Abstract
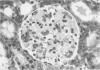
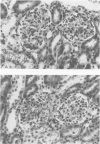
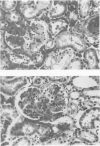
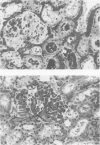
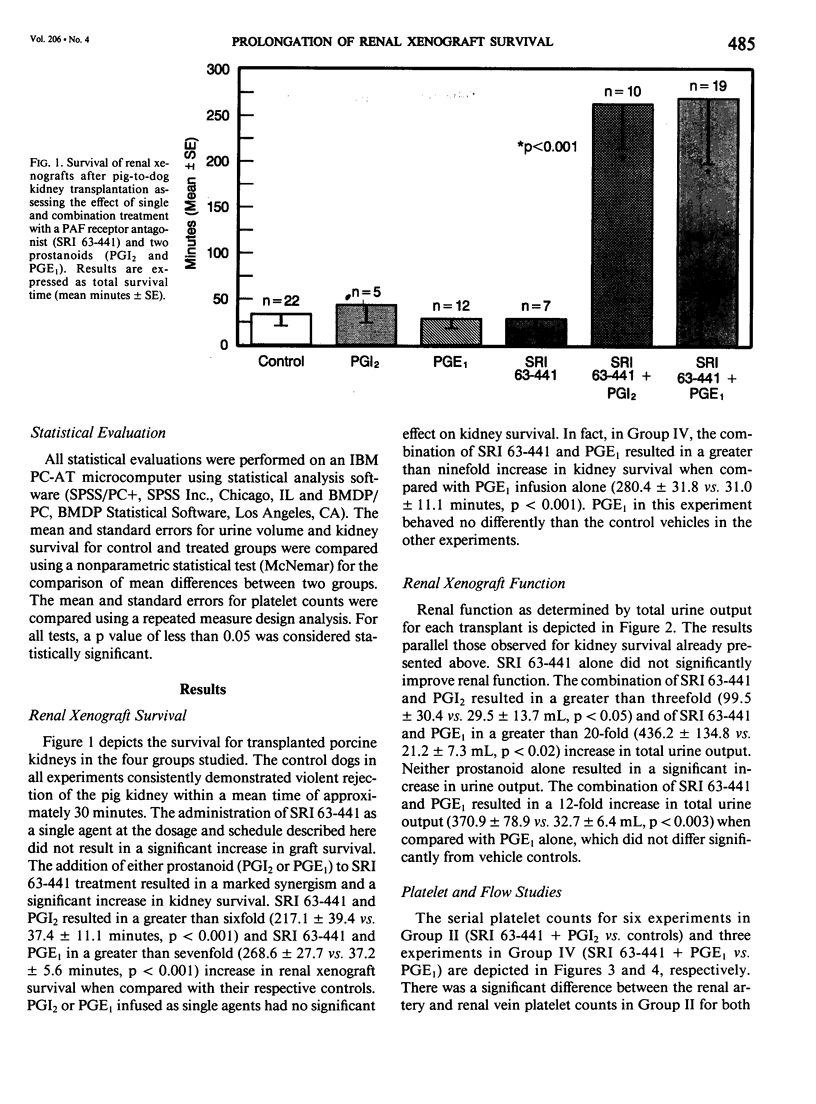
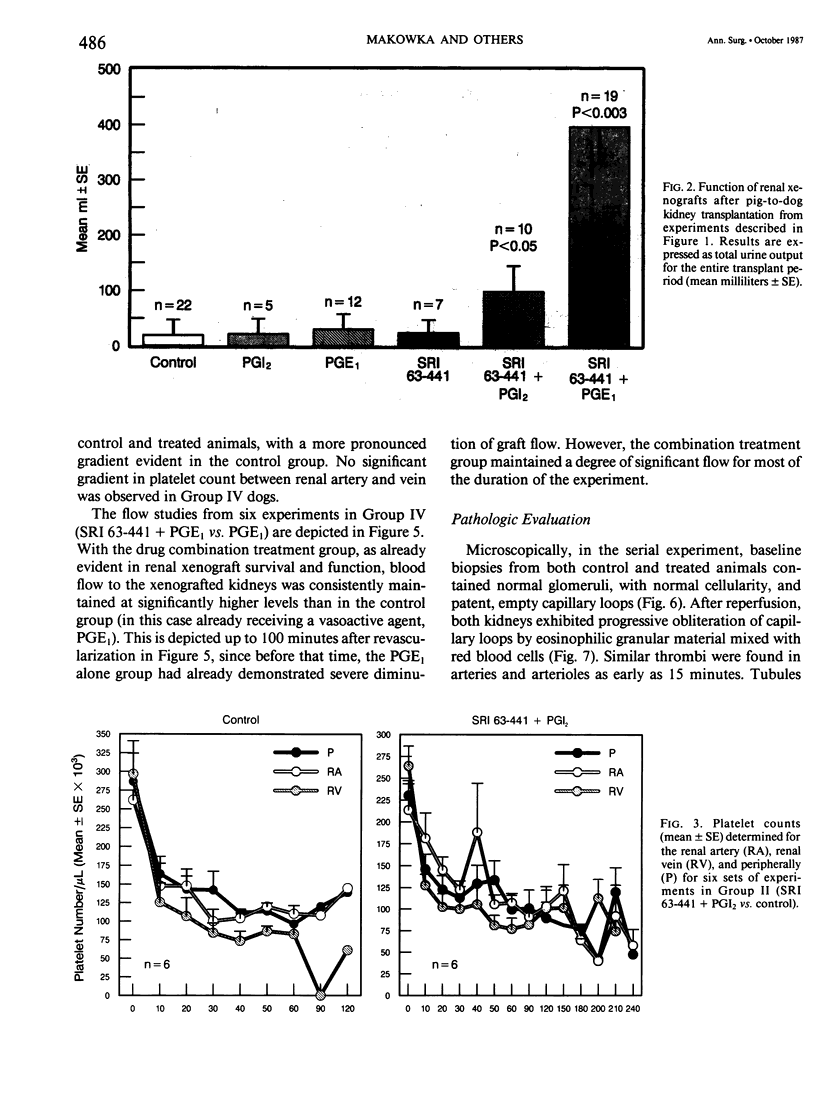
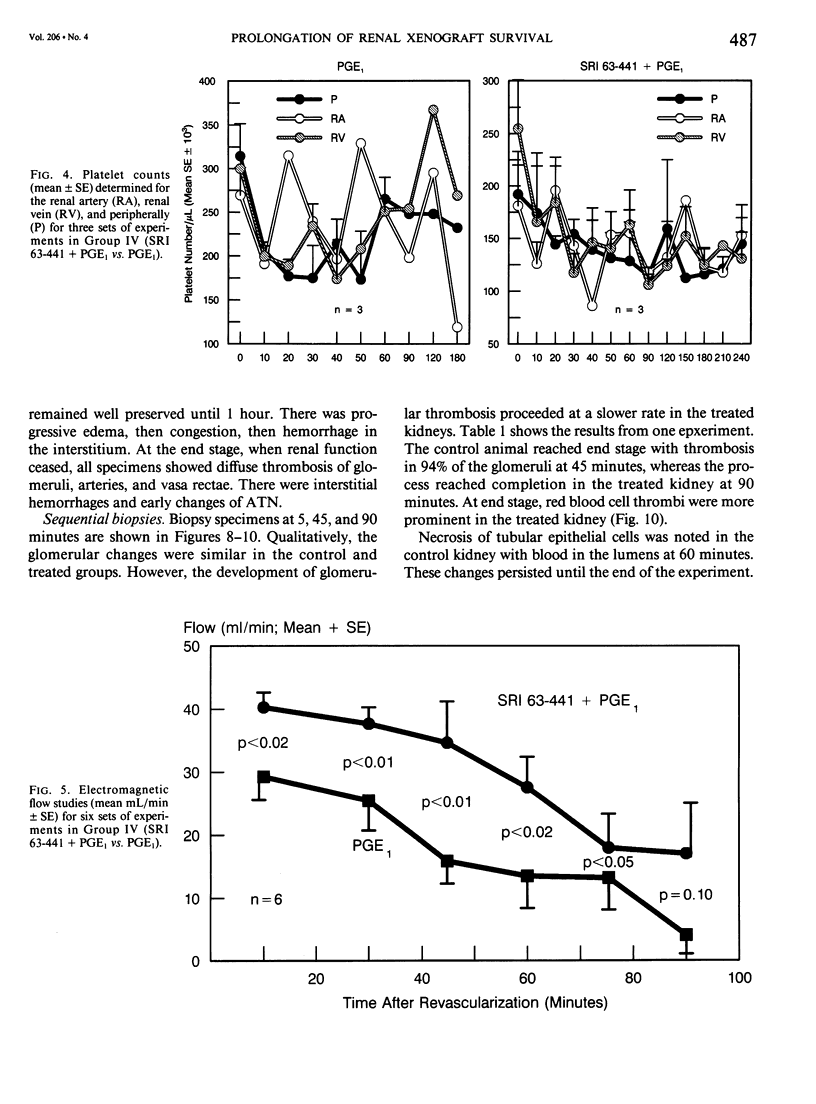
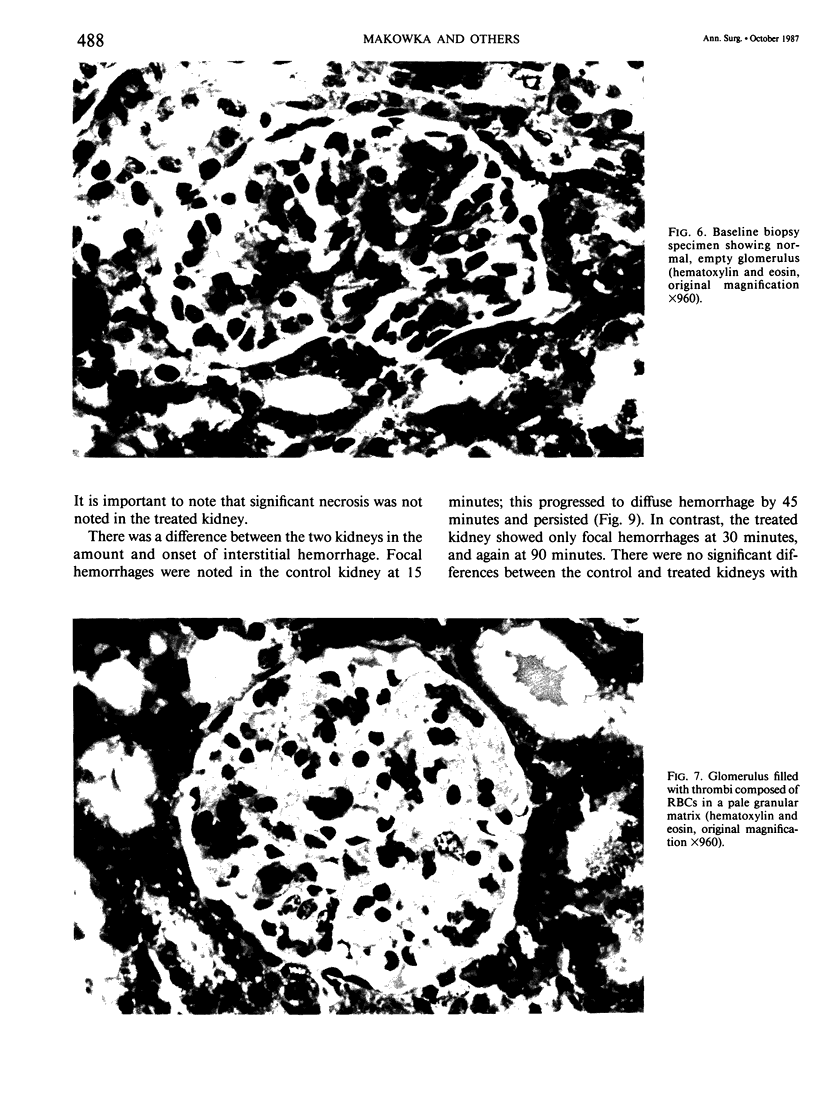
The pathogenesis of hyperacute renal rejection consists of a nonspecific effector cascade that invokes most of the components of a typical acute inflammatory response. Platelet-activating factor (PAF) represents the most recent and perhaps the most significant mediator and promoting agent of this phenomenon. These studies evaluated SRI 63-441, a novel, synthetic, and the most potent PAF receptor antagonist available, alone and in combination with other prostanoids, for their ability to influence this response and to prolong renal xenograft survival and function in a model of pig-to-dog heterotransplantation. Inhibition of PAF by SRI 63-441 alone, at the dosage and schedule used in these experiments, did not significantly prolong xenograft survival or function. However, the combination of SRI 63-441 with either prostacyclin (PGI2) or prostaglandin E1 (PGE1) infusion demonstrated significant synergism, and resulted in a 6-9-fold increase in kidney survival and a 3-20-fold increase in urine output. Neither PGI2 nor PGE1 infusions alone significantly influenced this xenograft model. Electromagnetic flow studies demonstrated significantly delayed diminution in renal artery blood flow in the combination-treated animals. Serial and end-stage histologic examination of kidneys receiving combination therapy demonstrated a delayed onset of the pathologic deterioration and an overall amelioration of the entire process. These studies demonstrate that significant abrogation of a rapid and violent form of hyperacute rejection can be achieved solely by the pharmacologic manipulation of the inflammatory mediator response.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boehmig H. J., Giles G. R., Amemiya H., Wilson C. B., Coburg A. J., Genton E., Bunch D. L., Dixon F. J., Starzl T. E. Hyperacute rejection of renal homografts: with particular reference to coaglation changes, humoral antibodies, and formed blood elements. Transplant Proc. 1971 Jun;3(2):1105–1117. [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Malavasi F., Tetta C., Piacibello W., Sanavio F., Bussolino F. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983 Nov;131(5):2397–2403. [PubMed] [Google Scholar]
- Camussi G. Potential role of platelet-activating factor in renal pathophysiology. Kidney Int. 1986 Feb;29(2):469–477. doi: 10.1038/ki.1986.23. [DOI] [PubMed] [Google Scholar]
- Forbes R. D., Guttmann R. D., Bazin H. Hyperacute rejection of cardiac allografts in a rat strain with a hereditary platelet function defect. Lab Invest. 1977 Aug;37(2):158–161. [PubMed] [Google Scholar]
- Forbes R. D., Kuramochi T., Guttmann R. D., Klassen J., Knaack J. A controlled sequential morphologic study of hyperacute cardiac allograft rejection in the rat. Lab Invest. 1975 Sep;33(3):280–288. [PubMed] [Google Scholar]
- Forbes R. D., Pinto-Blonde M., Guttmann R. D. The effect of anticomplementary cobra venom factor on hyperacute rat cardiac allograft rejection. Lab Invest. 1978 Nov;39(5):463–470. [PubMed] [Google Scholar]
- Giles G. R., Boehmig H. J., Lilly J., Amemiya H., Takagi H., Coburg A. J., Hathaway W. E., Wilson C. B., Dixon F. J., Starzl T. E. Mechanism and modification of rejection of heterografts between divergent species. Transplant Proc. 1970 Dec;2(4):522–538. [PMC free article] [PubMed] [Google Scholar]
- Handley D. A., Tomesch J. C., Saunders R. N. Inhibition of PAF-induced systemic responses in the rat, guinea pig, dog and primate by the receptor antagonist SRI 63-441. Thromb Haemost. 1986 Aug 20;56(1):40–44. [PubMed] [Google Scholar]
- Hefty T. R., Barry J. M. Renal transplantation in Saudi Arabia. Transplant Proc. 1986 Jun;18(3 Suppl 2):10–12. [PubMed] [Google Scholar]
- Ito S., Camussi G., Tetta C., Milgrom F., Andres G. Hyperacute renal allograft rejection in the rabbit. The role of platelet-activating factor and of cationic proteins derived from polymorphonuclear leukocytes and from platelets. Lab Invest. 1984 Aug;51(2):148–161. [PubMed] [Google Scholar]
- Jørgensen K. A., Kemp E., Barfort P., Starklint H., Larsen S., Munk-Anderson G. Platelet aggregation is not essential for xenograft rejection. Thromb Res. 1986 Jul 1;43(1):87–90. doi: 10.1016/0049-3848(86)90046-0. [DOI] [PubMed] [Google Scholar]
- Kissmeyer-Nielsen F., Olsen S., Petersen V. P., Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966 Sep 24;2(7465):662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- Kux M., Boehmig H. J., Amemiya H., Torisu M., Yokoyama T., Launois B., Popovtzer M., Wilson C. B., Dixon F. J., Starzl T. E. Modification of hyperacute canine renal homograft and pig-to-dog heterograft rejection by the intra-arterial infusion of citrate. Surgery. 1971 Jul;70(1):103–112. [PMC free article] [PubMed] [Google Scholar]
- Merkel F. K., Bier M., Beavers C. D., Merriman W. G., Wilson C., Starzl T. E. Modification of xenograft response by selective plasmapheresis. Transplant Proc. 1971 Mar;3(1):534–537. [PMC free article] [PubMed] [Google Scholar]
- Mundy A. R. Prolongation of cat to dog renal xenograft survival with prostacyclin. Transplantation. 1980 Sep;30(3):226–228. [PubMed] [Google Scholar]
- Pinckard R. N. The "new" chemical mediators of inflammation. Monogr Pathol. 1982;(23):38–53. [PubMed] [Google Scholar]
- Rosenberg J. C., Broersma R. J., Bullemer G., Mammen E. F., Lenaghan R., Rosenberg B. F. Relationship of platelets, blood coagulation, and fibrinolysis to hyperacute rejection of renal xenografts. Transplantation. 1969 Aug;8(2):152–161. doi: 10.1097/00007890-196908000-00008. [DOI] [PubMed] [Google Scholar]
- Shons A. R., Najarian J. S. Modification of xenograft rejection by aspirin, dextran, and cinanserin: the importance of platelets in hyperacute rejection. Transplant Proc. 1974 Dec;6(4):435–440. [PubMed] [Google Scholar]
- Starzl T. E., Lerner R. A., Dixon F. J., Groth C. G., Brettschneider L., Terasaki P. I. Shwartzman reaction after human renal homotransplantation. N Engl J Med. 1968 Mar 21;278(12):642–648. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. M., Hume D. M., Hudson R. P., Jr, Morris P. J., Kano K., Milgrom F. "Hyperacute" renal-homograft rejection in man. N Engl J Med. 1968 Sep 19;279(12):611–618. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]