Abstract
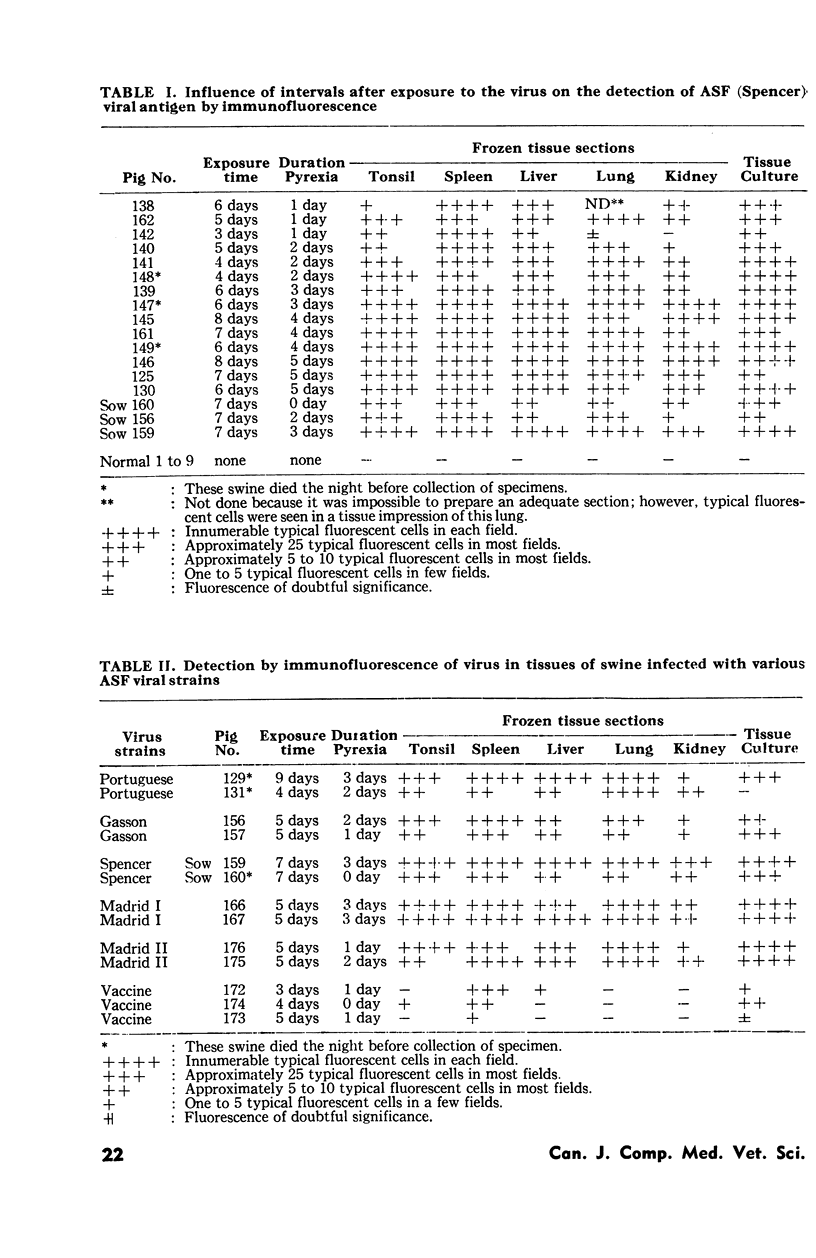
African swine fever immunofluorescent conjugates were prepared in swine and used successfully in the demonstration of viral antigen in frozen tissue sections and in inoculated tissue culture cells. Cross reactivity was observed with the six strains used in the inoculation of swine. The high antibody content of the serum of immune swine did not interfere with demonstration of the antigen in frozen tissue sections of certain of their organs. The localisation and extent of antigen varied with the stage of infection. The virus was demonstrated in spleen and other organs as early as after one day of pyrexia and until after death of the animal. A pool of hog cholera and African swine fever conjugates stained with dyes of different colours was used in the localisation of respective antigens in experimental mixed infection.
Full text
PDF




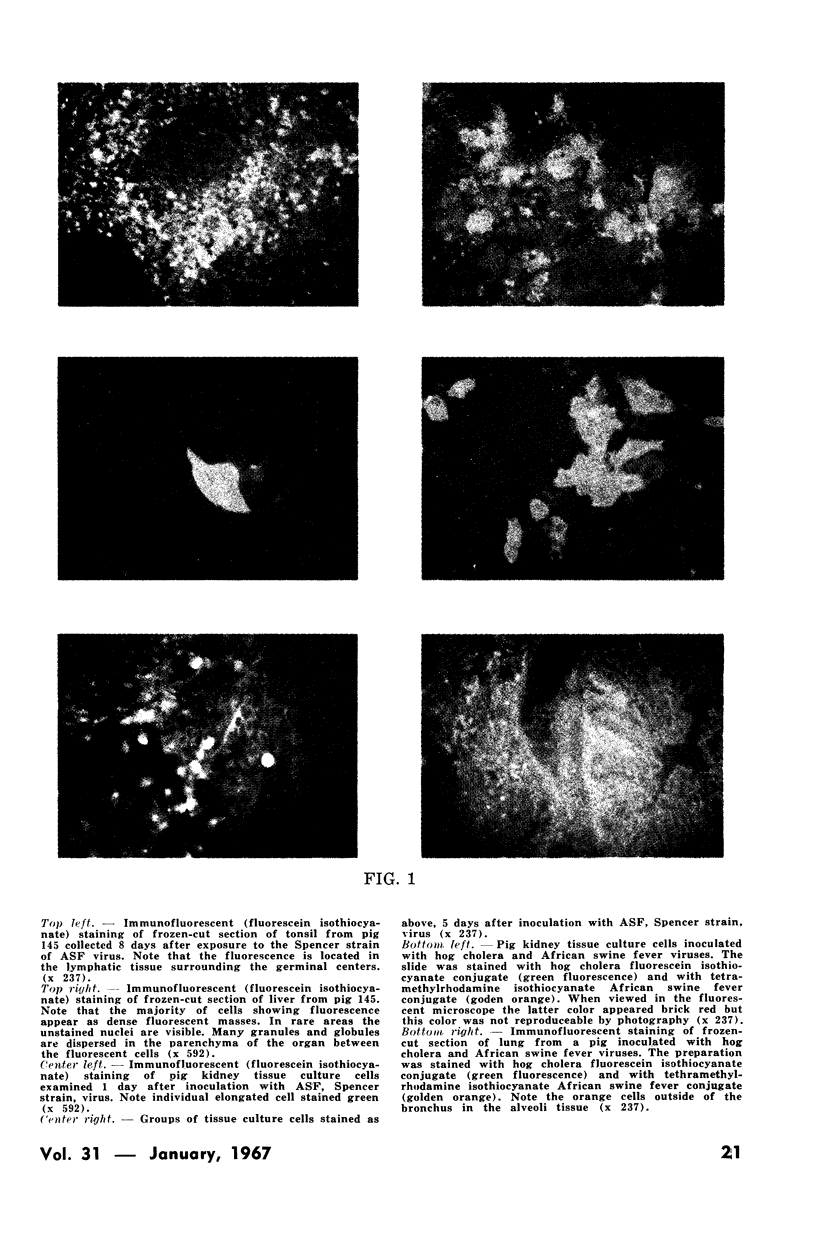

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- African swine fever. I. Antiserum production. Can J Comp Med Vet Sci. 1967 Jan;31(1):2–6. [PMC free article] [PubMed] [Google Scholar]
- BEAUREGARD M., BOULANGER P., WEBSTER W. A. THE USE OF FLUORESCENT ANTIBODY STAINING IN THE DIAGNOSIS OF RABIES. Can J Comp Med Vet Sci. 1965 Jun;29:141–147. [PMC free article] [PubMed] [Google Scholar]
- Boulanger P., Bannister G. L., Gray D. P., Ruckerbauer G. M., Willis N. G. African swine fever. 3. The use of the agar double-diffusion precipitation test for the detection of the virus in swine tissue. Can J Comp Med Vet Sci. 1967 Jan;31(1):12–15. [PMC free article] [PubMed] [Google Scholar]
- Boulanger P., Bannister G. L., Gray D. P., Ruckerbauer G. M., Willis N. G. African swine fever. II. Detection of the virus in swine tissues by means of the modified direct complement-fixation test. Can J Comp Med Vet Sci. 1967 Jan;31(1):7–11. [PMC free article] [PubMed] [Google Scholar]
- Boulanger P., Robertson A. Fluorescein-Labelled Antibody Technique for the Demonstration of Leptospira Pomona. Can J Comp Med Vet Sci. 1961 Dec;25(12):299–306. [PMC free article] [PubMed] [Google Scholar]
- Greig A. S., Boulanger P., Bannister G. L. African swine fever. V. Cultivation of the virus in primary pig kidney cells. Can J Comp Med Vet Sci. 1967 Jan;31(1):24–31. [PMC free article] [PubMed] [Google Scholar]
- Heuschele W. P., Coggins L., Stone S. S. Fluorescent antibody studies on African swine fever virus. Am J Vet Res. 1966 Mar;27(117):477–484. [PubMed] [Google Scholar]
- LEPINE P., SLIZEWICZ P., DANIEL P., PACCAUD M. Cultures cellulaires dans un milieu utilisant l'hydrolysat de caséine comme source d'acides aminés. Ann Inst Pasteur (Paris) 1956 May;90(5):654–656. [PubMed] [Google Scholar]
- Robertson A., Bannister G. L., Boulanger P., Appel M., Gray D. P. Hog cholera. V. Demonstration of the antigen in swine tissues by the fluorescent antibody technique. Can J Comp Med Vet Sci. 1965 Dec;29(12):299–305. [PMC free article] [PubMed] [Google Scholar]
- Robertson A., Greig A. S., Appel M., Girard A., Bannister G. L., Boulanger P. Hog cholera IV. Detection of the virus in tissue culture preparations by the fluorescent antibody technique. Can J Comp Med Vet Sci. 1965 Sep;29(9):234–241. [PMC free article] [PubMed] [Google Scholar]
- Ruckerbauer G. M., Robertson A., Bannister G. L., Boulanger P., Beauregard M. Toxoplasmosis I. Studies by the Fluorescein-labelled Antibody Technique. Can J Comp Med Vet Sci. 1963 Feb;27(2):27–33. [PMC free article] [PubMed] [Google Scholar]