Abstract
Recent evidence suggests a regulatory connection between cell volume, endoplasmic reticulum (ER) export, and stimulated Golgi-to-ER transport. To investigate the potential role of protein kinases we tested a panel of protein kinase inhibitors for their effect on these steps. One inhibitor, H89, an isoquinolinesulfonamide that is commonly used as a selective protein kinase A inhibitor, blocked both ER export and hypo-osmotic-, brefeldin A-, or nocodazole-induced Golgi-to-ER transport. In contrast, H89 did not block the constitutive ER Golgi-intermediate compartment (ERGIC)-to-ER and Golgi-to-ER traffic that underlies redistribution of ERGIC and Golgi proteins into the ER after ER export arrest. Surprisingly, other protein kinase A inhibitors, KT5720 and H8, as well as a set of protein kinase C inhibitors, had no effect on these transport processes. To test whether H89 might act at the level of either the coatomer protein (COP)I or the COPII coat protein complex we examined the localization of βCOP and Sec13 in H89-treated cells. H89 treatment led to a rapid loss of Sec13-labeled ER export sites but βCOP localization to the Golgi was unaffected. To further investigate the effect of H89 on COPII we developed a COPII recruitment assay with permeabilized cells and found that H89 potently inhibited binding of exogenous Sec13 to ER export sites. This block occurred in the presence of guanosine-5′-O-(3-thio)triphosphate, suggesting that Sec13 recruitment is inhibited at a step independent of the activation of the GTPase Sar1. These results identify a requirement for an H89-sensitive factor(s), potentially a novel protein kinase, in recruitment of COPII to ER export sites, as well as in stimulated but not constitutive Golgi-to-ER transport.
INTRODUCTION
The abundance of certain subcellular compartments is dramatically altered by changes in metabolic state (Nunnari, 1996); therefore, it seems reasonable that organelle biogenesis in general would be responsive to changes in cell physiology. Accumulating evidence in mammalian cells supports the notion that transport through the constitutive secretory pathway can be regulated according to the demands of cell physiology. Most notably, pharmacological agents that alter the activity of protein kinase A (PKA) and protein kinase C (PKC), as well as trimeric G proteins, have significant effects on trafficking both in vivo and in vitro (De Matteis et al., 1993; Hansen and Casanova, 1994; Ohashi and Huttner, 1994; Pimplikar and Simons, 1994; Muniz et al., 1996). Despite the data implicating key signal-transducing molecules in the regulation of constitutive transport, the physiological conditions that elicit such changes remain poorly understood, and the precise identity of these regulators as well as the mechanisms by which these regulators influence trafficking remains poorly understood.
Recently, we identified osmotically induced cell volume changes as one physiological stimulus that leads to dramatic alterations in both anterograde and retrograde transport between the endoplasmic reticulum (ER) and Golgi. Hypotonic conditions lead to an abrupt inhibition of ER export, but at the same time, retrograde transport from the Golgi to the ER is stimulated. The net result is the collapse of both the Golgi and ER-Golgi-intermediate compartment (ERGIC) into the ER (Lee and Linstedt, 1999). To begin to identify components of the signaling pathway mediating osmotic stress-induced alterations in transport, we screened a panel of pharmacological agents, including those directed against signaling pathways known to be activated by osmotic stress, for those that would inhibit the osmotically induced stimulation of retrograde transport. The panel included a variety of serine/threonine protein kinase inhibitors, tyrosine kinase inhibitors, a calcium ionophore, and wortmannin. From this screen, we uncovered a single agent, H89, that was a potent inhibitor of the hypotonially induced redistribution of Golgi residents to the ER (this study).
H89 is a member of the isoquinolinesulfonamide group of protein kinase inhibitors that also includes H8 and H7. These compounds tend to exhibit selective inhibition toward cyclic nucleotide-dependent protein kinases as well as PKC (Hidaka et al., 1984). They act as competitive inhibitors with respect to ATP and presumably achieve selectivity through their differential binding affinities for the ATP-binding site of various kinases. For example, 50 μM H8 inhibits PKA but not PKC in vivo (Lee and Chuong, 1997), whereas 500 μM H8 inhibits both (Hidaka et al., 1984). Inhibition of other kinases and ATP-binding proteins by H8 requires much higher concentrations; for example, 50-, 100-, 800-, and 600-fold higher for myosin light chain kinase, casein kinase I, casein kinase II, and myosin, respectively (Hidaka et al., 1984). In contrast to H8, H7 inhibits both PKA and PKC at 50 μM (Reich and Pfeffer, 1990; Xiao et al., 1997), but like H8, it has little effect on other kinases except at very high concentrations (Hidaka et al., 1984). Thus, an examination of the sensitivities of a process of interest to a number of these inhibitors can provide a powerful tool for deducing the potential involvement of a certain protein kinase. From this class, a derivative of H8 called H89 has emerged as the most specific inhibitor of PKA. Inhibition of PKA with H89 requires a 660-fold lower concentration than that required to inhibit PKC (Chijiwa et al., 1990). Thus, H89 has begun to be commonly used as a selective inhibitor of PKA (Chijiwa et al., 1990; Muniz et al., 1996, 1997). Interestingly, H89 has been reported to inhibit trans-Golgi network-to-cell surface transport, as well as ER-to-Golgi transport (Muniz et al, 1996, 1997). The inhibitory effect of H89 on these steps was attributed to the inhibition of PKA. More recently, however, H89 has been shown to inhibit Golgi vesiculation induced by the drug illimaquinone and the free Gβγ subunit of trimeric G proteins. Surprisingly, the target of H89 in the Golgi vesiculation reaction appears to be protein kinase D (PKD), an unusual PKC isoform, and not PKA (Jamora et al., 1999).
To extend the existing data, we undertook an analysis of the sensitivity of bidirectional ER-to-Golgi transport to H89 as well as to other protein kinase inhibitors, including other isoquinolinesulfonamides. Anterograde ER-to-Golgi transport, as well as stimulated, but not constitutive, retrograde transport originating from the Golgi was sensitive to H89. H89 appeared to block stimulated retrograde transport at an early step, prior to the appearance of tubular intermediates. ER-to-Golgi transport also was inhibited at an early step: the recruitment of the coatomer protein (COP)II component Sec13 to ER export sites was blocked by H89 both in vivo and in vitro. In contrast to H89, a number of other protein kinase inhibitors, including other isoquinolinesulfonamides, at concentrations reported to inhibit both PKA and PKC, did not inhibit either anterograde or retrograde transport. These results suggest that an H89-sensitive factor(s) distinct from PKA or PKC, and potentially a novel protein kinase, is required for bidirectional transport between the ER and Golgi.
MATERIALS AND METHODS
Cell Lines, Antibodies, and Reagents
HeLa cells were maintained in minimum essential medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum, 100 I.U./ml penicillin, and 100 μg/ml streptomycin (normal medium) at 37°C in a 5% CO2 incubator. The following antibodies were used: a mouse monoclonal antibody (mAb) against GPP130 (A1/118); a rabbit polyclonal antiserum against GM130 (provided by E. Sztul, University of Birmingham, Birmingham, AL); a mouse mAb against ERGIC 53 and a mouse mAb against p63 (provided by H.-P. Hauri, Biocenter, Basel, Switzerland); an affinity-purified rabbit antiserum against mammalian Sec13 (provided by B.L. Tang and W. Hong, National University of Singapore, Singapore); a mouse mAb against the hemagglutinin (HA) epitope (provided by J. Woolford, Carnegie Mellon University, Pittsburgh, PA); and a mouse mAb against vesicular stomatitis virus G (VSVG) protein (provided by P. Weidman, St. Louis University, St. Louis, MO). Fluorescein isothiocyanate- and rhodamine-conjugated secondary antibodies against mouse or rabbit immunoglobulins were purchased from Cappel (Organon Teknika, Durham, NC).
The pCDM8.1 plasmid encoding tsO45 VSVG was provided by J. Lippincott-Schwartz, National Institutes of Health, Bethesda, MD. Digitonin was purchased from Boehringer Mannheim, Indianapolis, IN. H89 and KT5720 were purchased from Calbiochem (San Diego, CA), and H8 was purchased from Toronto Research Chemicals (Ontario, Canada). H7, staurosporine, chelerythrine, wortmannin, A23187, brefeldin A (BFA), and nocodazole were purchased from Sigma. All drugs, with the following exceptions, were dissolved in dimethyl sulfoxide and maintained at −20°C as 1000× stocks: H89 and H8 were stored at 4°C and A23187 was stored at −80°C. The final concentrations of each drug were as follows: 50 μM H89, 120 μM H8, 60 μM H7, 1–10 μM chelerythrine, 9 μM KT5720, 1 μM wortmannin, 0.4 μg/ml A23187, 2.5 μg/ml BFA, and 10 μg/ml nocodazole.
Hypotonic and Drug Treatments
HeLa cells grown on 12-mm glass coverslips were transferred to wells of a 24-well plate containing 1 ml of hypotonic medium (20 mM HEPES, pH 7.2, 60 mM NaCl, 2.5 mM MgOAc) and incubated in a 37°C water bath. For the drug treatments, cells on coverslips were incubated in 1 ml of (HEPES-buffered, pH 7.2) normal medium or hypotonic medium into which the various drugs (1000× stocks in dimethyl sulfoxide) were diluted, and incubated in a 37°C water bath or in a 5%CO2 incubator for the indicated times.
Immunofluorescence Microscopy and Quantitation
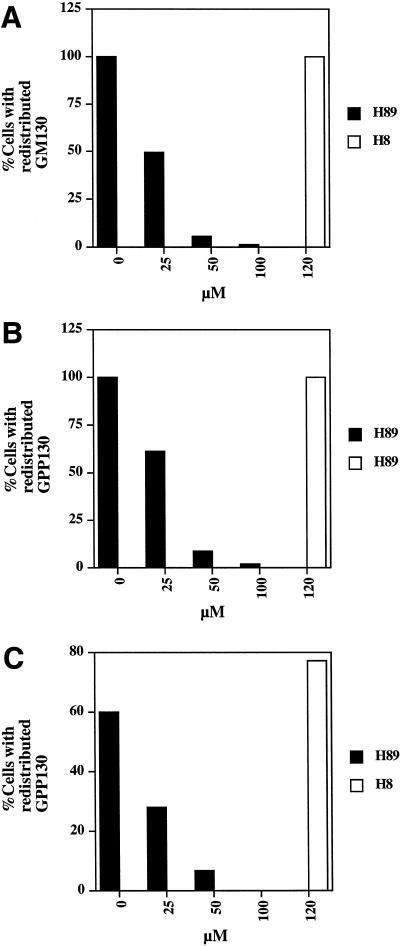
Cells were fixed for 20 min in 3% paraformaldehyede, and antibody staining was performed as described previously (Lee and Linstedt, 1999). Cells were analyzed with a fluorescence microscope (Nikon, Melville, NY) equipped with a Hamamatsu black-and-white cooled charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan). All images were taken with a 40× objective. Digital images were acquired in the program Photoshop (Adobe Systems, Mountain View, CA). For the quantitation of the H89 and H8 dose responses (Figure 4), 10 fields of ∼30 cells were examined and the percentage of cells with a redistributed Golgi resident-staining pattern determined. The average of two independent experiments was presented. For the quantitation of Sec13 recruitment assays (Figure 8), the fluorescence intensity in a rectangle of 304 pixels placed in a juxtanuclear position outside of the nucleus was measured with NIH image. Ten representative cells were measured and the average of two independent experiments was presented.
Figure 4.
H89 dose response for inhibition of hypotonically (A), BFA (B)-, and nocodazole (C)-induced redistribution. Cells were treated with hypotonic medium (210 mOsm), 2.5 μg/ml BFA, or 10 μg/ml nocodazole in the presence of 0, 25, 50, and 100 μM H89 or 120 μM H8. Hypotonically treated cells were stained with GM130 antibodies and BFA- and nocodazole-treated cells were stained with GPP130 antibodies. The averaged results of two independent experiments are presented.
Figure 8.
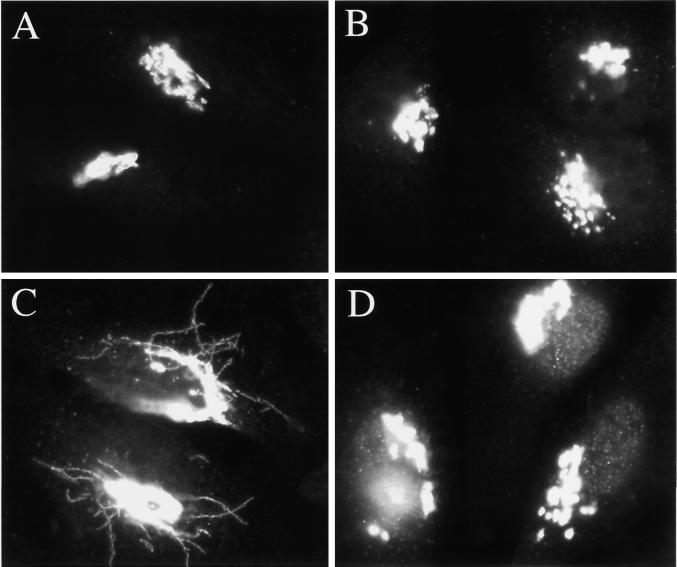
H89, but not H8, inhibits Sec13 recruitment. HeLa cells grown to 50% confluence on 12-mm glass coverslips were permeabilized in 30 μg/ml digitonin, washed, and incubated on ice as described in MATERIALS AND METHODS. After 20 min, cells were incubated in 50 μl of cytosol (A), buffer (B), cytosol + 50 μm H89 (C), or cytosol + 120 μM H8 (D) for 30 min at 32°C. All incubations included an ATP-regenerating system + 0.5 mM GTPγS. (E) Quantitation of A, C, and D. See MATERIALS AND METHODS for method of quantitation.
TsO45 VSVG Transport Assay
Cells were transiently transfected with tsO45 VSVG on a pCDM8.1 plasmid with calcium phosphate precipitation and passaged onto coverslips 1 d post transfection. Two days after transfection, cells were placed in normal medium (buffered with 25 mM HEPES, pH 7.2) in a water bath at 40°C. After 5 h, coverslips were transferred to normal medium or normal medium containing various protein kinase inhibitors (preequilibrated at 32°C) for 20 min at 32°C. Cells were fixed and stained with a mAb against the cytoplasmic tail of VSVG.
Generation of HASec13 Stable Cell Line
The following oligonucleotides were used to amplify by polymerase chain reaction (PCR) Sec13 from a HeLa cDNA library: 5′-GGAATTCGTGTCAGTAATTAACACTGTGG and 3′-GCTCTAGATCACTGCTCGTTCTGCTGGCCC. The PCR product was digested with EcoRI and XbaI and ligated to an EcoRI and XbaI-digested pCB6 vector containing an HA epitope in frame just upstream of the EcoRI site. The resulting construct was used to transfect HeLa cells by a standard calcium phosphate precipitation protocol. G418 (0.4 mg/ml) was used to select stable transfectants.
Digitonin Extraction and Immunoblot Assay
HeLa cells stably expressing HASec13 were grown to 50% confluence on 60-mm plates. Prior to extraction, cells were washed twice with 5 ml of permeabilization buffer or PB (25 mM HEPES, pH 7.2, 125 mM KOAc, 2.5 mM MgOAc, 5 mM EGTA) at room temperature. Cells were then extracted with 2 ml of PB containing 30 μg/ml digitonin + 1 mM dithiothreitol (DTT) + 10 μg/ml leupeptin + 10 μg/ml pepstatin for 6 min at room temperature. After removal of the digitonin extract, the permeabilized cells were dissolved in sample buffer, boiled, and resolved by 10% SDS-PAGE. Immunoblotting was performed as previously described (Lee and Linstedt, 1999).
Sec13 Recruitment Assay
Cells grown on glass coverslips were washed with 1 ml of PB, placed cell side up on parafilm, and incubated with 50 μl of PB + 1 mM DTT + 30 μg/ml digitonin for 6 min at room temperature
Permeabilized cells on coverslips were washed twice with ice cold PB + 1 mM DTT, and incubated on ice. After 20 min, coverslips were transferred to parafilm and incubated in transport buffer or TB (25 mM HEPES, pH 7.2, 75 mM KOAc, 5 mM MgOAc, 5 mM EGTA, 1.8 mM CaCl2) containing 1 mM DTT + 10 μg/ml leupeptin + 10 μg/ml pepstatin. In certain samples, an ATP-regenerating system (0.5 mM ATP, 0.5 mM UTP, 50 μM GTP, 5 mM creatine phosphate, 25 μg/ml creatine phosphokinase, 0.5 mM MgCl2, 0.05 mM EGTA), 0.5 mM guanosine-5′-O-(3-thio)triphosphate (GTPγS), and/or 2.5 mg/ml cytosol were included. Cells were incubated at 32°C for 30 min.
For the preparation of cytosol, 12 × 15-cm plates of HeLa cells were washed twice with ice cold PBS, scraped into PBS, and collected by centrifugation at 2000 rpm (SA600), 5 min. After washing in 10 volumes of TB, cells were resuspended in 2 volumes of TB + 10 μg/ml pepstatin + 10 μg/ml leupeptin + 1 mM DTT + 10 μM ATP + 5 μM GTP, and homogenized with a ball-bearing homogenizer. A postnuclear supernatant (1000 × g) was centrifuged at 100 K for 15 min in a TLA100.3 rotor. The supernatant, ∼8 mg/ml protein, was frozen and stored at −80°C.
RESULTS
H89 Inhibits Hypotonically induced-, BFA-induced-, and Nocodazole-induced Stimulation of Golgi-to-ER Transport
In a search for pharmacological agents that prevented the hypotonically induced stimulation of Golgi-to-ER retrograde transport, we tested a panel of drugs known to inhibit a variety of signaling molecules. Most agents were without effect. However, H89, commonly used as a potent and selective inhibitor of PKA (Muniz et al., 1996, 1997; Chijiwa et al., 1990), strongly inhibited the stimulation of Golgi resident redistribution to the ER that occurred upon incubation in hypotonic medium. As expected, the cis-Golgi resident GM130 (Nakamura et al., 1995) exhibited a characteristic perinuclear Golgi-staining pattern (Figure 1A). This pattern was not noticeably affected by the inclusion of 50 μM H89 in normal medium during a 20-min incubation (Figure 1B). As previously reported (Lee and Linstedt, 1999), hypotonic treatment led to the rapid (t1/2 = ∼10 min) redistribution of GM130 to the ER by 20 min (Figure 1C). Strikingly, inclusion of 50 μM H89 in the hypotonic medium prevented the redistribution response (Figure 1D). The inhibition of hypotonically induced Golgi-to-ER transport appeared to occur at an early step because tubules, whose kinetics of appearance and disappearance is consistent with their being intermediates in Golgi-to-ER transport (Lippincott-Schwartz et al., 1990; Sciaky et al., 1997), were prominent in control, hypotonically treated cells but were rarely seen in the presence of H89. This point is best illustrated with another cis-Golgi marker, GPP130 (Linstedt et al., 1997). As previously reported, the rate at which different Golgi residents redistribute to the ER upon hypotonic treatment varies significantly (Lee and Linstedt, 1999). GPP130 redistributes more slowly than GM130 (t1/2 = ∼60–120 min); thus, tubular intermediates are easily observable over a wide range of times. As expected, GPP130 almost never exhibited tubular staining in cells maintained in normal medium (Figure 2A), and addition of 50 μM H89 to normal medium for 10 min did not significantly alter the pattern of GPP130 staining (Figure 2B). In contrast, 10 min after transfer to hypotonic medium, 10–25% of the cells had GPP130 tubules emanating from the Golgi (Figure 2C). However, the presence of 50 μM H89 in the hypotonic medium prevented altogether the appearance of GPP130 tubules (Figure 2D). Although H89 is a potent inhibitor of PKA (Chijiwa et al., 1990), H89 did not appear to exert its inhibitory effect on hypotonically induced Golgi resident redistribution through the inhibition of PKA or conventional PKC isozymes because other selective inhibitors of PKA (120 μM H8 [Lee and Chuong, 1997; Figure 4 and 9 μM KT5720 [Linn et al., 1996]) and PKC (60 μM H7 [Reich and Pfeffer, 1990; Xiao et al., 1997] and 1–10 μM chelerythrine [Herbert et al., 1990]), all used at concentrations equal to or greater than those previously reported to be effective in HeLa cells, did not inhibit the response (our unpublished results).
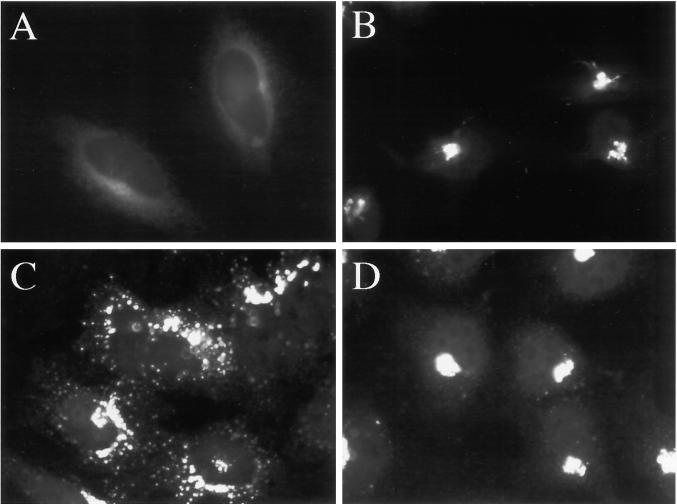
Figure 1.
H89 blocks hypotonically induced redistribution of GM130 to the ER. HeLa cells grown to 50% confluence on 12-mm glass coverslips were placed in 1 ml of normal medium (A), normal medium containing 50 μM H89 (B), hypotonic (210 mOsm) medium (C), or hypotonic medium containing 50 μM H89 (D) for 20 min at 37°C.
Figure 2.
H89 blocks hypotonically induced GPP130 tubules. HeLa cells grown to 50% confluence on 12-mm glass coverslips were placed in 1 ml of normal medium (A), normal medium containing 50 μM H89 (B), hypotonic (210 mOsm) medium (C), or hypotonic medium containing 50 μM H89 (D) for 10 min at 37°C.
To ascertain whether the inhibitory effect of H89 on Golgi resident redistribution was specific for the hypotonic response, we asked whether H89 also would affect Golgi-to-ER transport induced by BFA and nocodazole. As previously demonstrated for a number of Golgi markers (Doms et al., 1989; Lippincott-Schwartz et al., 1989, 1990), 2.5 μg/ml BFA induced GPP130 redistribution to the ER with a t1/2 of ∼5 min (Figure 3A; Figure 2A for the GPP130-staining pattern in untreated cells). Strikingly, the presence of 50 μM H89 blocked the BFA-induced redistribution of GPP130 (Figure 3B), as well as of GM130 and giantin (our unpublished results). As described above for the hypotonic response, H89 appeared to inhibit an early step in the redistribution response because the number of BFA-induced tubules was dramatically diminished in the presence of H89 (our unpublished results). Finally, we examined the effect of H89 on nocodazole-induced fragmentation of the Golgi as marked by GPP130 staining (Storrie and Yang, 1998). Recent work suggests that Golgi-to-ER transport is an obligatory step in the nocodazole-induced fragmentation of the Golgi into ministacks (Cole et al., 1996; Storrie et al., 1998; Drecktrah and Brown, 1999; see Shima et al. 1998 for a contrasting view). GPP130 redistribution to peripheral sites occurred with a t1/2 of ∼60–90 min as previously reported for other Golgi markers (Figure 3C). As observed for both hypotonic- and BFA-induced Golgi resident redistribution, 50 μM H89 was a potent inhibitor of nocodazole-induced GPP130 redistribution (Figure 3D), as well as GM130 redistribution (Figure 6, C and D) and giantin redistribution (our unpublished results). Tubulin staining confirmed that the effect of H89 was not a consequence of an alteration in the microtubule network (our unpublished results).
Figure 3.
H89 blocks both BFA- and nocodazole-induced redistribution of GPP130. HeLa cells grown to 50% confluence on 12-mm glass coverslips were incubated in 2.5 μg/ml BFA in the absence (A) or presence (B) of 50 μM H89 for 20 min at 37°C, or incubated in 10 μg/ml nocodazole in the absence (C) or presence (D) of 50 μM H89 for 60 min at 37°C.
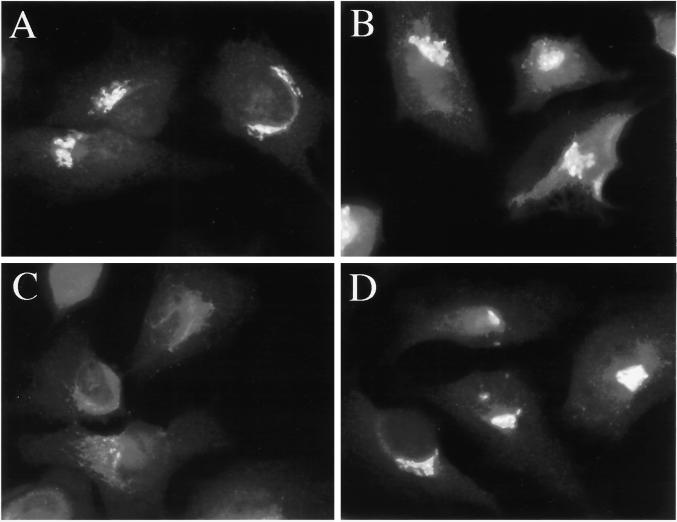
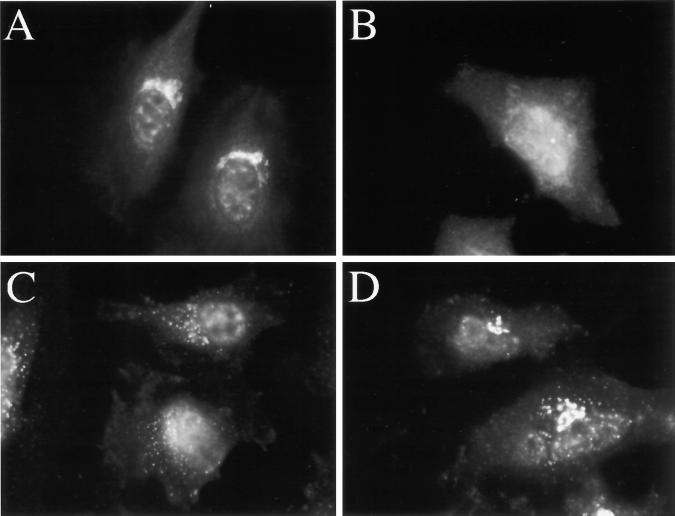
Figure 6.
H89 treatment induces, and does not block the constitutive recycling of GM130 to the ER (A and B), but does block the nocodazole-induced redistribution of GM130 (C and D). HeLa cells grown to 50% confluence on 12-mm glass coverslips were incubated in the absence (A) or presence (B) of 50 μm H89 for 120 min. In C and D, cells were incubated in the presence of 10 μg/ml nocodazole for 60 min (C), or in the presence of 50 μM H89 and 10 μg/ml nocodazole for 60 min (D).
A quantitative analysis of the effect of H89 on each of the three retrograde transport reactions at varying H89 concentrations indicated that all three stimulated Golgi-to-ER transport reactions shared a similar dose response to H89 (Figure 4,A–C). It should be noted that 50 μM H89, a concentration commonly used to inhibit PKA (Chijiwa et al., 1990) almost completely inhibited all three reactions. However, none of the reactions was inhibited by H8 (Hidaka et al., 1984), a structurally related PKA inhibitor (Figure 4, A–C).
H89 Does Not Inhibit Hypotonically Induced ERGIC-to-ER Transport, or Constitutive Retrograde Transport Originating from ERGIC and Golgi
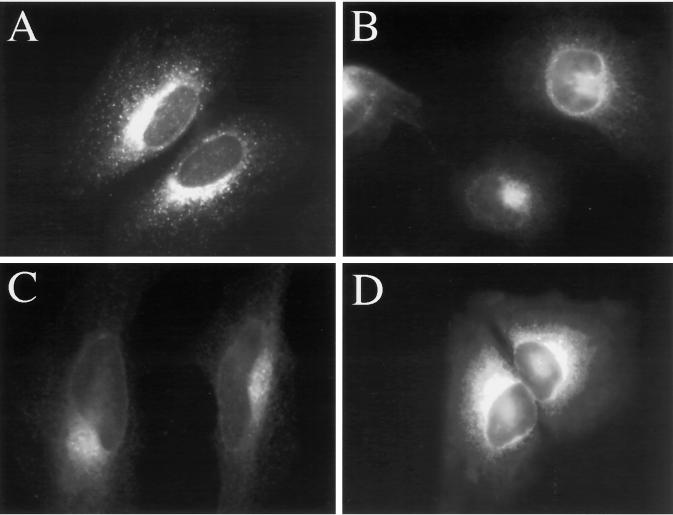
We previously reported that hypotonic treatment also leads to the apparent collapse of the ERGIC compartment, as marked by ERGIC 53 (Lee and Linstedt, 1999). Because BFA does not cause collapse of the ERGIC (Lippincott-Schwartz et al., 1990), the hypotonic response provided a unique opportunity to assess whether H89 also inhibited ERGIC-to-ER transport. As expected, ERGIC 53 staining of cells maintained in normal medium produced a characteristic punctate pattern (Figure 5A) that was completely redistributed to an ER-staining pattern in cells incubated for 20 min in hypotonic medium (Figure 5B). Interestingly, 50 μM H89 in the hypotonic medium did not inhibit this response (Figure 5C). In fact, 50 μM H89 alone in normal medium induced the redistribution of ERGIC 53 to the ER by 20 min (Figure 5D). These results suggested that although H89 was a potent inhibitor of hypotonic-, BFA-, and nocodazole-induced Golgi-to-ER transport, hypotonically induced ERGIC-to-ER transport was unaffected by H89.
Figure 5.
H89 does not block hypotonically induced ERGIC 53 redistribution to the ER, and H89 alone induces the redistribution of ERGIC 53 to the ER. HeLa cells grown to 50% confluence on 12-mm glass coverslips were placed in 1 ml of normal medium (A), hypotonic (210 mOsm) medium (B), hypotonic medium containing 50 μM H89 (C), or normal medium containing 50 μm H89 (D) at 37°C for 20 min.
A straightforward explanation for the induction of ERGIC-to-ER transport by H89 in normal medium was provided by a recent report that H89 blocks ER export (Jamora et al., 1999). From this perspective, H89 might cause the accumulation of ERGIC 53 in the ER by virtue of its ability to block ER export. Indeed, ERGIC and Golgi residents have been shown to redistribute to the ER in cells in which ER export is blocked by the dominant negative version of the Sar1 GTPase, presumably due to the constitutive recycling of ERGIC and Golgi residents through the ER (Cole et al., 1996; Storrie et al., 1998; Drecktrah and Brown, 1999; Zaal et al., 1999). If this were the case, long-term H89 treatment in normal medium also would be predicted to lead to the slow redistribution of Golgi residents to the ER. Indeed, although 50 μM H89 alone in normal medium did not cause any significant redistribution of Golgi residents during short incubation times (Figures 1B and 2B), longer incubation times resulted in the redistribution of GM130 from its characteristic Golgi pattern (Figure 6A) to an ER pattern (Figure 6B) by 2 h with a t1/2 of ∼1 h (compared with the relatively rapid t1/2 of ∼10′ accumulation induced by hypotonic treatment in Figure 1). Other Golgi residents, such as GPP130 and giantin, also redistributed to the ER in the presence of 50 μM H89 but with significantly slower kinetics (t1/2 = ∼2–3 h; our unpublished results). These results were consistent with the observation that H89 inhibited ER-to-Golgi transport (Jamora et al., 1999; see below), and importantly, it suggested that H89 only blocked the stimulated form of Golgi-to-ER transport, and not the constitutive recycling of ERGIC and Golgi residents to the ER.
Several investigators have proposed that nocodazole, rather than stimulating Golgi-to-ER transport, uncovers a constitutive recycling pathway. This is based on the kinetics of nocodazole-induced Golgi fragmentation, which is more similar to the kinetics of accumulation of Golgi residents in the ER upon treatment with dominant negative Sar1 than to the kinetics of Golgi-to-ER transport induced by BFA (Storrie et al., 1998). In contrast to this proposal, our observation that the nocodazole-induced Golgi-to-ER transport pathway was H89 sensitive (Figure 3, C and D) suggested that the nocodazole-induced retrograde pathway was mechanistically more related to both BFA- and hypotonically induced retrograde transport than to the constitutive Golgi-to-ER transport pathway. To rule out the possibility that the differential sensitivity of nocodazole-induced and constitutive recycling pathways to H89 was due to the use of different markers to assay the different pathways, we compared the H89 sensitivity of a single marker to both nocodazole-induced and constitutive retrograde transport. As shown in Figure 6C, nocodazole induced the complete dispersal of GM130 to ministacks by 60 min. However, the nocodazole-induced dispersal was inhibited by 50 μM H89 such that significant amounts of GM130 remained in its normal juxtanuclear location even at 60 min (Figure 6D). Therefore, the same H89 treatment that allowed the constitutive recycling of GM130 to the ER (Figure 6B) inhibited the nocodazole-induced retrograde transport of GM130. The partial redistribution of GM130 in the presence of nocodazole and H89 most likely reflected the beginnings of the H89-induced slow redistribution of GM130 to the ER (Figure 6B) due to the H89-induced ER export block (see below). Therefore, the nocodazole-induced retrograde pathway appeared mechanistically distinct from the constitutive retrograde pathway.
To further investigate the proposal that H89 blocks stimulated but not constitutive retrograde transport out of the Golgi, we took advantage of our previous observation that hypertonic conditions also block ER export and lead to the redistribution of Golgi residents to the ER, but significantly more slowly than hypotonic conditions (Lee and Linstedt, 1999). For instance, GM130 redistributes to the ER by 20 min in hypotonic medium, but redistribution to the ER in hypertonic medium requires longer incubation times (∼2 h). Because both conditions abruptly block ER export, we suggested previously that retrograde transport was stimulated under hypotonic, but not under hypertonic conditions. Also consistent with this proposal, extensive tubules containing Golgi residents were only observed under hypotonic conditions. We therefore asked whether H89 would inhibit Golgi resident redistribution under hypertonic conditions. As predicted, 50 μM H89 had no effect on the kinetics with which GM130 and GPP130 redistributed to the ER under hypertonic conditions (our unpublished results). Together, our results suggest that H89 specifically inhibits the stimulated, but not constitutive form of retrograde transport originating from the Golgi.
H89 Inhibits Sec13 Recruitment to ER Export Sites
The slow collapse of ERGIC and Golgi residents into the ER induced by H89 was consistent with a block in ER export. In addition, as mentioned above, H89 but not protein kinase inhibitor, a PKA-specific peptide inhibitor, was recently shown to inhibit ER export (Jamora et). These results suggested that a novel H89-sensitive factor, possibly the same factor required for stimulated retrograde transport out of the Golgi, also might play a role in ER export. To extend this finding, we first performed a morphological transport assay on cells transfected with tsO45 VSVG (Gallione and Rose, 1985) to confirm the H89-induced anterograde transport block. Indeed, we found that H89 was a potent inhibitor of ER export at concentrations similar to those required to inhibit retrograde transport between the Golgi and ER. That is, H89 inhibited ER export partially at 25 μM, almost completely at 50 μM, and completely at 100 μM (our unpublished results). In contrast, 60 μM H7 (Reich and Pfeffer, 1990; Xiao et al., 1997), 120 μM H8 (Lee and Chuong, 1997), 9 μM KT5720 (Linn et al., 1996), and 1 μM chelerythrine (Herbert et al., 1990), at concentrations reported to inhibit PKA and PKC in vivo, had no detectable effect on ER export of VSVG.
COPI and COPII recruitment are essential steps in anterograde and retrograde transport processes (Barlowe et al., 1994; Letourneur et al., 1994; Bednarak et al., 1995). Therefore, to explore the possible mechanism(s) by which H89 inhibited ER export and by which it inhibited stimulated retrograde transport, we examined the staining pattern of COPI and COPII components βCOP (Waters et al., 1991) and Sec13 (Barlowe et al., 1994; Tang et al., 1997), respectively, in H89-treated cells. BFA-induced Golgi collapse is known to require the dissociation of βCOP from the Golgi (Donaldson et al., 1990; Scheel et al., 1997). Significantly, the H89 treatment that blocked Golgi resident redistribution (Figure 3, A and B) did not block βCOP dissociation, indicating that the H89-mediated inhibition of BFA-induced Golgi resident redistribution was independent of an effect on COPI. Interestingly, we observed that βCOP binding to the Golgi in normal medium also was unaffected by 50 μM H89, although there was a diminution of peripheral βCOP staining associated with the ERGIC (our unpublished results). The loss of βCOP at peripheral, ERGIC-associated sites was consistent with the H89-induced collapse of the ERGIC into the ER as described above (Figure 5D).
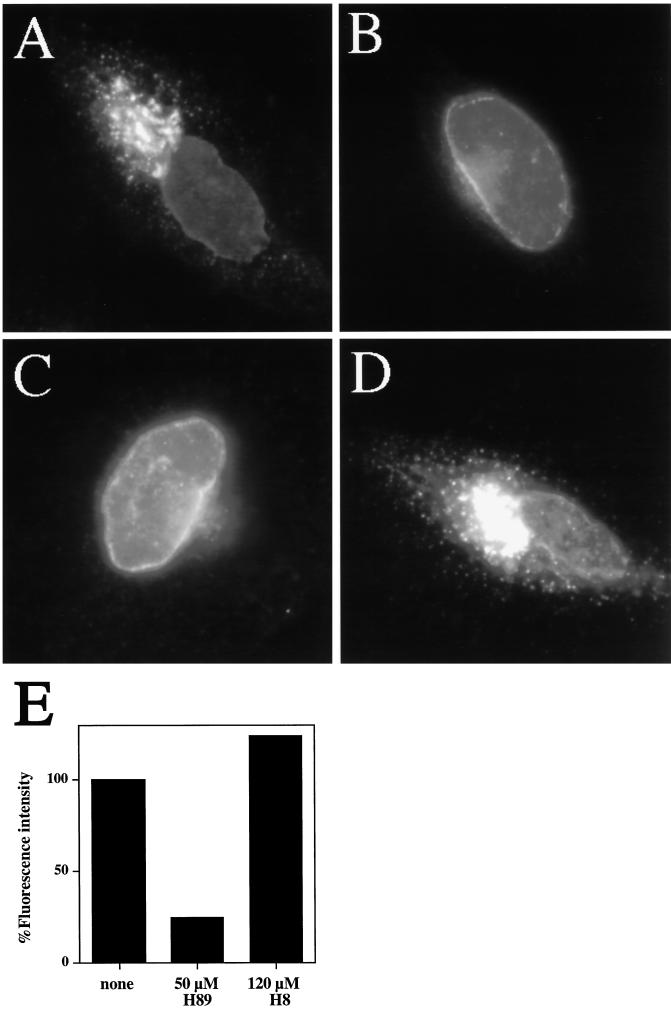
Next, we examined the binding of the mammalian homolog of Sec13 to ER export sites. As expected, Sec13 antibodies in untreated cells stained punctate, peripheral structures (Figure 7A) presumably corresponding to ER exit sites (Tang et al., 1997). Strikingly, 50 μM H89 led to a rapid reduction in Sec13 staining in peripheral ER exit sites over time such that by 10 min of H89 treatment, only ∼25% of the cells exhibited significant peripheral Sec13 staining (Figure 7B). The apparent increase in Sec13 in the nuclear or perinuclear region of cells with diminished peripheral Sec13 staining was most likely due to nonspecific binding of the polyclonal Sec13 antibody to the nucleus. This was determined by performing the same experiment in cells that had been stably transfected with an HA epitope-tagged version of Sec13. In this case, Sec13 could be detected with a mAb against the HA epitope. As can be seen in Figure 7C, in untreated transfectants, the HA antibody stained peripheral structures resembling those stained by the Sec13 antibody (compare Figure 7A and 7C). After treatment of the transfectants with 50 μM H89 for 10 min, staining of the peripheral structures was greatly diminished in most cells (Figure 7D). However, no significant increase in nuclear or perinuclear Sec13 staining was detected with the HA epitope antibody. These results suggested that H89 induced the redistribution of Sec13 from ER exit sites to the cytoplasm. Importantly, H89 did not affect the staining pattern of the ER marker p63 (Schweizer et al., 1995), indicating that ER morphology was generally unchanged (our unpublished results).
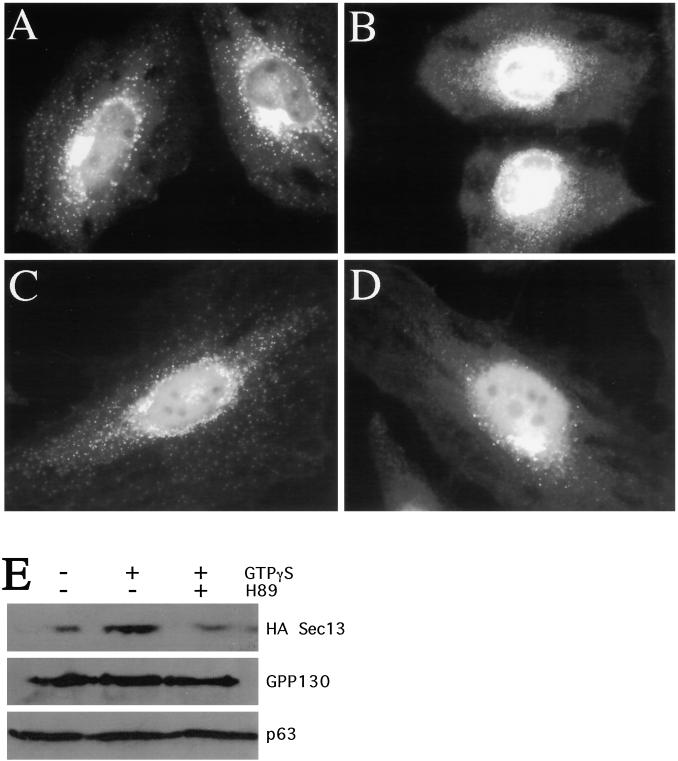
Figure 7.
H89 treatment leads to displacement of Sec13 from peripheral ER exit sites to a soluble cytosolic pool. HeLa cells grown to 50% confluence on 12-mm glass coverslips were incubated in the absence (A) or presence (B) of 50 μm H89 for 10 min, fixed, and stained with antibodies against Sec13. In C and D, HeLa cells stably transfected with HA-tagged Sec13 were grown to 50% confluence and incubated in the absence (C) or presence (D) of 50 μM H89 for 10 min, fixed, and stained with antibodies against the HA epitope. (E) Immunoblot of HASec13-expressing HeLa cells after digitonin extraction under the conditions indicated. H89-treated cells were preincubated for 10 min with 50 μM H89 prior to extraction in 50 μM H89. GTPγS was present at 1 mM where indicated. See MATERIALS AND METHODS for further details.
To further confirm that H89 treatment led to the displacement of membrane-associated Sec13 to a soluble cytosolic pool, we used a digitonin extraction and immunoblot assay. Digitonin permeabilization results in the extraction of cytoplasmic protein, leaving ER- and large membrane-associated constituents behind. Because Sec13 membrane association is transient and coupled to the hydrolysis of GTP by Sar1 (Matsuoka et al., 1998), we added the nonhydrolyzable GTP analogue GTPγS to the PB. As expected, cells extracted without GTPγS retained very little HASec13, whereas those extracted in the presence of GTPγS retained a significantly greater amount (Figure 7E, lanes 1 and 2). In contrast, cells pretreated with 50 μM H89 for 10 min prior to extraction in the presence of GTPγS only retained the low level of HASec13 seen after extraction in the absence of GTPγS (Figure 7E, lane 3). ER and Golgi membranes were not differentially extracted under these conditions as indicated by the recovery of the ER protein p63 and the Golgi protein GPP130 from the same cells (Figure 7E, lanes 1–3). Furthermore, the staining pattern of HASec13 in cells extracted with digitonin and GTPγS was the punctate, peripheral pattern characteristic of ER exit sites, whereas cells pretreated with H89, or extracted in the absence of GTPγS, yielded no staining (our unpublished results). Thus, H89 treatment led to the loss of Sec13 from apparent ER exit sites into the soluble cytosolic pool.
As described above, inclusion of GTPγS during permeabilization was necessary for the retention of Sec13 at presumptive ER exit sites. This suggested that Sec13 might undergo Sar1 GTPase-dependent cycles of dissociation and reassociation during permeabilizaton. Presumably, inclusion of GTPγS prevented Sec13 dissociation, a step that normally requires GTP hydrolysis by Sar1 (Aridor et al., 1995; Matsuoka et al., 1998). Because H89 inhibited the GTPγS-mediated stabilization of Sec13 at ER exit sites, we next considered the possibility that H89 might inhibit Sec13 recruitment. To test whether H89 inhibited COPII coat recruitment, we developed a Sec13 recruitment assay in digitonin-permeabilized cells. As described above, permeabilization of cells in the absence of added nucleotides led to extraction of Sec13. However, subsequent incubation of the cells with cytosol, ATP, and GTPγS led to the recruitment of Sec13 to structures that resembled the peripheral ER exit sites seen in intact, untreated cells (Figure 8A; compare with Figure 7A). No recruitment was observed in the absence of cytosol (Figure 8B), and recruitment was significantly reduced in the absence of GTPγS (our unpublished results). In addition, recruitment required ATP, even in the presence of GTPγS (our unpublished results; Barlowe et al., 1994). As would be expected if H89 inhibited the recruitment of COPII to ER exit sites, inclusion of 50 μM H89 in the reaction mixture inhibited the appearance of Sec13-staining peripheral structures in vitro (Figure 8C). The inhibition by H89 was uniform in that nearly 100% of the cells exhibited only low or undetectable levels of peripheral Sec13 staining. In contrast, 120 μM H8 had no inhibitory effect on Sec13 recruitment (Figure 8D). A quantitation of the effects of both kinase inhibitors is presented in Figure 8E. Unfortunately, because H89 is a competitive inhibitor with respect to ATP, its effects are reversible (Chijiwa et al., 1990); thus, we could not easily test whether the H89 sensitive activity was membrane-associated or cytosolic. Importantly, we found that under the conditions of our Sec13 recruitment assay, the stimulated retrograde transport pathway was inoperative. Even in the absence of H89, the addition of BFA did not induce the retrograde movement of Golgi residents (our unpublished results). This indicated that the recruitment of Sec13, at least under the conditions of our assay, was independent of retrograde transport. Therefore, the inhibition of Sec13 recruitment by H89 appeared to be the consequence of a direct, rather than indirect, effect of H89. Together, our results suggested that an H89-sensitive factor was required for an early step in stimulated, but not constitutive, Golgi-to-ER retrograde transport pathway, and was independently required for COPII recruitment to ER export sites.
DISCUSSION
The idea that Golgi residents, in common with proteins that function at the interface of the ER and Golgi complex, undergo a slow, constitutive recycling pathway through the ER was initially proposed by Lippincott-Schwartz and colleagues (Cole et al., 1998) and has been supported by a number of studies, predominantly using the dominant negative version of the Sar1 GTPase (Barlowe et al., 1993; Aridor et al., 1995), which blocks ER export and leads to the slow accumulation of Golgi residents in the ER (Storrie et al., 1998; Zaal et al., 1999). More recently, a certain subset of phospholipase A2 (PLA2) inhibitors also have been shown to cause the slow redistribution of Golgi residents to the ER through a block in ER export (Drecktrah and Brown, 1999). In contrast to Sar1 or PLA2 inhibition, the fast kinetics with which Golgi residents redistribute to the ER in the presence of BFA or under hypotonic conditions suggests that these treatments either turn on a novel retrograde pathway, or stimulate a slower, constitutive retrograde pathway. Consistent with this supposition is the observation that both treatments induce the appearance of extensive membrane tubule intermediates containing backwards moving Golgi residents. Our results indicate that an H89-sensitive activity, potentially a novel protein kinase, is required for a stimulated, but not constitutive form of retrograde transport originating from the Golgi. H89 treatment blocked retrograde transport of Golgi residents stimulated by three independent means: BFA, nocodazole, and hypotonic treatment. The fact that H89 opposed the effects of such differing treatments suggests that the H89-sensitive factor is intimately involved in stimulated retrograde transport from the Golgi, and not in neutralizing any particular perturbant. Also consistent with this idea is the fact that we observed no effect of H89 on either the BFA-induced dissociation of βCOP from the Golgi or the nocodazole-induced depolymerization of microtubules. H89 appeared to block the stimulated retrograde movement of Golgi residents at an early step because BFA and hypotonically induced tubules did not accumulate in the presence of H89, and in fact were rarely seen in the presence of H89. Furthermore, our finding that H89 actively induced the slow redistribution of ERGIC and Golgi residents to the ER under iso-osmotic conditions, coupled with our observation that H89 did not inhibit the slow, presumably unstimulated redistribution of Golgi residents to the ER under hypertonic conditions, suggests that the H89-sensitive factor is not required for retrograde transport, per se, but specifically required for a stimulated form of retrograde transport out of the Golgi.
It is puzzling that H89 blocks nocodazole-induced Golgi fragmentation because recent work from several laboratories suggest that the nocodazole-induced dispersal of the Golgi into ministacks occurs via the slow, constitutive Golgi-to-ER transport pathway (Cole et al., 1996; Storrie et al., 1998; Drecktrah and Brown, 1999; Zaal et al., 1999). The basis for this supposition is that the relatively slow kinetics with which nocodazole induces Golgi fragmentation is comparable to the kinetics with which Golgi residents accumulate in the ER upon imposition of an ER export block (Storrie et al., 1998). In contrast, our observations suggest that the nocodazole-induced Golgi-to-ER transport pathway is more similar to the BFA- and hypotonically induced Golgi-to-ER transport pathways, which are clearly stimulated, than to the constitutive Golgi-to-ER transport pathway.
One possible explanation is that there are multiple constitutive retrograde pathways, and H89, in addition to blocking a stimulated form of retrograde transport out of the Golgi, also blocks a subset of the constitutive retrograde transport pathways. Indeed, recent work has defined at least two constitutive retrograde transport pathways operating between the Golgi and ER: a COPI-dependent pathway and a COPI-independent, Rab6-dependent pathway (Girod et al., 1999). Rapidly recycling markers such as ERGIC 53 and the KDEL receptor take the COPI-dependent pathway, whereas more slowly recycling markers such as N-acetylglucosminyltransferase-1, N-acetylglucosaminyltransferase-2, and sialyltransferase appear to take the Rab6-dependent pathway. Our observations indicate that H89 does not block the COPI-dependent pathway because ERGIC 53 recycling proceeds in the presence of H89. Whether H89 blocks the Rab6-dependent pathway remains to be tested. Nonetheless, the idea that H89 blocks a subset of constitutive retrograde pathways seems rather unlikely because H89 blocks the nocodazole-induced redistribution of GM130, GPP130, and giantin, but not the constitutive retrograde movement of the same markers uncovered by two means of blocking ER export: hypertonic stress or H89 treatment. Thus, based on sensitivity to H89, we propose that the nocodazole-induced retrograde pathway is more mechanistically related to the BFA- and hypotonically induced pathways. Although the kinetics of nocodazole-induced retrograde transport are clearly slower than those of BFA- and hypotonically induced retrograde transport, a precise determination of the time that it takes for nocodazole-induced Golgi-to-ER transport for a particular marker may reveal kinetics that exceed that of constitutive Golgi-to-ER recycling of the same marker.
The slow ER accumulation of ERGIC and Golgi markers induced by H89 was consistent with the notion that H89, in addition to blocking the stimulated form of Golgi-to-ER transport, also might block ER export. Malhotra and colleagues have shown that H89 inhibits the export of VSVG protein out of the ER (Jamora et al., 1999). We confirmed this finding, and extended it by demonstrating that the inhibitory effect of H89 is on the recruitment of the COPII component Sec13/sec31 to ER export sites. The requirement for an H89-sensitive factor most likely occurs downstream of the recruitment of the Sar1 GTPase because the presence of GTPγS in the recruitment reaction did not bypass the requirement for an H89-sensitive factor. The potential requirement for a protein kinase activity in COPII recruitment, particularly downstream of Sar1, is intriguing in light of a recent report demonstrating that dephosphorylation of Sec13/sec31, but not Sec23/sec24 or Sar1, inhibits budding from the ER in vitro (Salama et al., 1997). Perhaps the H89-sensitive activity is required, either directly or indirectly, for the phosphorylation of Sec13/s31, which in turn is required for the recruitment of Sec13/s31 to export sites. We are currently investigating this issue.
The suggestion that a single H89-sensitive factor is required both for COPII recruitment and for retrograde transport from the Golgi is surprising because there is no indication at present that COPII plays any role in Golgi-to-ER transport. Thus, it raises the possibility that the requirement for an H89-sensitive factor in ER export is an indirect consequence of an H89-induced block in stimulated retrograde transport, or that the requirement for an H89-sensitive factor in stimulated retrograde transport is an indirect consequence of an H89-induced block in ER export. An argument against the former is that ER export occurs efficiently in the absence of incoming retrograde traffic from the Golgi, at least in vitro (Matsuoka et al., 1998). An argument against the latter is that nocodazole induces the redistribution of Golgi residents to the ER even in the presence of the dominant negative version of Sar1, which blocks ER export (Storrie et al., 1998). Furthermore, hypotonic conditions stimulate retrograde transport even when ER export is blocked (Lee and Linstedt, 1999). Thus, it is likely that both anterograde and retrograde transport between the ER and the Golgi are independently sensitive to H89. An alternative proposal is that H89 has two distinct targets, one required for ER export and the other required for stimulated retrograde transport from the Golgi. Our finding that both reactions are similarly sensitive to H89 and similarly insensitive to a variety of other related protein kinase antagonists would argue against this possibility. Nonetheless, this issue will only be resolved once the target of H89 in each reaction pathway has been identified.
In contrast to a previous report (Muniz et al., 1996), and in common with Malhotra and colleagues (Jamora et al., 1999), we found that the target of H89 in ER export is neither PKA nor PKC. Nor is PKA or PKC the relevant H89-sensitive factor in the stimulated Golgi-to-ER transport pathway. Several inhibitors of PKA and PKC had no effect on either ER export or stimulated retrograde transport from the Golgi. What then is the target of H89? One possibility is PKD, an unusual isoform of PKC (Johannes et al., 1994; Valverde et al., 1994). Malhotra and colleagues showed that a PKD-specific peptide substrate, but not specific peptide substrates of other PKC isoforms, inhibits illimaquinone and Gβγ-mediated fragmentation of the Golgi as well as ER export (Jamora et al., 1999). Thus, it seems reasonable that the requirement that we observed for both COPII recruitment and stimulated retrograde transport from the Golgi is for PKD. There are, however, caveats to consider. First, the isoquinolinesulfonamide H8, which has been reported to inhibit PKD in vitro (Johannes et al., 1995), albeit less effectively than H89, did not inhibit either COPII recruitment or retrograde transport from the Golgi. A threefold higher concentration of H8, relative to H89, is required to inhibit PKD in vitro (Johannes et al., 1995). However, we observed no inhibition of either process in the presence of concentrations of H8 five-to-sevenfold higher than the effective concentration of H89 either in vivo or in vitro. Second, previous studies have demonstrated that although PKD is abundantly expressed in certain human cell lines, such as HepG2, there is little or no detectable PKD message (Johannes et al., 1994) or protein (Prestle et al., 1996) in HeLa cells, in which all of our studies were performed. These observations would argue that the target of H89, at least in the processes described herein, is not PKD. Further studies will be required to confirm the identity of the H89-sensitive factor.
What is downstream of the H89-sensitive factor? As discussed above, it seems unlikely that a single transport factor would be required independently for both COPII recruitment and stimulated retrograde transport from the Golgi. Interestingly, as alluded to above, Brown and colleagues (Drecktrah and Brown, 1999) have recently reported that a certain subset of cytoplasmic PLA2 inhibitors inhibits ER export as well as BFA- and nocodazole-induced Golgi resident redistribution, but significantly lower concentrations of PLA2 antagonists were required to inhibit ER export compared with the concentrations required to inhibit BFA- or nocodazole-induced Golgi resident redistribution. The precise identity(ies) of these PLA2 activities remains unknown; however, it raises the intriguing possibility that distinct members of a single class of lipid-remodeling activities might be required for bidirectional transport between the ER and Golgi, and that this class of lipid remodelers is regulated by an H89-sensitive factor.
ACKNOWLEDGMENTS
These experiments were made possible by the generous contribution of antibodies and reagents from the following investigators: B.L. Tang and W. Hong (National University of Singapore, Singapore), E. Sztul (University of Birmingham, Birmingham, AL), H.-P. Hauri (Biocenter, Basel, Switzerland), J. Lippincott-Schwartz (National Institutes of Health, Bethesda, MD), and P. Weidman (St. Louis University, St. Louis, MO). This work was supported by a National Institutes of Health grant GM-56779-02 to A.D.L.
REFERENCES
- Aridor M, Bannvkh SI, Rowe T, Balch WE. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, d'Enfert C, Schekman R. Purification and characterization of Sar1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem. 1993;15:873–879. [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach M, Razzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by SEC proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bednarak SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI-coated and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Cole NB, Ellenberg J, Song J, DiEulis D, Lippincott-Schwartz J. Retrograde transport of Golgi-localized proteins to the ER. J Cell Biol. 1998;140:1–15. doi: 10.1083/jcb.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis MA, Santini G, Kahn RA, DiTullio G, Luini A. Receptor and protein kinase C-mediated regulation of ARF binding to the Golgi complex. Nature. 1993;26:818–821. doi: 10.1038/364818a0. [DOI] [PubMed] [Google Scholar]
- Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Lippincott-Schwartz J, Bloom GS, Kreiss TE, Klausner RD. Dissociation of a 100 kDa peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990;111:2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D, Brown WJ. Phospholipase A2 antagonists inhibit nocodazole-induced Golgi ministack formation: evidence of an ER intermediate and constitutive cycling. Mol Biol Cell. 1999;10:4021–4032. doi: 10.1091/mbc.10.12.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione CJ, Rose JK. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J Virol. 1985;54:374–382. doi: 10.1128/jvi.54.2.374-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod A, Storrie B, Simpson JC, Johannes L, Goud B, Roberts LM, Lord JM, Nilsson T, Pepperkok R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat Cell Biol. 1999;1:423–430. doi: 10.1038/15658. [DOI] [PubMed] [Google Scholar]
- Hansen SH, Casanova JE. Gsα stimulates transcytosis and apical secretion in MDCK cells through cAMP and protein kinase A. J Cell Biol. 1994;126:677–687. doi: 10.1083/jcb.126.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Jamora C, Yamanouye N, Van Lint JV, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V. Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Johannes FJ, Prestle J, Dieterich S, Oberhagemann P, Link G, Pfizenmaier K. Characterization of activators and inhibitors of protein kinase C mu. Eur J Biochem. 1995;227:303–307. doi: 10.1111/j.1432-1033.1995.tb20389.x. [DOI] [PubMed] [Google Scholar]
- Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCμ is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994;269:6140–6148. [PubMed] [Google Scholar]
- Lee TH, Linstedt AD. Osmotically induced cell volume changes alter anterograde and retrograde transport, Golgi structure, and COPI dissociation. Mol Biol Cell. 1999;10:1445–1462. doi: 10.1091/mbc.10.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Chuong CM. Activation of protein kinase A is a pivotal step involved in both BMP-2- and cyclic AMP-induced chondrogenesis. J Cell Physiol. 1997;170:153–165. doi: 10.1002/(SICI)1097-4652(199702)170:2<153::AID-JCP7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:119–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lin HY, Thacorf HR, Davis FB. Potentiation by thyroxine of interferon-gamma-induced antiviral state requires PKA and PKC activities. Am J Physiol. 1996;271:C1256–C1261. doi: 10.1152/ajpcell.1996.271.4.C1256. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Mehta A, Suhan J, Reggio H, Hauri H-P. Sequence and overexpression of GPP130/GIMPc: evidence for saturable pH-sensitive targeting of a type II early Golgi membrane protein. Mol Biol Cell. 1997;8:1073–1087. doi: 10.1091/mbc.8.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri H-P, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the ER in the presence of Brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- Muniz M, Alonso M, Hidalgo J, Velasco A. A regulatory role for cAMP-dependent protein kinase in protein traffic along the exocytic route. J Biol Chem. 1996;271:30935–30941. doi: 10.1074/jbc.271.48.30935. [DOI] [PubMed] [Google Scholar]
- Muniz M, Martin ME, Hidalgo J, Velasco A. Protein kinase A activity is required for the budding of constitutive transport vesicles from the trans-Golgi network. Proc Natl Acad Sci USA. 1997;94:14461–14466. doi: 10.1073/pnas.94.26.14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreiss TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J. Regulation of organelle biogenesis. Cell. 1996;84:389–394. doi: 10.1016/s0092-8674(00)81283-0. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Huttner WB. An elevation of cytosolic protein phosphorylation mediates trimeric G-protein regulation of secretory vesicle formation from the trans-Golgi network. J Biol Chem. 1994;269:24897–24905. [PubMed] [Google Scholar]
- Pimplikar SW, Simons K. Activators of protein kinase A stimulate apical but not basolateral transport in epithelial Madin-Darby canine kidney cells. J Biol Chem. 1994;269:19054–19059. [PubMed] [Google Scholar]
- Prestle J, Pfizenmaier K, Brenner J, Johannes F-J. Protein kinase Cμ is located at the Golgi compartment. J Cell Biol. 1996;134:1401–1410. doi: 10.1083/jcb.134.6.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich NC, Pfeffer LM. Evidence for involvement of protein kinase C in the cellular response to interferon? Proc Natl Acad Sci USA. 1990;87:8761–8765. doi: 10.1073/pnas.87.22.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama NR, Chuang JS, Schekman R. SEC31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell. 1997;8:205–217. doi: 10.1091/mbc.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel J, Pepperkok R, Lowe M, Griffiths G, Kreis TE. Dissociation of coatomer from membranes is required for brefeldin A-induced transfer of Golgi enzymes to the endoplasmic reticulum. J Cell Biol. 1997;137:319–333. doi: 10.1083/jcb.137.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Rohrer J, Slot JW, Geuze HJ, Kornfeld S. Reassessment of the subcellular localization of p63. J Cell Sci. 1995;108:2477–2485. doi: 10.1242/jcs.108.6.2477. [DOI] [PubMed] [Google Scholar]
- Sciaky N, Presley PJ, Smith C, Zaal KA, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima DT, Cabrera-Poch N, Pepperkok R, Warren G. An ordered inheritance strategy for the Golgi apparatus: visualization of mitotic disassembly reveals a role for the mitotic spindle. J Cell Biol. 1998;141:955–966. doi: 10.1083/jcb.141.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, White J, Rottger S, Stelzer EHK, Suganuma T, Nilsson T. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, Yang W. Dynamics of the interphase mammalian Golgi complex as revealed through drugs producing reversible Golgi disassembly. Biochim Biophys Acta. 1998;1404:127–138. doi: 10.1016/s0167-4889(98)00053-6. [DOI] [PubMed] [Google Scholar]
- Tang BL, Peter F, Krijnse-Locker J, Low SH, Griffiths G, Hong W. The mammalian homolog of yeast Sec13p is enriched in the intermediate compartment and is essential for protein transport from the endoplasmic reticulum to the Golgi apparatus. Mol Cell Biol. 1997;17:256–266. doi: 10.1128/mcb.17.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci USA. 1994;91:8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Serafini T, Rothman JE. “Coatomer”: a cytosolic protein complex containing subunits of nonclathrin-coated Golgi transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- Xiao G-H, Falkner C, Xie, Yiqiang, Lindahl RG, Prough RA. CAMP-dependent negative regulation of rat aldehyde dehydrogenase class 3 gene expression. J Biol Chem. 1997;272:3238–3245. doi: 10.1074/jbc.272.6.3238. [DOI] [PubMed] [Google Scholar]
- Zaal KJ, Smith CL, Polishchuk RS, Altan N, Cole NB, Ellenberg J, Hirschberg K, Presley JF, Roberts TH, Siggia E, Phair RD, Lippincott-Schwartz J. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell. 1999;99:589–601. doi: 10.1016/s0092-8674(00)81548-2. [DOI] [PubMed] [Google Scholar]