S. Double whole-cell patch-clamp characterization of gap junctional channels in isolated insect epidermal cell pairs. J Membrane Biol. 1993;135:165–180. doi: 10.1007/BF00231442. [DOI] [PubMed] [Google Scholar]
- Colman A. In: In: Transcription and Translation-A Practical Approach. Hames BD, Higgins SJ, editors. Oxford, UK: IRL; 1984. pp. 271–302. [Google Scholar]
- Crompton D, Todman M, Wilkin M, Ji S, Davies J. Essential and neural transcripts from the Drosophila shaking-B locus are differentially expressed in the embryonic mesoderm and pupal nervous system. Dev Biol. 1995;170:142–158. doi: 10.1006/dbio.1995.1203. [DOI] [PubMed] [Google Scholar]
- Curtin KD, Zhang Z, Wyman RJ. Drosophila has several genes for gap junction proteins. Gene. 1999;232:191–201. doi: 10.1016/s0378-1119(99)00123-7. [DOI] [PubMed] [Google Scholar]
- Ebihara L, Xu X, Oberti C, Beyer EC, Berthoud VM. Co-expression of lens fiber connexins modifies hemi-gap-junctional channel behavior. Biophys J. 1999;76:198–206. doi: 10.1016/S0006-3495(99)77189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MM, Buehler LK, Kumar NM, Gilula NB. Cell-free synthesis and assembly of connexins into functional gap junction membrane channels. EMBO J. 1997;16:2703–2716. doi: 10.1093/emboj/16.10.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina MD, Sanchez D, Herrera M, Bastiani MJ. Developmental expression and molecular characterization of two gap junction channel proteins expressed during embryogenesis in the grasshopper Schistocerca americana. Dev Genet. 1999;24:137–150. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<137::AID-DVG13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gho M. Voltage-clamp analysis of gap junctions between embryonic muscles in Drosophila. J Physiol. 1994;481:371–383. doi: 10.1113/jphysiol.1994.sp020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl DL, Nurminsky DI, Jones RW, Lozovskaya ER. Genome structure and evolution in Drosophila: applications of the framework P1 map. Proc Natl Acad Sci USA. 1994;91:6824–6829. doi: 10.1073/pnas.91.15.6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DS, Jiang JX, Taffet SM, Burt JM. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1999;96:6495–6500. doi: 10.1073/pnas.96.11.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennemann H, Dahl E, White JB, Schwarz HJ, Lalley PA, Chang S, Nicholson BJ, Willecke K. Two gap junction genes, connexin 31.1 and 30.3, are closely linked on mouse chromosome 4 and preferentially expressed in skin. J Biol Chem. 1992;267:17225–17233. [PubMed] [Google Scholar]
- Jiang JX, Goodenough DA. Heteromeric connexons in lens gap junction channels. Proc Natl Acad Sci USA. 1996;93:1287–1291. doi: 10.1073/pnas.93.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess RE, Rubin GM. Analysis of P transposable element functions in Drosophila. Cell. 1984;38:135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Krishnan SN, Frei E, Swain GP, Wyman RJ. Passover: a gene required for synaptic connectivity in the giant fiber system of Drosophila. Cell. 1993;73:967–977. doi: 10.1016/0092-8674(93)90274-t. [DOI] [PubMed] [Google Scholar]
- Landesman Y, White TW, Starich TA, Shaw JE, Goodenough DA, Paul DL. Innexin-3 forms connexin-like intercellular channel. J Cell Sci. 1999;112:2391–2396. doi: 10.1242/jcs.112.14.2391. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Rhee SK. Heteromeric gap junction channels in rat hepatocytes in which the expression of connexin26 is induced. Molecules and Cells. 1998;8:295–300. [PubMed] [Google Scholar]
- Lehmann R, Tautz D. In situ hybridization to RNA. Methods Cell Biol. 1994;44:575–598. doi: 10.1016/s0091-679x(08)60933-4. [DOI] [PubMed] [Google Scholar]
- Lennon G, Auffray C, Polymeropoulos M, Soares MB. The I.M.A.G.E. consortium: an integrated molecular analysis of genomes and their expression. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- Lipshitz HD, Kankel DR. Specificity of gene action during central nervous system development in Drosophila melanogaster: analysis of the lethal (1) optic ganglion reduced locus. Dev Biol. 1985;108:56–77. doi: 10.1016/0012-1606(85)90009-0. [DOI] [PubMed] [Google Scholar]
- Manning G, Krasnow MG. Development of the Drosophila tracheal system. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster, vol. I. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1993. pp. 609–685. [Google Scholar]
- Obaid AL, Socolar SJ, Rose B. Cell-to-cell channels with two independently regulated gates in series: analysis of junctional conductance modulation by membrane-potential, calcium, and pH. J Membrane Biol. 1983;73:69–89. doi: 10.1007/BF01870342. [DOI] [PubMed] [Google Scholar]
- Phelan P, Nakagawa M, Wilkin MB, Moffat KG, O'Kane CJ, Davies JA, Bacon JP. Mutations in shaking-B prevent electrical synapse formation in the Drosophila giant fiber system. J Neurosci. 1996;16:1101–1113. doi: 10.1523/JNEUROSCI.16-03-01101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P, Stebbings LA, Baines RA, Bacon JP, Davies JA, Ford C. Drosophila Shaking-B protein forms gap junctions in paired Xenopus oocytes. Nature. 1998a;391:181–184. doi: 10.1038/34426. [DOI] [PubMed] [Google Scholar]
- Phelan P, et al. Innexins: a family of invertebrate gap-junction proteins. Trends Genet. 1998b;14:348–349. doi: 10.1016/s0168-9525(98)01547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P. Gap junction communication in invertebrates: the innexin gene family. Curr Top Membranes. 2000;49:389–422. [Google Scholar]
- Ruangvoravat CP, Lo CW. Restrictions in gap junctional communication in the Drosophila larval epidermis. Dev Dynamics. 1992;193:70–82. doi: 10.1002/aja.1001930110. [DOI] [PubMed] [Google Scholar]
- Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–1407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MVL. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981;77:77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Herman RK, Shaw JE. Molecular and genetic analysis of unc-7, a Caenorhabditis elegans gene required for coordinated locomotion. Genetics. 1993;133:527–541. doi: 10.1093/genetics/133.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Lee RYN, Panzarella C, Avery L, Shaw JE. eat-5 and unc-7 represent a multigene family in Caenorhabditis elegans involved in cell-cell coupling. J Cell Biol. 1996;134:537–548. doi: 10.1083/jcb.134.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer KA. The gap junction proteins beta1 connexin (connexin 32) and beta2 connexin (connexin 26) can form heteromeric hemi-channels. J Biol Chem. 1995;270:6768–6772. [PubMed] [Google Scholar]
- Swenson KI, Jordan JR, Beyer EC, Paul DL. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989;57:145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todman MG, Baines RA, Stebbings LA, Davies JA, Bacon JP. Gap-junctional communication between developing Drosophila muscles is essential for their normal development. Dev Genet. 1999;24:57–68. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<57::AID-DVG7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Trimarchi JR, Murphey RK. The shaking-B2 mutation disrupts electrical synapses in a flight circuit in adult Drosophila. J Neurosci. 1997;17:4700–4710. doi: 10.1523/JNEUROSCI.17-12-04700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- Verselis VK, Bennett MVL, Bargiello TA. A voltage dependent gap junction in Drosophila melanogaster. Biophys J. 1991;59:114–126. doi: 10.1016/S0006-3495(91)82204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner AE, Lawrence PA. Permeability of gap junctions at the segmental border in insect epidermis. Cell. 1982;28:243–252. doi: 10.1016/0092-8674(82)90342-7. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kankel DR. Molecular cloning and analysis of l(1)ogre, a locus of Drosophila melanogaster with prominent effects on the postembryonic development of the central nervous system. Genetics. 1990;126:1033–1044. doi: 10.1093/genetics/126.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R, Levine E, Rabadan-Diehl C, Dahl G. Formation of hybrid cell-cell channels. Proc Natl Acad Sci USA. 1989;86:5380–5384. doi: 10.1073/pnas.86.14.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilders R, Jongsma HJ. Limitations of the dual voltage clamp method in assaying conductance and kinetics of gap junction channels. Biophys J. 1992;63:942–953. doi: 10.1016/S0006-3495(92)81664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RK. How the worm was won—the C. elegans genome sequencing project. Trends Genet. 1999;15:51–58. doi: 10.1016/s0168-9525(98)01666-7. [DOI] [PubMed] [Google Scholar]
- Yeager M, Nicholson BJ. Structure of gap junction intercellular channels. Curr Opin Structural Biol. 1996;6:183–192. doi: 10.1016/s0959-440x(96)80073-x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Curtin KD, Sun Y-A, Wyman RJ. Nested transcripts of gap junction gene have distinct expression patterns. J Neurobiol. 1999;40:288–301. doi: 10.1002/(sici)1097-4695(19990905)40:3<288::aid-neu2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]