Abstract
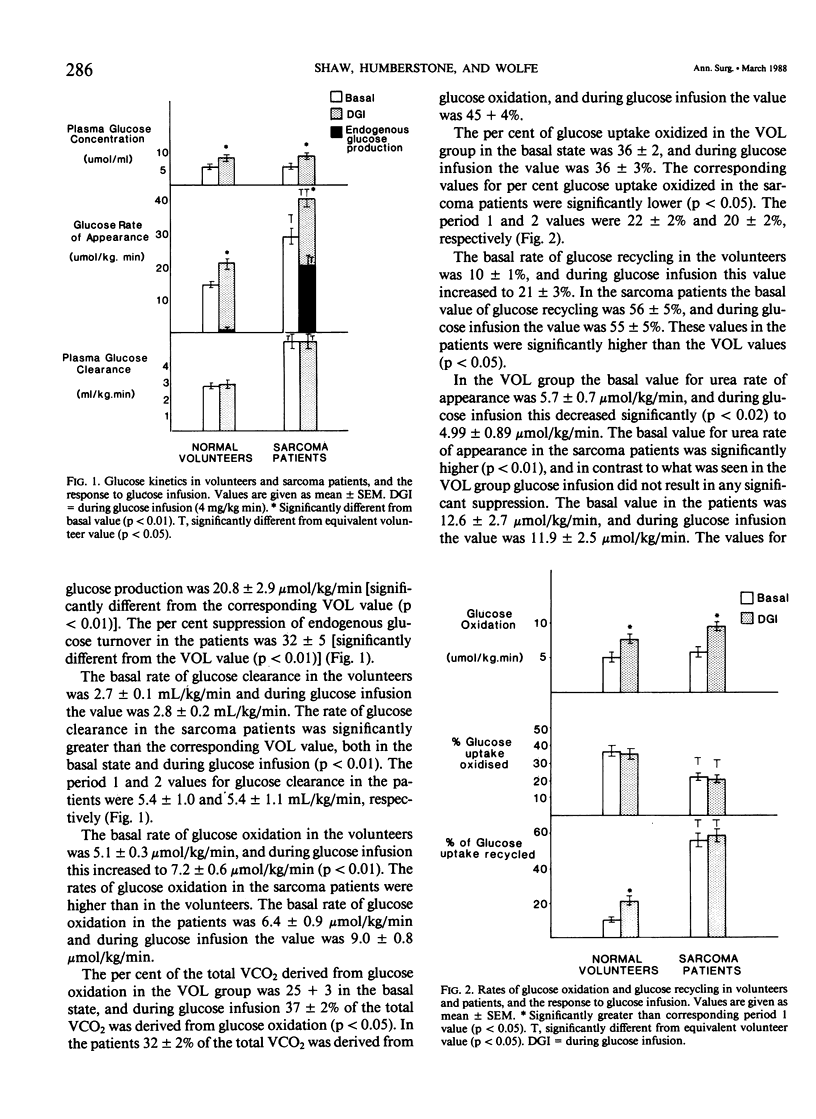
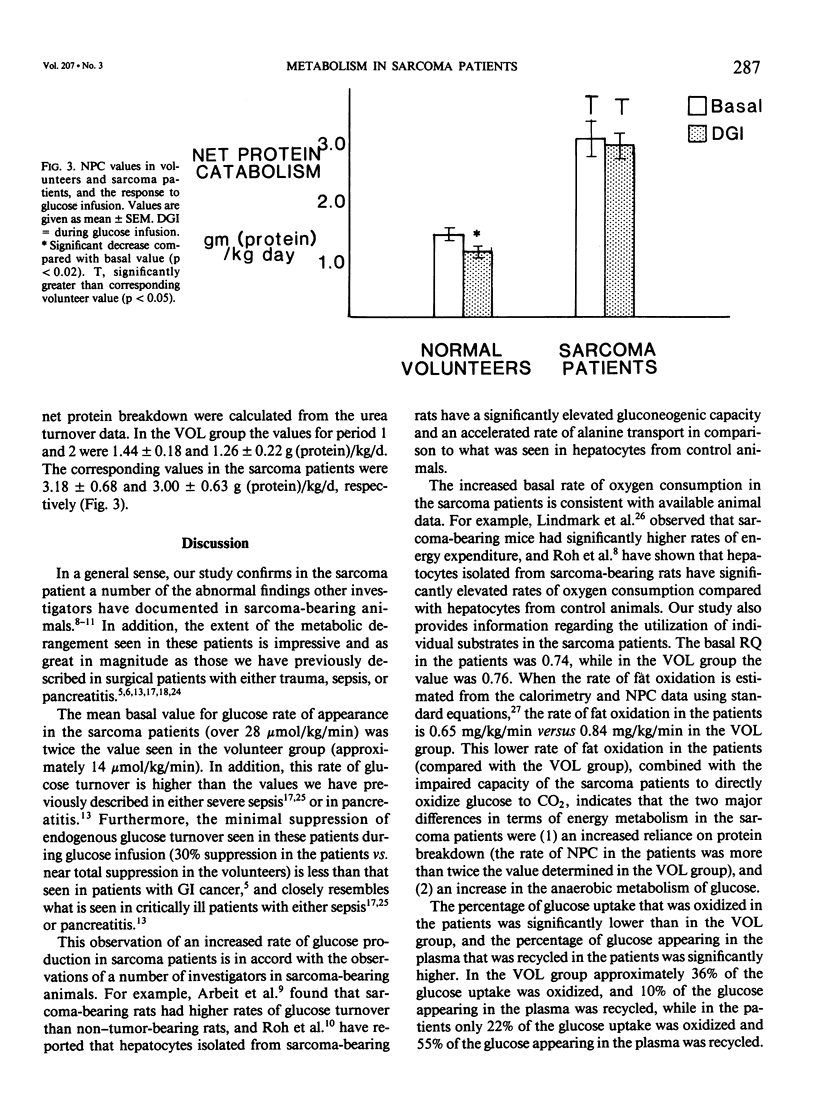
We have performed a series of isotopic infusions both in normal volunteers (N = 16) and in sarcoma patients (N = 7). Using the primed-constant infusion of stable or radioisotopes we have determined the rates of glucose turnover, glucose oxidation, glucose recycling, and net protein catabolism (NPC). In addition, we have measured VO2 and VCO2. The values for VCO2 and VO2 were higher in the patients than in the volunteers (mean VO2 values in volunteers and patients: 107 +/- 13 and 158 +/- 13 mumol/kg/min, respectively). The basal rate of glucose appearance in the sarcoma patients was twice the value seen in the volunteers (28.3 +/- 3.5 vs. 13.9 +/- 0.3 mumol/kg/min). Glucose infusion in the volunteers resulted in virtually total suppression of endogenous glucose production, while in the patients glucose infusion induced only a 30% suppression of endogenous glucose production (p less than 0.01). The rate of glucose clearance in the patients was approximately twice the value seen in the volunteers [5.4 +/- 1.0 vs. 2.7 +/- 0.1 mL/kg/min (p less than 0.01)]. The per cent of glucose uptake oxidized in the patients was significantly less than in the volunteers [22% vs. 36% (p less than 0.05)], and the per cent of glucose uptake that was recycled was significantly higher in the patients [55% vs. 10% (p less than 0.01)]. In addition, the basal rate of net protein loss was increased twofold in the patients [3.2 +/- 0.5 vs. 1.4 +/- 0.4 g (protein)/kg/d (p less than 0.01)], and in contrast to the situation in the volunteers there was no suppression in the rate of net protein loss when the patients were infused with glucose. The basal insulin concentration and the insulin response to glucose infusion in the patients were similar to that of the volunteers, but the plasma cortisol level was similar in volunteers and in the patients (p less than 0.05). We conclude from these studies the following: (1) Sarcoma patients have significantly elevated rates of glucose production and glucose clearance, but they have an impaired capacity to directly oxidize either endogenous or infused glucose, coupled with an increased rate of glucose recycling. (2) Sarcoma patients have an elevated metabolic rate and are catabolic. In addition, in contrast with normal volunteers, glucose infusion does not result in a suppression of protein loss.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Allsop J. R., Wolfe R. R., Burke J. F. Tracer priming the bicarbonate pool. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):137–139. doi: 10.1152/jappl.1978.45.1.137. [DOI] [PubMed] [Google Scholar]
- Arbeit J. M., Burt M. E., Rubinstein L. V., Gorschboth C. M., Brennan M. F. Glucose metabolism and the percentage of glucose derived from alanine: response to exogenous glucose infusion in tumor-bearing and non-tumor-bearing rats. Cancer Res. 1982 Dec;42(12):4936–4942. [PubMed] [Google Scholar]
- Brennan M. F. Total parenteral nutrition in the cancer patient. N Engl J Med. 1981 Aug 13;305(7):375–382. doi: 10.1056/NEJM198108133050705. [DOI] [PubMed] [Google Scholar]
- Dempsey D. T., Feurer I. D., Knox L. S., Crosby L. O., Buzby G. P., Mullen J. L. Energy expenditure in malnourished gastrointestinal cancer patients. Cancer. 1984 Mar 15;53(6):1265–1273. doi: 10.1002/1097-0142(19840315)53:6<1265::aid-cncr2820530609>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Frayn K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Karlberg H. I., Kern K. A., Fischer J. E. Albumin turnover in sarcoma-bearing rats in relation to cancer anorexia. Am J Surg. 1983 Jan;145(1):95–101. doi: 10.1016/0002-9610(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Lindmark L., Edström S., Ekman L., Karlberg I., Lundholm K. Energy metabolism in nongrowing mice with sarcoma. Cancer Res. 1983 Aug;43(8):3649–3654. [PubMed] [Google Scholar]
- Norton J. A., Maher M., Wesley R., White D., Brennan M. F. Glucose intolerance in sarcoma patients. Cancer. 1984 Dec 15;54(12):3022–3027. doi: 10.1002/1097-0142(19841215)54:12<3022::aid-cncr2820541234>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Norton J. A., Shamberger R., Stein T. P., Milne G. W., Brennan M. F. The influence of tumor-bearing on protein metabolism in the rat. J Surg Res. 1981 May;30(5):456–462. doi: 10.1016/0022-4804(81)90090-1. [DOI] [PubMed] [Google Scholar]
- Roh M. S., Ekman L. G., Jeevanandam M., Brennan M. F. Elevated energy expenditure in hepatocytes from tumor-bearing rats. J Surg Res. 1985 May;38(5):407–415. doi: 10.1016/0022-4804(85)90055-1. [DOI] [PubMed] [Google Scholar]
- Roh M. S., Ekman L., Jeevanandam M., Brennan M. F. Gluconeogenesis in tumor-influenced hepatocytes. Surgery. 1984 Aug;96(2):427–434. [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Klein S., Wolfe R. R. Assessment of alanine, urea, and glucose interrelationships in normal subjects and in patients with sepsis with stable isotopic tracers. Surgery. 1985 May;97(5):557–568. [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Determinations of glucose turnover and oxidation in normal volunteers and septic patients using stable and radio-isotopes: the response to glucose infusion and total parenteral feeding. Aust N Z J Surg. 1986 Oct;56(10):785–791. doi: 10.1111/j.1445-2197.1986.tb02327.x. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Fatty acid and glycerol kinetics in septic patients and in patients with gastrointestinal cancer. The response to glucose infusion and parenteral feeding. Ann Surg. 1987 Apr;205(4):368–376. doi: 10.1097/00000658-198704000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Glucose and urea kinetics in patients with early and advanced gastrointestinal cancer: the response to glucose infusion, parenteral feeding, and surgical resection. Surgery. 1987 Feb;101(2):181–191. [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Glucose, fatty acid, and urea kinetics in patients with severe pancreatitis. The response to substrate infusion and total parenteral nutrition. Ann Surg. 1986 Dec;204(6):665–672. doi: 10.1097/00000658-198612000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Jeevanandam M., Brennan M. F. Protein synthesis in the tumor-influenced hepatocyte. Surgery. 1985 Aug;98(2):275–282. [PubMed] [Google Scholar]
- Waterhouse C., Kemperman J. H. Carbohydrate metabolism in subjects with cancer. Cancer Res. 1971 Sep;31(9):1273–1278. [PubMed] [Google Scholar]
- Waterhouse C. Lactate metabolism in patients with cancer. Cancer. 1974 Jan;33(1):66–71. doi: 10.1002/1097-0142(197401)33:1<66::aid-cncr2820330113>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W. Hormonal responses and their effect on metabolism. Surg Clin North Am. 1976 Oct;56(5):999–1018. doi: 10.1016/s0039-6109(16)41029-7. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Allsop J. R., Burke J. F. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979 Mar;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Durkot M. J. Evaluation of the role of the sympathetic nervous system in the response of substrate kinetics and oxidation to burn injury. Circ Shock. 1982;9(4):395–406. [PubMed] [Google Scholar]
- Wolfe R. R. Measurement of urea kinetics in vivo by means of a constant tracer infusion of di-15N-urea. Am J Physiol. 1981 Apr;240(4):E428–E434. doi: 10.1152/ajpendo.1981.240.4.E428. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Wolfe M. H., Nadel E. R., Shaw J. H. Isotopic determination of amino acid-urea interactions in exercise in humans. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jan;56(1):221–229. doi: 10.1152/jappl.1984.56.1.221. [DOI] [PubMed] [Google Scholar]


