Abstract
PAG/Cbp (hereafter named PAG) is a transmembrane adaptor molecule found in lipid rafts. In resting human T cells, PAG is tyrosine phosphorylated and associated with Csk, an inhibitor of Src-related protein tyrosine kinases. These modifications are rapidly lost in response to T-cell receptor (TCR) stimulation. Overexpression of PAG was reported to inhibit TCR-mediated responses in Jurkat T cells. Herein, we have examined the physiological relevance and the mechanism of PAG-mediated inhibition in T cells. Our studies showed that PAG tyrosine phosphorylation and association with Csk are suppressed in response to activation of normal mouse T cells. By expressing wild-type and phosphorylation-defective (dominant-negative) PAG polypeptides in these cells, we found that the inhibitory effect of PAG is dependent on its capacity to be tyrosine phosphorylated and to associate with Csk. PAG-mediated inhibition was accompanied by a repression of proximal TCR signaling and was rescued by expression of a constitutively activated Src-related kinase, implying that it is due to an inactivation of Src kinases by PAG-associated Csk. We also attempted to identify the protein tyrosine phosphatases (PTPs) responsible for dephosphorylating PAG in T cells. Through cell fractionation studies and analyses of genetically modified mice, we established that PTPs such as PEP and SHP-1 are unlikely to be involved in the dephosphorylation of PAG in T cells. However, the transmembrane PTP CD45 seems to play an important role in this process. Taken together, these data provide firm evidence that PAG is a bona fide negative regulator of T-cell activation as a result of its capacity to recruit Csk. They also suggest that the inhibitory function of PAG in T cells is suppressed by CD45. Lastly, they support the idea that dephosphorylation of proteins on tyrosine residues is critical for the initiation of T-cell activation.
T-cell activation is initiated by the interaction of the T-cell receptor (TCR) for antigens with antigenic peptides complexed to major histocompatibility complex molecules (37). TCR engagement by antigens triggers the tyrosine phosphorylation of a short sequence, the immunoreceptor tyrosine-based activation motif, present in the TCR-associated CD3-ζ subunits (7, 23). Such immunoreceptor tyrosine-based activation motifs function by orchestrating the sequential activation of the Src-related protein tyrosine kinases (PTKs) Lck and FynT, which initiate TCR signaling, followed by that of the Zap-70/Syk PTKs, which amplify the response (7). These various PTKs induce tyrosine phosphorylation of several polypeptides, including the transmembrane adaptor LAT, the adaptor SLP-76, and enzymatic effectors such as phospholipase C (PLC)-γ (9, 24, 27, 28). Protein tyrosine phosphorylation subsequently leads to the activation of several other signaling pathways, which ultimately induce effector functions.
PAG (phosphoprotein associated with glycosphingolipid-enriched microdomains), or Cbp (Csk-binding protein), is an ∼80-kDa transmembrane protein expressed in all cell types, including T cells (2, 20). It possesses a short extracellular domain (16 to 18 amino acids), a single transmembrane segment, and a long intracytoplasmic domain bearing multiple tyrosine-based motifs (9 in mouse PAG and 10 in human PAG). Interestingly, PAG/Cbp (hereafter termed PAG) is concentrated in membrane microdomains referred to as lipid rafts. These microdomains are rich in signaling molecules such as Src-related PTKs, LAT, G proteins, and glycophosphatidyl inositol-linked receptors and are proposed to play an important role in cell signaling (19, 23).
Previous studies revealed that PAG is tyrosine phosphorylated in unstimulated human T cells, seemingly as a result of the action of Src kinases (2, 32). This phosphorylation is accompanied by the association of PAG with significant amounts of Csk, a Src homology 2 (SH2) domain-bearing cytoplasmic PTK expressed ubiquitously (23, 30, 35). Interestingly, PAG becomes rapidly dephosphorylated and is dissociated from Csk in response to activation of human T cells (2, 17, 32). Since Csk inhibits Src-related enzymes by phosphorylating their inhibitory tyrosine [tyrosine (Y) 505 in Lck; Y528 in FynT], it was proposed that PAG might mediate a negative signal in lipid rafts which is aimed at preventing or terminating T-cell activation. In support of this idea, transient overexpression of PAG was reported to cause an inhibition (∼2-fold) of TCR-mediated activation of nuclear factor of activated T cells and interleukin-2 (IL-2) production in Jurkat T cells (2, 17). Whether a similar inhibitory function exists for endogenous PAG molecules expressed in normal T cells was not established.
The mechanism of PAG-mediated inhibition remains to be clarified. While inactivation of Src kinases by PAG-associated Csk molecules is a plausible explanation, other possibilities exist. Along these lines, it is noteworthy that the cytoplasmic domain of PAG bears several protein-protein interaction motifs, including the aforementioned tyrosines, proline-rich motifs, and a carboxyl-terminal PDZ-binding sequence. While one of the tyrosines, tyrosine 314 in mouse PAG, was reported to be responsible for Csk binding in transiently transfected Cos cells, there is evidence that one or more other tyrosines are also phosphorylated (2, 20). These residues might mediate the binding of additional SH2 domain-bearing molecules, thus allowing recruitment of other inhibitory effectors to PAG. PAG also bears several proline-rich sequences in its cytoplasmic regions, which might permit binding of SH3 domain-containing molecules. Lastly, the PDZ-binding motif of PAG was reported to allow a physical interaction between PAG and EBP-50, a cytoskeletal linker (3, 17). This association appears to be critical for PAG-mediated inhibition, at least in Jurkat T cells (17).
CD45 is a transmembrane protein tyrosine phosphatase (PTP) abundantly expressed in all nucleated hemopoietic cells (26, 31). Previous studies showed that CD45 expression is required for the initiation of TCR signaling. This function is thought to reflect the ability of CD45 to dephosphorylate the inhibitory tyrosine of Src kinases Lck and FynT. As a consequence, CD45 antagonizes the inhibitory impact of Csk, thereby favoring T-cell activation. However, given the extreme abundance of CD45 in T-cell membranes, it is plausible that CD45 has additional targets that explain its permissive effect on T-cell activation.
In this study, we attempted to elucidate the physiological relevance and the mechanism of action of PAG in T cells. Our data provided evidence that PAG is involved in the negative regulation of T-cell activation in normal T cells. They also indicated that the inhibitory effect of PAG on T-cell activation is dependent on its ability to be tyrosine phosphorylated, to associate with Csk, and to inactivate Src-related kinases. Finally, they suggested that CD45, but not PTPs such as PEP and SHP-1, promotes the dephosphorylation of PAG in T cells. This effect might help explain the functional importance of CD45 during T-cell activation.
MATERIALS AND METHODS
Cells.
The CD45-positive mouse T-cell line YAC-1 and Cos-1 cells were obtained from the American Type Culture Collection, Rockville, Md. The CD45-negative YAC-1 variant, YACN1 (36), was provided by Jonathan Ashwell, National Institutes of Health, Bethesda, Md.
cDNAs and constructs.
The wild-type mouse pag cDNA (EST clone AI882478) was obtained from Genome Systems, Inc., St. Louis, Mo. Variants in which all tyrosines in the cytoplasmic domain (9Y→F) or Y314 alone were replaced by phenylalanines were produced using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). All cDNAs were fully sequenced to ensure that they contained no unwanted mutations (data not shown). The cDNAs coding for constitutively activated FynT (FynT Y528F), myristylated CD45 (wild type or phosphatase inactive [C→S]), and ΔSH2 Y505F Lck were described elsewhere (6, 13, 25).
Antibodies and reagents.
Antibodies directed against the cytoplasmic domain of PAG were produced in rabbits by immunization with a glutathione S-transferase fusion protein encompassing amino acids 352 to 425 of the cytoplasmic domain of mouse PAG. In some experiments, a rabbit polyclonal antibody directed against the cytoplasmic domain of human PAG and cross-reacting with mouse PAG was used. Rabbit antibodies against Csk, PEP, PTP-PEST, SHP-1, SHP-2, FynT, Lck, and phosphotyrosine (P.tyr) were generated in our laboratory. Antiphosphotyrosine monoclonal antibody (MAb) 4G10 was purchased from Upstate Biotechnology Inc., Lake Placid, N.Y. Anti-LAT antibodies were provided by Larry Samelson (National Institutes of Health, Bethesda, Md.), while those reacting against the cytoplasmic domain of mouse CD45 were obtained from Phil Branton, McGill University, Montreal, Quebec, Canada. Purified anti-CD3 MAb 145-2C11 and anti-CD28 MAb 37.51 were prepared in our laboratory. Anti-TCR MAb H57-597, biotinylated MAb 145-2C11, and biotinylated MAb H57-597 were purchased from BD Pharmingen, Mississauga, Ontario, Canada. Cholera toxin (B subunit) conjugated to horseradish peroxidase was purchased from Sigma, St. Louis, Mo.
Mice.
For production of PAG transgenics, pag cDNAs were cloned in the pCD2 construct, which contains the human CD2 promoter (obtained from Dimitris Kioussis, London, England). To create transgenic mice expressing constitutively activated FynT, the cDNA coding for FynT Y528F was inserted into the vector plckHGH (provided by Roger Perlmutter, University of Washington, Seattle, Wash.) (12). This vector bears the proximal lck promoter and is active mostly in thymocytes. Transgenic mice were produced according to established protocols by the IRCM Transgenic Service. At least two independent founders of each transgenic type were used in our studies. Mice lacking expression of CD45 (4) or SHP-1 (motheaten) (33) were obtained from the Jackson Laboratory, Bar Harbor, Maine. Those lacking PEP were obtained from Matt Thomas (Washington University, St. Louis, Mo.). They were created by replacing most of the phosphatase domain of PEP with a neomycin resistance cassette (M. Thomas, personal communication). These mice lacked functional PEP protein and exhibited no obvious defect in T-cell development.
Cell stimulation.
Typically, thymocytes (30 × 106) were stimulated for the indicated periods of time at 37°C with biotinylated anti-CD3 MAb 145-2C11 (10 μg) or anti-TCR H57-597 (10 μg) and avidin (14 μg) in a volume of 200 μl. Unstimulated controls were incubated at 37°C with avidin alone. After lysis in buffer containing maltoside (1% n-dodecyl-β-d-maltoside, 50 mM Tris [pH 7.6], 150 mM NaCl, 2 mM EDTA) supplemented with protease and phosphatase inhibitors (13), postnuclear lysates were processed for immunoprecipitation or immunoblotting. In some experiments, lysates were separated by sucrose density gradient centrifugation (see below).
Immunoprecipitations and immunoblots.
Unless specified, immunoprecipitations and immunoblottings were performed according to previously described protocols (13, 34), with the exception that maltoside-containing buffer was used.
Functional assays.
Using magnetic columns (Stem Cell Technologies, Vancouver, British Columbia, Canada), CD4+ or CD8+ T cells were purified from thymus, spleen, or lymph nodes of individual mice. The purity of the cell preparations was verified by flow cytometry and was consistently greater than 90% (data not shown). Using anti-CD3 MAb 145-2C11 (1 or 3 μg/ml) coated on plastic, with or without soluble anti-CD28 MAb 37.51 (1 μg/ml), T cells were activated in vitro for 40 to 48 h. In some experiments, recombinant IL-2 (20 U/ml) was added to the culture medium. Controls were stimulated with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (100 ng/ml). After stimulation, proliferation was measured by assaying for [3H]thymidine incorporation, while cytokine production was revealed by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minn.). All assays were done in triplicate, and experiments were repeated at least three times.
Cell fractionation.
Cells (150 × 106) were lysed in 1 ml of Brij 58-containing buffer (1% Brij 58, 25 mM Tris [pH 7.6], 150 mM NaCl, 5 mM EDTA) supplemented with protease and phosphatase inhibitors. Lysates were then mixed with 1 ml of 80% sucrose (made in the same buffer without detergent) and overlaid sequentially with 2 ml of 30% sucrose and 1 ml of 5% sucrose. After centrifugation at 200,000 × g for 16 h at 4°C, 0.5-ml fractions were collected from the top of the gradient. Typically, fractions 2 to 4 contained the lipid rafts while fractions 7 to 10 contained the soluble proteins. Individual fractions were analyzed by immunoblotting or immunoprecipitation, after solubilization using 1% maltoside. In some cases, fractions were pooled prior to analysis.
Intracellular calcium fluxes.
Ex vivo thymocytes (2 × 106) were loaded with Indo-1 (10 μM; Molecular Probes, Eugene, Oreg.) for 45 min at 37°C and stained for 10 min at room temperature with phycoerythrin-coupled anti-CD4 and fluorescein-isothiocyanate-coupled anti-CD8 (purchased from BD Pharmingen). After the cells were washed, they were stimulated at 37°C with biotinylated anti-TCR H57-597 and avidin. Changes in intracellular calcium over time were monitored using a cell sorter (MoFlo; Cytomation, Fort Collins, Colo.). CD4+ single-positive cells were selectively analyzed by gating on CD4+ CD8− thymocytes. As control, cells were stimulated with the calcium ionophore ionomycin (100 ng/ml).
Substrate-trapping experiment.
Using Lipofectamine (Life Technologies, Burlington, Ontario, Canada), Cos-1 cells were transfected with the indicated cDNAs. After lysis in substrate-trapping buffer (11), lysates were processed for immunoprecipitation and immunoblotting according to standard protocols.
RESULTS
Rapid dephosphorylation of PAG in antigen receptor-stimulated mouse T cells.
Previously, it was reported that PAG tyrosine phosphorylation, as well as its association with Csk, was rapidly reduced following activation of human T cells with anti-CD3 antibodies (2, 17, 32). To investigate whether a similar phenomenon exists in mouse T cells, ex vivo mouse thymocytes were activated for various periods of time with biotinylated anti-CD3 MAb 145-2C11 and avidin. Thymocyte preparations were chosen for these experiments, because they yield large numbers of pure T cells without requiring prolonged in vitro manipulations that can alter protein tyrosine phosphorylation. After stimulation, cells were lysed in Brij 58-containing buffer and lysates were fractionated by sucrose density gradient centrifugation. Following solubilization of lipid raft fractions with maltoside, PAG was recovered by immunoprecipitation with a polyclonal rabbit anti-PAG serum and its tyrosine phosphorylation was monitored by immunoblotting with anti-P.tyr antibodies (Fig. 1A, top panel).
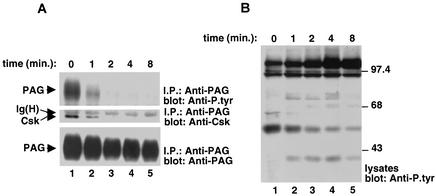
FIG. 1.
Effect of TCR stimulation on PAG tyrosine phosphorylation and association with Csk in normal mouse T cells. (A) PAG immunoprecipitations. Mouse thymocytes were stimulated for the indicated periods of time at 37°C with biotinylated anti-CD3 MAb 145-2C11 and avidin. Unstimulated controls were incubated with avidin alone. Unstimulated and stimulated cells were lysed in Brij 58-containing buffer and subjected to sucrose density gradient centrifugation. Lipid raft fractions (fractions 2 and 3) were recovered, solubilized in maltoside, and immunoprecipitated with anti-PAG antibodies. Tyrosine phosphorylation of PAG was monitored by probing of anti-PAG immunoprecipitates with anti-P.tyr antibodies (top panel). The association of PAG with Csk was determined by reprobing the immunoblot membrane with anti-Csk (center panel), while the abundance of PAG was verified by reprobing with anti-PAG (bottom panel). (B) Overall protein tyrosine phosphorylation. Total cell lysates from the experiment depicted in Fig. 1A were probed by anti-P.tyr immunoblotting.
This analysis showed that PAG was prominently tyrosine phosphorylated in unstimulated thymocytes (Fig. 1A, lane 1). By contrast, upon antigen receptor stimulation (Fig. 1A, lanes 2 to 5), PAG tyrosine phosphorylation became markedly reduced. Such a diminution occurred rapidly (within 1 min; Fig. 1A, lane 2) and was essentially complete by 2 min (lane 3). It correlated temporally with the induction of overall protein tyrosine phosphorylation in thymocyte lysates (Fig. 1B). A similar phenomenon was observed in ex vivo splenic T cells (data not shown). The association of PAG with Csk was also examined (Fig. 1A, center panel). We found that significant amounts of Csk were associated with PAG in unstimulated mouse thymocytes (Fig. 1A, lane 1). However, this interaction was quickly eliminated following antigen receptor stimulation (Fig. 1A, lanes 2 to 5). Hence, these findings demonstrated that the reduction in PAG tyrosine phosphorylation and association with Csk seen in response to TCR engagement occurred in normal mouse T cells.
Expression of wild-type and phosphorylation-defective PAG molecules in normal mouse T cells.
Considering these observations, we addressed further the role of PAG, and the impact of its tyrosine phosphorylation, in the regulation of T-cell activation. To this end, using a CD2 promoter-driven construct, various PAG polypeptides were expressed in transgenic mice. In addition to wild-type PAG, we studied phosphorylation-defective PAG mutants in which either all nine tyrosines in the cytoplasmic region, or the major Csk-binding site (Y314) alone (2, 20, 30), were mutated to phenylalanines. The two PAG mutants were chosen with the expectation that they might also behave as dominant-negative molecules and help establish the role of endogenous PAG polypeptides in T-cell functions. The expression of dominant-negative variants of signaling molecules in transgenic mice has been validated as a useful tool to elucidate the biochemical pathways regulating T-cell activation (5). In keeping with the fact that the CD2 promoter is active both in immature and in mature T cells, the different PAG polypeptides were found to be overexpressed in thymocytes, splenic T cells, and lymph node T cells (Fig. 2A and data not shown).
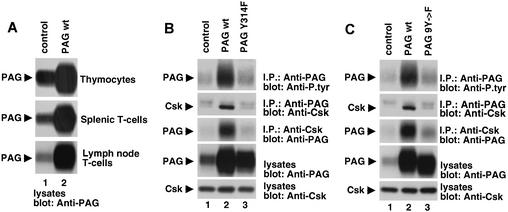
FIG. 2.
Overexpression of wild-type PAG and dominant-negative PAG mutants in transgenic mice. (A) Overexpression of PAG in various T-cell populations. Purified T cells from normal control mice or transgenic mice overexpressing wild-type PAG (PAG wt) were probed by immunoblotting of total cell lysates with anti-PAG. Flow cytometry analyses confirmed that >90% of cells in all preparations were T cells (data not shown). Similar results were obtained with transgenic mice expressing PAG Y314F and PAG 9Y→F (data not shown). (B and C) Tyrosine phosphorylation of PAG and its association with Csk. PAG was immunoprecipitated from lipid raft fractions isolated from thymocytes of the indicated mice, and its tyrosine phosphorylation was determined by immunoblotting with anti-P.tyr antibodies (top panels). The association of PAG with Csk was ascertained by reprobing of the immunoblot membrane with anti-Csk (second panels from the top) or by immunoblotting of anti-Csk immunoprecipitates with anti-PAG (third panels from the top). The abundance of PAG (fourth panels from the top) and Csk (fifth panels from the top) was verified by immunoblotting of total cell lysates with anti-PAG and anti-Csk, respectively. Note that in Fig. 2B and C, the duration of the autoradiographic exposures was much shorter than that used for Fig. 1A. This explains the weaker signal of PAG tyrosine phosphorylation (top panels) and PAG-associated Csk (second panels from the top) in control thymocytes. Both immunoreactive products were more clearly seen upon longer autoradiographic exposures (data not shown). The upper band seen in the anti-Csk immunoblots of PAG immunoprecipitates is the heavy chain of immunoglobulin.
The ability of the PAG molecules to undergo tyrosine phosphorylation and associate with Csk was examined first (Fig. 2B and C). We found that thymocytes overexpressing wild-type PAG (lanes 2) contained greater amounts of tyrosine-phosphorylated PAG (top panels) and PAG-associated Csk (second from the top) than control thymocytes (lanes 1). However, no such increases were observed in thymocytes expressing PAG Y314F (Fig. 2B, lane 3) or PAG 9Y→F (Fig. 2C, lane 3). While a small enhancement of PAG tyrosine phosphorylation and PAG-associated Csk was seen in thymocytes expressing the two PAG mutants, these changes were possibly caused by an influence of the mutant PAG molecules on the phosphorylation of the endogenous PAG polypeptides. In any case, these results implied that Y314 is the predominant site of PAG tyrosine phosphorylation in normal T cells and that it is critical for the ability of PAG to recruit Csk in these cells. Whether the other eight tyrosines in the cytoplasmic region of mouse PAG are phosphorylated in T-lymphocytes remains to be demonstrated.
Expression of the PAG transgenes had no appreciable impact on thymocyte numbers or subpopulations. Moreover, it had no influence on the numbers or proportions of CD4+ and CD8+ T cells in spleen and lymph nodes or on the levels of TCR expression at the cell surface (data not shown).
Tyrosine 314-dependent inhibition of TCR-induced proliferation and IL-2 secretion by PAG.
To ascertain the impact of PAG on TCR signaling, CD4+ splenic T cells were purified from the various mice and were stimulated with anti-CD3 alone or in combination with anti-CD28. T-cell proliferation was then monitored by measuring the incorporation of tritiated thymidine (Fig. 3A and B). This assay showed that overexpression of wild-type PAG caused a pronounced inhibition of thymidine incorporation in response to stimulation with anti-CD3 or anti-CD3 plus anti-CD28. Similar results were obtained with CD4+ thymocytes, CD4+ lymph node T cells, or CD8+ splenic T cells or when anti-TCR MAb H57-597 or anti-Thy-1 antibody was used for stimulation (data not shown). In contrast, expression of PAG Y314F provoked an increase in the proliferative response to the presence of anti-CD3 or anti-CD3 plus anti-CD28 (Fig. 3A). In addition to showing that the Csk-binding site was required for the inhibitory effect of PAG, this observation confirmed that PAG Y314F had a dominant-negative effect in T cells. A similar effect was seen with PAG 9Y→F (Fig. 3B). Importantly, the differences in TCR-mediated proliferation between these various mice were not due to global variations in cell responsiveness, as all cells responded equally well to PMA plus ionomycin (Fig. 3A and B).
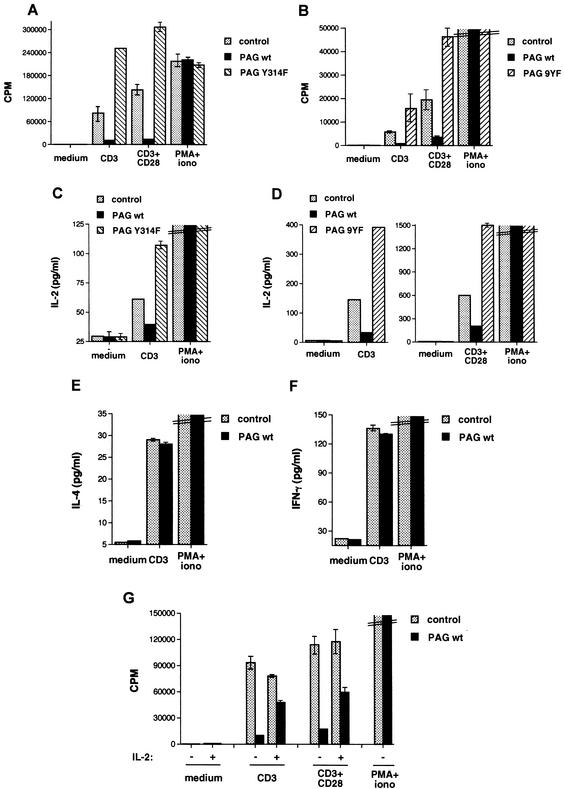
FIG. 3.
Impact of PAG on antigen receptor-induced proliferation and cytokine production. CD4+ splenic T cells were isolated from the indicated mice and stimulated for 40 to 48 h with medium alone, immobilized anti-CD3 alone (1 or 3 μg/ml), immobilized anti-CD3 (1 or 3 μg/ml) plus soluble anti-CD28 (1 μg/ml), or the combination of PMA (50 ng/ml) plus ionomycin (iono) (100 ng/ml). wt, wild type. (A and B) Thymidine incorporation. All assays were done in triplicate, and average values are shown. (C and D) IL-2 secretion; (E) IL-4 production; (F) IFN-γ production. (G) The experiment was performed as described for Fig. 3A, except that the proliferation assays were in the absence or in the presence of recombinant IL-2 (20 U/ml). For panels C to G, all assays were done in duplicate and average values are shown.
The influence of PAG expression on antigen receptor-induced cytokine production was also evaluated (Fig. 3C to F). Cells were stimulated as outlined above, and the release of IL-2, gamma interferon (IFN-γ), or IL-4 in the supernatant was monitored by enzyme-linked immunosorbent assay. These studies revealed that wild-type PAG provoked a significant reduction of IL-2 secretion in response to anti-CD3 or anti-CD3 plus anti-CD28 (Fig. 3C and D). Conversely, PAG Y314F (Fig. 3C) and PAG 9Y→F (Fig. 3D) caused an increase in IL-2 production. Intriguingly, however, PAG had no influence on the production of IL-4 (Fig. 3E) or IFN-γ (Fig. 3F), even when lower concentrations of anti-CD3 were used for stimulation (data not shown).
Since T cells utilize IL-2 to sustain their growth, we examined whether the inhibitory effect of PAG on IL-2 secretion was the basis for the reduction in their proliferation (Fig. 3G). To this end, T cells were stimulated with anti-CD3 alone or in combination with anti-CD28, in the presence or in the absence of exogenous IL-2. Proliferation was then measured as described earlier. We found that addition of IL-2 only partially corrected the inhibitory effect of PAG on proliferation. Thus, while part of the inhibitory effect of PAG on proliferation can be ascribed to reduced IL-2 production, it is likely that additional factors are also involved.
Inhibition of proximal TCR-mediated signaling events by PAG.
To establish the biochemical mechanism responsible for PAG-mediated inhibition, we assessed the effect of PAG on TCR-induced protein tyrosine phosphorylation, the earliest event of T-cell activation (Fig. 4). Thymocytes from the various transgenic mice were stimulated with biotinylated anti-TCR MAb H57-597 and avidin, and the induction of protein tyrosine phosphorylation was monitored by immunoblotting of total cell lysates with anti-P.tyr antibodies (Fig. 4A). We observed that cells overexpressing wild-type PAG (lanes 6 to 10) exhibited a decrease in TCR-induced protein tyrosine phosphorylation in comparison to cells from control mice (lanes 1 to 5). This diminished tyrosine phosphorylation involved mostly a polypeptide of 36 kDa (p36), which was confirmed by immunoprecipitation to be LAT, a lipid raft-associated transmembrane adaptor required for TCR signaling (38) (data not shown). In addition, a less marked reduction of tyrosine phosphorylation of proteins of 120, 100, 76 and 70 kDa was observed. In contrast to wild-type PAG, PAG Y314F (Fig. 4A, lanes 11 to 15) had no inhibitory effect on antigen receptor-triggered protein tyrosine phosphorylation. However, in all experiments, this mutant had a small stimulatory effect on the tyrosine phosphorylation of LAT (for example, compare lanes 3 through 5 to lanes 13 through 15; data not shown). Similar results were obtained with PAG 9Y→F (data not shown).
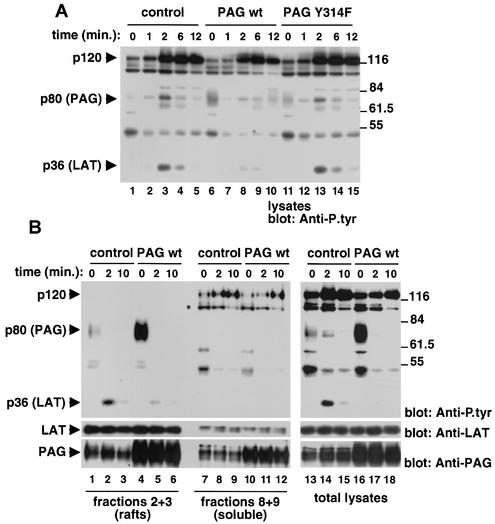
FIG. 4.
Regulation of TCR-induced protein tyrosine phosphorylation by PAG. wt, wild type. (A) Overall protein tyrosine phosphorylation. Thymocytes from the indicated mice were stimulated as outlined for Fig. 1, except that biotinylated anti-TCR MAb H57-597 plus avidin was used. Changes in protein tyrosine phosphorylation were monitored by immunoblotting of total cell lysates with anti-P.tyr antibodies. (B) Cell fractionation. Cells were stimulated as described for panel A, except that lysates were fractionated by sucrose density gradient centrifugation. Lysates corresponding to equal cell numbers were obtained from fractions 2 and 3 (lipid raft fractions) or fractions 8 and 9 (soluble fractions) and were probed by immunoblotting with anti-P.tyr (top panel), anti-LAT (center panel), or anti-PAG (bottom panel) antibodies. Total cell lysates were analyzed in lanes 13 to 18.
In addition to the induction of intracellular protein tyrosine phosphorylation events, TCR stimulation resulted in the dephosphorylation of an ∼80-kDa tyrosine-phosphorylated substrate, most evident in thymocytes overexpressing wild-type PAG (Fig. 4A, lanes 6 to 10). This product, which represented PAG (data not shown), was detectable in unstimulated cells (lane 6) but disappeared within 1 min of TCR stimulation (lane 7). Interestingly, such a decrease seemed to precede the induction of overall protein tyrosine phosphorylation by TCR stimulation.
Because PAG is primarily located in lipid rafts (2, 20), we wanted to exclude the possibility that its overexpression was inhibiting TCR signaling simply by displacing LAT from the rafts (Fig. 4B). To this end, cells were activated as described above but were lysed in Brij 58-containing buffer. Lysates were subsequently fractionated by sucrose density gradient centrifugation, and aliquots from lipid raft (fractions 2 and 3) and soluble (fractions 8 and 9) fractions were probed by anti-P.tyr (Fig. 4B, top panel) or anti-LAT (center panel) immunoblotting. As expected, PAG overexpression caused a decrease in p36/LAT tyrosine phosphorylation in the lipid rafts (top panel; compare lanes 2 and 5). Importantly, however, reprobing with anti-LAT antibodies showed that this diminution was not due to a reduction of the abundance of LAT in the rafts (center panel).
In addition to the decrease in lipid raft-associated p36/LAT tyrosine phosphorylation, PAG overexpression provoked a reduction of the tyrosine phosphorylation of polypeptides found solely in the soluble fractions, such as p120 (Fig. 4B; compare lanes 8 and 11). This finding indicated that PAG was able to inhibit protein tyrosine phosphorylation not only inside but also outside the rafts. It is possible that this effect was caused by the pool of PAG molecules (∼20% of total) situated in the soluble fractions (bottom panel, lanes 7 to 12). However, because PAG tyrosine phosphorylation occurred exclusively inside the rafts (top panel, lanes 1 to 6), it seems more plausible that this inhibition was also effected by the raft-associated PAG.
Next, we tested the effect of PAG on TCR-induced calcium fluxes, a proximal signaling event known to be highly dependent on LAT tyrosine phosphorylation (27) (Fig. 5). Thymocytes were loaded with the calcium indicator dye Indo-1 and were stimulated with biotinylated anti-TCR MAb H57-597 and avidin. Changes in levels of intracellular calcium over time were subsequently monitored in CD4+ single-positive thymocytes by flow cytometry. This analysis showed that compared to normal cells (Fig. 5A), T cells overexpressing wild-type PAG (Fig. 5B) exhibited a pronounced reduction of the TCR-induced increase in intracellular calcium levels. In contrast, T cells expressing PAG Y314F (Fig. 5C) demonstrated a more sustained calcium signal than control thymocytes (Fig. 5A). Nonetheless, all cells responded equally well to the calcium ionophore ionomycin (data not shown). Since wild-type PAG and PAG Y314F influenced calcium fluxes within a few minutes of TCR stimulation, these results further supported the notion that PAG acted proximally on the TCR signaling cascade. Moreover, they implied that the small increase in LAT tyrosine phosphorylation seen in cells expressing PAG Y314F (Fig. 4A and data not shown) was likely to be biologically significant.
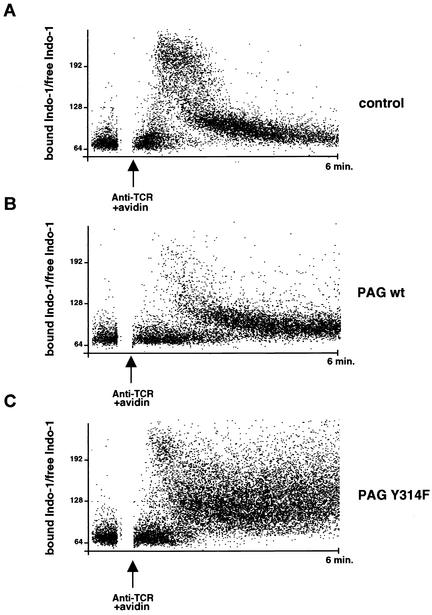
FIG. 5.
Regulation of TCR-induced calcium fluxes by PAG. Thymocytes were loaded with Indo-1 and were stimulated at 37°C with biotinylated anti-TCR MAb H57-597 and avidin. Changes in intracellular calcium were monitored, using a cell sorter, by gating on CD4+ single-positive thymocytes. The ratio of bound Indo-1/free Indo-1 is shown on the ordinate. The arrow corresponds to the moment at which the biotinylated anti-TCR antibody and avidin were present and represents time 0. Cells were observed for 6 min. Similar results were obtained when calcium changes were analyzed in total thymocytes (data not shown). In comparison to normal cells, considerably fewer cells overexpressing wild-type (wt) PAG exhibited a calcium response (20.2% versus 4.6%).
Rescue of PAG-mediated inhibition by a constitutively activated Src kinase.
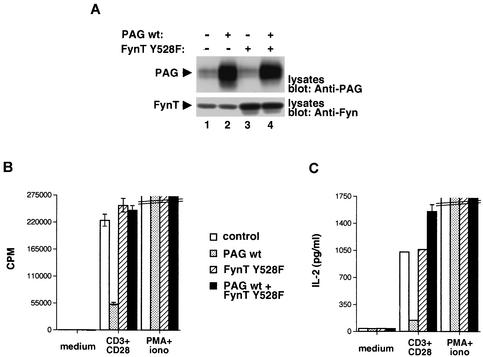
Considering that the aptitude of PAG to inhibit T-cell activation correlated with its ability to bind Csk and inhibit proximal TCR signaling events, it was reasonable to propose that this effect is due to an inactivation of Src kinases. To test this idea, we examined whether the inhibitory impact of PAG could be rescued by expression of a Src kinase mutant that was refractory to Csk-mediated inhibition. To this end, transgenic mice expressing a mutated version of the Src-related kinase FynT, in which the inhibitory tyrosine (Y528) is replaced by phenylalanine, were created. This mutated Src kinase was selected for these studies because it had been shown previously to have no appreciable effect on T-cell development (12). Once generated, mice expressing FynT Y528F were crossed with those overexpressing wild-type PAG. Adequate expression of the two transgenes was confirmed by immunoblotting of thymocyte lysates with anti-PAG (Fig. 6A, top panel) or anti-Fyn (bottom panel) antibodies.
FIG. 6.
Impact of constitutively activated Src kinase on PAG-mediated inhibition. Mice overexpressing wild-type PAG were crossed with transgenic mice expressing a constitutively activated version of FynT (FynT Y528F). wt, wild type. (A) Expression of PAG and FynT. Lysates from thymocytes were probed by immunoblotting with anti-PAG (top panel) or anti-Fyn (bottom panel). (B) Thymidine incorporation; (C) IL-2 secretion. Cells were stimulated and assayed as detailed for Fig. 3.
CD4+ thymocytes from these animals were stimulated with anti-CD3 plus anti-CD28, and cell proliferation and IL-2 production were measured as described for Fig. 3. As expected, wild-type PAG inhibited the proliferative response to anti-CD3 plus anti-CD28 (Fig. 6B). A similar effect was seen on IL-2 release (Fig. 6C). More importantly, while constitutively activated FynT alone had no measurable impact on these responses, it abolished the inhibitory influence of wild-type PAG (Fig. 6B and C). Therefore, these data demonstrated that a mutant Src kinase that was refractory to Csk-mediated inhibition was able to bypass the suppressive effect of PAG in normal T cells.
Regulation of PAG tyrosine phosphorylation by PTPs.
Since tyrosine phosphorylation of PAG appears to be necessary for its ability to inhibit T-cell activation, we sought to identify the PTP(s) involved in counteracting this phosphorylation. By dephosphorylating PAG, this PTP could presumably have a permissive effect in TCR signaling. Several candidates were considered. First, the proline-rich phosphatases PEP and PTP-PEST might be involved, given that both have been reported to bind Csk via the Csk SH3 domain (10, 14). Second, the SH2 domain-containing PTP SHP-1, as well as its relative SHP-2, might contribute, as SHP-1 was found to be recruited to lipid rafts in response to TCR stimulation (22). And third, we estimated that CD45 was a candidate, since it is extremely abundant in T-cell membranes and is known to be a positive regulator of TCR signaling (31).
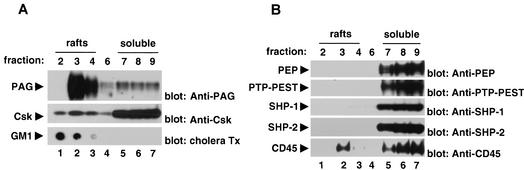
We first ascertained whether these PTPs were present in lipid raft fractions of T cells (Fig. 7), hypothesizing that the PTP involved in PAG regulation was likely to accumulate at least partially in lipid rafts. In agreement with previous reports, PAG (Fig. 7A, top panel) and GM1 gangliosides (bottom panel) were present in large quantities in the lipid raft fractions of mouse thymocytes (lanes 1 to 3). Likewise, ∼20% of Csk (center panel) was localized in these fractions, presumably due to its interaction with PAG. In contrast, PTPs such as PEP (Fig. 7B, top panel), PTP-PEST (second panel from top), SHP-1 (third panel from top), and SHP-2 (fourth panel from top) were present exclusively in the soluble fractions (lanes 5 to 7). This was not the case for CD45 (fifth panel from top), however, which was detectable in moderate amounts (∼5 to 10%) in the lipid raft fractions (lanes 1 to 3).
FIG. 7.
Cell fractionation experiments. (A and B) Sucrose density gradient centrifugation. The distribution of several molecules in mouse thymocytes was examined by sucrose density gradient centrifugation. Polypeptides were detected by immunoblotting with the indicated antibodies, utilizing aliquots obtained from the various fractions. Lipid rafts corresponded to fractions 2 to 4, while soluble proteins were present in fractions 7 to 9. In the case of GM1 gangliosides, detection was achieved using a dot blot and probing of the membrane with cholera toxin (Tx) coupled to horseradish peroxidase. Similar results were obtained when lysates were prepared with Triton X-100 (data not shown).
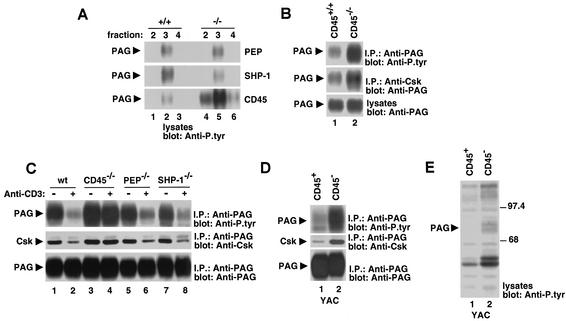
To further examine the nature of the PTP(s) responsible for PAG dephosphorylation in T cells, thymocytes were isolated from mice lacking PEP, SHP-1, or CD45 and then cell lysates were separated by sucrose density gradient centrifugation. Fractions corresponding to lipid rafts were probed by immunoblotting with anti-P.tyr antibodies (Fig. 8A). This experiment revealed that an ∼80-kDa protein consistent with PAG was tyrosine phosphorylated to a normal extent in lipid raft fractions from PEP-deficient (top panel) or SHP-1-deficient (center panel) thymocytes. However, the phosphotyrosine content of this product was increased in CD45-deficient thymocytes (bottom panel). Immunoprecipitation with anti-PAG antibodies confirmed that this polypeptide was PAG (Fig. 8B and C, top panels). The enhanced PAG tyrosine phosphorylation in CD45-deficient thymocytes was accompanied by an increase in the amount of PAG-associated Csk (Fig. 8B, center panel). Next, the involvement of these PTPs in the ability of PAG to undergo dephosphorylation (Fig. 8C, top panel) and dissociate from Csk (center panel) in response to TCR stimulation was ascertained. We observed that these responses were normal in thymocytes lacking PEP (lanes 5 and 6) or SHP-1 (lanes 7 and 8). By contrast, there was little or no PAG dephosphorylation and dissociation from Csk in TCR-stimulated thymocytes lacking CD45 (lanes 3 and 4).
FIG. 8.
Involvement of PTPs in dephosphorylation of PAG in T cells. (A) Extent of PAG tyrosine phosphorylation in phosphatase-deficient thymocytes. Thymocytes were isolated from the indicated mice, and lysates were fractionated by sucrose density gradient centrifugation. The extent of tyrosine phosphorylation of PAG was evaluated by immunoblotting of fractions corresponding to lipid rafts (fractions 2 to 4) with anti-P.tyr antibodies. (B) PAG tyrosine phosphorylation and association with Csk in CD45-deficient thymocytes. PAG was immunoprecipitated from cell lysates with anti-PAG, and its phosphotyrosine content was examined by anti-P.tyr immunoblotting (top panel). The extent of association of PAG with Csk was determined by immunoblotting of anti-Csk immunoprecipitates with anti-PAG (center panel). The abundance of PAG was verified by immunoblotting of total cell lysates with anti-PAG (bottom panel). (C) TCR-induced dephosphorylation of PAG in phosphatase-deficient thymocytes. Cells were stimulated for 1 min with biotinylated anti-CD3 MAb 145-2C11 and avidin. Unstimulated controls were incubated with avidin alone. Tyrosine phosphorylation of PAG was assessed by probing of anti-PAG immunoprecipitates with anti-P.tyr immunoblotting (top panel). The association with Csk was examined by reprobing the immunoblot membrane with anti-Csk (center panel), whereas the abundance of PAG was verified by reprobing of the immunoblot with anti-PAG (bottom panel). (D) PAG tyrosine phosphorylation and association with Csk in a CD45-negative T-cell line. CD45-positive and CD45-negative derivatives of the mouse T-cell line YAC-1 were examined. PAG was immunoprecipitated from cell lysates with anti-PAG, and its phosphotyrosine content was examined by anti-P.tyr immunoblotting (top panel). The extent of association of PAG with Csk was determined by immunoblotting of anti-PAG immunoprecipitates with anti-Csk (center panel), while the abundance of PAG was verified by immunoblotting of anti-PAG immunoprecipitates with anti-PAG (bottom panel). (E) Overall protein tyrosine phosphorylation in CD45-positive and CD45-negative derivatives of YAC-1. Overall protein tyrosine phosphorylation was determined by immunoblotting of total cell lysates with anti-P.tyr antibodies.
Because thymocyte maturation is arrested at the double-positive stage in CD45-deficient mice (4, 21), it was possible that the increased baseline PAG phosphorylation in these animals was due to a change in thymocyte subpopulations. To help exclude this possibility, PAG tyrosine phosphorylation was studied in CD45-positive and CD45-negative variants of the mouse T-cell line YAC-1 (36) (Fig. 8D). As was observed in CD45-deficient thymocytes (Fig. 8A and 8B), the phosphotyrosine content of PAG was prominently increased in CD45-negative YAC-1 cells (Fig. 8D, top panel, lane 2) compared to CD45-positive cells (lane 1). A concomitant augmentation of the association of PAG with Csk was noted (center panel). The enhanced PAG tyrosine phosphorylation in CD45-negative YAC-1 cells appeared to be quite selective, as most other polypeptides found in total cell lysates exhibited no increase in tyrosine phosphorylation (Fig. 8E).
Hence, based on the data of Fig. 7 and 8, it seems unlikely that PTPs such as PEP, SHP-1, and possibly PTP-PEST and SHP-2 are involved in inhibiting PAG tyrosine phosphorylation in T cells. However, it appears that CD45 has a role in this process. To assess further whether PAG was a direct substrate of CD45, a substrate-trapping experiment was performed (Fig. 9). This experiment is based on the principle that PTPs, in which the catalytic site is mutated and rendered inactive, can stably interact with their substrates in transfected cells (16). Cos-1 cells were transfected with cDNAs encoding PAG and activated Lck (to induce PAG tyrosine phosphorylation), in the presence of either wild-type or inactive CD45. A myristylated form of CD45 (Src-CD45) was used in these studies, to facilitate the membrane targeting of CD45. Immunoblots of total cell lysates confirmed that all proteins were adequately expressed in the transfected cells (Fig. 9A).
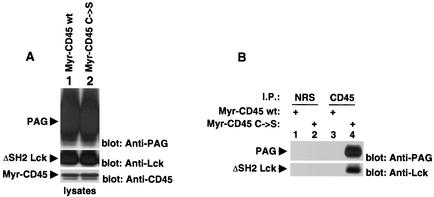
FIG. 9.
Substrate-trapping experiment. Cos-1 cells were transiently transfected with the indicated cDNAs, as detailed in the text. (A) Expression levels of the various polypeptides. The abundance of PAG (top panel), ΔSH2 Y505F Lck (center panel) and the two Src-CD45 variants (bottom panel) in total cell lysates was assessed by immunoblotting with the indicated antibodies. (B) Association of PAG with inactive, but not active, CD45. Lysates were immunoprecipitated with the specified antisera and then probed by immunoblotting with the indicated antibodies. NRS, normal rabbit serum.
This experiment showed that PAG was easily detected in anti-CD45 immunoprecipitates obtained from cells expressing the inactive form of CD45 (Fig. 9B, top panel, lane 4) but not those expressing wild-type CD45 (lane 2). No PAG was found in immunoprecipitates obtained with normal rabbit serum (lanes 1 and 3). A similar association was seen between activated Lck and CD45 (bottom panel), in keeping with the previously published data indicating that activated Lck is also a substrate of CD45 (31). Hence, the results of this study suggested that, like Lck, PAG might be a direct target of CD45.
DISCUSSION
In this report, we have examined the function and regulation of the lipid raft-associated transmembrane adaptor PAG in T cells. First, as previously reported for human T cells (2, 17, 32), we observed that PAG was extensively tyrosine phosphorylated and associated with Csk in ex vivo mouse thymocytes. Furthermore, following antigen receptor stimulation on these cells, PAG underwent rapid dephosphorylation and became dissociated from Csk. In time-course analyses, PAG dephosphorylation temporally coincided with, or perhaps even preceded, the overall intracellular protein tyrosine phosphorylation signal induced by TCR engagement. Taken together, these findings supported the earlier idea that PAG dephosphorylation and dissociation from Csk are early events of T-cell activation and that they might be required for TCR signaling to proceed normally.
To address the mechanism of action of PAG, wild-type PAG and phosphorylation-defective PAG mutants were expressed in normal mouse T cells via transgenesis. Our analyses of these mice revealed that overexpression of wild-type PAG caused a striking inhibition of TCR-induced proliferation and IL-2 production. This effect was observed in several T-cell populations, namely, CD4+ splenic T cells, CD8+ splenic T cells, CD4+ thymocytes, and CD4+ lymph node T cells. In contrast to wild-type PAG, the phosphorylation-defective PAG mutants PAG Y314F and PAG 9Y→F caused an enhancement of these TCR-triggered responses. In addition to demonstrating the importance of tyrosine phosphorylation for the inhibitory function of PAG, the dominant-negative effect of these mutants implied that the inhibitory impact of wild-type PAG was not a spurious effect of overexpression. Rather, it reflected the true function of endogenous PAG molecules.
Several lines of evidence indicated that PAG inhibits T-cell activation primarily by recruiting Csk and inactivating Src kinases. First, we found that the inhibitory influence of PAG was eliminated by mutation of Y314, the major Csk-binding site of PAG (20, 30). Obviously, the possibility that this site was also implicated in recruiting other SH2 domain-containing molecules to PAG cannot be excluded. Second, it was noted that augmented PAG expression resulted in an inhibition of TCR-induced protein tyrosine phosphorylation, an effect analogous to that observed upon overexpression of Csk (8). And lastly, PAG-mediated inhibition was rescued by expression of a Src kinase mutant that is refractory to the effect of Csk (FynT Y528F). While this last finding is in keeping with our model, it is worth mentioning that the activated FynT might also function by causing enhanced phosphorylation of proteins other than PAG.
While PAG overexpression inhibited TCR-induced proliferation and IL-2 secretion, it is noteworthy that it had no impact on the production of IL-4 and IFN-γ. This finding suggested that the intensity and/or nature of the TCR signals required for release of IL-2 and proliferation might be distinct from those needed for production of IL-4 and IFN-γ. Interestingly, a similar alteration in the profile of cytokine production was reported for anergic T cells. Like PAG-overexpressing cells, these cells have severe defects in TCR-induced proliferation and IL-2 secretion but tend to exhibit normal secretion of IL-4 or IFN-γ (1, 15). This qualitative difference was proposed to reflect a hierarchy in the TCR signaling thresholds required for production of the various cytokines (18). It is possible that a similar phenomenon explains the differential effects of PAG on cytokine production. Given the similarities between anergic and PAG-overexpressing T cells, it is also tempting to speculate that PAG is involved in the pathophysiology of T-cell anergy.
A surprising finding in our studies was that expression of the dominant-negative PAG molecules had no appreciable effect on thymocyte development. This is in striking contrast to the previously described severe effects of Csk deficiency on T-cell maturation (29). A possible explanation for this distinction is that PAG-independent mechanisms exist for membrane recruitment of Csk. Along these lines, it was reported that the Csk SH2 domain can interact with other molecules such as Dok-related adaptors, paxillin, and focal adhesion kinase (35). Alternatively, the expression levels of the phosphorylation-defective PAG polypeptides might have been insufficient to obliterate fully the physiological function of endogenous PAG molecules. While the creation of PAG-deficient mouse T cells should help distinguish between these possibilities, it seems probable, based on the available evidence, that additional mechanisms of Csk recruitment exist.
Considering the importance of PAG tyrosine phosphorylation for its inhibitory function, we attempted to identify the PTPs regulating this process. By analyzing T cells lacking various PTPs, evidence was adduced that PEP and SHP-1 were not involved in controlling PAG tyrosine phosphorylation. The lack of effect of PEP on PAG tyrosine phosphorylation was also confirmed by analyses of transgenic mice overexpressing wild-type PEP or phosphatase-inactive versions of PEP (our unpublished results). The observation that PEP had no apparent effect on PAG tyrosine phosphorylation was unexpected, given that PEP associates with Csk by way of the Csk SH3 domain (10). Nonetheless, we recently obtained indications that the pool of Csk molecules associated with PEP does not interact simultaneously with PAG (our unpublished results). Therefore, PAG might not be accessible to PEP-mediated dephosphorylation.
However, our results provided an indication that CD45 is involved in inhibiting PAG tyrosine phosphorylation in T cells. In support of this idea, CD45, but not PTPs like PEP and SHP-1, partially colocalized with PAG in lipid raft fractions. Moreover, we found that the phosphotyrosine content of PAG was increased in lipid raft fractions of CD45-deficient thymocytes as well as in a CD45-negative variant of the mouse T-cell line YAC-1. While it is impossible with the currently available technologies to prove that CD45 was acting directly on PAG, this notion was suggested by the finding that a substrate-trapping mutant of CD45 can interact with tyrosine-phosphorylated PAG in transiently transfected Cos-1 cells. On the other hand, it is also plausible that CD45 regulated PAG phosphorylation by an indirect mechanism, for instance by inactivating Src kinases through dephosphorylation of their activating tyrosine (31). The development of new methodologies capable of identifying enzyme-substrate interactions in vivo is needed to resolve these issues. Lastly, it should be pointed out that, in addition to CD45, other PTPs are likely to be involved in regulating PAG tyrosine phosphorylation. This is definitely true for nonhemopoietic cells, which express PAG but lack CD45.
The finding that CD45 is involved, directly or indirectly, in regulating PAG tyrosine phosphorylation is likely to be important. It suggests that CD45 sets the threshold of TCR signaling by at least two mechanisms. First, as documented in the past, CD45 dephosphorylates the inhibitory tyrosine of Src kinases (31). And second, as reported herein, it promotes the dephosphorylation of PAG, thereby diminishing the amount of Csk located in lipid rafts. Both effects converge on increasing the catalytic activity of Src kinases, and their combination might be critical to the generation of sufficient Src kinase activation to allow productive TCR signaling to occur.
In summary, the data reported in this work provide compelling evidence that PAG is a negative regulator of T-cell activation in normal T cells as a result of its capacity to recruit Csk and inactivate Src kinases. They also support the idea that the dephosphorylation of PAG is a pivotal event during the initiation of T-cell activation. In the light of these results, additional studies are warranted to elucidate the mechanism responsible for PAG dephosphorylation upon TCR engagement. One possibility is that TCR stimulation activates or alters the cellular localization of PTPs like CD45 and others. Alternatively, triggering of the TCR might inactivate or sequester the PTK(s) responsible for PAG phosphorylation. An understanding of this phenomenon would undoubtedly provide valuable insights into the molecular changes responsible for initiating T-cell activation.
Acknowledgments
We thank Sylvain Latour and members of our laboratory for comments on the manuscript as well as Larry Samelson, Phil Branton, and Jon Ashwell for gifts of reagents.
This work was supported by grants from the National Cancer Institute of Canada and the Canadian Institutes of Health Research (to A.V.) as well as from the Center of Molecular and Cellular Immunology and the Wellcome Trust (to V.H.). A.V. is a Senior Investigator of the Canadian Institutes of Health Research and holds a Canada Research Chair.
REFERENCES
- 1.Blish, C. A., S. R. Dillon, A. G. Farr, and P. J. Fink. 1999. Anergic CD8+ T cells can persist and function in vivo. J. Immunol. 163:155-164. [PubMed] [Google Scholar]
- 2.Brdicka, T., D. Pavlistova, A. Leo, E. Bruyns, V. Korinek, P. Angelisova, J. Scherer, A. Shevchenko, A. Shevchenko, I. Hilgert, J. Cerny, K. Drbal, Y. Kuramitsu, B. Kornacker, V. Horejsi, and B. Schraven. 2000. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 191:1591-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brdickova, N., T. Brdicka, L. Andera, J. Spicka, P. Angelisova, S. L. Milgram, and V. Horejsi. 2001. Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton. FEBS Lett. 507:133-136. [DOI] [PubMed] [Google Scholar]
- 4.Byth, K. F., L. A. Conroy, S. Howlett, A. J. Smith, J. May, D. R. Alexander, and N. Holmes. 1996. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J. Exp. Med. 183:1707-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantrell, D. A. 2002. Transgenic analysis of thymocyte signal transduction. Nat. Rev. Immunol. 2:20-27. [DOI] [PubMed] [Google Scholar]
- 6.Caron, L., N. Abraham, T. Pawson, and A. Veillette. 1992. Structural requirements for enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Mol. Cell. Biol. 12:2720-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, A. C., D. M. Desai, and A. Weiss. 1994. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu. Rev. Immunol. 12:555-592. [DOI] [PubMed] [Google Scholar]
- 8.Chow, L. M., M. Fournel, D. Davidson, and A. Veillette. 1993. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature 365:156-160. [DOI] [PubMed] [Google Scholar]
- 9.Clements, J. L., and G. A. Koretzky. 1999. Recent developments in lymphocyte activation: linking kinases to downstream signaling events. J. Clin. Investig. 103:925-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cloutier, J. F., and A. Veillette. 1996. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 15:4909-4918. [PMC free article] [PubMed] [Google Scholar]
- 11.Cloutier, J. F., and A. Veillette. 1999. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J. Exp. Med. 189:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke, M. P., K. M. Abraham, K. A. Forbush, and R. M. Perlmutter. 1991. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell 65:281-291. [DOI] [PubMed] [Google Scholar]
- 13.Davidson, D., L. M. Chow, M. Fournel, and A. Veillette. 1992. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J. Exp. Med. 175:1483-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson, D., J. F. Cloutier, A. Gregorieff, and A. Veillette. 1997. Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells. J. Biol. Chem. 272:23455-23462. [DOI] [PubMed] [Google Scholar]
- 15.Evavold, B. D., and P. M. Allen. 1991. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science 252:1308-1310. [DOI] [PubMed] [Google Scholar]
- 16.Flint, A. J., T. Tiganis, D. Barford, and N. K. Tonks. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh, K., M. Sakakibara, S. Yamasaki, A. Takeuchi, H. Arase, M. Miyazaki, N. Nakajima, M. Okada, and T. Saito. 2002. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J. Immunol. 168:541-544. [DOI] [PubMed] [Google Scholar]
- 18.Itoh, Y., and R. N. Germain. 1997. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cytokine responses of CD4+ T cells. J. Exp. Med. 186:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janes, P. W., S. C. Ley, A. I. Magee, and P. S. Kabouridis. 2000. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 12:23-34. [DOI] [PubMed] [Google Scholar]
- 20.Kawabuchi, M., Y. Satomi, T. Takao, Y. Shimonishi, S. Nada, K. Nagai, A. Tarakhovsky, and M. Okada. 2000. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404:999-1003. [DOI] [PubMed] [Google Scholar]
- 21.Kishihara, K., J. Penninger, V. A. Wallace, T. M. Kundig, K. Kawai, A. Wakeham, E. Timms, K. Pfeffer, P. S. Ohashi, and M. L. Thomas. 1993. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell 74:143-156. [DOI] [PubMed] [Google Scholar]
- 22.Kosugi, A., J. Sakakura, K. Yasuda, M. Ogata, and T. Hamaoka. 2001. Involvement of SHP-1 tyrosine phosphatase in TCR-mediated signaling pathways in lipid rafts. Immunity 14:669-680. [DOI] [PubMed] [Google Scholar]
- 23.Latour, S., and A. Veillette. 2001. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr. Opin. Immunol. 13:299-306. [DOI] [PubMed] [Google Scholar]
- 24.Myung, P. S., N. J. Boerthe, and G. A. Koretzky. 2000. Adapter proteins in lymphocyte antigen-receptor signaling. Curr. Opin. Immunol. 12:256-266. [DOI] [PubMed] [Google Scholar]
- 25.Niklinska, B. B., D. Hou, C. June, A. M. Weissman, and J. D. Ashwell. 1994. CD45 tyrosine phosphatase activity and membrane anchoring are required for T-cell antigen receptor signaling. Mol. Cell. Biol. 14:8078-8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penninger, J. M., J. Irie-Sasaki, T. Sasaki, and A. J. Oliveira-dos-Santos. 2001. CD45: new jobs for an old acquaintance. Nat. Immunol. 2:389-396. [DOI] [PubMed] [Google Scholar]
- 27.Rudd, C. E. 1999. Adaptors and molecular scaffolds in immune cell signaling. Cell 96:5-8. [DOI] [PubMed] [Google Scholar]
- 28.Samelson, L. E. 2002. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 20:371-394. [DOI] [PubMed] [Google Scholar]
- 29.Schmedt, C., K. Saijo, T. Niidome, R. Kuhn, S. Aizawa, and A. Tarakhovsky. 1998. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature 394:901-904. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi, S., Y. Takayama, A. Ogawa, K. Tamura, and M. Okada. 2000. Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J. Biol. Chem. 275:29183-29186. [DOI] [PubMed] [Google Scholar]
- 31.Thomas, M. L., and E. J. Brown. 1999. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol. Today 20:406-411. [DOI] [PubMed] [Google Scholar]
- 32.Torgersen, K. M., T. Vang, H. Abrahamsen, S. Yaqub, V. Horejsi, B. Schraven, B. Rolstad, T. Mustelin, and K. Tasken. 2001. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J. Biol. Chem. 276:29313-29318. [DOI] [PubMed] [Google Scholar]
- 33.Tsui, H. W., K. A. Siminovitch, L. de Souza, and F. W. Tsui. 1993. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat. Genet. 4:124-129. [DOI] [PubMed] [Google Scholar]
- 34.Veillette, A., M. A. Bookman, E. M. Horak, and J. B. Bolen. 1988. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55:301-308. [DOI] [PubMed] [Google Scholar]
- 35.Veillette, A., S. Latour, and D. Davidson. 2002. Negative regulation of immunoreceptor signaling. Annu. Rev. Immunol. 20:669-707. [DOI] [PubMed] [Google Scholar]
- 36.Volarevic, S., B. B. Niklinska, C. M. Burns, H. Yamada, C. H. June, F. J. Dumont, and J. D. Ashwell. 1992. The CD45 tyrosine phosphatase regulates phosphotyrosine homeostasis and its loss reveals a novel pattern of late T cell receptor-induced Ca2+ oscillations. J. Exp. Med. 176:835-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss, A., and D. R. Littman. 1994. Signal transduction by lymphocyte antigen receptors. Cell 76:263-274. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]