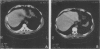
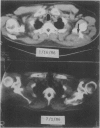
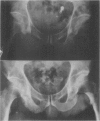
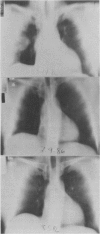
Abstract
Recent increases in knowledge of cellular immunology, combined with developments in biotechnology, have provided new opportunities for the development of immunotherapies for the treatment of cancer in humans. One approach to therapy is that of adoptive immunotherapy, that is, the transfer to the tumor bearing host of lymphoid cells with antitumor reactivity that can mediate antitumor responses. Several lymphocyte subpopulations have now been identified that may be suitable for use in adoptive immunotherapy. Resting lymphocytes incubated in interleukin-2 (IL-2) give rise to lymphokine activated killer (LAK) cells that can lyse malignant cells, but not normal cells. Clinical studies in patients with advanced cancer have revealed that treatment with high dose IL-2 alone or in combination with LAK cells can mediate the complete or partial regression of cancer in selected patients. Other approaches are currently undergoing investigation, including the adoptive transfer of tumor infiltrating lymphocytes, which, in animal models, have antitumor reactivity 50-100 times more potent than do LAK cells. Other new approaches to immunotherapy include the use of combination of lymphokines, such as the use of tumor necrosis factor or alpha interferon in conjunction with IL-2. The availability of recombinant lymphokines that provide large amounts of biologically active materials can hopefully lead to the development of effective new therapies for cancer in humans.
Full text
PDF
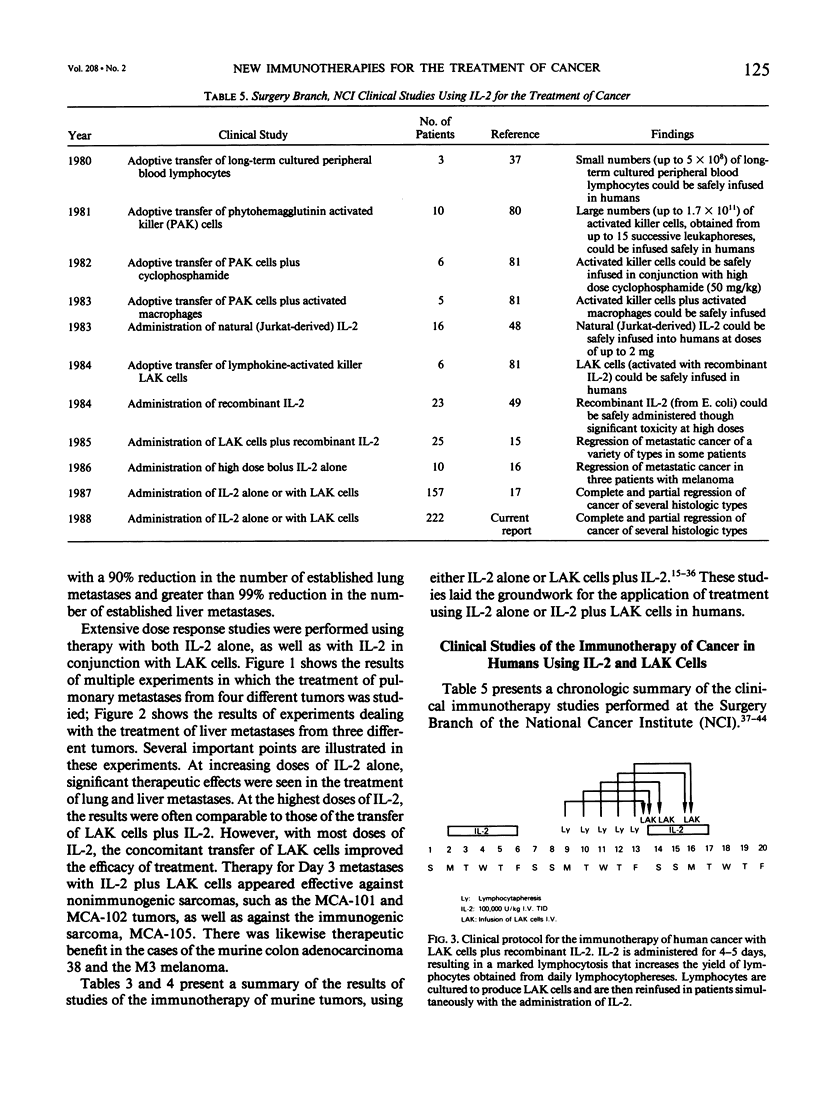
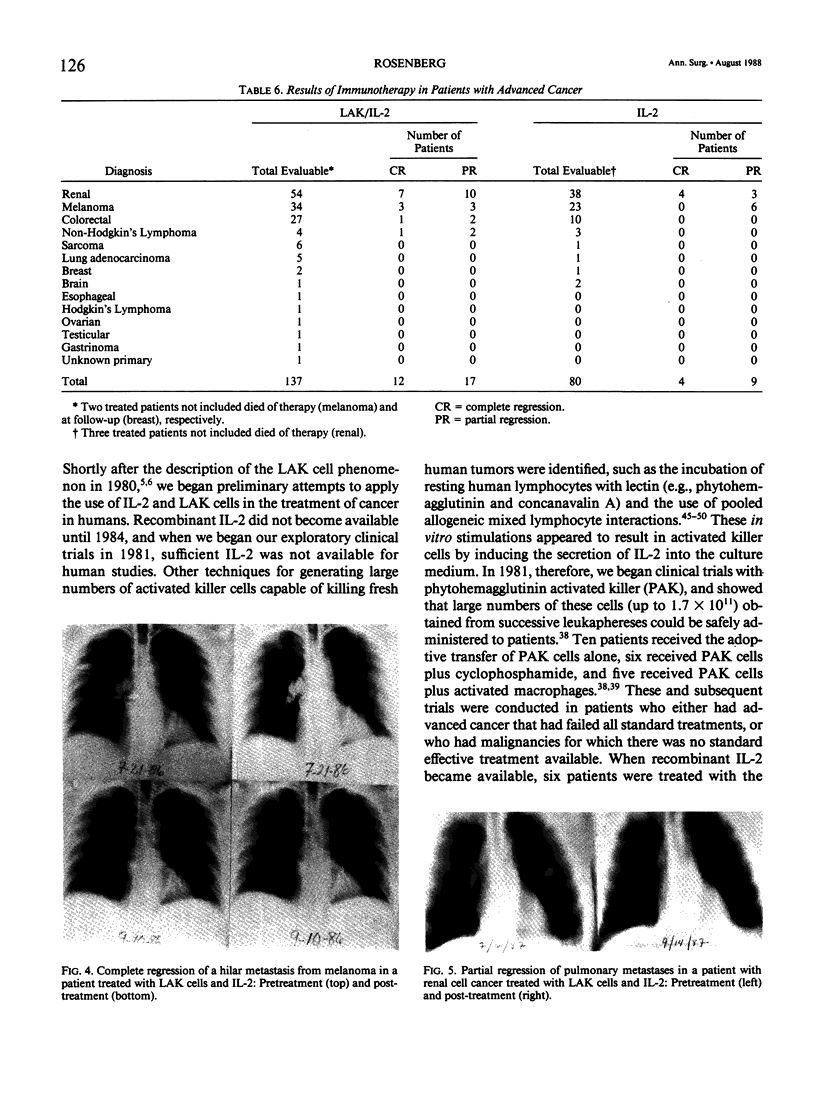
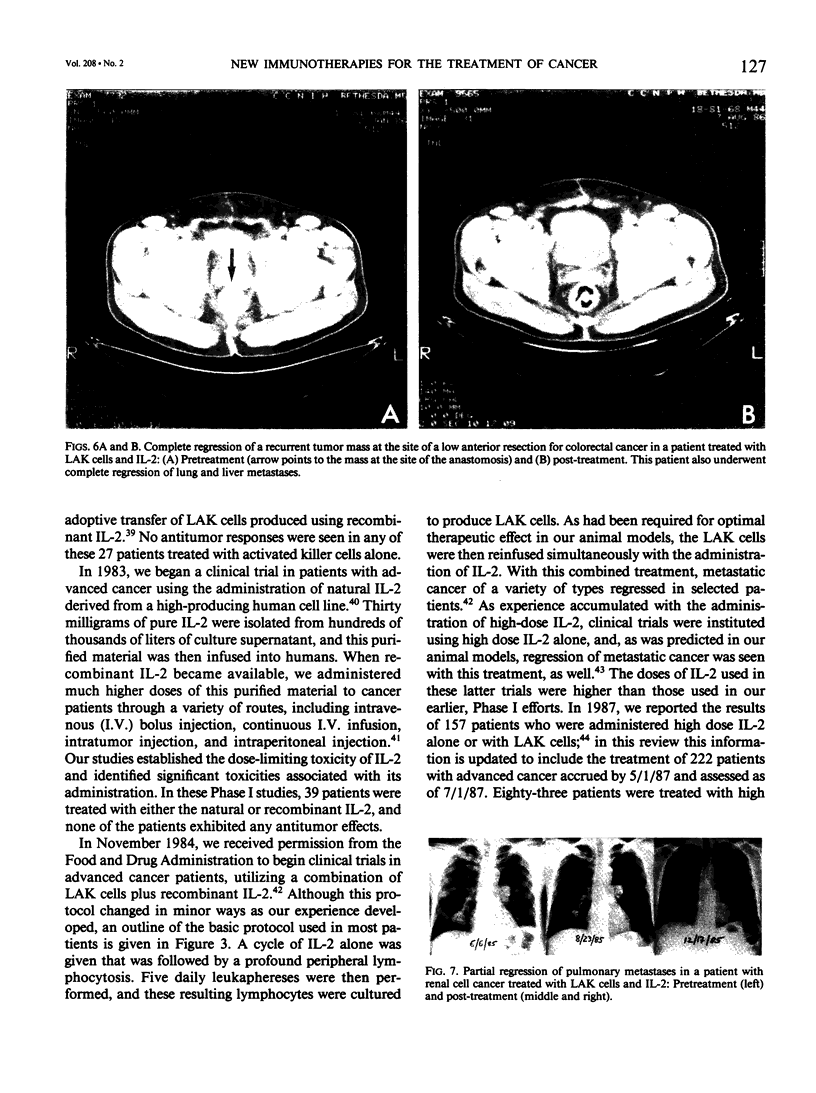
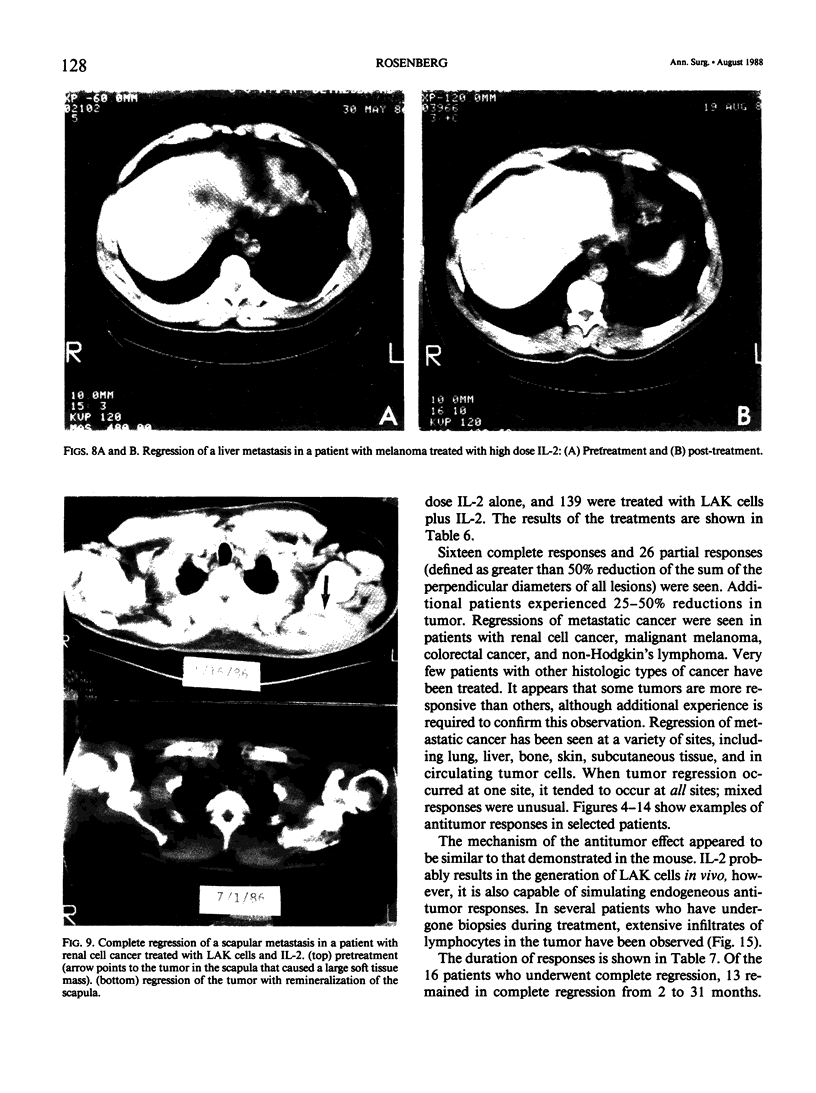



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belldegrun A., Muul L. M., Rosenberg S. A. Interleukin 2 expanded tumor-infiltrating lymphocytes in human renal cell cancer: isolation, characterization, and antitumor activity. Cancer Res. 1988 Jan 1;48(1):206–214. [PubMed] [Google Scholar]
- Belldegrun A., Webb D. E., Austin H. A., 3rd, Steinberg S. M., White D. E., Linehan W. M., Rosenberg S. A. Effects of interleukin-2 on renal function in patients receiving immunotherapy for advanced cancer. Ann Intern Med. 1987 Jun;106(6):817–822. doi: 10.7326/0003-4819-106-6-817. [DOI] [PubMed] [Google Scholar]
- Denicoff K. D., Rubinow D. R., Papa M. Z., Simpson C., Seipp C. A., Lotze M. T., Chang A. E., Rosenstein D., Rosenberg S. A. The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med. 1987 Sep;107(3):293–300. doi: 10.7326/0003-4819-107-2-293. [DOI] [PubMed] [Google Scholar]
- Donohue J. H., Rosenstein M., Chang A. E., Lotze M. T., Robb R. J., Rosenberg S. A. The systemic administration of purified interleukin 2 enhances the ability of sensitized murine lymphocytes to cure a disseminated syngeneic lymphoma. J Immunol. 1984 Apr;132(4):2123–2128. [PubMed] [Google Scholar]
- Eisenthal A., Lafreniere R., Lefor A. T., Rosenberg S. A. Effect of anti-B16 melanoma monoclonal antibody on established murine B16 melanoma liver metastases. Cancer Res. 1987 Jun 1;47(11):2771–2776. [PubMed] [Google Scholar]
- Ettinghausen S. E., Lipford E. H., 3rd, Mulé J. J., Rosenberg S. A. Recombinant interleukin 2 stimulates in vivo proliferation of adoptively transferred lymphokine-activated killer (LAK) cells. J Immunol. 1985 Nov;135(5):3623–3635. [PubMed] [Google Scholar]
- Ettinghausen S. E., Lipford E. H., 3rd, Mulé J. J., Rosenberg S. A. Systemic administration of recombinant interleukin 2 stimulates in vivo lymphoid cell proliferation in tissues. J Immunol. 1985 Aug;135(2):1488–1497. [PubMed] [Google Scholar]
- Ettinghausen S. E., Moore J. G., White D. E., Platanias L., Young N. S., Rosenberg S. A. Hematologic effects of immunotherapy with lymphokine-activated killer cells and recombinant interleukin-2 in cancer patients. Blood. 1987 Jun;69(6):1654–1660. [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Ramsey K. M., Mazumder A., Wilson D. J., Djeu J. Y., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. II. Precursor phenotype is serologically distinct from peripheral T lymphocytes, memory cytotoxic thymus-derived lymphocytes, and natural killer cells. J Exp Med. 1983 Mar 1;157(3):884–897. doi: 10.1084/jem.157.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Tilden A. B., Balch C. M. Interleukin 2 activation of cytotoxic T-lymphocytes infiltrating into human metastatic melanomas. Cancer Res. 1986 Jun;46(6):3011–3017. [PubMed] [Google Scholar]
- Kurnick J. T., Kradin R. L., Blumberg R., Schneeberger E. E., Boyle L. A. Functional characterization of T lymphocytes propagated from human lung carcinomas. Clin Immunol Immunopathol. 1986 Mar;38(3):367–380. doi: 10.1016/0090-1229(86)90247-3. [DOI] [PubMed] [Google Scholar]
- Lafreniere R., Rosenberg S. A. Adoptive immunotherapy of murine hepatic metastases with lymphokine activated killer (LAK) cells and recombinant interleukin 2 (RIL 2) can mediate the regression of both immunogenic and nonimmunogenic sarcomas and an adenocarcinoma. J Immunol. 1985 Dec;135(6):4273–4280. [PubMed] [Google Scholar]
- Lafreniere R., Rosenberg S. A. Successful immunotherapy of murine experimental hepatic metastases with lymphokine-activated killer cells and recombinant interleukin 2. Cancer Res. 1985 Aug;45(8):3735–3741. [PubMed] [Google Scholar]
- Lotze M. T., Chang A. E., Seipp C. A., Simpson C., Vetto J. T., Rosenberg S. A. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA. 1986 Dec 12;256(22):3117–3124. [PubMed] [Google Scholar]
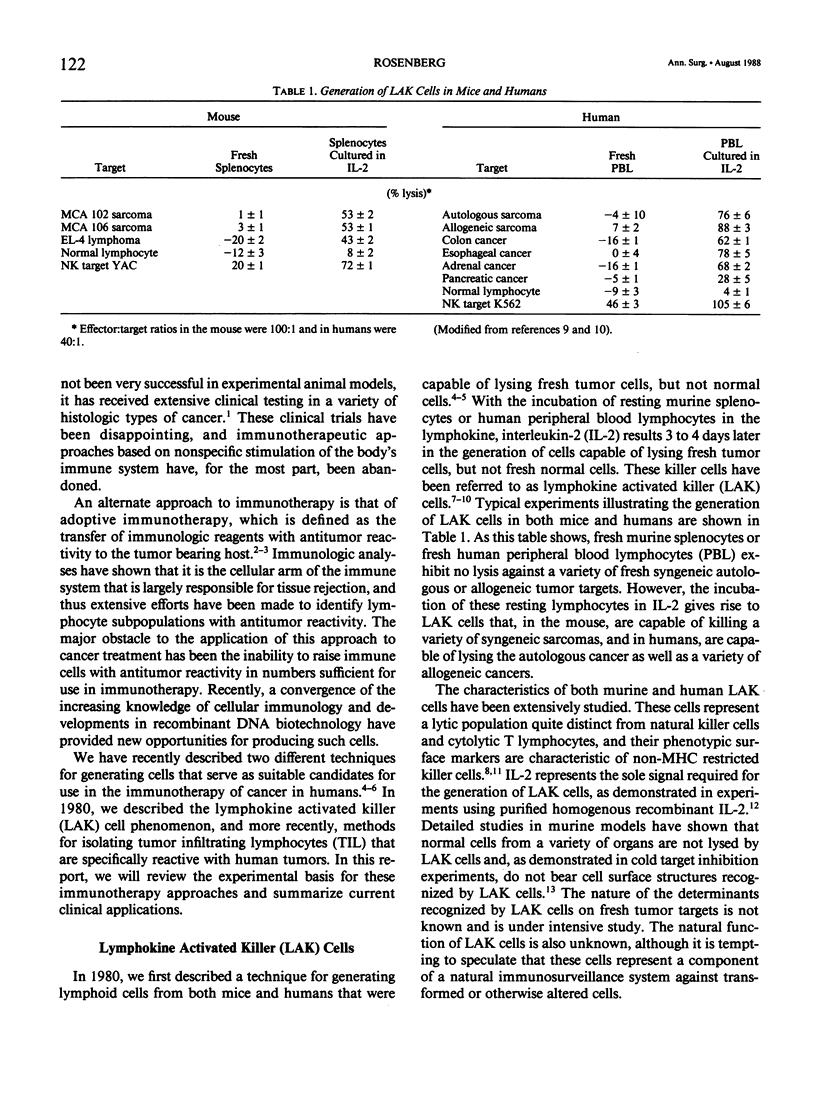
- Lotze M. T., Frana L. W., Sharrow S. O., Robb R. J., Rosenberg S. A. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol. 1985 Jan;134(1):157–166. [PubMed] [Google Scholar]
- Lotze M. T., Grimm E. A., Mazumder A., Strausser J. L., Rosenberg S. A. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981 Nov;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- Lotze M. T., Line B. R., Mathisen D. J., Rosenberg S. A. The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implications for the adoptive immunotherapy of tumors. J Immunol. 1980 Oct;125(4):1487–1493. [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Ettinghausen S. E., Rayner A. A., Sharrow S. O., Seipp C. A., Custer M. C., Rosenberg S. A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–2875. [PubMed] [Google Scholar]
- Mazumder A., Eberlein T. J., Grimm E. A., Wilson D. J., Keenan A. M., Aamodt R., Rosenberg S. A. Phase I study of the adoptive immunotherapy of human cancer with lectin activated autologous mononuclear cells. Cancer. 1984 Feb 15;53(4):896–905. doi: 10.1002/1097-0142(19840215)53:4<896::aid-cncr2820530414>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Mazumder A., Grimm E. A., Rosenberg S. A. Characterization of the lysis of fresh human solid tumors by autologous lymphocytes activated in vitro with phytohemagglutinin. J Immunol. 1983 Feb;130(2):958–964. [PubMed] [Google Scholar]
- Mazumder A., Grimm E. A., Rosenberg S. A. Lysis of fresh human solid tumor cells by autologous lymphocytes activated in vitro by allosensitization. Cancer Immunol Immunother. 1983;15(1):1–10. doi: 10.1007/BF00199454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Grimm E. A., Zhang H. Z., Rosenberg S. A. Lysis of fresh human solid tumors by autologous lymphocytes activated in vitro with lectins. Cancer Res. 1982 Mar;42(3):913–918. [PubMed] [Google Scholar]
- Mazumder A., Rosenberg S. A. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med. 1984 Feb 1;159(2):495–507. doi: 10.1084/jem.159.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Rosenstein M., Rosenberg S. A. Lysis of fresh natural killer-resistant tumor cells by lectin-activated syngeneic and allogeneic murine splenocytes. Cancer Res. 1983 Dec;43(12 Pt 1):5729–5734. [PubMed] [Google Scholar]
- Mulé J. J., Ettinghausen S. E., Spiess P. J., Shu S., Rosenberg S. A. Antitumor efficacy of lymphokine-activated killer cells and recombinant interleukin-2 in vivo: survival benefit and mechanisms of tumor escape in mice undergoing immunotherapy. Cancer Res. 1986 Feb;46(2):676–683. [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Rosenberg S. A. The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo. J Immunol. 1985 Jul;135(1):646–652. [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Schwarz S. L., Rosenberg S. A. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984 Sep 28;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- Mulé J. J., Yang J. C., Afreniere R. L., Shu S. Y., Rosenberg S. A. Identification of cellular mechanisms operational in vivo during the regression of established pulmonary metastases by the systemic administration of high-dose recombinant interleukin 2. J Immunol. 1987 Jul 1;139(1):285–294. [PubMed] [Google Scholar]
- Mulé J. J., Yang J., Shu S., Rosenberg S. A. The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo: direct correlation between reduction of established metastases and cytolytic activity of lymphokine-activated killer cells. J Immunol. 1986 May 15;136(10):3899–3909. [PubMed] [Google Scholar]
- Muul L. M., Spiess P. J., Director E. P., Rosenberg S. A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987 Feb 1;138(3):989–995. [PubMed] [Google Scholar]
- Ottow R. T., Steller E. P., Sugarbaker P. H., Wesley R. A., Rosenberg S. A. Immunotherapy of intraperitoneal cancer with interleukin 2 and lymphokine-activated killer cells reduces tumor load and prolongs survival in murine models. Cell Immunol. 1987 Feb;104(2):366–376. doi: 10.1016/0008-8749(87)90038-4. [DOI] [PubMed] [Google Scholar]
- Papa M. Z., Vetto J. T., Ettinghausen S. E., Mulé J. J., Rosenberg S. A. Effect of corticosteroid on the antitumor activity of lymphokine-activated killer cells and interleukin 2 in mice. Cancer Res. 1986 Nov;46(11):5618–5623. [PubMed] [Google Scholar]
- Papa M. Z., Yang J. C., Vetto J. T., Shiloni E., Eisenthal A., Rosenberg S. A. Combined effects of chemotherapy and interleukin 2 in the therapy of mice with advanced pulmonary tumors. Cancer Res. 1988 Jan 1;48(1):122–129. [PubMed] [Google Scholar]
- Rayner A. A., Grimm E. A., Lotze M. T., Chu E. W., Rosenberg S. A. Lymphokine-activated killer (LAK) cells. Analysis of factors relevant to the immunotherapy of human cancer. Cancer. 1985 Mar 15;55(6):1327–1333. doi: 10.1002/1097-0142(19850315)55:6<1327::aid-cncr2820550628>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Roberts K., Lotze M. T., Rosenberg S. A. Separation and functional studies of the human lymphokine-activated killer cell. Cancer Res. 1987 Aug 15;47(16):4366–4371. [PubMed] [Google Scholar]
- Rosenberg S. A. Adoptive immunotherapy of cancer: accomplishments and prospects. Cancer Treat Rep. 1984 Jan;68(1):233–255. [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A. Immunotherapy of cancer by systemic administration of lymphoid cells plus interleukin-2. J Biol Response Mod. 1984 Oct;3(5):501–511. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Mulé J. J., Spiess P. J., Reichert C. M., Schwarz S. L. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985 May 1;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Rosenstein M., Yron I., Kaufmann Y., Rosenberg S. A. Lymphokine-activated killer cells: lysis of fresh syngeneic natural killer-resistant murine tumor cells by lymphocytes cultured in interleukin 2. Cancer Res. 1984 May;44(5):1946–1953. [PubMed] [Google Scholar]
- Shiloni E., Eisenthal A., Sachs D., Rosenberg S. A. Antibody-dependent cellular cytotoxicity mediated by murine lymphocytes activated in recombinant interleukin 2. J Immunol. 1987 Mar 15;138(6):1992–1998. [PubMed] [Google Scholar]
- Shiloni E., Eisenthal A., Sachs D., Rosenberg S. A. Antibody-dependent cellular cytotoxicity mediated by murine lymphocytes activated in recombinant interleukin 2. J Immunol. 1987 Mar 15;138(6):1992–1998. [PubMed] [Google Scholar]
- Shiloni E., Lafreniere R., Mulé J. J., Schwarz S. L., Rosenberg S. A. Effect of immunotherapy with allogeneic lymphokine-activated killer cells and recombinant interleukin 2 on established pulmonary and hepatic metastases in mice. Cancer Res. 1986 Nov;46(11):5633–5640. [PubMed] [Google Scholar]
- Shu S. Y., Chou T., Rosenberg S. A. Generation from tumor-bearing mice of lymphocytes with in vivo therapeutic efficacy. J Immunol. 1987 Jul 1;139(1):295–304. [PubMed] [Google Scholar]
- Shu S., Chou T., Rosenberg S. A. In vitro sensitization and expansion with viable tumor cells and interleukin 2 in the generation of specific therapeutic effector cells. J Immunol. 1986 May 15;136(10):3891–3898. [PubMed] [Google Scholar]
- Strausser J. L., Mazumder A., Grimm E. A., Lotze M. T., Rosenberg S. A. Lysis of human solid tumors by autologous cells sensitized in vitro to alloantigens. J Immunol. 1981 Jul;127(1):266–271. [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Muul L. M., Solomon D., Rosenberg S. A. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods. 1987 Aug 24;102(1):127–141. doi: 10.1016/s0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- Vetto J. T., Papa M. Z., Lotze M. T., Chang A. E., Rosenberg S. A. Reduction of toxicity of interleukin-2 and lymphokine-activated killer cells in humans by the administration of corticosteroids. J Clin Oncol. 1987 Mar;5(3):496–503. doi: 10.1200/JCO.1987.5.3.496. [DOI] [PubMed] [Google Scholar]
- West W. H., Tauer K. W., Yannelli J. R., Marshall G. D., Orr D. W., Thurman G. B., Oldham R. K. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987 Apr 9;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- Yron I., Wood T. A., Jr, Spiess P. J., Rosenberg S. A. In vitro growth of murine T cells. V. The isolation and growth of lymphoid cells infiltrating syngeneic solid tumors. J Immunol. 1980 Jul;125(1):238–245. [PubMed] [Google Scholar]
- Zarling J. M., Robins H. I., Raich P. C., Bach F. H., Bach M. L. Generation of cytotoxic T lymphocytes to autologous human leukaemia cells by sensitisation to pooled allogeneic normal cells. Nature. 1978 Jul 20;274(5668):269–271. doi: 10.1038/274269a0. [DOI] [PubMed] [Google Scholar]