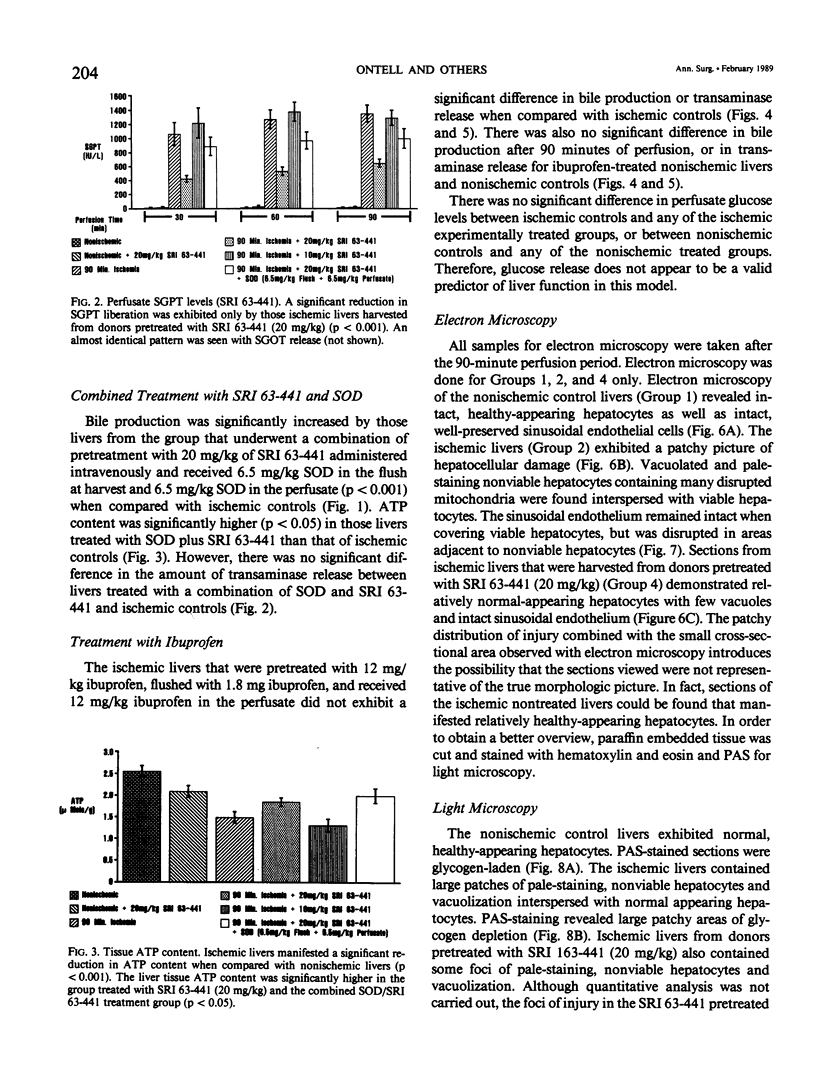
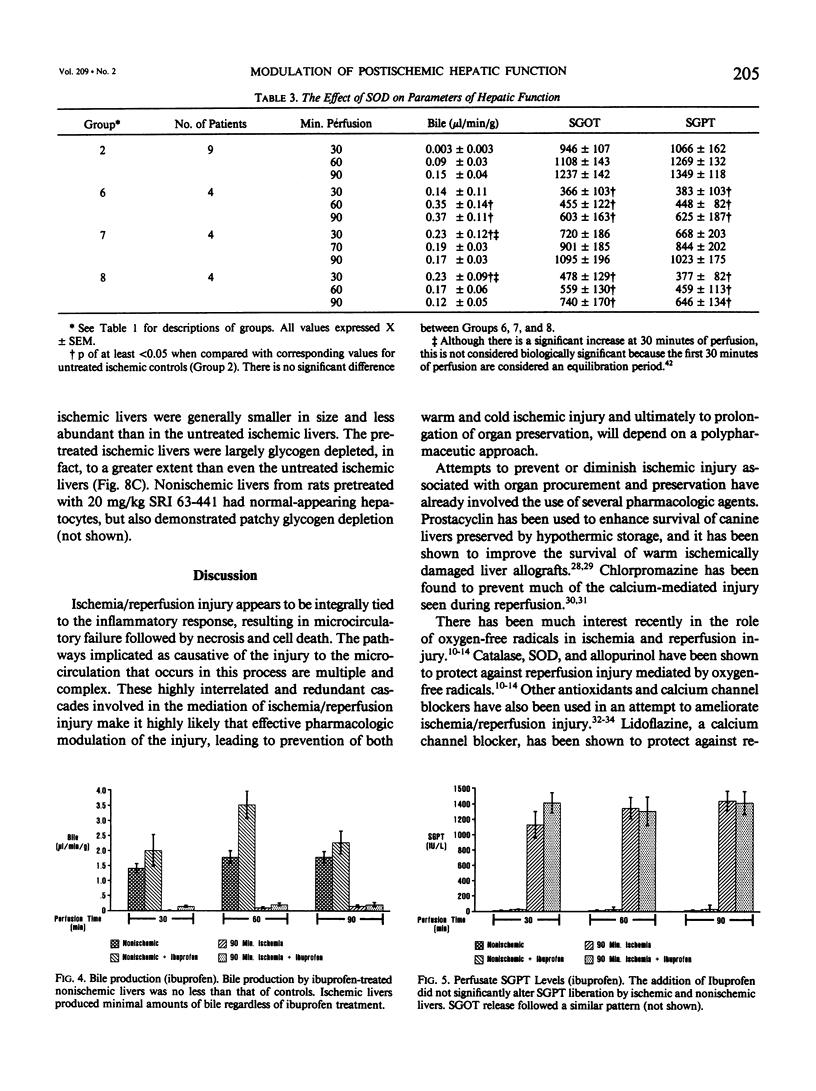
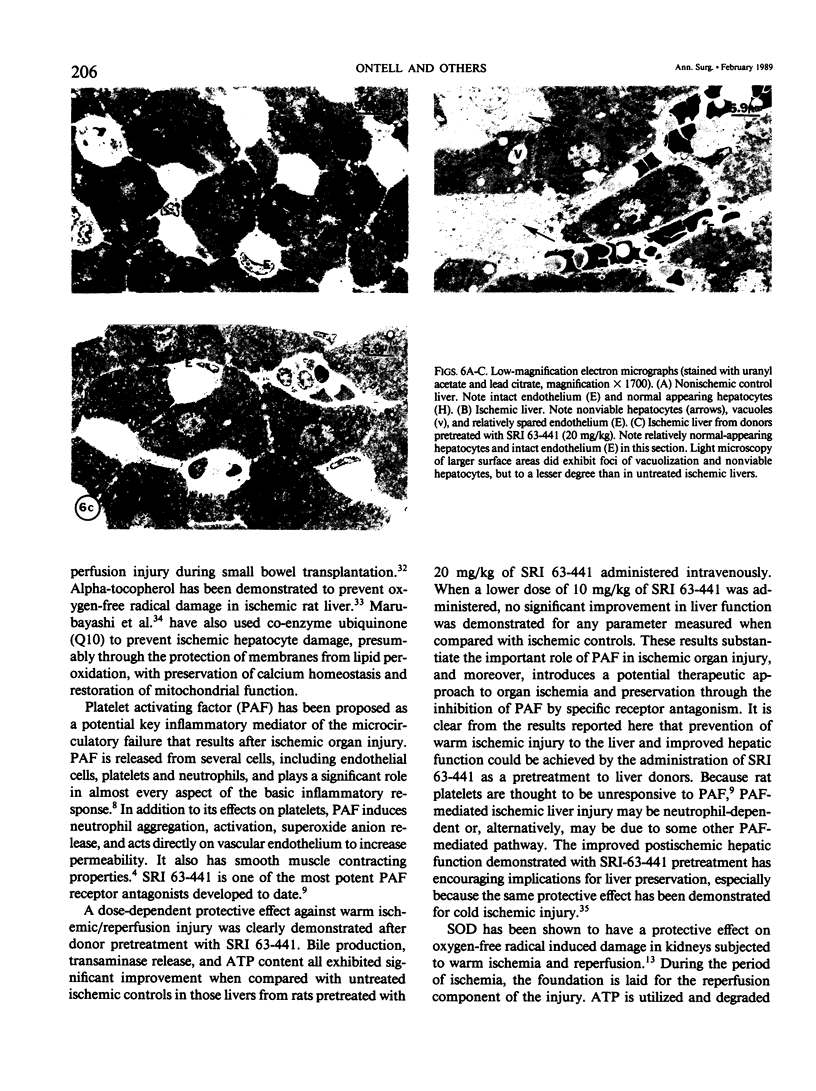
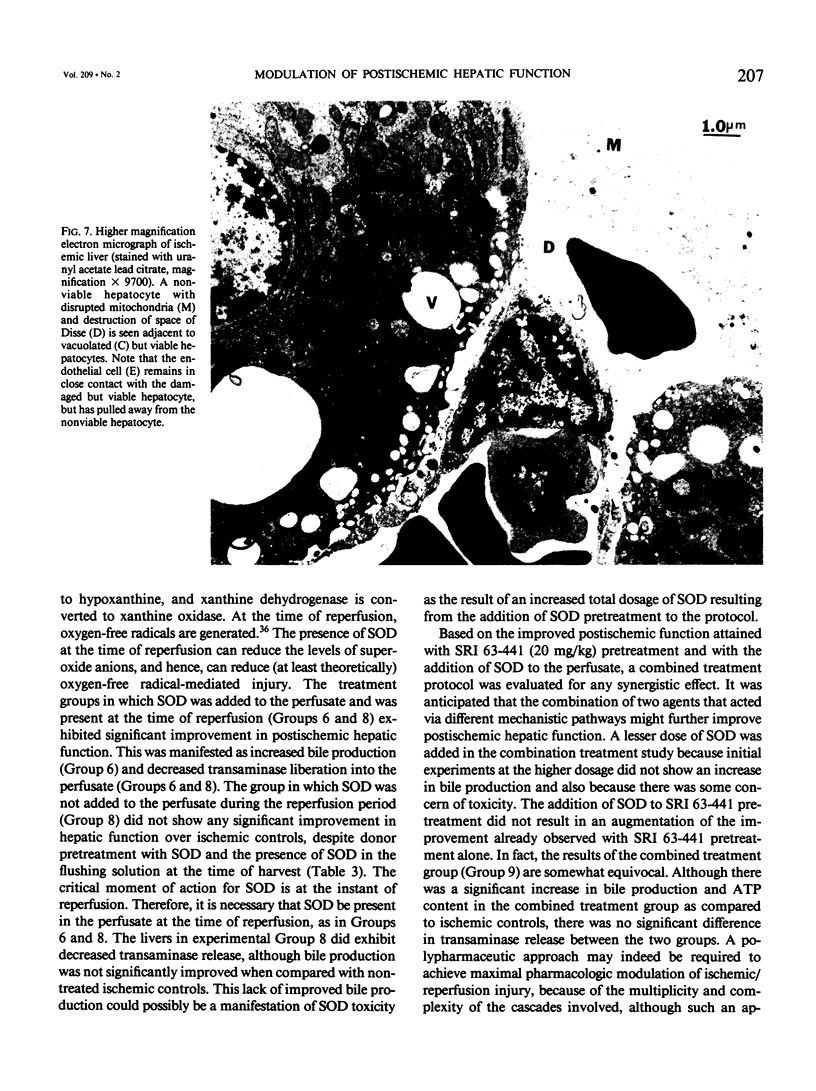
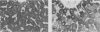Abstract
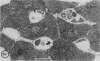
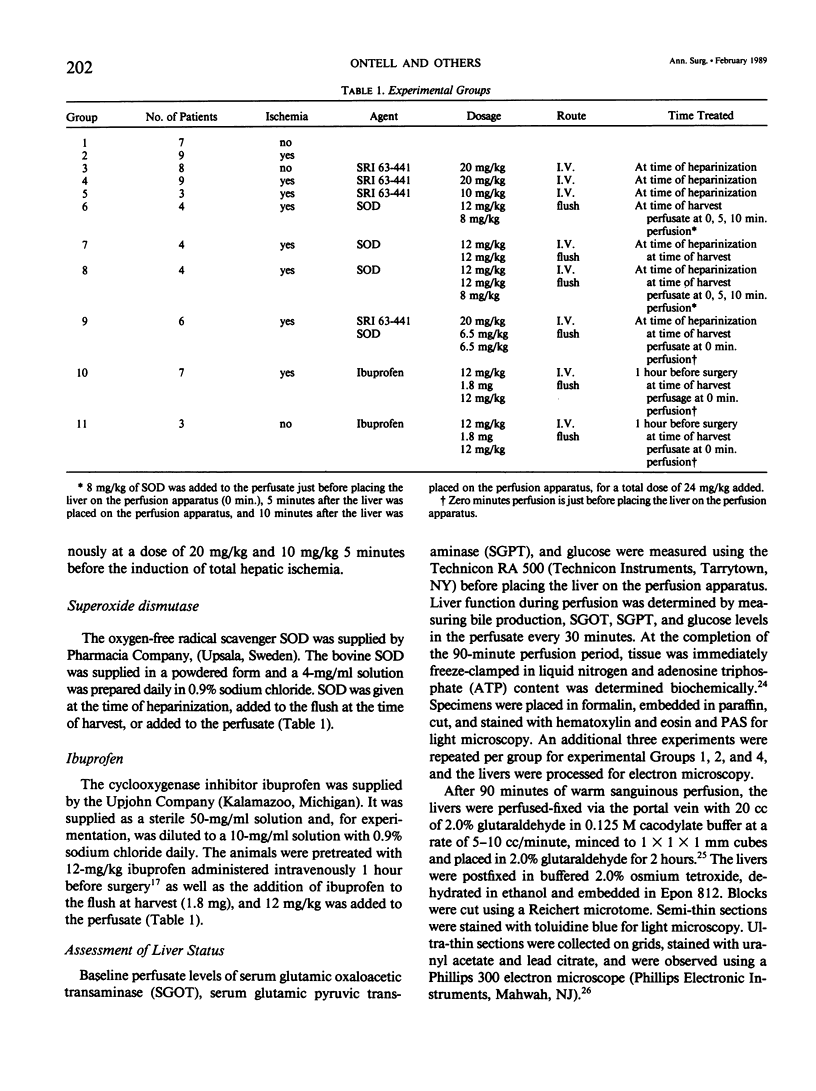
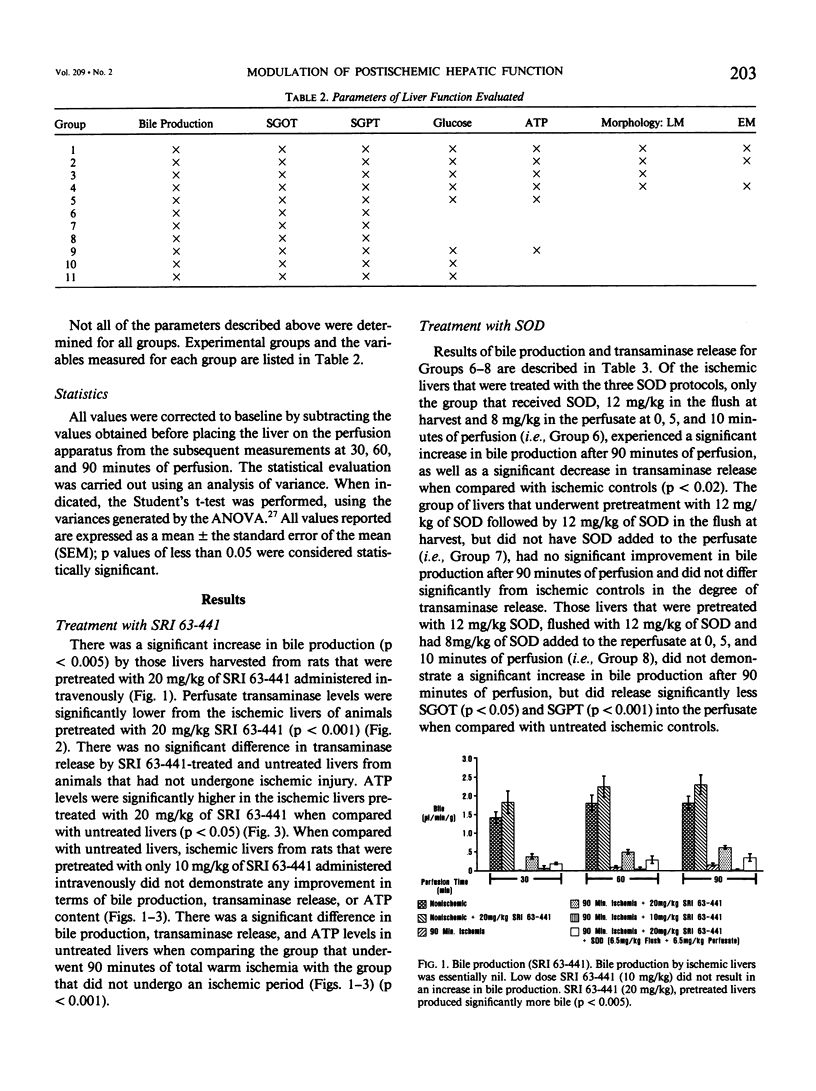
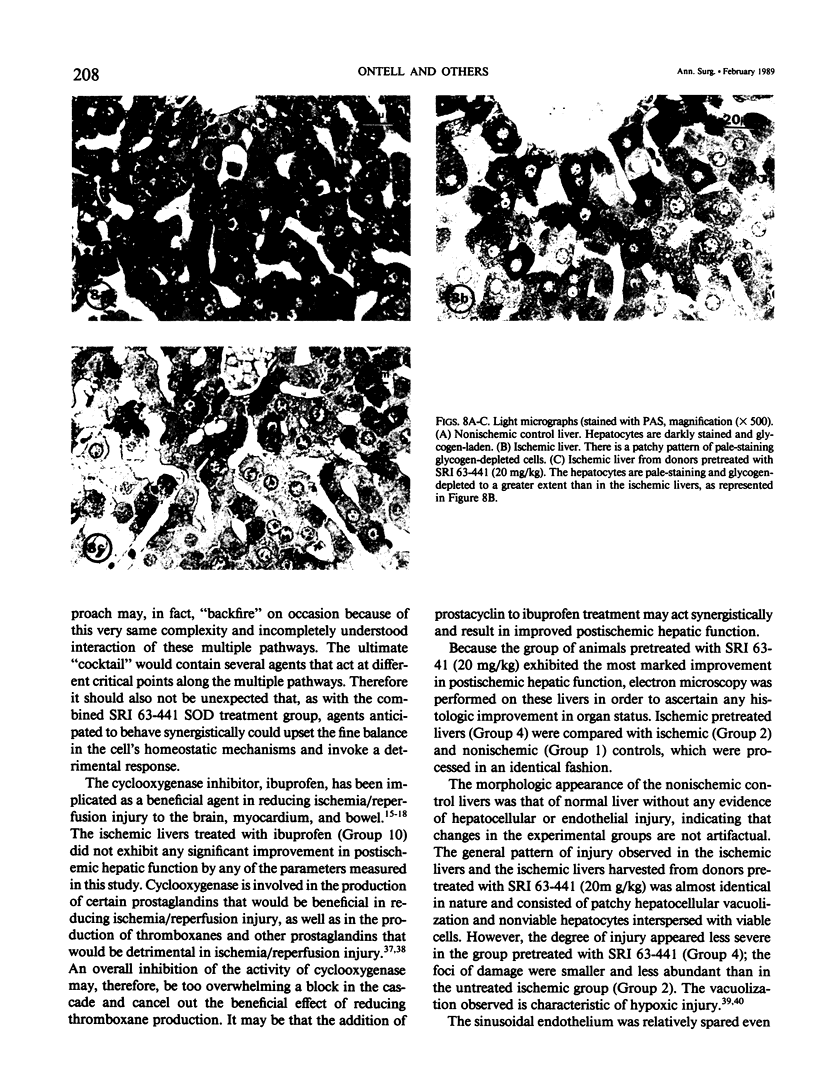
The present study evaluated and compared the effects of SRI 63-441, a potent platelet activating factor antagonist, superoxide dismutase (SOD), an oxygen free radical scavenger, and ibuprofen, a cyclooxygenase inhibitor on hepatic function after 90 minutes of warm ischemia. After warm ischemia, livers were harvested and underwent 90 minutes of warm, oxygenated, sanguinous perfusion on an isolated liver perfusion apparatus. Pretreatment of donor animals with 20 mg/kg intravenous (I.V.) SRI 63-441 5 minutes before induction of total hepatic ischemia resulted in significantly increased bile production, a significant decrease in transaminase release, and a higher tissue adenosine triphosphate (ATP) content when compared with ischemic nontreated controls. SOD resulted in improved bile production and decreased transaminase liberation only when present in the perfusate at the time of in vitro reperfusion. Ibuprofen did not improve postischemic hepatic function in this model. Electron microscopy revealed patchy hepatocellular vacuolization with an intact sinusoidal endothelium in all ischemic livers. However, the degree of damage was less severe in the livers from those rats pretreated with 20 mg/kg SRI 63-441. This study demonstrates that SRI 63-441 pretreatment significantly reduces hepatic warm ischemic injury, and in the present model, appears superior to two other agents that have been advanced in the treatment of ischemic injury. The use of such agents singly or in combinations have important implications as regards gaining a better understanding of the basic mechanisms in organ ischemia, and moreover, for therapeutic applications in organ ischemia and preservation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHFORD T. P., BURDETTE W. J. RESPONSE OF THE ISOLATED PERFUSED HEPATIC PARENCHYMA TO HYPOXIA. Ann Surg. 1965 Aug;162:191–207. doi: 10.1097/00000658-196508000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M. J., Bentley I. S., Tanner A. R., Saunders P. K., Millward-Sadler G. H., Wright R. Oxygen-derived free radicals promote hepatic injury in the rat. Gastroenterology. 1985 Nov;89(5):1114–1122. doi: 10.1016/0016-5085(85)90218-5. [DOI] [PubMed] [Google Scholar]
- Atalla S. L., Toledo-Pereyra L. H., MacKenzie G. H., Cederna J. P. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation. 1985 Dec;40(6):584–590. doi: 10.1097/00007890-198512000-00002. [DOI] [PubMed] [Google Scholar]
- Baker G. L., Corry R. J., Autor A. P. Oxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion. Protective effect of superoxide dismutase. Ann Surg. 1985 Nov;202(5):628–641. doi: 10.1097/00000658-198511000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Biffignandi P., Arese P. Platelet-activating factor--a powerful lipid autacoid possibly involved in microangiopathy. Acta Haematol. 1986;75(3):129–140. doi: 10.1159/000206106. [DOI] [PubMed] [Google Scholar]
- Buxton D. B., Shukla S. D., Hanahan D. J., Olson M. S. Stimulation of hepatic glycogenolysis by acetylglyceryl ether phosphorylcholine. J Biol Chem. 1984 Feb 10;259(3):1468–1471. [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Pfau R. G., Farber J. L. Prevention by chlorpromazine of ischemic liver cell death. Am J Pathol. 1977 Sep;88(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- Fahimi H. D. Perfusion and immersion fixation of rat liver with glutaraldehyde. Lab Invest. 1967 May;16(5):736–750. [PubMed] [Google Scholar]
- Farber J. L., Chien K. R., Mittnacht S., Jr Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981 Feb;102(2):271–281. [PMC free article] [PubMed] [Google Scholar]
- Grosfeld J. L., Kamman K., Gross K., Cikrit D., Ross D., Wolfe M., Katz S., Weber T. R. Comparative effects of indomethacin, prostaglandin E1, and ibuprofen on bowel ischemia. J Pediatr Surg. 1983 Dec;18(6):738–742. doi: 10.1016/s0022-3468(83)80015-3. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Berry M. N., Williams M. C., Severinghaus E. M. A simple and inexpensive membrane "lung" for small organ perfusion. J Lipid Res. 1974 Mar;15(2):182–186. [PubMed] [Google Scholar]
- Jugdutt B. I., Hutchins G. M., Bulkley B. H., Becker L. C. Salvage of ischemic myocardium by ibuprofen during infarction in the conscious dog. Am J Cardiol. 1980 Jul;46(1):74–82. doi: 10.1016/0002-9149(80)90608-6. [DOI] [PubMed] [Google Scholar]
- Koyama I., Bulkley G. B., Williams G. M., Im M. J. The role of oxygen free radicals in mediating the reperfusion injury of cold-preserved ischemic kidneys. Transplantation. 1985 Dec;40(6):590–595. doi: 10.1097/00007890-198512000-00003. [DOI] [PubMed] [Google Scholar]
- Kuhn J. E., Steimle C. N., Zelenock G. B., D'Alecy L. G. Ibuprofen improves survival and neurologic outcome after resuscitation from cardiac arrest. Resuscitation. 1986 Dec;14(4):199–212. doi: 10.1016/0300-9572(86)90064-x. [DOI] [PubMed] [Google Scholar]
- LEVINE R. A., PESCH L. A., KLATSKIN G., GIARMAN N. J. EFFECT OF SEROTONIN ON GLYCOGEN METABOLISM IN ISOLATED RAT LIVER. J Clin Invest. 1964 May;43:797–809. doi: 10.1172/JCI104966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer A. M., Polansky E. W. Beneficial effects of ibuprofen in acute myocardial ischemia. Cardiology. 1979;64(5):265–279. doi: 10.1159/000170624. [DOI] [PubMed] [Google Scholar]
- Lelcuk S., Alexander F., Kobzik L., Valeri C. R., Shepro D., Hechtman H. B. Prostacyclin and thromboxane A2 moderate postischemic renal failure. Surgery. 1985 Aug;98(2):207–212. [PubMed] [Google Scholar]
- MILLER L. L., BLY C. G., WATSON M. L., BALE W. F. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J Exp Med. 1951 Nov;94(5):431–453. doi: 10.1084/jem.94.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubayashi S., Dohi K., Ochi K., Kawasaki T. Role of free radicals in ischemic rat liver cell injury: prevention of damage by alpha-tocopherol administration. Surgery. 1986 Feb;99(2):184–192. [PubMed] [Google Scholar]
- Marubayashi S., Dohi K., Yamada K., Kawasaki T. Changes in the levels of endogenous coenzyme Q homologs, alpha-tocopherol, and glutathione in rat liver after hepatic ischemia and reperfusion, and the effect of pretreatment with coenzyme Q10. Biochim Biophys Acta. 1984 Jan 24;797(1):1–9. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Monden M., Fortner J. G. Twenty-four- and 48-hour canine liver preservation by simple hypothermia with prostacyclin. Ann Surg. 1982 Jul;196(1):38–42. doi: 10.1097/00000658-198207000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K., Fukumoto O., Fujimoto K., Fujikawa Y. Development of hypoxic change of the liver cells as revealed by the isolated perfused rat liver. Acta Pathol Jpn. 1971 Aug;21(3):313–328. doi: 10.1111/j.1440-1827.1971.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Ontell S. J., Colella M. S., Horowitz J., Makowka L., Trager J., Starzl T. E. Applications of the isolated perfused rat liver in transplantation research. J Invest Surg. 1988;1(1):25–27. doi: 10.3109/08941938809141072. [DOI] [PubMed] [Google Scholar]
- Parks W. M., Hoak J. C., Czervionke R. L. Comparative effect of ibuprofen on endothelial and platelet prostaglandin synthesis. J Pharmacol Exp Ther. 1981 Nov;219(2):415–419. [PubMed] [Google Scholar]
- Pinckard R. N. Platelet-activating factor. Hosp Pract (Off Ed) 1983 Nov;18(11):67–76. doi: 10.1080/21548331.1983.11702680. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G. L., Scholle J. L., Shepherd A. P., Ward W. F. Effects of hematocrit on oxygenation of the isolated perfused rat liver. Am J Physiol. 1983 Dec;245(6):G769–G774. doi: 10.1152/ajpgi.1983.245.6.G769. [DOI] [PubMed] [Google Scholar]
- Saunders R. N., Handley D. A. Platelet-activating factor antagonists. Annu Rev Pharmacol Toxicol. 1987;27:237–255. doi: 10.1146/annurev.pa.27.040187.001321. [DOI] [PubMed] [Google Scholar]
- Snyder F. Chemical and biochemical aspects of platelet activating factor: a novel class of acetylated ether-linked choline-phospholipids. Med Res Rev. 1985 Jan-Mar;5(1):107–140. doi: 10.1002/med.2610050105. [DOI] [PubMed] [Google Scholar]
- Toledo-Pereyra L. H. Role of prostaglandins (PGI2) in improving the survival of ischemically damaged liver allografts. Trans Am Soc Artif Intern Organs. 1984;30:390–394. [PubMed] [Google Scholar]
- Vargaftig B. B., Chignard M., Benveniste J., Lefort J., Wal F. Background and present status of research on platelet-activating factor (PAF-acether). Ann N Y Acad Sci. 1981;370:119–137. doi: 10.1111/j.1749-6632.1981.tb29727.x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Chignard M., Lefort J., Benveniste J. Platelet-tissue interaction: role of platelet-activating factor (PAF-acether). Agents Actions. 1980 Dec;10(6):502–506. doi: 10.1007/BF02024151. [DOI] [PubMed] [Google Scholar]