Abstract
The formation of small vesicles is mediated by cytoplasmic coats the assembly of which is regulated by the activity of GTPases, kinases, and phosphatases. A heterotetrameric AP-3 adaptor complex has been implicated in the formation of synaptic vesicles from PC12 endosomes (Faundez et al., 1998). When the small GTPase ARF1 is prevented from hydrolyzing GTP, we can reconstitute AP-3 recruitment to synaptic vesicle membranes in an assembly reaction that requires temperatures above 15°C and the presence of ATP suggesting that an enzymatic step is involved in the coat assembly. We have now found an enzymatic reaction, the phosphorylation of the AP-3 adaptor complex, that is linked with synaptic vesicle coating. Phosphorylation occurs in the β3 subunit of the complex by a kinase similar to casein kinase 1α. The kinase copurifies with neuronal-specific AP-3. In vitro, purified casein kinase I selectively phosphorylates the β3A and β3B subunit at its hinge domain. Inhibiting the kinase hinders the recruitment of AP-3 to synaptic vesicles. The same inhibitors that prevent coat assembly in vitro also inhibit the formation of synaptic vesicles in PC12 cells. The data suggest, therefore, that the mechanism of AP-3-mediated vesiculation from neuroendocrine endosomes requires the phosphorylation of the adaptor complex at a step during or after AP-3 recruitment to membranes.
INTRODUCTION
Membrane proteins are carried from donor membranes to acceptor membranes by two steps, the vesiculation of a carrier vesicle from the donor and the fusion of the carrier vesicle with the acceptor. Vesiculation of the donor membrane can itself be divided into five steps: recognition of cargo proteins; recruitment of coating molecules; the imposition of curvature on the forming vesicle membrane; fission of the carrier vesicle from the donor membrane; and, finally, the loss of the vesicle coat (Springer et al., 1999). To discover the molecular events that take place at each step, several laboratories have reconstituted either the entire process of vesiculation or individual steps in vitro.
The first two steps of vesiculation, cargo recognition and coating, can be regulated by phosphorylation. Membrane proteins are recognized as cargo because they contain sorting domains. Adaptors interact with a tyrosine or a dileucine motif (Schmid, 1997; Kirchhausen et al., 1997; Bonifacino and Dell'Angelica, 1999). The recognition of cargo molecules can be regulated by the phosphorylation of sites close to the sorting domains; for example, in the trafficking of furin (Wan et al., 1998; Teuchert et al., 1999; Molloy et al., 1999), CD4 (Pitcher et al., 1999), CTLA-4 (Shiratori et al., 1997), MHC-II-Ii complex (Anderson and Roche, 1998), and pIgAR (Casanova et al., 1990; Okamoto et al., 1994; Luton et al., 1998). The cargo molecules are recruited into a newly forming carrier vesicle by cytoplasmic coat molecules (Matsuoka et al., 1998; Bremser et al., 1999). The recruitment of two of the known coats, COPI and COPII, is regulated by small ARF-like GTPases and appears to be a relatively simple process (Lowe and Kreis, 1998; Springer et al., 1999). COPII coat recruitment also is regulated by phosphorylation, since serines of the sec31p component of the COPII coat need to be phosphorylated for full coating activity (Salama et al., 1997). Another form of coating involves the clathrin triskelion. The function of clathrin heavy and light chains is modulated by phosphorylation. Clathrin heavy chain recruitment is regulated by a single tyrosine phosphorylation (Wilde et al., 1999), whereas light chains are regulated by multiple serine-phosphorylation events (Chu et al., 1999). Clathrin-mediated coating requires a heterotrimeric adaptor complex, AP-1 for Golgi and AP-2 for plasma membrane vesiculation, that links cargo to clathrin. In contrast to the results for COPII, phosphorylation of serines in AP-1 and AP-2 inhibits their recruitment to membranes (Wilde and Brodsky, 1996).
Vesiculation from endosomes also can be reconstituted in vitro and has been found to be relatively simple. When the donor membranes are early endosomes from the neuroendocrine cell line PC12, small vesicles are formed in vitro that have a size, density, and composition that is similar to brain synaptic vesicles (Lichtenstein et al., 1998). The formation of PC12 synaptic vesicles, sometimes called synaptic-like microvesicles, can be reconstituted in vitro by adding two cytoplasmic factors, the small GTPase, ARF1, and a third form of adaptor complex, AP-3, but no added clathrin (Faundez et al., 1997, 1998; Shi et al., 1998). The identification of AP-3 as a coating factor was a result of our ability to mimic the first two of the five steps of vesiculation from endosomes, cargo recognition and coat recruitment, by using a novel “reverse reaction,” in which purified synaptic vesicles are coated with AP-3 by incubation in the presence of GTP-ARF (Faundez et al., 1998). The coating reaction replicated the budding process since it also requires temperatures above 15°C and hydrolyzable ATP (Faundez et al., 1998). The experimental simplicity of the coating reaction allowed us to investigate the ATP-dependent step.
We have now found an enzymatic reaction, the phosphorylation of the AP-3 adaptor complex, that is associated with synaptic vesicle coating. Phosphorylation is by a kinase similar to casein kinase Iα that copurifies with AP-3 and phosphorylates the β3 subunit at its hinge domain. Inhibiting the phosphorylation inhibits the coating process, suggesting that phosphorylation helps the recruitment of AP-3 complexes or the stabilization of AP-3 coats. The phosphorylation appeared to have physiological significance since inhibiting casein kinase inhibits the recruitment of AP-3 to endosomes and also synaptic vesicle production in PC12 cells.
MATERIALS AND METHODS
Materials
[125I]Na and ECL reagents were obtained from Amersham Corp (Arlington Heights, IL). [35S]ATPγS and [35S]GTPγS were obtained from NEN (Boston, MA). All the nucleotides, creatine phosphate, creatine kinase, and Sephadex G25 were purchased from Boehringer Mannheim (Indianapolis, IN). Protein G-Sepharose 4 Fast Flow and glutathione-Sepharose 4B were obtained from Pharmacia Biotech (Uppsala, Sweden). Brefeldin A was purchased from Epicenter Technologies (Madison, WI). Lithium heparin, staurosporine, DRB (5,6-Dichloro-1-β-d-ribofuranosylbenzimidazole), and the catalytic tryptic fragment of brain protein kinase C were acquired from Calbiochem (San Diego, CA). Purified casein kinases I and II and the catalytic subunit of the cAMP-dependent kinase were purchased from Promega (Madison, WI). The casein kinase inhibitor CKI-7 was purchased from Seikagaku America (Ijamsville, MD). All drugs were dissolved in methanol. Cell culture media and reagents were obtained from the University of California Cell Culture Facility (San Francisco, CA). Geniticin (G418) and IPTG were obtained from Life Technologies (Gaithersburg, MD). All the other reagent-grade chemicals were purchased from Sigma (St. Louis, MO), Fisher Chemical (Fairlawn, NJ), or Calbiochem.
Antibodies
Monoclonal antibodies against synaptophysin (SY38) were purchased from Boehringer Mannheim. Polyclonal antibodies against three subunits of AP-3, β-NAP647–796, which recognize both β3A and β3B, μ3/p47, and ς3 were generated as described in Dell'Angelica et al. (1997b). Anti-ARF antibodies used in this study were polyclonal antibodies raised in rabbits immunized with myristoylated recombinant human mutant ARF1 Q71L. Isotype-specific antibodies against casein kinase Iα (78 19) and Iδ (N19) reactive against casein kinase Iδ−ε were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Affinity-purified anti-casein kinase Iα antibody (RA) was a generous gift of Dr. R.A. Anderson (Department of Pharmacology, University of Wisconsin-Madison Medical School) (Gross et al., 1995).
Animals and Brain Cytosol Preparation
Female Sprague Dawley rats were from Bantin and Kingman (Fremont, CA). C57BL/6J, mocha STOCK gr+/+ AP-3dmh, and pearl C57BL/6J-pe mice were from the Jackson Laboratory (Bar Harbor, ME). Pale ear mice (ep/ep) and pearl C57BL/6J-pe mice were a gift of Dr. R. Swank (Roswell Park Cancer Institute, Buffalo, NY). Either rat or mouse brain cytosol were prepared as described (Clift-O'Grady et al., 1998). When required, each milliliter of cytosol was dialyzed overnight at 4°C against 1000 ml of intracellular buffer (38 mM potassium aspartate, 38 mM potassium glutamate, 38 mM potassium gluconate, 20 mM potassium MOPS, pH 7.2, 5 mM reduced glutathione, 5 mM sodium carbonate, and 2.5 mM magnesium sulfate) supplemented with an antiprotease mixture (Clift-O'Grady et al., 1998).
Expression and Purification of Recombinant Proteins
Q71L human ARF1 cDNA, kindly provided by Dr. D. Shields (Albert Einstein College of Medicine, Bronx, NY), was used to generate recombinant protein as described (Faundez et al., 1997). Plasmids encoding GST and GST-fusion proteins encompassing the trunk of β3A (residues 1–287 and 288–642), the hinge domain of β3A (residues 643–809) and β3B (residues 647–796), the ear domain of β3A (residues 810–1094), or the hinge–COOH terminal region of β2 (residues 592–951) were gifts of Dr. E. Dell'Angelica (NIH) (Dell'Angelica et al., 1997a,b), and they were purified, concentrated in Centriprep 30 (Amicon, Beverly, MA), and extensively dialyzed against intracellular buffer
Synaptic-like Microvesicles Coating Assay and Affinity Chromatography
PC12 synaptic-like microvesicles (SVs) were isolated from the PC12/N49A cell line transfected with an epitope-tagged version of the synaptic vesicle protein VAMP2, mutant N49A (Faundez et al., 1998). Cell-free synaptic vesicle-coating assays were performed in 250 μl total volume in intracellular buffer, using PC12/N49A 125I-KT3-labeled vesicles as described (Faundez et al., 1998). Reconstituted mixtures in the absence or presence of drugs were kept at 0°C for 15 min. Coating reactions were started by warming to 37°C. Reactions were stopped at 0°C for 10 min and loaded on the top of continuous 10–45% (wt/vol) sucrose gradients buffered in 20 mM MOPS-KOH, pH 7.4, and 0.5 mM MgCl2. Sucrose gradients were centrifuged at 183,000 × g for 150 min in a SW55 rotor. Fractions (27–28) were collected from the bottom of the gradient and counted in a gamma counter. Sucrose concentration at the peak was determined by refractometry.
Antibodies against the cytosolic tail of synaptophysin (SY38, 0.5 μg) were bound to 25 μl of protein G Sepharose. SVs were bound to the matrix for 3 h or overnight at 0°C, and the unbound vesicles were washed away in intracellular buffer supplemented with 0.1% ovalbumin. Reaction mixtures containing 3 mg/ml rat brain cytosol, in the absence or presence of either drugs or different nucleotides, were incubated for 15 min at 0°C followed by warming to 37°C for 40 min with periodic resuspension of the beads. After arresting the reactions at 0°C, the matrix was washed in intracellular buffer, and the retained proteins were eluted with Laemmli sample buffer and resolved in 8–18% gradient PAGE–SDS gels before immunoblotting (Faundez et al., 1998).
To thiophosphorylate coated synaptic vesicles, AP-3 recruitment was triggered by adding [35S]-ATPγS (0.5 μCi/μmol) and incubating at 37°C for 40 min. Reactions were stopped at 0°C, and bound complexes were washed extensively in intracellular buffer. Coated SVs were solubilized from the column by either sample buffer to directly resolve the proteins in SDS-PAGE, or in buffer B (50 mM Tris, pH 7.4; 300 mM NaCl; 5 mM EDTA, 1% Triton X-100) incubated for 30 min at 4°C, from which AP-3 β subunits were immunoprecipitated and immunocomplexes were washed and analyzed as described.
AP-3 Recruitment Assays to PC12-Donor Endosomes
A PC12 N49A membrane fraction enriched in synaptic vesicle endosome donor and devoid of plasma membrane (50,000-g membranes) was prepared as described (Lichtenstein et al., 1998; de Wit et al., 1999). To isolate radiolabeled donor endosomes, cell were labeled as described (vide infra) and the 50K-g membranes were resolved by sucrose gradient centrifugation as described (Lichtenstein et al., 1998). Briefly, PC12N49A cells were incubated for 45 min at 15°C before homogenization. Homogenate was sedimented at 1000 × g for 5 min, and the supernatants were sequentially spun at 10,000 × g for 10 min and at 27,000 × g for 35 min (donor enriched membranes). Donor membranes were resuspended in intracellular buffer at ∼2–4 mg/ml. Assays were made with 50 μg of membranes and 2 mg/ml dialyzed rat brain cytosol in the absence or presence of either ATPγS or CKI-7. Reaction mixtures were incubated in ice for 15 min to be transferred to 37°C for 20 min and stopped in ice for 5 min. Soluble and membrane-bound proteins were separated by sedimenting the reaction mixture through a 600-μl 12.5% sucrose cushion prepared in intracellular buffer at 27,000 × g for 45 min. Pellets were washed in cold intracellular buffer and were resuspended in sample buffer to be analyzed by immunoblot with antibodies directed to β3. The background AP-3 binding to membranes was determined in complete reactions in the absence of any added nucleotide. Membrane load was determined by immunoblotting with antibodies against synaptophysin (SY38). Immunoblot quantitation was performed as described (Faundez et al., 1997). Usually AP-3 recruitment to membranes increased 5.1 ± 3.3-fold (range, 2–10-fold; n = 5) over the background in the presence of ATP-regenerating system and 20 μM GMP-PNP .
Immunoprecipitations and Phosphorylation Assays
Brain AP-3 was immunoprecipitated from either rat brain cytosol or mouse brain cytosol in native conditions or was purified to homogeneity as described (Faundez et al., 1998). Preimmune sera or affinity-purified anti-ς3 antibodies were prebound to protein G-Sepharose, and free antibody was washed away. Cytosol (0.25–0.6 mg per assay) was diluted in either intracellular buffer or buffer A (10 mM HEPES, pH 7.4; NaCl 150 mM; EGTA 1 mM; and MgCl2 0.1 mM) and incubated overnight with the antibody–Sepharose complex. Unbound material was removed by extensively washing either in intracellular buffer or buffer A supplemented with Triton X-100 0.1% followed by a wash in intracellular buffer. Bead-bound immune complexes were resuspended in 250 μl of intracellular buffer. Before thiophosphorylation, beads were incubated with or without drugs for 15 min at 0°C. Reactions were initiated at 24°C with 20 μCi [35S]ATPγS (65 Ci/mmol)) for the specified times. Assays were stopped with 800 μl of ice-cold buffer A, 20 mM EDTA, and the free nucleotide was washed away in buffer A, Triton X-100 0.1%. Thiophosphorylated proteins were eluted with Laemmli sample buffer and were resolved by SDS-PAGE.
Purified bovine brain AP-3 complex (6 μg) was incubated in intracellular buffer in the absence or presence of different drugs at 0°C for 15 min. Thiophosphorylation was performed as previously described except that after stopping the assays in buffer A, 20 mM EDTA, proteins were TCA-precipitated from the reaction mixture and analyzed by SDS-PAGE.
Histone IIA, GST, and GST-fusion proteins (20 μg) were thiophosphorylated in vitro in intracellular buffer (50 μl per assay) with liver-purified casein kinase I or II (10 U/assay), catalytic subunit of the cAMP-dependent kinase (10U/assay), or the catalytic tryptic fragment of brain protein kinase C (20 ng/assay). Thiophosphorylation reactions were performed for 20 min at 24°C in the presence of 20 μCi [35S]-ATPγS. Reactions were stopped in buffer A, 20 mM EDTA, and proteins were TCA-precipitated from the reaction mixture and analyzed by SDS-PAGE.
Protein labeling was analyzed in a STORM Phosphorimager, and the results were quantified using ImageQuant, version 1.2, software (Molecular Dynamics, Sunnyvale, CA).
Cell Labeling and Fractionation
PC12 cells containing the VAMP-TAg N49A mutation were labeled with [125I]KT3 monoclonal antibody against the T-antigen epitope tag as described (Clift-O'Grady et al., 1998; Faundez et al., 1998). Briefly, cells were labeled at 0°C for 15 min and then were transferred at 15°C for 40 min. To examine the effect of reagents on the in vivo generation of SVs, cells were first labeled at 15°C then were treated in DME H-21, 10 mM HEPES, pH 7.4, with either brefeldin A for 15 min at 0°C (Faundez et al., 1997) or with kinase inhibitors for 20 min at 15°C. In vivo vesicle production was resumed by heating the cells at 37°C. Reactions were stopped at 0°C. Homogenization, cell fractionation, and synaptic vesicle production assessment were performed as described (Faundez et al., 1997; Clift-O'Grady et al., 1998).
Other Procedures
KT3 monoclonal antibodies were iodinated by chloramine-T (Faundez et al., 1992). Protein assays were performed using the Bio-Rad Protein Assay Dye Reagent (Bio-Rad, Richmond, CA) using BSA as standard.
RESULTS
AP-3 Recruitment to Synaptic Vesicles Requires ATP
AP-3 coat recruitment to synaptic vesicles is inhibited by temperatures of ≤ 15°C (Faundez et al., 1998). In contrast, AP-2 adaptor recruitment to synaptic vesicles occurs at 4°C and in the absence of nucleotides (Zhang et al., 1994). To address the presence of novel regulatory enzymatic mechanisms, we analyzed the nucleotide requirements for AP-3 recruitment to synaptic vesicles.
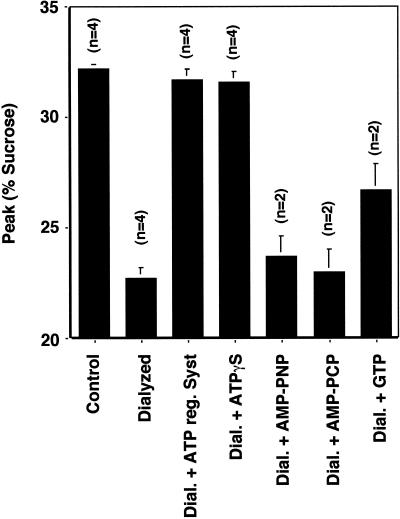
The ATP requirement for AP-3 recruitment can be measured readily using isolated synaptic vesicles. Labeled PC12 synaptic vesicles were incubated with rat brain cytosol in the absence or presence of different nucleotides and the GTP mutant form of ARF1 (Q71L). AP-3 recruitment was assessed as before (Faundez et al., 1998) as a shift in vesicle sedimentation in sucrose velocity gradients. In the presence of an ATP-regenerating system and ARF1Q71L, protein from brain cytosol was bound to synaptic vesicles (Figure 1). Removing nucleotides by cytosol dialysis inhibited the process in a reversible manner. Adding back an ATP-regenerating system restored coat binding to membranes. Coating activity was supported equally well by the partially hydrolyzable ATP analog ATPγS (200 μM), but not by the nonhydrolyzable ATP analogs AMP-PCP and AMP-PNP at similar concentrations (Figure 1). Higher nonhydrolyzable nucleotide analog concentrations or longer incubation times did not restore the coating activity (data not shown). Since ATPγS can substitute for ATP as a substrate for protein kinases (Gratecos and Fischer, 1974; Nichols et al., 1990), these results suggest that a phosphorylation reaction is required for the AP-3 coat recruitment to synaptic vesicles.
Figure 1.
Nucleotide requirements for the ARF-dependent protein recruitment onto SV. Synaptic vesicle coating reactions were performed with synaptic vesicles isolated from 125I-KT3-labeled PC12N49A cells. Synaptic vesicles were incubated in the presence of 3 mg/ml normal or dialyzed cytosol plus 30 μM Q71L ARF1 mutant. Dialyzed cytosol was supplemented with either an ATP-regenerating system (O'Grady et al. 1998) or with different nucleotides at 200 μM concentration, except for GTP, which was at a final concentration of 1 mM. Samples were warmed to 37°C for 30 min, and reactions were stopped at 0°C. Vesicle sedimentation was analyzed in 10–45% continuous sucrose gradients, and the sucrose concentration was measured at the peak of radioactivity. Only the poorly hydrolyzable ATP analog ATPγS could fully replace the ATP requirements of the coating reaction and shifts the density from 22 to 32% sucrose.
β3 Subunit of the AP-3 Complex Is Thiophosphorylated
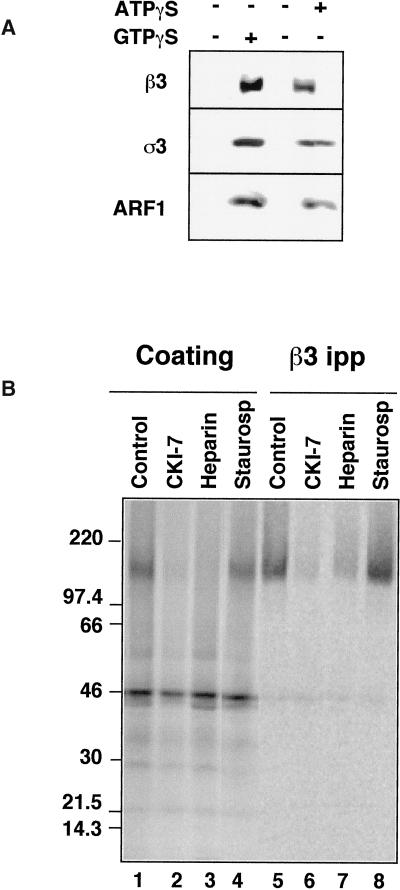
We performed labeling experiments with [35S]ATPγS to identify putative phosphorylated substrates that might account for the ATP/ATPγS requirements in the coating reaction. We first modified the reaction to establish the minimal concentration of nucleotides required for coating synaptic vesicles. In the presence of an ATP-regenerating system (1 mM ATP), GTPγS (20 μM) induced an ARF1-dependent AP-3 recruitment to vesicles (Figure 2a; compare lanes 1 and 2). Likewise, AP-3 translocation to synaptic vesicles occurred in the presence of ATPγS at concentrations as low as 150 μM, even in the absence of GTPγS (Figure 2a; compare lanes 3 and 4). Since ARF1 does not bind ATP (Kahn and Gilman, 1986); this could represent an interconversion of ATPγS to GTPγS by a nucleoside diphosphokinase activity present in rat brain cytosol (Seifert et al., 1988; Wieland and Jakobs, 1992).
Figure 2.
Recruitment of AP-3 to immobilized synaptic vesicles. (A) AP-3 is recruited to SVs in the presence of ATPγS. Coating assays were performed using unlabeled PC12 N49A synaptic vesicles attached to an antisynaptophysin-protein G-Sepharose affinity column. Reactions were performed with rat brain cytosol, supplemented (lanes 1 and 2) or not (lanes 3 and 4) with an ATP-regenerating system. AP-3 recruitment to SV was induced at 37°C for 30 min either by GTPγS (20 μM, lane 2) or solely by ATPγS (200 μM, lane 4). AP-3 recruitment to synaptic vesicles was assessed by immunoblot with antibodies against either β3 or ς3 subunits of the complex. Similarly, ARF1 binding to membranes was determined with an anti-ARF1 antibody. (B) The β3 subunit of the synaptic vesicle-bound AP-3 complex is thiophosphorylated by acasein kinase activity. Immunoimmobilized PC12 N49A synaptic vesicles were coated in vitro in the presence of dialyzed rat brain cytosol. Coating was triggered by adding [35S]-ATPγS (100 μCi) and warming the reactions at 37°C for 40 min. Reactions were stopped at 4°C, and bound complexes were washed extensively in intracellular buffer. Coated SVs were solubilized from the column by either sample buffer to directly resolve the proteins in SDS-PAGE (lanes 1–4) or in buffer B (lanes 5–8), from which AP-3 β subunits were immunoprecipitated and immunocomplexes were analyzed by SDS-PAGE. The intensity of the β3 thiophosphorylated band was reduced by the casein kinase inhibitors CKI-7 (500 μM, lanes 2 and 6) and heparin (10 μg/ml, lanes 4 and 9) but not by a generic serine–threonine kinase inhibitor staurosporine (10 μM, lanes 4 and 8).
When AP-3 recruitment to immunoimmobilized synaptic vesicles was triggered in the presence of [35S]ATPγS, a prominent thiophosphorylated band of 150 kDa could be detected if there were synaptic vesicles on the beads (Figure 2b, lane 1); in the absence of synaptic vesicles a 46-kDa thiophophorylated band was nonspecifically bound to the protein G matrix. The 150-kDa band could be immunoprecipitated by antibodies against the β3 subunit of the AP-3 complex (Figure 2b, lane 5). No other thiophosphorylated bands were apparent after solubilizing the coated synaptic vesicles.
We compared the effects of a broad-spectrum inhibitor of serine–threonine kinases, staurosporine (Meggio et al., 1995), to the selective casein kinase inhibitor CKI-7 (Chijiwa et al., 1989). CKI-7 was studied since genetic interactions between AP-3 complex subunits and casein kinase I genes have been reported in yeast (Panek et al., 1997). The presence of the thiophosphorylated β3 subunit was decreased by CKI-7 or heparin, another inhibitor of casein kinases (Zhai et al., 1995) (Figure 2b, lanes 2 and 3, 6 and 7) but not by high concentrations of staurosporine (Figure 2b, lanes 4 and 8), which is unable to inhibit casein kinases (Meggio et al., 1995). Identical results were obtained whether the total proteins were recruited to synaptic vesicles (Figure 2b, lanes 1–4) or the immunoprecipitated β3 subunit were analyzed (Figure 2b, lanes 5–8). Thus, the ATP requirement for coating could be to allow the phosphorylation of the β subunit of the AP-3 complex.
β3 Subunits Are Selectively Thiophosphorylated in the Hinge Region by Casein Kinase
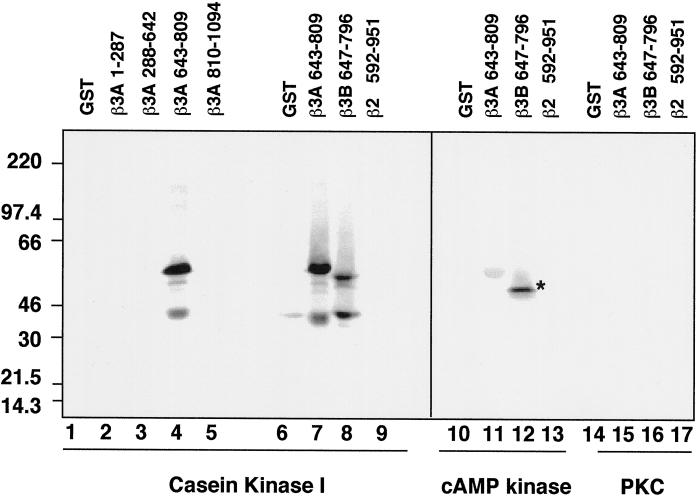
Fusion proteins encompassing different segments of β3A and β3B were used to define the regions phosphorylated by the protein kinases. Sequence analysis predicted 22 D/EXXS consensus sites for casein kinase I (Songyang et al., 1996) clustered in the acidic hinge regions of β3A, and 13 in β3B. Only fusion proteins encompassing the hinge domains of both β3 subunits, amino acids 643–809 and 647–796 of β3A and β3B, respectively, were thiophosphorylated by purified casein kinase I (Figure 3, lanes 4, 7 and 8). Neither GST alone (Figure 3, lanes 1 and 6) nor the trunk (amino acids 1–642 of β3A; Figure 3, lanes 2 and 3) nor the clathrin binding region of the ear (amino acids 810–1094 and 799–1081 of β3A and β3B, respectively (Figure 3, lanes 5 and data not shown) were substrates of the purified kinase. Although the corresponding subunits of AP-1 and AP-2, the β1, β2 adaptins, are phosphorylated at the hinge regions (Wilde and Brodsky, 1996), they do not contain casein kinase I consensus sites. Consistently, no thiophosphorylation by casein kinase I above the level of GST was detected when a fusion protein of the hinge–COOH terminal domain of β2 adaptin (amino acids 592–951) was used as a substrate (Figure 3; compare lanes 6 and 9). Purified casein kinase II also thiophosphorylated the hinge domain of β3A, although three times less efficiently. The hinge domain of β3B was thiophosphorylated with similar efficiency by both casein kinases (data not shown). Neither cAMP-dependent kinase (Figure 3, lanes 11 and 12) nor protein kinase C (Figure 3, lane 15 and 16) thiophosphorylated the β3 hinge regions. Under identical conditions, histone H2A was efficiently thiophosphorylated by both of these kinases (data not shown). These data show that the hinge regions of β3 chains are selectively thiophosphorylated by casein kinase.
Figure 3.
The hinge domain of β3A and β3B are selectively thiophosphorylated by casein kinase I. GST and GST fusion proteins encompassing the trunk of β3A (residues 1–287 and 288–642; lanes 2 and 3, respectively), the hinge domain of β3A (residues 643–809; lanes 4, 7, 11, and 15) and β3B (residues 647–796; lanes 8, 12, and 16), the ear domain of β3A (residues 810–1094, lane 5), or the hinge–COOH terminal region of β2 (residues 592–951; lanes 9, 13, and 17) were thiophosphorylated as described with purified casein kinase I (10 U/assay; lanes 1–9), catalytic subunit of the cAMP-dependent kinase (10U/assay; lanes 10–13), or the catalytic tryptic fragment of brain protein kinase C (20 ng/assay; lanes 14–17). Recombinant proteins were TCA-precipitated from the reaction mixture and were analyzed by SDS-PAGE. Only the hinge domains of β3A and β3B were thiophosphorylated by casein kinase I. The asterisk in lane 12 corresponds to a bacterial contaminant band.
A Casein Kinase I-like Activity Is Associated with the AP-3 Complex
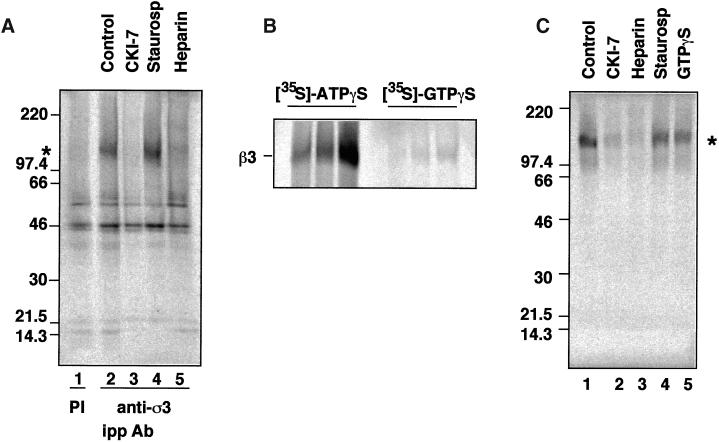
An unidentified kinase activity has been reported to coimmunoprecipitate with antibodies against β3B (Newman et al., 1995). To determine whether the kinase activity associated with the AP-3 complex behaved like a casein kinase, brain AP-3 heterooligomers were immunoprecipitated with antibodies against ς3 subunits, and the immune complexes were [35S]ATPγS thiophosphorylated in vitro in the absence or presence of kinase inhibitors. Anti-ς3 antibodies brought down the complex together with a kinase able to thiophosphorylate the β3 subunit. No such band was observed when preimmune antibodies were used (Figure 4a; compare lanes 1 and 2). The kinase activity associated with the AP-3 complex was sensitive to CKI-7 and heparin (Figure 4a, lanes 3 and 5, respectively; and Figure 5b, dotted bar) but not to staurosporine (Figure 4a, lane 4; Figure 5b, striped bar), characteristics of a casein kinase. The kinase activity immunoisolated with the AP-3 complex thiophosphorylated an exogenous substrate, which is a GST fusion protein containing the hinge domain of β3B (data not shown). Casein kinase II possesses a similar affinity for both ATP and GTP, whereas casein kinase I uses ATP preferentially as a substrate (Edelman et al., 1987; Tuazon and Traugh, 1991). To determine whether casein kinase I or II is associated with AP-3, we performed thiophosphorylation reactions using either [35S]ATPγS or [35S]GTPγS as substrates. [35S]ATPγS was an effective substrate for the casein kinase activity associated with the complex (Figure 4b), whereas no significant incorporation of the label into the β3 subunit was detected using [35S]GTPγS. Both nucleotide analogs thiophosphorylated the GST-β3B hinge fusion protein indistinguishably when assayed as substrates for purified casein kinase II (data not shown).
Figure 4.
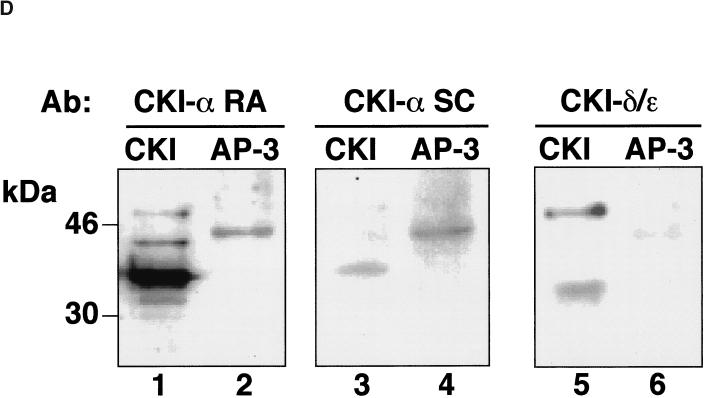
The AP-3 coat complex is tightly associated with casein kinase I. (A) Casein kinase I activity coimmunoprecipitates with the AP-3 complex. Native AP-3 complex was immunoprecipitated from rat brain cytosol with antibodies against ς3 subunits (lanes 2–5) with preimmune sera (lane 1) as a control. Immunocomplexes were reconstituted at 4°C for 15 min in intracellular buffer in the absence (lanes 1 and 2) or the presence of CKI-7 (500 μM, lane 3), staurosporine (10 μM, lane 4), or heparin (10 μg/ml, lane 5). Thiophosphorylation was started at 24°C by adding 20 μCi [35S]-ATPγS. Reactions were stopped in ice by adding buffer A plus 20 mM EDTA. Immunocomplexes were extensively washed in buffer A before SDS-PAGE. An asterisk indicates the position of phosphorylated β chain. (B) Immunoprecipitated brain AP-3 complex was thiophosphorylated for 5, 10, and 20 min, using either 20 μCi [35S]-ATPγS or 20 μCi [35S]-GTPγS, as previously described. Only [35S]-ATPγS was effective in thiophosphorylating the β3 subunit of the AP-3 complex. (C) Purified brain AP-3 complex is tightly associated with the casein kinase I activity. MonoS-purified bovine brain AP-3 complex in intracellular buffer was reconstituted at 4°C for 15 min in the absence (lane 1) or presence of CKI-7 (500 μM, lane 2), heparin (10 μg/ml, lane 3), staurosporine (300 nM, lane 4), or GTPγS (20 μM, lane 5). Thiophosphorylation reactions were performed as described in the presence of 20 μCi [35S]-ATPγS. Thiophosphorylated AP-3 complex was TCA-precipitated from the reaction mixture and was analyzed by SDS-PAGE. Both the immunoprecipitated and purified AP-3 complex displayed a similar inhibition profile. (D) A Iα-like casein kinase is associated with the AP-3 complex. Either 5.6 μg of liver-purified casein kinase I (lanes 1, 3, and 5) or 6 μg of bovine brain-purified AP-3 complex were resolved in SDS-PAGE gels and immunoblotted with affinity-purified anti-casein kinase Iα antibodies (CKI-α RA and CKI-α SC) or anti-δ/ε casein kinase I isoforms. Only the antibodies directed against the Iα isoform, RA and SC, cross-reacted with a 45-kDa band present in the AP-3 complex.
Figure 5.
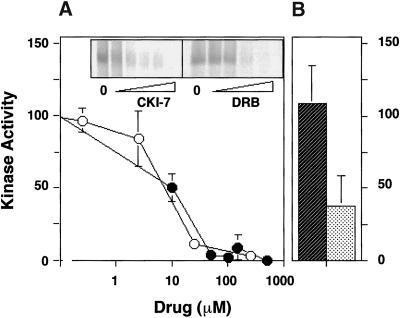
Casein kinase inhibitors hinder β3 thiophosphorylation in a dose-dependent manner. (A) Native AP-3 complex was immunoprecipitated from rat brain cytosol with antibodies against ς3 subunits. Thiophosphorylation reactions were performed as described. Before adding 20 μCi [35S]-ATPγS, the reaction mixtures were preincubated at 4°C for 15 min in intracellular buffer in the absence or presence of increasing concentrations of either CKI-7 (closed circles) or DRB (5,6-Dichloro-1-β-d-rybofuranosylbenzimidazole; open circles). Thiophosphorylation was stopped with ice-cold buffer A plus 20 mM EDTA. Immunocomplexes were extensively washed in buffer A to then be resolved in SDS-PAGE gels. Bands were quantified by PhosphorImager. The IC50 for CKI-7 and DRB were both ∼10 μM (n = 2). (B) Depicted is the quantitation of the effects of 300 nM staurosporine (diagonally striped bar) or 10 μg/ml heparin (n = 3) on the kinase coimmunoprecipitated with the AP-3 complex.
Casein kinase I activity remained associated with AP-3, even when purified to homogeneity in vitro by standard conditions (Faundez et al., 1998). After the final MonoS purification step, Coomassie staining of pure AP-3 showed only five bands that corresponded to the δ, β3, μ3, and ς3A and ς3B subunits (Faundez et al., 1998). When incubated with [35S]ATPγS AP-3, the pure complex still incorporated label in the β subunit as determined by its molecular weight (Figure 4c, lane 1). As expected for a casein kinase I, this endogenous phosphorylation was significantly sensitive to CKI-7 and heparin (Figure 4c, lanes 2 and 3) but not to staurosporine or to an excess of cold GTPγS (Figure 4c, lanes 4 and 5).
The casein kinase I isoform in the purified AP-3 complex could be identified with isoform-specific antibodies (Figure 4d). Two affinity-purified antibodies against casein kinase Iα (RA and SC) recognized the 37-kDa variant present in liver-purified casein kinase Iα (Figure 4d, lanes 1 and 3). The RA antibody also detected two additional bands, probably corresponding to Iα splicing variants (Figure 4d, lane 1; Gross and Anderson, 1998). Both antibodies cross-reacted with a 45-kDa band present in purified AP-3 (Figure 4d, lanes 2 and 4). Neither the 37-kDa isoform of casein kinase Iα nor casein kinase Iδ−ε (Figure 4d, lane 6) was detected in purified AP-3 complex. Thus, the kinase activity in pure AP-3 is a 45-kDa Ια-like casein kinase.
We further characterized pharmacologically the Ια-like AP-3-associated casein kinase using DRB (5,6-Dichloro-1-β-d-ribofuranosylbenzimidazole), an inhibitor of casein kinases but not of protein kinases A and C (Meggio et al., 1990). Although DRB is structurally unrelated to CKI-7, it inhibits at an IC50 of ∼10 μM, a value close to that reported for purified casein kinase I (Figure 5a) (Chijiwa et al., 1989; Meggio et al., 1990).
The Neuronal AP-3 Complex Is Associated with Casein Kinase I
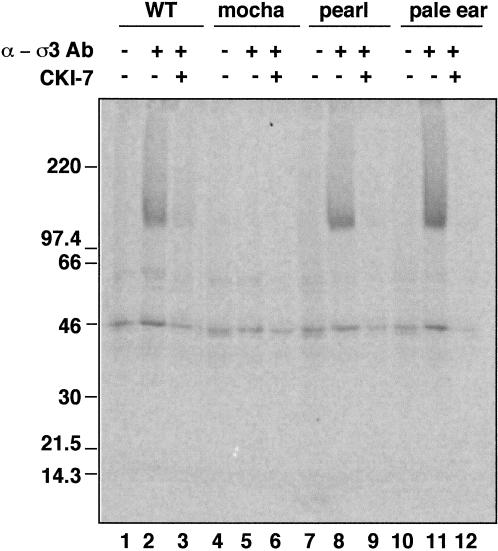
ATP-dependent AP-3 recruitment to synaptic vesicles is observed using cytosol derived from the brain. Both the neuronal-specific β3 subunit (β3B) and the ubiquitous β3A are expressed in the brain, whereas the liver has only the ubiquitous form. We took advantage of two members of the storage pool disease family of mutant mice to show that neuronal AP-3 has a casein kinase 1α-like activity. Mocha mice that do not express the δ subunit lack both AP-3 complexes (Kantheti et al., 1998), whereas the pearl mice lacking β3A have only the brain form (Feng et al., 1999). As a control, we used another Hermansky–Pudlak-like family member, pale ear, defective in a soluble protein that does not interact with AP-3 (Gardner et al., 1997; Dell'Angelica et al., 2000). Antibodies against the ς3 subunit immunoprecipitate AP-3 complex with associated CKI-7-sensitive kinase activity from normal brain cytosol (Figure 6, lanes 1–3). As expected, no thiophosphorylated β3 subunit was detected when immunoprecipitations were performed from mocha brain cytosol that was defective in both kinds of AP-3 complex. Pearl or pale ear AP-3 complexes were both thiophosphorylated by the CKI-7-sensitive casein kinase I (Figure 6, lanes 8 and 9, 11 and 12). No thiophosphorylation differences were detected among wild-type-, pearl-, and pale ear-derived AP-3 complexes. Thus, since pearl brain has only the neuronal AP-3, the neuronal AP-3 complex must be associated with, and modified by, casein kinase I.
Figure 6.
The neuronal AP-3 complex is associated with casein kinase I. AP-3 complexes from mouse brain cytosol (250 μg/assay) prepared from C57BL6 mice (lanes 1–3), or the Hermansky–Pudlak-like mice mutants mocha (lanes 4–6), pearl (lanes 7–9), and pale ear (lanes 10–12) were immunoprecipitated with preimmune sera (lanes 1, 4, 7, and 10) or antibodies against ς3 subunits. Immunocomplexes were reconstituted at 4°C for 15 min in intracellular buffer in the absence or presence of CKI-7 (250 μM, lanes 3, 6, 9, and 12). Thiophosphorylation was started at 24°C by adding 20 μCi [35S]-ATPγS. Reactions were stopped in ice by adding buffer A plus 20 mM EDTA, were extensively washed, and the complexes were resolved in SDS–PAGE gels. AP-3 complexes immunoprecipitated from pearl mutants that only have the neuronal form of AP-3 were thiophosphorylated. No β3-phosphorylated bands were detected in mocha mice brain cytosol that lacks both neuronal and nonneuronal AP-3 complexes.
The casein kinase Iα-like activity that copurifies with AP-3 is likely to be responsible for the phosphorylation of the β subunit of AP-3 on its hinge domain. To determine whether it was also responsible for coat assembly, we returned to coat-recruitment assays.
If the requirement for hydrolyzable ATP reflects a need for casein kinase I activity, then kinase inhibitors should inhibit the density shift. The AP-3 recruitment required to bud synaptic vesicles from transferrin receptor-positive donor endosomes (Lichtenstein et al., 1998) was discovered using purified synaptic vesicles (Faundez et al., 1998). Synaptic vesicles selectively bind AP-3 in a GTP-dependent manner (Faundez et al., 1998), a process that greatly increases their sedimentation rate in sucrose gradients.
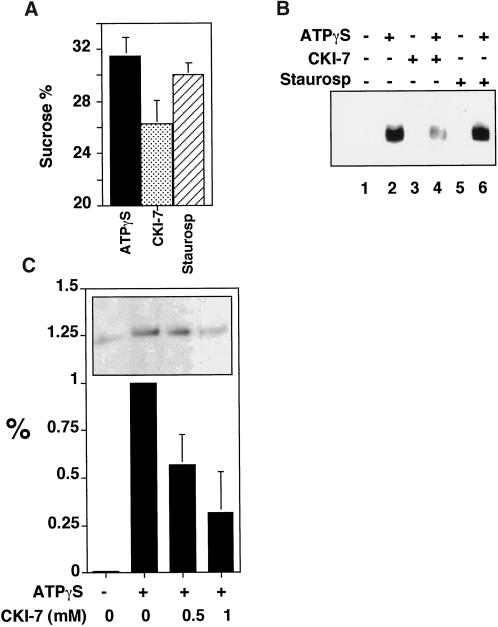
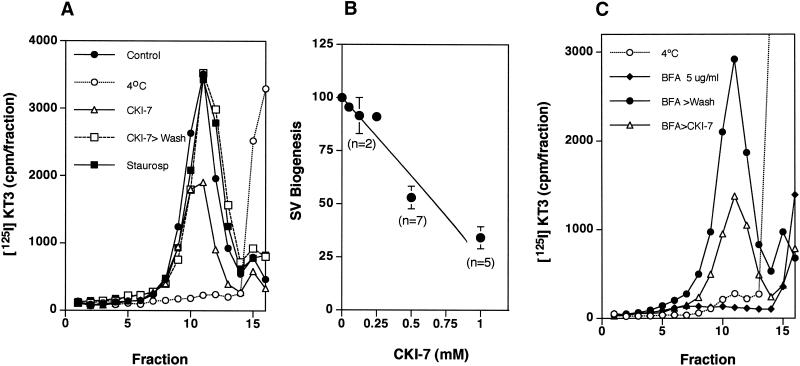
The ATPγS-dependent AP-3 translocation from cytosol to synaptic vesicle membranes was repeated either in the absence or presence of kinase inhibitors. Although the density shift was insensitive to staurosporine, it was decreased by 50% in the presence of CKI-7 (Figure 7a). AP-3 also can be recruited to synaptic vesicles attached to a matrix (Faundez et al., 1998). When ATPγS was used with matrix-bound vesicles, AP-3 recruitment, which was measured by the presence of β3 subunit, was inhibited by 40 ± 17% (SE) (n = 7) by CKI-7, but not by staurosporine (Figure 7b). To determine whether AP-3 recruitment to endosomes was similarly inhibited, we used a 50K-g membrane fraction. This fraction is free of synaptic vesicles but contains transferrin receptor-positive donor endosome membranes, which are enriched 4-fold in synaptic vesicle budding activity compared with total cell homogenates (Lichtenstein et al. 1998; de Wit et al. 1999). AP-3 binding to membranes was determined with antibodies against β3. As with synaptic vesicles, ATPγS induced the recruitment of AP-3 to donor-enriched membranes. CKI-7 prevented it to a similar extent (Figure 7c). These results again suggest that casein kinase I activity is required to recruit AP-3 to membranes. The residual activity in the presence of inhibitors could be due to already phosphorylated AP-3 complexes in the brain cytosol.
Figure 7.
Characterization of inhibition by CK1–7. (A) Casein kinase I activity is required for the coat recruitment. Synaptic vesicle coating reactions were performed with radioactive SVs from 125I-KT3 endocytically labeled PC12N49A cells in the presence of 3 mg/ml dialyzed cytosol plus 150 μM ATPγS. Before adding the nucleotide, reaction mixtures were incubated for 15 min at 37°C in the absence or presence of CKI-7 (500 μM, n = 4) or staurosporine (10 μM, n = 4). CKI-7 concentrations < 200 μM were ineffective. Coating reactions were triggered by ATPγS at 37°C for 30 min then were stopped at 0°C. Radioactive vesicle sedimentation was analyzed as before in 10–45% continuous sucrose gradients. CKI-7 partially inhibited the coating reaction. (B) Coating assays were performed using unlabeled PC12 N49A synaptic vesicles bound to an antisynaptophysin-protein G-Sepharose affinity column. Reactions were performed at 37°C with dialyzed rat brain cytosol. Before the addition (even lanes) or not (odd lanes) of ATPγS (150 μM), the coating mixtures were preincubated at 37°C for 15 min in the absence (lanes 1 and 2) or in the presence of either CKI-7 (500 μM, lanes 3 and 4) or staurosporine (10 μM, lanes 5 and 6). Coating was stopped at 4°C, and the adsorbed coated SV was extensively washed in intracellular buffer. Complexes were resolved in SDS–PAGE gels, and the AP-3 content was assessed by immunoblot with antibodies against the β3 subunit. CKI-7 reduced the AP-3 recruited to SV to 40 ± 17% of the control reactions (n = 7). (C) ATPγS-dependent AP-3 recruitment to 50K-g membrane fractions is reduced by casein kinase inhibition. A donor membrane-enriched fraction was incubated in the absence or presence of ATPγS (150 μM). ATPγS-treated assays either were added or not to CKI-7. Reaction mixtures were incubated as described in panel B. Reactions were stopped by chilling down and sedimentation through a sucrose cushion. Blot analysis was performed as in panel B. CKI-7 reduced AP-3 recruitment to donor-enriched membranes by 58 ± 16% (n = 5) and 32 ± 21 (n = 6) at 0.5 and 1 mM, respectively. All data represent average ± SE.
The donor endosomes have not yet been purified, and so recruitment of AP-3 to them cannot be measured. Although it would be preferable to measure binding to purified precursor membranes rather than product, it is currently only possible to purify the product. We attempted to measure a sedimentation chase in radioactively labeled donor endosomes. Radiolabeled VAMP N49A TAg donor endosomes had only slightly increased sedimentation in the presence of nonhydrolyzable GTP (from 29.6 ± 0.3% to 32.3 ± 0.7% sucrose, n = 3), presumably because a smaller fraction of their membranes is coated. This density shift was not, however, entirely due to AP-3 recruitment, since donor endosomes also changed their sedimentation if AP-3-deficient mocha brain cytosol was used (31.2 ± 0.1% sucrose, n = 3). This is not surprising since transferrin-containing vesicles can bud from the same precursor endosomes in an AP-3-independent manner (Lichtenstein and Kelly, unpublished observations).
Casein Kinase I Inhibition Hinders In Vivo Synaptic Vesicle Formation From Endosomes
If phosphorylation of the β3 subunit by casein kinase I is important for synaptic vesicle production, the CKI-7 inhibitor should inhibit synaptic vesicle formation from labeled endosomes in living cells. To test this prediction, PC12N49A cells expressing an epitope-tagged mutant VAMP, were labeled with [125I] KT3 anti-TAg antibodies at 15°C. After washing away the unbound antibody, cells were incubated at 15°C in the absence or presence of kinase inhibitors. To allow synaptic vesicle formation, cells were warmed to 37°C then homogenized. A high-speed supernatant was analyzed by glycerol velocity gradient centrifugation to measure synaptic vesicle production. Vesicle biogenesis was initiated from the 15°C-labeled endosomes at 37°C (Figure 8a, closed circles) but not at 4°C (Figure 8a, open circles). The casein kinase I inhibitor CKI-7 (500 μM) inhibited vesicle generation ∼50% (Figure 8a, open triangles). After washing the drug out, the CKI-7-mediated block was completely reversed after 20 min (Figure 8a, open squares). The fast reversibility of the block argues against a nonspecific toxic effect of the drug. Staurosporine, which does not inhibit AP-3-associated casein kinase I, did not affect vesicle budding (Figure 8a, closed squares). The CKI-7-mediated inhibition of vesicle production was dose-dependent with an IC50 of ∼500 μM (Figure 8b, n = 7), a concentration 50 times higher than that required to inhibit casein kinase I activity in vitro (Figure 5a). It is similar, however, to concentrations that are needed to block casein kinase I in vivo (Beyaert et al., 1995; Bioukar et al., 1999). A possible explanation of the difference in IC50 between the in vitro phosphorylation and the in vivo vesicle production is the 100-fold higher ATP concentration in intact cells, since the inhibitors interact with the ATP-binding site (Xu et al., 1996).
Figure 8.
CKI-7 inhibits in vivo SV budding from PC12 cell endosomes. PC12 N49A cells were labeled with 125I-KT3 at 15°C as described. Unbound radiolabeled antibodies were extensively washed at 4°C, and cells were incubated for 30 min at 15°C in the absence or presence of CKI-7 (500 μM, panel A, or staurosporine (300 nM). In (B) cells were incubated in increasing concentrations of CK1–7. Cells were warmed at 37°C for 20 min to allow SV formation, and the reactions were stopped by chilling down the cells. Recovery experiments (n = 2) were performed after extensively washing the cells with DME media at 4°C before returning them for 20 min to 37°C. Cells were homogenized and fractionated as previously described, and the SV production was assessed in glycerol velocity gradients. CKI-7 inhibited SV budding with an IC50 of 500 μM (B). No inhibition was detected by staurosporine (n = 5). (C) CKI-7 inhibits in vivo endosomal SV budding at a step close to coat recruitment. PC12 N49A cells were labeled with 125I-KT3 at 15°C, unbound label was washed away at 4°C to then incubate the cells either at 4°C (open circles) or for 15 min at 37°C in DME H21 media supplemented with 5 μg/ml brefeldin A to release coat from the membranes. Brefeldin A was kept (closed diamonds) or washed away at 4°C, and the cells were incubated at 15°C for 30 min in the absence (closed circles) or presence of 500 μM CKI-7 (open triangles). Without removing the inhibitor cells, they were warmed at 37°C for 20 min to allow SV formation, and the reactions were stopped by chilling down the cells. Cells were homogenized and fractionated as previously described, and the SV content was assessed in glycerol velocity gradients. CKI-7 hindered SV formation from brefeldin A-treated endosomes as effectively as it inhibited SV formation for endosomes labeled at 15°C. Depicted is a representative experiment of two.
Brefeldin A also reversibly blocks the generation of synaptic vesicles from endosomes, presumably because GTP-ARF is needed to recruit the AP-3 coat (Faundez et al., 1997, 1998). If casein kinase-mediated phosphorylation is needed for coat recruitment, then inhibiting the kinase activity should inhibit synaptic vesicle formation after brefeldin A is removed.
To determine where the in vivo CKI-7 block occurs with respect to the brefeldin A-mediated endosomal budding block, PC12N49A cells were labeled with [125I] KT3 anti-TAg antibody at 15°C, were washed of uninternalized antibody, and were incubated in brefeldin A for 30 min at 37°C to release coats from endosomes and to prevent vesicle formation. As previously reported, brefeldin A blocked vesicle production reversibly (Figure 8c). However, if brefeldin A was removed in the presence of CKI-7, vesicle biogenesis again was inhibited by 50% (Figure 8c). These results show that casein kinase I is required in a step during or after AP-3 recruitment to membranes.
DISCUSSION
The AP-3-associated Kinase Activity
The first indication of an AP-3 adaptor complex was the discovery of subunits with similarities to the μ (Pevsner et al., 1994) and β chains (Newman et al., 1995) of AP-1 and AP-2. Subsequently δ and ς subunits of AP-3 were identified (Dell'Angelica et al., 1997a,b; Ooi et al., 1997; Simpson et al., 1997). Deletions of AP-3 subunits rescued endocytosis of Ste3p in yeast mutants defective in yeast casein kinase genes yck1 and yck2 (Panek et al., 1997) and also inhibited the traffic of membrane proteins to yeast vacuolar elements (Cowles et al., 1997; Stepp et al., 1997). Consistent with a role for AP-3 in endosomal trafficking, mutant AP-3 in Drosophila, mice, and humans affected organelles such as melanosomes or platelet-dense granules, the synthesis of which has been linked to the endosomal trafficking pathway (Simpson et al., 1997; Kantheti et al., 1998; Ooi et al., 1997; Dell'Angelica et al., 1999; Feng et al., 1999). A link between AP-3 and a population of synaptic vesicles is provided by the mocha mouse. The mocha mouse, which lacks neuronal as well as nonneuronal AP-3, fails to make a subclass of synaptic vesicles (Kantheti et al., 1998) or to make vesicles of incorrect composition.
Genetic and biochemical data link AP-3 strongly to membrane traffic, which is not surprising given the similarity in composition between AP-3 and the AP-1 and AP-2 adaptor complexes. All AP-3 complexes have a large δ subunit and a small ς3A or ς3B subunit. Nonneuronal forms of AP-3 have μ3A and β3A subunits, whereas neuronal forms have μ3B and β3B subunits (reviewed in Odorizzi et al., 1998). Both β3A and β3B subunits were known from earlier work to be phosphorylated (Newman et al., 1995; Dell'Angelica et al., 1997b). Here we have shown that phosphorylation can be attributed to a kinase that copurifies with AP-3 and that phosphorylates both β3A and β3B, but not the β subunit of AP-2, on their hinge regions. The kinase might not have been detected in protein stains of purified AP-3 because its mobility is too close to that of the μ3 subunit (45 kDa). Alternatively, the kinase may be substoichiometric. The kinase can act in trans, since adding β3 hinge regions to immunopurified AP-3 causes hinge phosphorylation.
The kinase activity complexed with AP-3 has properties similar to casein kinase Iα. Neither cAMP-dependent protein kinase nor protein kinase C were capable of phosphorylating the hinge regions. Furthermore, the AP-3 kinase activity was insensitive to staurosporine, a promiscuous inhibitor of serine–threonine kinases (Meggio et al., 1995). The casein kinases were attractive candidates because they prefer to phosphorylate near acidic amino acids (Tuazon and Traugh, 1991), which are rich in the β3 hinge region, because of the genetic interactions in yeast between AP-3 and casein kinase (Panek et al., 1997) and because casein kinases are insensitive to staurosporine (Meggio et al.,1995). Inhibition of kinase activity by CKI-7 and DRB at 10−5 M is also diagnostic of casein kinases. The activity is more like casein kinase I than II because it cannot use GTP (Tuazon and Traugh, 1991) and is recognized by antibodies to casein kinase Ια. Seven isoforms of casein kinase I have been identified (α, β, γ1–3, ε, and δ) (Gross and Anderson, 1998), and casein kinase 1α has at least four different splicing variants (Green and Bennett, 1998). The diversity may account for the wide range of substrates and cellular locations of the casein kinase 1 family (Gross and Anderson, 1998). The activity that copurifies with AP-3 is 45 kDa and, so, differs from the 37-kDa isoform already described in synaptic vesicles (Gross et al., 1995).
Physiological Significance of AP-3 Phosphorylation
The AP-3-associated kinase activity appears to have physiological significance. AP-3-mediated coating of purified synaptic vesicles was found to be inhibited by agents that inhibited casein kinase I activity. It is likely that the hinge region of the β subunit of AP-3 is the major site of phosphorylation during the coating event. Thus, an attractive model is that phosphorylation of AP-3 changes its conformation, allowing it to become active in coating. The inhibition of PC12 synaptic vesicle formation from endosomes labeled in the presence of brefeldin A suggests that coating by phosphorylated AP-3 is needed for vesicle production. The lack of specificity for forms of AP-3 may mean that CKI-7 may interfere with AP-3-mediated events even in nonneuronal cells. At present, we cannot discriminate between a phosphorylation requirement for initial AP-3 recruitment and AP-3 coat assembly. Nor can we eliminate the possibility that low levels of cargo phosphorylation or inositol phospholipid phosphorylation (Gaidarov and Keen, 1999) also are required.
The activation of AP-3 by casein kinase provides a means of regulating how much of the synaptic vesicle production is from endosomes. Although casein kinase I was originally defined as a constitutive enzyme (Tuazon and Traugh, 1991), independent of upstream regulators, increasing evidence links it to regulators such as G-protein-coupled receptors, tyrosine kinases, phosphatases, and inositol phospholipids (Gross et al., 1995; Cegielska et al., 1998; Bioukar et al., 1999). Phosphorylation of muscarinic receptors and rhodopsin, for example, by casein kinase I is stimulus-dependent (Tobin et al., 1997; Waugh et al., 1999), and the binding of insulin to its receptor activates casein kinase I activity (Cobb and Rosen, 1983). Alternatively, however, casein kinase I activity could be constitutive in PC12 cells, and the AP-3 pathway could be regulated via a phosphatase activity.
Both plasma membrane and the cytosolic components required to form clathrin-coated vesicles are likely to be regulated by phosphorylation. The polyphosphoinositide-binding site of the α subunit of AP-2 is required for targeting to clathrin-coated pits (Gaidarov and Keen, 1999), implying that lipid kinases could modulate adaptor recruitment. Consistently, adaptor-dependent, de novo-coated pit formation requires ATP, although it is presently unknown whether this reflect lipid or protein modification (McLauchlan et al., 1997). Cytosolic AP-2 is phosphorylated, whereas AP-2 on membranes is not (Wilde and Brodsky, 1996), implying that AP-2 dephosphorylation could be necessary for recruitment to its donor compartment. ATP and ATPγS, but not AMP-PNP, can release AP-2 that has been recruited to synaptotagmin, suggesting that phosphorylation may reverse the coating steps (Zhang et al., 1994). Dephosphorylation of components of endocytic machinery also appears to be necessary in vivo. When calcium enters the nerve terminal, calcineurin triggers the dephosphorylation of dynamin, amphiphysin, synaptojanin, epsin, and eps15, allowing them to assemble with AP-2 into a macromolecular complex, which presumably is required for the internalization of plasma membrane (Bauerfeind et al., 1997; Slepnev et al., 1998; Chen et al., 1999). The dephosphorylation of β-arrestin also is required for its association with clathrin (Lin et al., 1997).
ATP and a casein kinase Iα-like activity appear to be required for AP-3 recruitment to membranes and for synaptic vesicle formation from endosomes. Now that coating can be studied in a relatively well-defined system, it should be possible to define what regulates phosphorylation and dephosphorylation and the exact steps in the coating process at which they occur.
ACKNOWLEDGMENTS
We wish to thanks Drs. D. Shields, E. Dell'Angelica, R. Anderson and R. Swank for gifts of antibodies, DNAs, and mice. The research was supported by National Institute of Health grants NS09878, NS15927, and DA10154.
REFERENCES
- Anderson HA, Roche PA. Phosphorylation regulates the delivery of MHC class II invariant chain complexes to antigen processing compartments. J Immunol. 1998;160:4850–4858. [PubMed] [Google Scholar]
- Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem. 1997;272:30984–30992. doi: 10.1074/jbc.272.49.30984. [DOI] [PubMed] [Google Scholar]
- Beyaert R, Vanhaesebroeck B, Declercq W, Van Lint J, Vandenabele P, Agostinis P, Vandenheede JR, Fiers W. Casein kinase-1 phosphorylates the p75 tumor necrosis factor receptor and negatively regulates tumor necrosis factor signaling for apoptosis. J Biol Chem. 1995;270:23293–23299. doi: 10.1074/jbc.270.40.23293. [DOI] [PubMed] [Google Scholar]
- Bioukar EB, Marricco NC, Zuo D, Larose L. Serine phosphorylation of the ligand-activated beta-platelet-derived growth factor receptor by casein kinase I-gamma2 inhibits the receptor's autophosphorylating activity. J Biol Chem. 1999;274:21457–21463. doi: 10.1074/jbc.274.30.21457. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Dell'Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Sollner TH, Rothman JE, Wieland FT. Coupling of coat assembly and vesicle budding in packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Casanova JE, Breitfeld PP, Ross SA, Mostov KE. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990;248:742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Cegielska A, Gietzen KF, Rivers A, Virshup DM. Autoinhibition of casein kinase I epsilon (CKI epsilon) is relieved by protein phosphatases and limited proteolysis. J Biol Chem. 1998;273:1357–1364. doi: 10.1074/jbc.273.3.1357. [DOI] [PubMed] [Google Scholar]
- Chen H, Slepnev VI, Di Fiore PP, De Camilli P. The interaction of epsin and Eps15 with the clathrin adaptor A.P-2 is inhibited by mitotic phosphorylation and enhanced by stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem. 1999;274:3257–3260. doi: 10.1074/jbc.274.6.3257. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Hagiwara M, Hidaka H. A. newly synthesized selective casein kinase I inhibitor: N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J Biol Chem. 1989;264:4924–4927. [PubMed] [Google Scholar]
- Chu DS, Pishvaee B, Payne GS. A modulatory role for clathrin light chain phosphorylation in Golgi membrane protein location during vegetative growth and during the mating response of S. cerevisiae. Mol Biol Cell. 1999;10:713–726. doi: 10.1091/mbc.10.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift-O'Grady L, Desnos C, Lichtenstein Y, Faundez V, Horng JT, Kelly RB. Reconstitution of synaptic vesicle biogenesis from P.C12 membranes. Methods. 1998;16:150–159. doi: 10.1006/meth.1998.0662. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Rosen OM. Description of a protein kinase derived from insulin-treated 3T3–L1 cells that catalyzes the phosphorylation of ribosomal protein S6 and casein. J Biol Chem. 1983;258:12472–12481. [PubMed] [Google Scholar]
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- de Wit H, Lichtenstein Y, Geuze HJ, Kelly RB, van der Sluijs P, Klumperman J. Synaptic vesicles form by budding from tubular extensions of sorting endosomes in PC12 cells. Mol Biol Cell. 1999;10:4163–4176. doi: 10.1091/mbc.10.12.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Ooi CE, Bonifacino JS. Beta3A-adaptin, a subunit of the adaptor-like complex AP-3. J Biol Chem. 1997a;272:15078–15084. doi: 10.1074/jbc.272.24.15078. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997b;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Edelman AM, Blumenthal DK, Krebs EG. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Faundez V, Horng JT, Kelly RB. ARF1 is required for synaptic vesicle budding in PC12 cells. J Cell Biol. 1997;138:505–515. doi: 10.1083/jcb.138.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez V, Horng JT, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Faundez V, Krauss R, Holuigue L, Garrido J, Gonzalez A. Epidermal growth factor receptor in synaptic fractions of the rat central nervous system. J Biol Chem. 1992;267:20363–20370. [PubMed] [Google Scholar]
- Feng L, Seymour AB, Jiang S, To A, Peden AA, Novak EK, Zhen L, Rusiniak ME, Eicher EM, Robinson MS, Gorin MB, Swank RT. The beta3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. Hum Mol Genet. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Keen JH. Phosphoinositide–AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol. 1999;146:755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, Wildenberg SC, Keiper NM, Novak EK, Rusiniak ME, Swank RT, Puri N, Finger JN, Hagiwara N, Lehman AL, Gales TL, Bayer ME, King RA, Brilliant MH. The mouse pale ear (ep) mutation is the homologue of human Hemansky-Pudlak syndrome. Proc Natl Acad Sci USA. 1997;94:9238–9243. doi: 10.1073/pnas.94.17.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratecos D, Fischer EH. Adenosine 5′-O(3-thiotriphosphate) in the control of phosphorylase activity. Biochem Biophys Res Commun. 1974;58:960–967. doi: 10.1016/s0006-291x(74)80237-8. [DOI] [PubMed] [Google Scholar]
- Green CL, Bennett GS. Identification of four alternatively spliced isoforms of chicken casein kinase I alpha that are all expressed in diverse cell types. Gene. 1998;216:189–195. doi: 10.1016/s0378-1119(98)00291-1. [DOI] [PubMed] [Google Scholar]
- Gross SD, Anderson RA. Casein kinase I: spatial organization and positioning of a multifunctioning protein kinase family. Cell Signal. 1998;10:699–711. doi: 10.1016/s0898-6568(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Gross SD, Hoffman DP, Fisette PL, Baas P, Anderson RA. A phosphatidylinositol 4,5-bisphosphate-sensitive casein kinase I alpha associates with synaptic vesicles and phosphorylates a subset of vesicle proteins. J Cell Biol. 1995;130:711–724. doi: 10.1083/jcb.130.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, Gilman AG. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986;261:7906–7911. [PubMed] [Google Scholar]
- Kantheti P, Qiao X, Diaz ME, Peden AA, Meyer GE, Carskadon SL, Kapfhamer D, Sufalko D, Robinson MS, Noebels JL, Burmeister M. Mutation in A.P-3 delta in the mocha mouse links endosome transport to storage deficiency in platelets, melanosomes and synaptic vesicles. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Bonifacino JS, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- Lichtenstein Y, Desnos C, Faundez V, Kelly RB, Clift-O'Grady L. Vesiculation and sorting from PC12-derived endosomes in vitro. Proc Natl Acad Sci USA. 1998;95:11223–11228. doi: 10.1073/pnas.95.19.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, Lefkowitz RJ. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem. 1997;272:31051–31057. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- Lowe M, Kreis TE. Regulation of membrane traffic in animal cells by COPI. Biochim Biophys Acta. 1998;1404:53–66. doi: 10.1016/s0167-4889(98)00046-9. [DOI] [PubMed] [Google Scholar]
- Luton F, Cardone MH, Zhang M, Mostov KE. Role of tyrosine phosphorylation in ligand induced regulation of transcytosis of the polymeric Ig receptor. Mol Biol Cell. 1998;9:1787–1802. doi: 10.1091/mbc.9.7.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Morimitsu Y, Uchida K, Schekman R. Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Mol Cell. 1998;2:703–708. doi: 10.1016/s1097-2765(00)80168-9. [DOI] [PubMed] [Google Scholar]
- McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1997;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Meggio F, Shugar D, Pinna LA. Ribofuranosyl-benzimidazole derivatives as inhibitors of casein kinase-2 and casein kinase-1. Eur J Biochem. 1990;187:89–94. doi: 10.1111/j.1432-1033.1990.tb15280.x. [DOI] [PubMed] [Google Scholar]
- Meggio F, Donella DA, Ruzzene M, Brunati AM, Cesaro L, Guerra B, Meyer T, Mett H, Fabbro D, Furet P. Different susceptibility of protein kinases to staurosporin inhibition. Kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur J Biochem. 1995;234:317–322. doi: 10.1111/j.1432-1033.1995.317_c.x. [DOI] [PubMed] [Google Scholar]
- Molloy SS, Anderson ED, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- Newman LS, McKeever MO, Okano HJ, Darnell RB. Beta-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell. 1995;82:773–783. doi: 10.1016/0092-8674(95)90474-3. [DOI] [PubMed] [Google Scholar]
- Nichols RA, Sihra TS, Czernik AJ, Nairn AC, Greengard P. Calcium/calmodulin-dependent protein kinase II increases glutamate and noradrenaline release from synaptosomes. Nature. 1990;343:647–651. doi: 10.1038/343647a0. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colors. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Okamoto CT, Song W, Bomsel M, Mostov KE. Rapid internalization of the polymeric immunoglobulin receptor requires phosphorylated serine 726. J Biol Chem. 1994;269:15676–15682. [PubMed] [Google Scholar]
- Ooi CE, Moreira JE, Dell'Angelica EC, Poy G, Wassarman DA, Bonifacino JS. Altered expression of a novel adaptin leads to defective pigment granule biogenesis in the Drosophila eye color mutant garnet. EMBO J. 1997;16:4508–4518. doi: 10.1093/emboj/16.15.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek HR, Stepp JD, Engle HM, Marks KM, Tan PK, Lemmon SK, Robinson LC. Suppressors of YCK-encoded yeast casein kinase I deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner J, Volknandt W, Wong BR, Scheller RH. Two rat homologs of clathrin-associated adaptor proteins. Gene. 1994;146:279–283. doi: 10.1016/0378-1119(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Pitcher C, Honing S, Fingerhut A, Bowers K, Marsh M. Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation. Mol Biol Cell. 1999;10:677–691. doi: 10.1091/mbc.10.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama NR, Chuang JS, Schekman RW. SEC31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell. 1997;8:205–217. doi: 10.1091/mbc.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Seifert R, Rosenthal W, Schultz G, Wieland T, Gierschick P, Jakobs KH. The role of nucleoside-diphosphate kinase reactions in G. protein activation of N.A.D.P.H. oxidase by guanine and adenine nucleotides. Eur J Biochem. 1988;175:51–55. doi: 10.1111/j.1432-1033.1988.tb14165.x. [DOI] [PubMed] [Google Scholar]
- Shi G, Faundez V, Roos J, Dell'Angelica E, Kelly RB. Neuroendocrine synaptic vesicles are formed in vitro by both clathrin-dependent and clathrin-independent pathways. J Cell Biol. 1998;143:947–955. doi: 10.1083/jcb.143.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T, Miyaake S, Ohno H, Nakaseko C, Isono K, Bonifacino JS, Saito T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997;6:583–589. doi: 10.1016/s1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- Simpson F, Peden AA, Christopoulou L, Robinson MS. Characterization of the adaptor-related protein complex, AP-3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev VI, Ochoa GC, Butler MH, Grabs D, De Camilli P. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, DeMaggio AJ, Hoekstra MF, Blenis J, Hunter T, Cantley LC. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylasae kinaes, calmodulin-dependent kinase II, CKD5 and ErkI. Mol Cell Biol. 1996;16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Spang S, Schekman R. A primer on vesicle budding. Cell. 1999;97:145–148. doi: 10.1016/s0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuchert M, Schafer W, Berghofer S, Hoflack B, Klenk HD, Garten W. Sorting of furin at the trans-Golgi network. Interaction of the cytoplasmic tail sorting signals with A.P-1 Golgi-specific assembly proteins. J Biol Chem. 1999;274:8199–8207. doi: 10.1074/jbc.274.12.8199. [DOI] [PubMed] [Google Scholar]
- Tobin AB, Totty NF, Sterlin AE, Nahorski SR. Stimulus-dependent phosphorylation of G-protein-coupled receptors by casein kinase 1alpha. J Biol Chem. 1997;272:20844–20849. doi: 10.1074/jbc.272.33.20844. [DOI] [PubMed] [Google Scholar]
- Tuazon PT, Traugh JA. Casein kinase I and II–multipotential serine protein kinases: structure, function and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- Waugh MG, Challiss RA, Bernstein G, Nahorski SR, Tobin AB. Agonist-induced desensitization and phosphorylation of m1-muscarinic receptors. Biochem J. 1999;338:175–183. [PMC free article] [PubMed] [Google Scholar]
- Wieland T, Jakobs KH. Evidence for nucleoside diphosphokinase-dependent channeling of guanosine 5′-(gamma-thio) triphosphate to guanine nucleotide-binding proteins. Mol Pharmacol. 1992;42:731–735. [PubMed] [Google Scholar]
- Wilde A, Brodsky FM. In vivo phosphorylation of adaptors regulates their interaction with clathrin. J Cell Biol. 1996;135:635–645. doi: 10.1083/jcb.135.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A, Beattie EC, Lem L, Riethof DA, Liu SH, Mobley WC, Soriano P, Brodsky FM. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- Xu R-M, Carmel G, Kuret J, Cheng C. Structural basis for selectivity of the isoquinoline sulfonamide family of protein kinase inhibitors. Proc Natl Acad Sci USA. 1996;93:6308–6313. doi: 10.1073/pnas.93.13.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Graves PR, Robinson LC, Italiano M, Culbertson MR, Rowles J, Cobb MH, DePaoli-Roach AA, Roach PJ. Casein kinase I gamma subfamily: molecular cloning, expression and characterization of three mammalian isoforms and complementation of defects in the S. cerevisiae YCK genes. J Biol Chem. 1995;270:12717–12724. doi: 10.1074/jbc.270.21.12717. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Davletov BA, Sudhof TC, Anderson RG. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]