Abstract
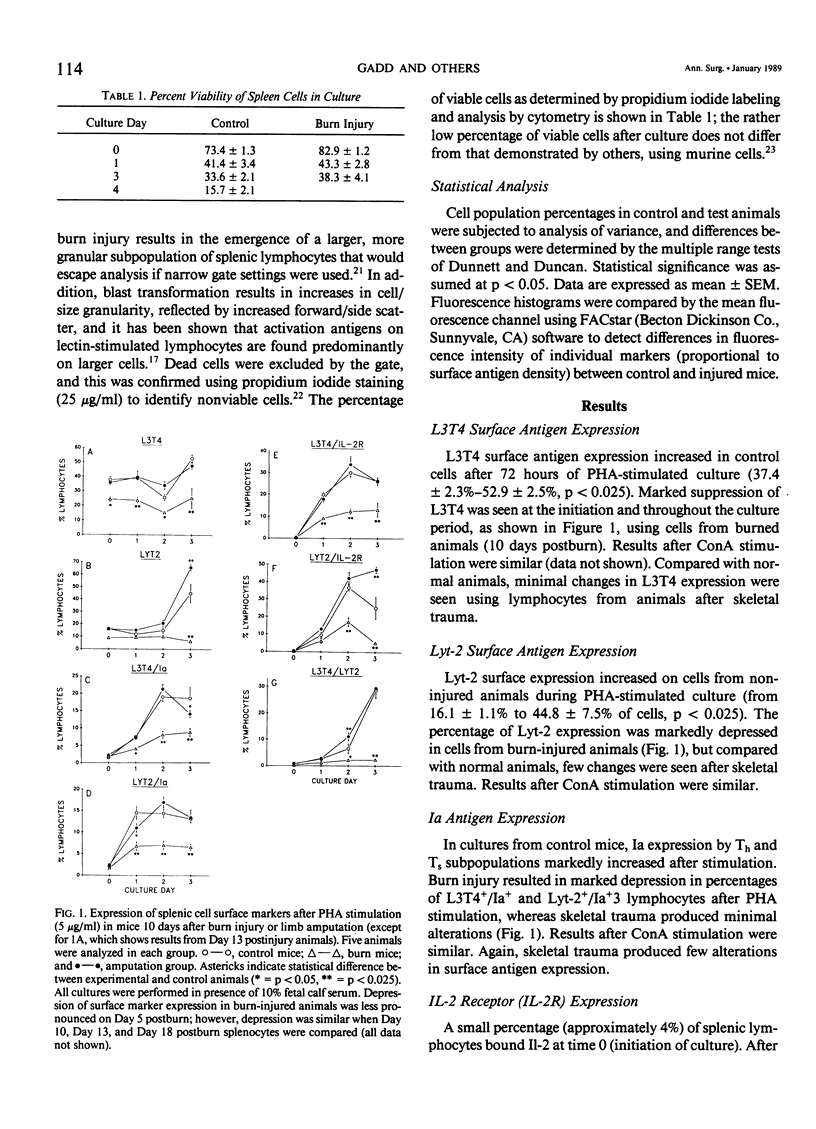
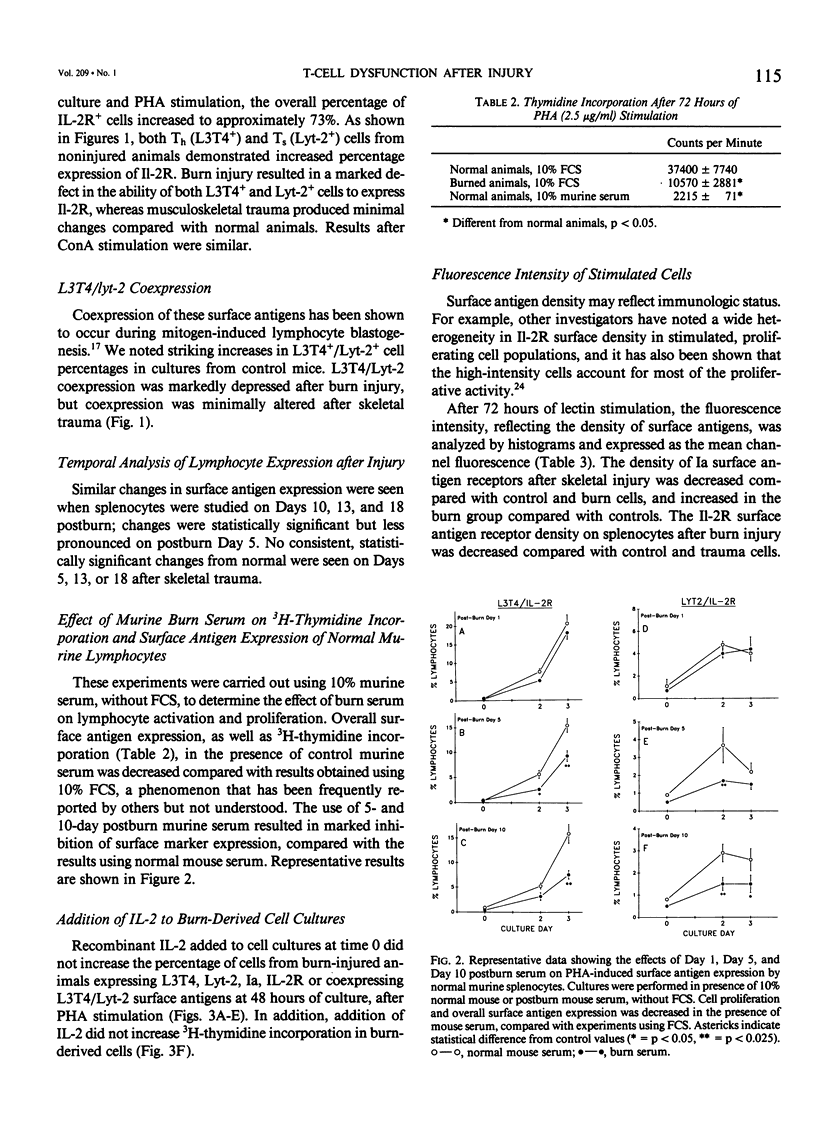
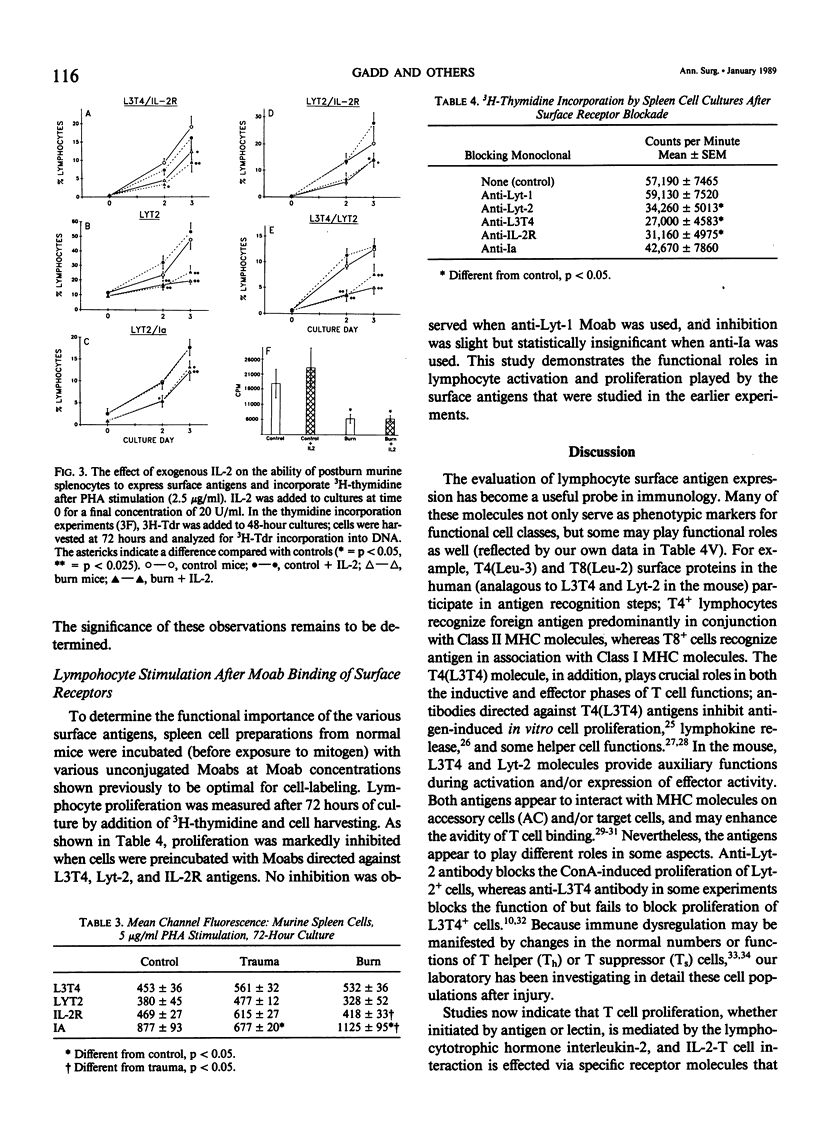
Murine spleen T-cell activation in lectin-stimulated cultures after 25% body surface area burn injury or hind-limb amputation was studied by measuring the temporal expression of cell surface markers using monoclonal antibodies and two-color flow cytometry. Lymphocyte activation has been shown to be accompanied by the appearance of new surface antigens, including Interleukin-2 (IL-2) deceptor (IL-2R) and Ia, and emergence of cells that coexpress helper (Th) and suppressor (Ts) surface markers. IL-2R has been shown to appear early on stimulated cells, before DNA synthesis, whereas Ia appears later. Surface markers (L3T4, Lyt2, Ia, and IL-2R) were analyzed at time 0 and after 24, 48, and 72 hours of mitogen-stimulated culture. The appearance of IL-2R and Ia on Th (L3T4+) and Ts (Lyt-2+) populations was markedly depressed after burn injury, but minimal changes were seen after musculoskeletal injury. In addition, coexpression of L3T4/Lyt2 antigens was markedly reduced in burn-derived cells. Serum from burn-injured animals caused depression of surface antigen expression by stimulated normal cells. Recombinant IL-2, when added to burn-derived cell cultures, did not increase expression of these surface markers during culture, nor did it improve proliferation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Miller C. L., Trunkey D. D., Lim R. C., Jr Identity of mononuclear cells which compromise the resistance of trauma patients. J Surg Res. 1979 May;26(5):478–487. doi: 10.1016/0022-4804(79)90037-4. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Reinherz E. L., Poppema S., McCluskey R. T., Schlossman S. F. Location of T cell and major histocompatibility complex antigens in the human thymus. J Exp Med. 1980 Oct 1;152(4):771–782. doi: 10.1084/jem.152.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddison W. E., Rao P. E., Talle M. A., Goldstein G., Shaw S. Possible involvement of the T4 molecule in T cell recognition of class II HLA antigens: evidence from studies of proliferative responses to SB antigens. J Immunol. 1983 Jul;131(1):152–157. [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Schlossman S. F. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985 Apr;134(4):2281–2286. [PubMed] [Google Scholar]
- Boudet R. A., Morse P. A., Jr, Watts L. M. An analysis of phytohemagglutinin lymphoblastogenesis in whole blood. Surg Gynecol Obstet. 1978 Apr;146(4):609–616. [PubMed] [Google Scholar]
- Braylan R. C., Benson N. A., Nourse V., Kruth H. S. Correlated analysis of cellular DNA, membrane antigens and light scatter of human lymphoid cells. Cytometry. 1982 Mar;2(5):337–343. doi: 10.1002/cyto.990020511. [DOI] [PubMed] [Google Scholar]
- Broome J. D., Jeng M. W. Promotion of replication in lymphoid cells by specific thiols and disulfides in vitro. Effects on mouse lymphoma cells in comparison with splenic lymphocytes. J Exp Med. 1973 Sep 1;138(3):574–592. doi: 10.1084/jem.138.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. R. Regulation of autoimmune disease physiological and therapeutic. Immunol Rev. 1986 Dec;94:5–21. doi: 10.1111/j.1600-065x.1986.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Cotner T., Williams J. M., Christenson L., Shapiro H. M., Strom T. B., Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983 Feb 1;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K. Regulation of human polymorphonuclear neutrophil (PMN) activity against Candida albicans by large granular lymphocytes via release of a PMN-activating factor. J Immunol. 1987 Oct 15;139(8):2761–2767. [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist E., Mewes A., Baker C. C., Strasser T., Alkan S. S., Rieber P., Heberer G. Prostaglandin E2 (PGE2)-dependent suppression of interleukin alpha (IL-2) production in patients with major trauma. J Trauma. 1987 Aug;27(8):837–848. doi: 10.1097/00005373-198708000-00001. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Variability of sequential studies of lymphocyte blastogenesis in normal adults. Clin Exp Immunol. 1976 Jul;25(1):28–35. [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Flood P. M., Gershon R. K. Immunoregulatory T-cell pathways. Annu Rev Immunol. 1983;1:439–463. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- Gutstein N. L., Wofsy D. Administration of F(ab')2 fragments of monoclonal antibody to L3T4 inhibits humoral immunity in mice without depleting L3T4+ cells. J Immunol. 1986 Dec 1;137(11):3414–3419. [PubMed] [Google Scholar]
- Hansbrough J. F., Peterson V., Kortz E., Piacentine J. Postburn immunosuppression in an animal model: monocyte dysfunction induced by burned tissue. Surgery. 1983 Mar;93(3):415–423. [PubMed] [Google Scholar]
- Hansbrough J. F., Soderberg C., Field T. O., Jr, Swisher S., Brahme J., Zapata-Sirvent R. L., Tonks M., Gadd M. A. Analysis of murine lymphocyte subpopulations by dual-color flow cytometry: technical considerations and specificities of monoclonal antibodies directed against surface markers. J Surg Res. 1988 Feb;44(2):121–136. doi: 10.1016/0022-4804(88)90040-6. [DOI] [PubMed] [Google Scholar]
- Helderman J. H., Strom T. B. Role of protein and RNA synthesis in the development of insulin binding sites on activated thymus-derived lymphocytes. J Biol Chem. 1979 Aug 10;254(15):7203–7207. [PubMed] [Google Scholar]
- Kern D. E., Lachmann L. B., Greenberg P. D. Lyt-2+ cells. Requirements for concanavalin A-induced proliferation and interleukin 2 production. J Immunol. 1987 Nov 1;139(9):2880–2887. [PubMed] [Google Scholar]
- Marrack P., Endres R., Shimonkevitz R., Zlotnik A., Dialynas D., Fitch F., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med. 1983 Oct 1;158(4):1077–1091. doi: 10.1084/jem.158.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Palacios R. Concanavalin A triggers T lymphocytes by directly interacting with their receptors for activation. J Immunol. 1982 Jan;128(1):337–342. [PubMed] [Google Scholar]
- Payne J., Hayden P., Meyer H. J., Walls R. S. Observed differences in postoperative lymphocyte transformation are explained by patient and population variations. Am J Surg. 1984 Feb;147(2):237–242. doi: 10.1016/0002-9610(84)90097-7. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Boldt D., Kornfeld S., Skinner A., Valeri C. R. Excretion of deoxyribonucleic acid by lymphocytes stimulated with phytohemagglutinin or antigen. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1685–1689. doi: 10.1073/pnas.69.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogozinski L., Bass A., Glickman E., Talle M. A., Goldstein G., Wang J., Chess L., Thomas Y. The T4 surface antigen is involved in the induction of helper function. J Immunol. 1984 Feb;132(2):735–739. [PubMed] [Google Scholar]
- Sarmiento M., Dialynas D. P., Lancki D. W., Wall K. A., Lorber M. I., Loken M. R., Fitch F. W. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Sasaki D. T., Dumas S. E., Engleman E. G. Discrimination of viable and non-viable cells using propidium iodide in two color immunofluorescence. Cytometry. 1987 Jul;8(4):413–420. doi: 10.1002/cyto.990080411. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Davignon D., Ho M. K., Kürzinger K., Martz E., Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Dialynas D. P., Fitch F. W., English M. Monoclonal antibody to L3T4 blocks the function of T cells specific for class 2 major histocompatibility complex antigens. J Immunol. 1984 Mar;132(3):1118–1123. [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. IV. Induction of suppressor cells within the OKT4+ population. J Exp Med. 1981 Aug 1;154(2):459–467. doi: 10.1084/jem.154.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Umiel T., Daley J. F., Bhan A. K., Levey R. H., Schlossman S. F., Reinherz E. L. Acquisition of immune competence by a subset of human cortical thymocytes expressing mature T cell antigens. J Immunol. 1982 Sep;129(3):1054–1060. [PubMed] [Google Scholar]
- Wang B. S., Heacock E. H., Wu A. V., Mannick J. A. Generation of suppressor cells in mice after surgical trauma. J Clin Invest. 1980 Aug;66(2):200–209. doi: 10.1172/JCI109845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbart R. H., Lusis A. J., Kacena A., Spolter L., Eggena P., Golde D. W. Neutrophil migration inhibition factor from T lymphocytes (NIF-T): partial purification by antibody affinity chromatography and further characterization. Clin Immunol Immunopathol. 1982 Mar;22(3):408–418. doi: 10.1016/0090-1229(82)90058-7. [DOI] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Zapata-Sirvent R., Hansbrough J. F., Bartle E. J. Prevention of posttraumatic alterations in lymphocyte subpopulations in mice by immunomodulating drugs. Arch Surg. 1986 Jan;121(1):116–122. doi: 10.1001/archsurg.1986.01400010130018. [DOI] [PubMed] [Google Scholar]
- van Agthoven A., Terhorst C., Reinherz E., Schlossman S. Characterization of T cell surface glycoproteins T 1 and T 3 present on all human peripheral T lymphocytes and functionally mature thymocytes. Eur J Immunol. 1981 Jan;11(1):18–21. doi: 10.1002/eji.1830110105. [DOI] [PubMed] [Google Scholar]


