Abstract
During ongoing C-type retrovirus infection, the probability of leukemia caused by insertional gene activation is markedly increased by the emergence of recombinant retroviruses that repeatedly infect host cells. The murine mink cell focus-inducing (MCF) viruses with this property have acquired characteristic changes in the N-terminal domain of their envelope glycoprotein that specify binding to a different receptor than the parental ecotropic virus. In this report, we show that MCF virus infection occurs through binding to this receptor (termed Syg1) and, remarkably, by a second mechanism that does not utilize the Syg1 receptor. By the latter route, the N-terminal domain of the ecotropic virus glycoprotein expressed on the cell surface in a complex with its receptor activates the fusion mechanism of the MCF virus in trans. The rate of MCF virus spread through a population of permissive human cells was increased by establishment of trans activation, indicating that Syg1 receptor-dependent and -independent pathways function in parallel. Also, trans activation shortened the interval between initial infection and onset of cell-cell fusion associated with repeated infection of the same cell. Our findings indicate that pathogenic retrovirus infection may be initiated by virus binding to cell receptors or to the virus envelope glycoprotein of other viruses expressed on the cell surface. Also, they support a broader principle: that cooperative virus-virus interactions, as well as virus-host interactions, shape the composition and properties of the retrovirus quasispecies.
A critical step in the pathogenesis of retrovirus-induced leukemia is provirus integration adjacent to genes, such as myc and myb, that regulate cell growth and/or apoptosis (7, 17, 20, 23, 33, 34). The deleterious effects of provirus integration create strong selection pressure on the host to avoid infection and, in turn, on the virus to adapt. As a consequence of this genetic interplay, at least 10 groups of leukemogenic retroviruses that use distinct receptors have been identified in mammals (32, 41, 45).
The N-terminal segment of the surface subunit (SU) is the portion of the mammalian leukemia virus envelope glycoprotein that binds to its receptor (5, 12, 21). This segment forms a discrete domain that is tethered to the remainder of SU by a proline-rich region, which may be flexible (36). Indeed, chimeric envelope glycoproteins, created by reciprocal exchange in the proline-rich region, are usually functional and bind to the receptor corresponding to its N-terminal domain (19, 36). In the case of Friend leukemia virus, this domain binds to its receptor, mCAT1, with 1:1 stoichiometry (12). The diversification of mammalian leukemia viruses has occurred by linkage of distinct receptor binding domains (RBDs) to a conserved glycoprotein that contains the components required for membrane fusion and infection.
Comparison of the atomic resolution structures of RBDs from Friend murine leukemia virus (MLV) and subgroup B feline leukemia virus (FeLV) reveals how these distinct RBDs are organized (4a; J. M. Cunningham, unpublished data). Both RBDs contain nine β-strands that assemble into similar barrel-like scaffolds. They also contain two prominent loops that project over the top of the scaffold and form the receptor-specific subdomain. Although the sequences of these loops differ markedly among retrovirus classes, a platform formed by four structurally conserved residues including two adjacent disulfide linkages has been identified that links the loops to each other and to the scaffold. Through this organization, receptor diversification is accommodated by the conserved mechanism of fusion.
The postreceptor function of the RBD has been revealed by studies of mutant viruses in which fusion and infection are uncoupled from receptor binding (2). Remarkably, infection by these viruses is restored by addition of the RBD (separated from the remainder of the viral glycoprotein [sRBD]) to the culture medium (27). Mapping and competition experiments indicate that trans activation of mutant virus infection requires binding of virus RBD and sRBD to their cognate receptors, which may be different (3, 4, 4a, 25, 26). A feline retrovirus that causes immunodeficiency has been identified in cats that is strictly dependent on trans activation by an sRBD encoded by an endogenous provirus (1). Recently, we have shown that the requirement for virus binding to the receptor for trans activation is circumvented by deletion of the RBD from the virus envelope glycoprotein (3, 4). This result strongly suggests that the virus fusion mechanism is activated by a direct interaction with the receptor-bound sRBD. Moreover, it indicates that the presence of the RBD is not required for the assembly of a fusion-competent virus envelope glycoprotein and also that virus binding to the receptor is not strictly required for trans activation. These observations suggested the possibility that naturally occurring viruses might exist that are targets for trans activation in the absence of receptor binding.
In virus-infected cells, newly synthesized retrovirus envelope glycoproteins bind to their cognate receptor and interfere with further infection by the same class of viruses (9, 37, 38). However, the envelope glycoprotein-receptor complexes that reach the cell surface might, paradoxically, enhance infection by retroviruses that are sensitive to trans activation. In this report, we identify a murine retrovirus with this property. Infection by this mink cell focus-inducing (MCF) virus is initiated through binding to its traditional receptor or by the trans activation mechanism. The MCF virus is a recombinant that is derived from an ecotropic parent that specifically supports infection by trans activation. The emergence of MCF viruses is a critical event in the pathogenesis of leukemia by insertional gene activation (7, 10, 11, 17, 22, 43). Therefore, the establishment of trans activation may play a significant role in the natural history of retrovirus infection in vivo.
MATERIALS AND METHODS
Cell lines and virus infection.
The derivation and propagation of the human 293 cell line (293mCAT1) and the Chinese hamster CHO-K1-derived cell line (CHTGmCAT1) expressing the ecotropic Friend virus receptor, mCAT1, as well as the NIH 3T3 cell line that expresses high levels of mCAT1 (NFM), have been described previously (13).
MCF247 virus was produced by a baby hamster kidney-derived cell line that contains a single MCF247 provirus (Christie Holland, Children's Hospital, Washington, D.C.). Mouse fibroblasts that produce the xenotropic NZB virus were obtained from Janet Hartley, National Institutes of Health, Bethesda, Md.
Virus infection was initiated by exposing 293mCAT1 cells (106) to 50 μl of virus-containing medium overnight (multiplicity of infection, <0.01). Three days later, 10% of these cells were transferred to each of two plates, one of which was propagated in medium containing the purified soluble Friend RBD (sFr-RBD) (20 nM). Every 3 to 4 days, cells from the RBD-treated and control cultures were harvested. Ten percent of these cells were replated on fresh culture plates, and fresh sFr-RBD was added to the medium of the previously treated cells. The remainder of the cells were pelleted, washed in phosphate-buffered saline, and frozen. After completion of the protocol (90 days), aliquots of frozen cells were used as the source of DNA for analysis of acquired provirus content by PCR. In addition, the time course of cell-cell fusion in both RBD-treated and control cells was confirmed by repeating the protocol with cells frozen 30 days after initial MCF247 virus infection.
Virus vectors.
Viral vectors were prepared by using 80% confluent plates of human 293 cells that were transfected with 20 μg of pMD.old.gagpol, encoding MLV gag/pol; 20 μg of pBABE-lacZ, encoding Escherichia coli lacZ; and 20 μg of the expression construct encoding the desired envelope glycoprotein (env), as described previously (42). The ecotropic Friend 57 (pCMV-Frgp85), amphotropic 4070A (pSV-MLV-A), and xenotropic NZB (pCMV-Xenogp85) env expression constructs have been described previously (3, 31). The expression plasmid encoding MCF247 env (pMCF-Fr-5′UT) was constructed as follows. The MCF247 env gene was amplified by PCR with an MCF247 provirus (provided by Christie Holland) as the template and cloned into the pCDNA3 expression vector (Invitrogen) to produce the pCMV-MCFgp85 plasmid. A SalI site (underlined) was introduced into pCMV-MCFgp85 immediately upstream of the initiating ATG by PCR-based mutagenesis (5′ primer, 5′-ACGCGTCGACATGGAAGGTCCAGCGTTC-3′; 3′ primer, SP6). The upstream untranslated region was then removed from the pCMV-Frgp85 plasmid and inserted upstream of the MCF247 env gene by using the HindIII and SalI restriction sites. This alteration of pCMV-MCFgp85 increased the expression level of MCF247 env.
FeLV-T vectors were produced by transfecting human 293 cells with pBABE-lacZ and the EECCψ−provirus obtained from Julie Overbaugh (University of Washington) (35). On the day following transfection, the culture medium was replaced, and after an additional 24 h, virus-containing medium was collected and filtered (0.45 μm). Virus infection of indicator cell lines was measured by counting cells that acquired β-galactosidase activity 2 days after exposure to virus supernatant. Virus titer (infectious units [i.u.] per milliliter) was determined by endpoint dilution on six-well plates.
The plasmids encoding murine or human Syg1 receptors (pCDNA3-mSYG1 and pCDNA3-hSYG1, respectively) have been described previously (47). Hamster Syg1 was isolated from CHTG cell mRNA by reverse transcription-PCR and cloned into pCDNA3 in a similar fashion. These Syg1 isoforms were expressed transiently in CHTG cells by using SuperFect reagent (Qiagen). An expression plasmid encoding the Friend envelope glycoprotein was stably expressed in CHTG and CHTGmCAT1 cells by calcium phosphate-mediated transfection with pCMV-Frgp85 DNA and selection in G418-containing medium.
Provirus DNA measurement.
DNA was isolated (DNeasy Tissue Kit; Qiagen) from infected cells, and the number of acquired proviruses was determined by quantitative real-time PCR with an iCycler IQ detection system (Bio-Rad). The primers and probe used for detection of the MCF247 provirus are located in the envelope gene (forward primer, TACCAACAGGGTGTGGAGGG; reverse primer, TGCCTGTCCAGTGGTCTCAC; probe, 6-FAM-CGAGAGAGGGCTACTGTGGCAAATGG-BHQ-1 [Biosearch Technologies]). In a typical assay, 200 ng of genomic DNA was amplified in triplicate (Platinum Quantitative PCR SuperMix-UDG; Invitrogen) in reaction mixtures containing the forward and reverse primers (each at 400 nM) and the probe (200 nM). Amplification was performed by denaturation of the template at 94°C for 20 s, followed by primer annealing at 60°C (20 s) and extension at 72°C (30 s) for 44 cycles. A standard curve was generated by spiking uninfected 293mCAT1 cell DNA with linear plasmid DNA containing the MCF247 envelope gene at calculated concentrations of 0.01, 0.1, 1, 10, and 100 copies per haploid genome. Calculations were based on an approximation of 3 × 109 bp per haploid human cell genome (hcg). The limit of detection for MCF247 provirus DNA was 0.005 copy per hcg.
Sequence of MCF247 virus envelope glycoprotein.
DNA was prepared from 293mCAT1 cells after MCF247 infection and propagation in the presence or absence of the sFr-RBD. The region encoding the MCF247 virus envelope glycoprotein was amplified by PCR from 293mCAT1 cell DNA obtained 77 days after initial infection with the proofreading DeepVent polymerase (New England Biolabs) and cloned into the TOPO-TA vector (Invitrogen) after addition of single 3′ adenine residues with Taq DNA polymerase (72°C, 30 min). Clones were sequenced with a set of nine primers spanning the entire gene, and the sequences were compared to those of two independent clones of this gene obtained from the proviral DNA in the original virus producer cell line.
Purification of RBDs.
The protocol for purification of the RBDs of Friend, amphotropic 4070, MCF247, and xenotropic NZB MLVs and subgroup B FeLV from the supernatant of baculovirus-infected insect cells has been described elsewhere (12).
RESULTS
trans activation of infection in the absence of virus receptor.
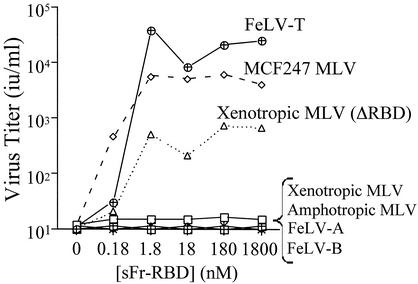
The hypothesis that trans activation provides an alternative to the virus receptor-dependent infection pathway was tested by performing a screen with the Chinese hamster-derived cell line CHTG. CHTG cells were chosen for study because they lack functional receptors for nearly all classes of mammalian leukemia viruses (46). The Friend leukemia virus receptor, mCAT1, was stably introduced into this cell line, and infection by several distinct mammalian type C retroviruses was measured as a function of the sFr-RBD concentration (0 to 1,800 nM) in the medium (Fig. 1). The sFr-RBD was chosen because in previous studies, the Fr-RBD/mCAT1 complex supported infection by mutant viruses from several different classes of MLV (3, 4; Barnett et al., unpublished). Addition of the sFr-RBD to the medium was not sufficient to establish infection by subgroup A or B FeLV or by amphotropic 4070A or xenotropic NZB MLV (Fig. 1). Susceptibility to xenotropic NZB retrovirus infection by the sFr-RBD was observed after deletion of the RBD from the virus envelope glycoprotein (ΔRBD), confirming the presence of trans activation activity. Two viruses, MCF247 and FeLV-T, were identified that did not require modification for sFr-RBD-dependent infection. The maximum titer of infection exceeded 103 i.u./ml for the MCF247 virus and 104 i.u./ml for FeLV-T. The infection profiles observed for these viruses are similar to the relationship between RBD concentration and infection observed in previous studies of trans activation of mutant viruses (3, 4, 4a).
FIG. 1.
trans activation of leukemogenic retrovirus infection of nonpermissive hamster cells by an sFr-RBD-receptor complex. The hamster-derived cell line CHTGmCAT1 was challenged with retroviral vectors containing E. coli lacZ and bearing the envelope glycoprotein from FeLV-A, FeLV-B, FeLV-T, or the murine amphotropic MCF247 or xenotropic NZB virus. A virus expressing an altered xenotropic envelope glycoprotein lacking an RBD (ΔRBD) was also studied. Infection was measured as a function of the concentration of the purified sFr-RBD added to the culture medium and scored by enumerating the foci of cells expressing β-galactosidase 2 days after exposure to virus. Virus infection titers, shown in infectious units per milliliter of virus-containing medium (i.u. per milliliter), were determined by endpoint dilution.
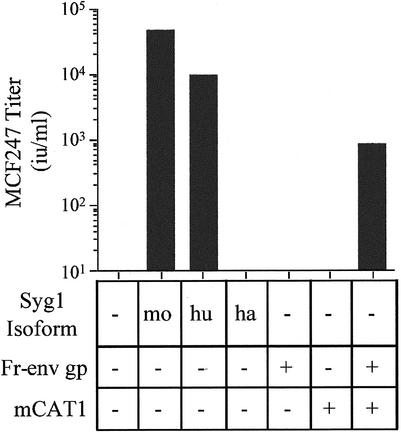
FeLV-T contains a proline residue in place of the critical histidine residue in the envelope glycoprotein required for receptor binding-induced activation of fusion (2, 35), and therefore, infection by this virus is strictly dependent on trans activation. A receptor for this virus has not been identified, and therefore, the possible requirement for virus binding in sFr-RBD-dependent trans activation cannot be directly assessed for FeLV-T. However, the MCF virus receptor has been identified as the integral membrane protein Syg1 (6, 44, 47). Expression of the hamster isoform of Syg1 in CHTG cells did not confer infection by the MCF247 virus under conditions in which both the human and mouse isoforms were functional (Fig. 2), affirming that the endogenous Syg1 protein in hamster cells is not a functional MCF247 virus receptor (30, 47). The titer of MCF247 virus infection of CHTG cells expressing murine Syg1 exceeded that achieved with human Syg1 by fivefold, consistent with the greater binding affinity of the mSyg1 receptor for the MCF247 virus envelope glycoprotein (D. L. Wensel, unpublished data). These experiments indicate that sFr-RBD-dependent infection of CHTG cells by the MCF247 virus does not require binding to the Syg1 receptor.
FIG. 2.
Expression of the Syg1 receptor or Friend leukemia virus envelope glycoprotein and the mCAT1 receptor confers susceptibility to MCF247 virus infection on nonpermissive hamster cells. CHTG cells expressing the murine (mo), human (hu), or hamster (ha) isoform of the MCF247 receptor (Syg1), the Friend virus receptor (mCAT1), and/or the Friend virus envelope glycoprotein (Fr-env gp) were studied. Syg1 isoforms were expressed in CHTG cells, and Fr-env gp was expressed in CHTG and CHTGmCAT1 cells by transient transfection. Two days later, these cells were exposed to the MCF247 viral vector containing E. coli lacZ and infection was scored (i.u. per milliliter) by counting the foci of cells expressing β-galactosidase 2 days later.
MCF viruses are a distinct class of retroviruses that are created by recombination during ongoing ecotropic virus (including Friend virus) infection in mice (15, 16, 43). Once formed, these recombinant viruses are selected in an environment where many host cells express the envelope glycoprotein of the ecotropic parent virus on the cell surface. MCF247 virus infection of CHTGmCAT1 cells was conferred by expression of the Friend virus envelope glycoprotein (Fig. 2), indicating that the intact glycoprotein is equivalent to the sFr-RBD in supporting trans activation. Like the sFr-RBD, the trans activation activity of the Friend virus envelope glycoprotein is strictly dependent on expression of mCAT1, but not Syg1.
The trans activation of MCF247 virus infection by other viral envelope glycoproteins was assessed by introduction of their receptors into CHTG cells and MCF247 virus challenge in the presence of cognate RBDs. We observed that only Fr-RBD/mCAT1, but not the feline B virus (FeB) RBD/Pit1 receptor or the amphotropic MLV RBD/Pit2 receptor, was active (data not shown). Also, in a separate experiment, we studied trans activation of a defective MCF247 virus on human 293mCAT1 cells that express the Syg1 and mCAT1 receptors. This virus, which carries a mutation in the critical histidine residue near the N terminus of SU (2), binds to the Syg1 receptor and is able to complete infection in the presence of the sFr-RBD but not in the presence of the FeB RBD. Therefore, in this small survey, trans activation of MCF247 virus infection was limited to the sFr-RBD. This observation suggests that MCF247 virus may be specifically adapted for trans activation by its parent ecotropic virus envelope glycoprotein in vivo.
trans activation accelerates MCF247 virus infection and cell-cell fusion.
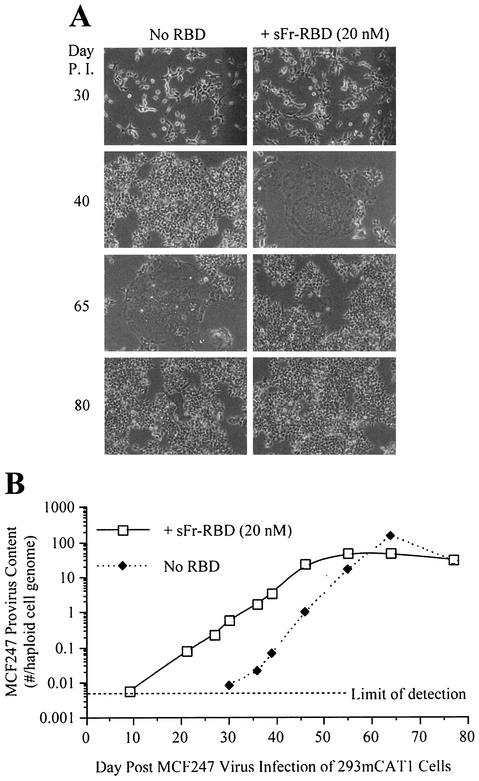
We were unable to achieve stable long-term expression of the Syg1 receptor in CHTG cells and therefore could not study chronic MCF247 virus infection in these cells. Also, the presence of many endogenous MCF-related proviruses in the genome of mouse NIH 3T3 cells confounded the measurement of infection of these cells by the use of a quantitative PCR for detection of acquired MCF247 proviruses (43). Therefore, human 293mCAT1 cells were used to assess the significance of trans activation on MCF247 virus infection of cells that express a functional Syg1 receptor (29). In a single round of infection, exposure to the sFr-RBD caused a small but reproducible increase in MCF247 virus infection (1 × 104 to 3 × 104 i.u./ml) of these cells. To study chronically infected cells, a single culture plate of human 293mCAT1 cells was exposed to the MCF247 virus at a low multiplicity of infection (<0.01). Two days later, the cells on this plate were divided equally and propagated in parallel in the presence or absence of the sFr-RBD for 90 days. With each passage (usually 1:10 every 3 to 4 days), cells were inspected for virus-induced injury and aliquots were harvested for measurement of MCF247 provirus DNA. During the first 10 passages after the initial infection, no detectable change in cell growth or morphology was observed (day 37). Remarkably, at passage 11 (day 40), large, flat, multinucleated cells indicative of cell-cell fusion (syncytia) were identified among sFr-RBD-treated cells (Fig. 3A). This phenotype increased such that 1 day after passage 14 (day 52), >90% of the cell nuclei were located in syncytia. The behavior of the cells that were not exposed to the sFr-RBD was similar to that of treated cells, but the onset (passage 14) and the peak (passage 17) of syncytium formation occurred three passages (12 days) later. For both cultures, the extent of syncytium formation was correlated with the degree of virus infection measured by a quantitative PCR assay for provirus DNA (Fig. 3B). The onset of cell-cell fusion corresponded to the acquisition of 3 to 4 MCF247 proviruses per hcg. The threshold of detection of MCF247 provirus DNA (0.005 provirus per hcg) was first exceeded 10 days after infection in sFr-RBD-treated cells and 30 days after infection in untreated cells (Fig. 3B). This indicates that the early replication of MCF247 virus in these cultures was enhanced by sFr-RBD.
FIG. 3.
MCF247 virus infection is enhanced by trans activation. Permissive human 293mCAT1 cells were exposed to MCF247 virus and cultured in the presence or absence of the purified sFr-RBD at a concentration of 20 nM. (A) Phase-contrast photomicrographs were obtained at a magnification of 200× at the indicated times postinfection (P. I.). (B) MCF247 provirus DNA was measured by quantitative PCR with high-molecular-weight DNA from MCF247 virus-infected cells as the template under the conditions described in Materials and Methods. Measurements were calibrated against a standard curve obtained by spiking 293mCAT1 cell DNA with a linear plasmid containing the MCF247 env gene at calculated concentrations of 0.01, 0.1, 1, 10, and 100 copies per haploid 293mCAT1 cell genome. The number of MCF247 proviruses per 293mCAT1 cell haploid genome is plotted as a function of time (days) after infection (average of triplicate measurements from one experiment). The MCF247 provirus detection threshold was one copy in 200 haploid genomes.
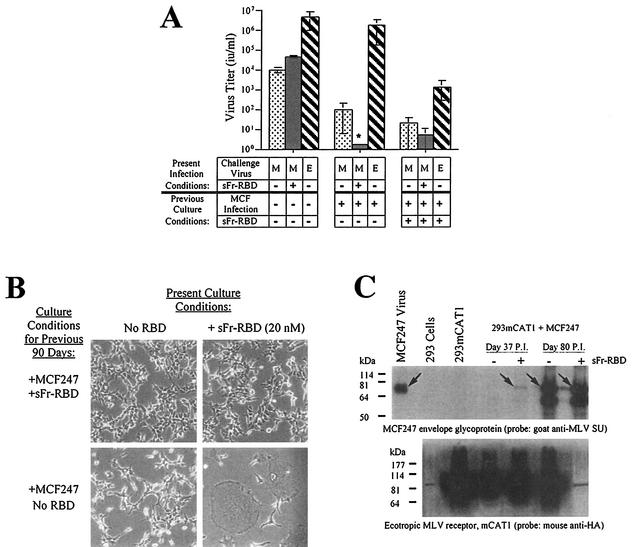
After 48 days in the sFr-RBD-treated cell culture, the frequency of new syncytia declined and the number of acquired proviruses stabilized at 35 proviruses per hcg. In the untreated cells, 100 proviruses per hcg were present at the peak of syncytium formation, which occurred 65 days after initial infection. With the resolution of syncytium formation on these cells, the number of proviruses per hcg also stabilized at 35. A small number of cells resistant to MCF247 virus-induced syncytium formation overgrew both cultures such that 80 days after initial infection, cell-cell fusion among both sFr-RBD-treated and untreated control cells was uncommon (Fig. 3A). The susceptibility of these cells to an additional single round of MCF247 virus infection was reduced more than 100-fold compared to that of parental human 293mCAT1 cells (Fig. 4A). Marked expression of MCF247 virus envelope glycoprotein was present in both cultures (Fig. 4C), suggesting that the stabilization of provirus number, the decrease in cell-cell fusion, and the reduction in susceptibility to infection were caused by loss of functional Syg1 receptor activity, likely caused by binding to MCF247 envelope glycoprotein (37, 38). The first exposure of the chronically infected cells to the sFr-RBD caused massive cell-cell fusion within hours, preventing accurate measurement of MCF247 virus infection (Fig. 4B and C). As expected, exposure to the fresh sFr-RBD had no effect on chronically infected cells that had been continuously exposed to the sFr-RBD (Fig. 4B). Remarkably, the sFr-RBD no longer supported MCF247 virus infection of these cells (Fig. 4A). An immunoblot revealed that mCAT1 expression was specifically lost in sFr-RBD-treated cells from the postfusion period (Fig. 4C). This finding suggests that trans activation of the MCF247 envelope glycoprotein by the sFr-RBD/mCAT1 complex on the cell surface selected against cell survival, likely by enhancing cell-cell fusion.
FIG. 4.
Properties of human 293mCAT1 cells chronically infected with MCF247 virus in the presence or absence of trans activation by sFr-RBD. (A) Susceptibility to virus infection. Chronically MCF247 virus-infected human 293mCAT1 cells (80 days) were challenged with MCF247 (M) or ecotropic Friend (E) viral vectors containing E. coli lacZ as indicated. An MCF247 virus challenge was also conducted in the presence of the sFr-RBD (40 nM). Two days after the challenge, infection was scored (i.u. per milliliter) by counting the foci of cells expressing β-galactosidase. The asterisk indicates that the MCF247 virus infection could not be accurately determined because of widespread cell-cell fusion after exposure to sFr-RBD (see the text and panel B). (B) Effect of sFr-RBD. Side-by-side plates of chronically infected cells (90 days) previously cultured in the presence (top panels) or absence (bottom panels) of trans activation conditions were examined 24 h after addition of sFr-RBD (20 nM) to the culture medium (right panels). Photomicrographs were prepared by phase-contrast microscopy (magnification, 200×) (C) Virus envelope glycoprotein and mCAT1 receptor expression. The steady-state levels of the MCF247 virus envelope glycoprotein and mCAT1 receptor were measured by immunoblotting of lysates prepared from MCF247 virus-infected 293mCAT1 cells. Lysates were prepared 37 and 80 days after infection. Day 37 corresponds to one passage prior to detectable cell-cell fusion by the sFr-RBD-treated cells. At day 80, the sFr-RBD-treated (+) and control (−) cultures were populated by cells that survived the preceding period of cell-cell fusion. The immunoblots were prepared after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with goat antibody that recognizes the virus envelope glycoprotein SU (top panel) or a mouse antibody, 12CA5, that recognizes the influenza virus-derived epitope tag attached at the COOH terminus of the mCAT1 receptor (bottom panel). The position of the mature MCF247 envelope glycoprotein is indicated by arrows in the top panel. In the lower panel, mCAT1 is detected as a smear from 60 to 200 kDa due to extensive glycosylation of the protein. Samples were loaded in every other lane in the same order for both blots, as indicated above the top blot, and blots were overexposed to detect low levels of protein expression. The signal present in unloaded lanes represents spillover from adjacent lanes, and the discrete band corresponding to 85 kDa in the lane containing the day 80, sFr-RBD-treated (+) lysate on the blot probed with anti-hemagglutinin is a ubiquitous cross-reacting species also seen in 293 cells without mCAT1. Molecular size markers are shown on the left.
The amino acid sequence of MCF247 virus glycoproteins in postfusion (day 77) cells was derived from the sequence of proviral DNA obtained by PCR amplification. Two independent analyses revealed that the MCF247 envelope glycoprotein encoded by proviruses from sFr-RBD-treated cells was not altered during the course of infection. This indicates that the presence of prolonged trans activation did not supply significant selection pressure to the MCF247 envelope glycoprotein. However, we cannot formally exclude the possibility that altered envelope glycoproteins arose during the period of cell-cell fusion (days 42 to 56) that were subsequently lost before day 77. Unlike the apparent behavior of MCF247 virus envelope glycoprotein in sFr-RBD-treated cells, we observed that the envelope glycoproteins encoded by two MCF247 proviruses obtained from untreated control cells were altered over the course of the experiment. Both proviruses encoded the D198G substitution. This residue is located in a short helical turn in the RBD scaffold that, to date, has not been implicated in receptor binding or fusion activation (18). Also, one of these two proviruses also contained an R435Q substitution near the C terminus of SU. These changes may reflect adaptation of the MCF247 virus to replication in the absence of trans activation.
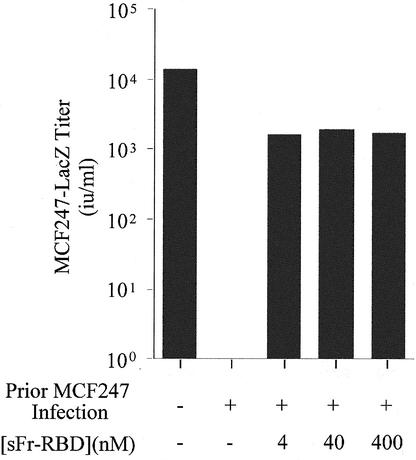
The massive cell-cell fusion associated with MCF247 virus infection of human 293mCAT1 cells was unexpected. In previous studies of mouse cells, infection was associated with establishment of superinfection interference that did not require a period of virus-induced cell-cell fusion (37). Therefore, it is possible that the behavior of human cells is not representative of conditions in vivo. As an additional test of the relevance of trans activation to MCF virus infection, its capacity to influence infection of a murine NIH 3T3-derived cell line (NFM) was assessed (13). Establishment of trans activation by the sFr-RBD on these cells did not significantly enhance MCF247 infection when the Syg1-dependent entry pathway was functional (data not shown). After chronic infection with the MCF247 virus was established, the susceptibility of these cells to a single round of infection was determined (Fig. 5). The titer of an MCF247-based virus vector decreased from 2 × 104 i.u./ml on uninfected control cells to less than 10 i.u./ml on chronically infected cells, indicative of down-regulation of Syg1 receptor activity caused by binding to newly synthesized MCF247 virus envelope glycoprotein. The titer of MCF247 virus infection on these chronically infected cells increased by more than 103 in the presence of sFr-RBD (4 to 400 nM), consistent with activation of virus infection in trans. Furthermore, within 24 h after exposure to the sFr-RBD, numerous syncytia indicative of cell-cell fusion were observed among the chronically infected cells. These findings indicate that the presence of the MCF247 virus envelope glycoprotein on the cell surface does not disrupt productive interaction of sFr-RBD/mCAT1 complexes with MCF247 virus envelope glycoproteins. Moreover, these findings suggest that trans activation is suffi-cient to activate virus and cell membrane fusion by the MCF247 virus envelope glycoprotein under conditions of classical superinfection interference associated with downregulation of functional Syg1 receptors. Therefore, the function of the sFr-RBD/mCAT1 complex in trans activation is not demonstrably influenced by changes in Syg1 receptor function caused by MCF247 virus infection and envelope glycoprotein expression.
FIG. 5.
Trans activation by sFr-RBD circumvents interference to MCF247 virus infection of chronically infected NIH 3T3 fibroblasts. NIH 3T3 fibroblasts that express high levels of mCAT1 (NFM cells) were infected with MCF247 virus and cultured for 30 days. These cells and uninfected controls were then challenged with the MCF247 viral vector containing E. coli lacZ in the presence or absence of the sFr-RBD at the indicated concentrations. Infection was scored 2 days later (i.u. per milliliter) by counting the foci of cells expressing β-galactosidase.
DISCUSSION
Alternative infection pathway for MCF virus.
Previous studies identified membrane protein Syg1 (6, 43, 46) as the receptor for the class of recombinant retroviruses, termed MCF viruses, that cause leukemia in mice (7, 17, 24, 32, 33). In this report, we provide evidence of an alternative mechanism of MCF virus infection that does not utilize Syg1 receptor. By this route, MCF virus infection is triggered in trans by the envelope glycoprotein of its parent ecotropic virus expressed on the cell surface and bound to its receptor, mCAT1.
Studies of mutant retroviruses in which fusion and entry are uncoupled from receptor binding provided the rationale for the experiments that led to this discovery (3, 4, 25, 27). These viruses are defective, but infection is restored in the presence of RBDs from wild-type viruses that are supplied in trans. RBD is active when expressed as a recombinant protein and added to the cell culture medium or as part of an intact envelope glycoprotein expressed on the target cell membrane. Receptor binding primes the trans activation activity of RBD, perhaps by inducing a change in conformation and/or by recruiting a required cofactor(s) (4, 27). The result is the formation of an active complex on the host cell membrane that likely interacts directly with the envelope glycoprotein of the virus to trigger fusion.
In the initial studies of defective ecotropic and amphotropic virus infection, binding of both the virus RBD and the trans-activating RBD to the receptor was required to restore infection. The requirement for virus receptor binding was circumvented by deletion of the RBD from the virus envelope glycoprotein (3, 4). In this regard, the viral RBD behaves as an appendage that is not required for assembly of a fusion-competent virus envelope glycoprotein and, prior to receptor binding, sterically interferes with activation of the virus fusion mechanism by the receptor-activated RBD supplied in trans.
The flexibility of the proline-rich segment that links the RBD to the remainder of the envelope glycoprotein may explain how receptor binding is able to displace the viral RBD and thereby permit the trans-activating RBD to gain access to the virus fusion mechanism. Moreover, these studies raised the possibility that naturally occurring viruses exist in which the RBD is present and functional but does not sterically block the access of the trans-activating RBD to the virus fusion mechanism. The MCF247 virus (and perhaps FeLV-T) was identified in a screen designed to detect such a virus. Three observations support the conclusion that binding of the MCF247 virus to its receptor is not required for trans activation of infection by the sFr-RBD. First, expression of the hamster Syg1 protein does not support MCF247 virus infection of CHTG cells under conditions in which the mouse and human Syg1 proteins are functional receptors (30; Fig. 2). Studies of chimeric Syg1 proteins designed to identify the receptor determinants of virus infection indicate that the hamster SYG1 protein does not bind to MCF or to xenotropic virus sRBDs (6; Wensel, unpublished), thereby explaining the failure of hamster Syg1 to support MCF247 infection. Second, the Fr-RBD receptor, mCAT1, is unrelated to Syg1 and does not support MCF247 virus infection alone. In addition, the viral targets for the trans activation activity of the sFr-RBD/mCAT1 complex are not restricted to the MCF class of retroviruses (3, 26; Fig. 1). Therefore, it is unlikely that mCAT1 has cryptic MCF virus receptor activity. Third, trans activation by sFr-RBD restored MCF247 virus infection of mouse fibroblasts in which superinfection interference was established by chronic MCF247 virus infection (Fig. 5). In these cells, the Syg1 receptor is down-regulated by expression of the MCF247 virus envelope glycoprotein. On the basis of these findings, we concluded that MCF247 virus infection is triggered by an RBD-receptor complex that can be supplied by either of two independent means: (i) through binding of the virus RBD to the Syg1 receptor or (ii) through trans activation by the ecotropic Fr-RBD bound to its receptor, mCAT1.
FeLV-T and trans activation.
The screening protocol for trans activation-dependent viruses also identified FeLV-T, which, like MCF247, is also a recombinant virus that is highly cytopathic (14, 35). FeLV-T was isolated from a cat that developed T-cell depletion and an AIDS-like syndrome after initial infection with nonpathogenic FeLV-A. The tropism of FeLV-T is restricted to T lymphocytes that express a truncated portion of an envelope glycoprotein that contains a functional RBD and is encoded by an endogenous provirus (1). Recent studies have indicated that FeLV-T is defective for receptor-dependent entry and the requirement for the truncated envelope glycoprotein is indicative of trans activation by the RBD (4a). FeLV-T contains a proline residue in place of histidine in the SerProHisGln motif that is present near the N terminus of all functional MLV envelope glycoproteins (35). Mutant MLVs that lack this His residue exhibit the postreceptor block to infection (2) that is bypassed by sFr-RBD-dependent trans activation (3, 4, 27). A receptor for FeLV-T has not been definitively identified, and therefore it is not possible to determine its role, if any, in sFr-RBD-dependent trans activation. However, on the basis of the behavior of the MCF247 virus, we speculate that binding of the FeLV-T envelope glycoprotein to its receptor may not be required to establish susceptibility to trans activation.
Contribution of trans activation to infection of Syg1 receptor-bearing cells.
Human 293mCAT1 cells were studied to assess the contribution of trans activation to MCF247 virus infection in cells with an intact Syg1 receptor-mediated entry pathway. A threefold increase in the titer of a single round of MCF247 virus infection of these cells was observed in the presence of the sFr-RBD. Moreover, the MCF247 virus spread more rapidly through the culture when the sFr-RBD was present in the medium. These findings indicate that the contribution of trans activation to MCF247 virus replication is significant even when the mechanism for receptor-dependent entry is intact. Thirty to 35 days after initial infection, the rate of MCF247 virus spreading increased in the cells propagated without the sFr-RBD, possibly reflecting virus adaptation to replication in the absence of trans activation. Consistent with this possibility, changes in the amino acid sequence of the virus envelope glycoprotein were identified. The effects of these changes on virus growth in the presence and absence of the sFr-RBD are being tested.
The marked cytopathic effect of MCF247 virus infection in chronically infected cells was not anticipated but provided a useful parameter with which to follow the course of infection. The onset of cell-cell fusion occurred 2 weeks earlier in sFr-RBD-treated cells and was correlated with expression of the MCF247 envelope glycoprotein. Analysis of chronically infected cells that survived the period of intense virus-induced cell-cell fusion (40 to 70 days after initial infection) indicated that both sFr-RBD-treated and untreated control cells were markedly resistant to further infection by the MCF247 virus, consistent with down-regulation of functional Syg1 receptors. A single exposure to the sFr-RBD caused rapid and extensive cell-cell fusion of chronically infected cells prepared in the absence of trans activation, indicating that these cells express functional MCF247 envelope glycoproteins and mCAT1 receptors on the surface membrane. In contrast, chronically infected cells that had been continuously exposed to the sFr-RBD were resistant to additional sFr-RBD-induced fusion and this property was correlated with loss of mCAT1 receptor expression (Fig. 4C). Therefore, resistance to cell-cell fusion in sFr-RBD-treated cells was specifically associated with down-regulation of trans activation activity through loss of mCAT1 receptor expression. The human 293mCAT1 cells used in these experiments were originally prepared by G418 drug selection of a polyclonal population of human 293 cells transfected with an expression plasmid coding for mCAT1 and for neomycin phosphotransferase (13). It is likely that a small number of G418-resistant clones that do not express mCAT1 were present in the initial selected culture. We propose that these clones survive the period of cell-cell fusion because they have inactivated the traditional entry pathway mediated by the Syg1 receptor and were unable to support the trans activation pathway mediated by the sFr-RBD/mCAT1 receptor complex.
The maturation state of viral envelope glycoproteins strongly regulates their function. In an infected cell, envelope glycoproteins that form complexes with receptors in the plasma membrane can trigger additional infection by trans activation. Formation of the trans activation complex does not trigger the fusion mechanism of envelope glycoproteins in the plasma membrane. These proteins are only rendered fusion-competent after incorporation into budding virions, which promotes cleavage of 16 residues from their C termini by the viral protease. It is likely that the cell-cell fusion observed in our studies of chronically infected cells was mediated by envelope glycoproteins on virions in the late stages of budding that had completed maturation.
Properties of envelope glycoproteins that contribute to virus-induced pathogenesis.
More than 90% of susceptible strains of mice that inherit or acquire a replication-active ecotropic virus develop leukemia before reaching 10 months of age (10, 11, 39, 40). In most strains of mice, the onset of leukemia is preceded by the appearance of MCF viruses that are first detected within 3 to 6 months after ecotropic virus infection (16, 17). Coinjection of newborn mice with ecotropic and MCF viruses decreases the interval between infection and onset of leukemia from months to weeks (10). Also, mouse strains that are unable to create or support the replication of MCF viruses are resistant to virus-induced leukemia (11). Apoptosis of MCF virus-infected hematopoietic cells prior to the onset of leukemia has been well documented (8, 17). This may occur because of cell-cell fusion, as observed in the in vitro studies in this report and/or as part of the cell response to unintegrated proviral DNA, as has been observed in mink cells chronically infected with the MCF13 virus (48). Analysis of virus-transformed leukemic cells indicates that they are derived from a single cell clone and almost always contain at least one MCF provirus inserted adjacent to a gene implicated in leukemic transformation (17). Activation of these genes may allow clonal escape from apoptotic signals associated with chronic MCF virus infection and thereby provide the substrate for additional genetic changes that result in leukemic transformation.
In one careful study, at least 6 to 10 new MCF proviruses per leukemic cell were identified (22). The presence of multiple acquired proviruses per cell was also observed in our studies of MCF247 virus infection of human 293mCAT1 cells. The capacity of MCF viruses to repeatedly infect host cells is distinct from that of ecotropic and other nonpathogenic MLVs. For these viruses, expression of envelope glycoprotein from a single provirus is sufficient to down-regulate the receptor and prevent additional infection. In this regard, the role of MCF viruses in the pathogenesis of leukemia is likely directly related to the increased probability of insertional gene activation caused by repeated infection.
The repeated infection of cells by MCF viruses may be caused by the failure of envelope glycoprotein expression to efficiently down regulate the Syg1 receptor from the cell surface, perhaps because of low binding affinity (30; Wensel, unpublished). In addition, trans activation may also favor repeated infection since the ecotropic envelope glycoprotein-receptor complex is not susceptible to down regulation by expression of the immature MCF virus envelope glycoprotein. An unresolved question is whether the MCF-RBD/Syg1 complex can trans-activate MCF virus infection.
Cats infected with FeLV-T develop immunodeficiency caused by the premature T-cell death associated with the accumulation of as many as 100 proviruses per cell (14). Since the traditional receptor-mediated entry pathway is blocked for this virus, repeated infection is strictly mediated by trans activation. Therefore, both reduced affinity of the virus RBD for the receptor and enhanced susceptibility to trans activation contribute to pathogenesis associated with multiple infections. The outcome of repeated infection, e.g., oncogenic transformation versus premature cell death, may reflect quantitative differences in provirus accumulation (28, 48).
trans activation as an example of virus-virus cooperation.
trans activation of MCF247 virus is specific to the sFr-RBD, and conversely, FeLV-T infection is more robust in the presence of the FeB RBD than in the presence of the sFr-RBD. In addition, the sRBD derived from amphotropic murine virus that binds to the Pit2 receptor did not support infection by either virus (data not shown). Therefore, both FeLV-T and the MCF247 virus may be adapted to trans activation by a specific RBD. FeLV-T and the MCF247 virus are the products of recombination with endogenous proviruses that express mRNAs that are copackaged into virions. Recombination between the dual virus genomes of retroviruses serves as a sifting mechanism that segregates the frequent errors introduced by reverse transcriptase. This process maintains core features of virus structure and function while allowing rapid selection of advantageous changes dictated by the host environment. Because of the frequency of mutation and recombination, at any one moment, an infected host contains a large number of distinct but closely related retroviruses, such as MCF variants. The observation that at least three recombination events are required to create an MCF virus suggests that they are specifically selected from this population (43). Susceptibility to trans activation may contribute to the selective advantage. The observation that FeLV-T replication is also dependent on trans activation suggests that this phenomenon may be a common property among recombinant retroviruses that emerge during ongoing infection and may be a critical determinant of viral pathogenesis. The discovery of trans activation raises the possibility that additional cooperative virus-virus interactions that have not yet been identified also shape the composition and properties of the retrovirus quasispecies.
Acknowledgments
We acknowledge Anna Barnett for providing RBDs, Julie Overbaugh for providing FeLV-T plasmids, Weining Lu for advice on quantitative PCR, and Jason Smith for suggestions on the manuscript.
This work was supported by the Howard Hughes Medical Institute (J.M.C.). D.L.W. is a Howard Hughes Medical Institute predoctoral fellow.
REFERENCES
- 1.Anderson, M. M., A. S. Lauring, C. C. Burns, and J. Overbaugh. 2000. Identification of a cellular cofactor required for infection by feline leukemia virus. Science 287:1828-1830. [DOI] [PubMed] [Google Scholar]
- 2.Bae, Y., S. M. Kingsman, and A. J. Kingsman. 1997. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J. Virol. 71:2092-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett, A. L., and J. M. Cunningham. 2001. Receptor binding transforms the surface subunit of the mammalian C-type retrovirus envelope protein from an inhibitor to an activator of fusion. J. Virol. 75:9096-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett, A. L., R. A. Davey, and J. M. Cunningham. 2001. Modular organization of the Friend murine leukemia virus envelope protein underlies the mechanism of infection. Proc. Natl. Acad. Sci. USA 98:4113-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Barnett, A. L., D. L. Wensel, W. Li, D. Fass, and J. M. Cunningham. 2003. Structure and mechanism of a coreceptor for infection by a pathogenic feline retrovirus. J. Virol. 77:2717-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battini, J. L., O. Danos, and J. M. Heard. 1995. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J. Virol. 69:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battini, J. L., J. F. Rasko, and A. D. Miller. 1999. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc. Natl. Acad. Sci. USA 96:1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belli, B., L. Wolff, V. Nazarov, and H. Fan. 1995. Proviral activation of the c-myb proto-oncogene is detectable in preleukemic mice infected neonatally with Moloney murine leukemia virus but not in resulting end stage T lymphomas. J. Virol. 69:5138-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonzon, C., and H. Fan. 1999. Moloney murine leukemia virus-induced preleukemic thymic atrophy and enhanced thymocyte apoptosis correlate with disease pathogenicity. J. Virol. 73:2434-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesebro, B., and K. Wehrly. 1985. Different murine cell lines manifest unique patterns of interference to superinfection by murine leukemia viruses. Virology 141:119-129. [DOI] [PubMed] [Google Scholar]
- 10.Cloyd, M. W., J. W. Hartley, and W. P. Rowe. 1980. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J. Exp. Med. 151:542-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cloyd, M. W., J. W. Hartley, and W. P. Rowe. 1981. Genetic study of lymphoma induction by AKR mink cell focus-inducing virus in AKR × NFS crosses. J. Exp. Med. 154:450-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davey, R. A., C. A. Hamson, J. J. Healey, and J. M. Cunningham. 1997. In vitro binding of purified murine ecotropic retrovirus envelope surface protein to its receptor, MCAT-1. J. Virol. 71:8096-8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davey, R. A., Y. Zuo, and J. M. Cunningham. 1999. Identification of a receptor-binding pocket on the envelope protein of Friend murine leukemia virus. J. Virol. 73:3758-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donahue, P. R., S. L. Quackenbush, M. V. Gallo, C. M. deNoronha, J. Overbaugh, E. A. Hoover, and J. I. Mullins. 1991. Viral genetic determinants of T-cell killing and immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J. Virol. 65:4461-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elder, J. H., J. W. Gautsch, F. C. Jensen, R. A. Lerner, J. W. Hartley, and W. P. Rowe. 1977. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc. Natl. Acad. Sci. USA 74:4676-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans, L. H., and M. W. Cloyd. 1985. Friend and Moloney murine leukemia viruses specifically recombine with different endogenous retroviral sequences to generate mink cell focus-forming viruses. Proc. Natl. Acad. Sci. USA 82:459-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan, H. 1997. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol. 5:74-82. [DOI] [PubMed] [Google Scholar]
- 18.Fass, D., R. A. Davey, C. A. Hamson, P. S. Kim, J. M. Cunningham, and J. M. Berger. 1997. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science 277:1662-1666. [DOI] [PubMed] [Google Scholar]
- 19.Han, L., T. Hofmann, Y. Chiang, and W. F. Anderson. 1995. Chimeric envelope glycoproteins constructed between amphotropic and xenotropic murine leukemia retroviruses. Somat. Cell Mol. Genet. 21:205-214. [DOI] [PubMed] [Google Scholar]
- 20.Hayward, W. S., B. G. Neel, and S. M. Astrin. 1981. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature 290:475-480. [DOI] [PubMed] [Google Scholar]
- 21.Heard, J. M., and O. Danos. 1991. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J. Virol. 65:4026-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herr, W., and W. Gilbert. 1984. Free and integrated recombinant murine leukemia virus DNAs appear in preleukemic thymuses of AKR/J mice. J. Virol. 50:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland, C. A., J. W. Hartley, and N. Hopkins. 1984. Viral integration near c-myc in 10 to 20% of MCF 247-induced AKR lymphomas. Proc. Natl. Acad. Sci. USA 81:6808-6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavignon, M., and L. Evans. 1996. A multistep process of leukemogenesis in Moloney murine leukemia virus-infected mice that is modulated by retroviral pseudotyping and interference. J. Virol. 70:3852-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavillette, D., B. Boson, S. J. Russell, and F. L. Cosset. 2001. Activation of membrane fusion by murine leukemia viruses is controlled in cis or in trans by interactions between the receptor-binding domain and a conserved disulfide loop of the carboxy terminus of the surface glycoprotein. J. Virol. 75:3685-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavillette, D., A. Ruggieri, B. Boson, M. Maurice, and F. L. Cosset. 2002. Relationship between SU subdomains that regulate the receptor-mediated transition from the native (fusion-inhibited) to the fusion-active conformation of the murine leukemia virus glycoprotein. J. Virol. 76:9673-9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavillette, D., A. Ruggieri, S. J. Russell, and F. L. Cosset. 2000. Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments. J. Virol. 74:295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, Q. X., and H. Fan. 1990. Combined infection by Moloney murine leukemia virus and a mink cell focus-forming virus recombinant induces cytopathic effects in fibroblasts or in long-term bone marrow cultures from preleukemic mice. J. Virol. 64:3701-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loiler, S. A., N. L. DiFronzo, and C. A. Holland. 1997. Gene transfer to human cells using retrovirus vectors produced by a new polytropic packaging cell line. J. Virol. 71:4825-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marin, M., C. S. Tailor, A. Nouri, S. L. Kozak, and D. Kabat. 1999. Polymorphisms of the cell surface receptor control mouse susceptibilities to xenotropic and polytropic leukemia viruses. J. Virol. 73:9362-9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markowitz, D., S. Goff, and A. Bank. 1988. Construction and use of a safe and efficient amphotropic packaging cell line. Virology 167:400-406. [PubMed] [Google Scholar]
- 32.Miller, A. D., and G. Wolgamot. 1997. Murine retroviruses use at least six different receptors for entry into Mus dunni cells. J. Virol. 71:4531-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nusse, R. 1986. The activation of cellular oncogenes by retroviral insertion. Trends Genet. 2:244-247. [Google Scholar]
- 34.Nusse, R., A. van Ooyen, D. Cox, Y. K. Fung, and H. Varmus. 1984. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature 307:131-136. [DOI] [PubMed] [Google Scholar]
- 35.Overbaugh, J., P. R. Donahue, S. L. Quackenbush, E. A. Hoover, and J. I. Mullins. 1988. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science 239:906-910. [DOI] [PubMed] [Google Scholar]
- 36.Peredo, C., L. O'Reilly, K. Gray, and M. J. Roth. 1996. Characterization of chimeras between the ecotropic and amphotropic 4070A envelope proteins. J. Virol. 70:3142-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rein, A. 1982. Interference grouping of murine leukemia viruses: a distinct receptor for the MCF-recombinant viruses in mouse cells. Virology 120:251-257. [DOI] [PubMed] [Google Scholar]
- 38.Rein, A., and A. Schultz. 1984. Different recombinant murine leukemia viruses use different cell surface receptors. Virology 136:144-152. [DOI] [PubMed] [Google Scholar]
- 39.Rowe, W. P. 1972. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J. Exp. Med. 136:1272-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe, W. P., and J. W. Hartley. 1983. Genes affecting mink cell focus-inducing (MCF) murine leukemia virus infection and spontaneous lymphoma in AKR F1 hybrids. J. Exp. Med. 158:353-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sommerfelt, M. A., and R. A. Weiss. 1990. Receptor interference groups of 20 retroviruses plating on human cells. Virology 176:58-69. [DOI] [PubMed] [Google Scholar]
- 42.Soneoka, Y., P. M. Cannon, E. E. Ramsdale, J. C. Griffiths, G. Romano, M. S. Kingsman, and A. J. Kingsman. 1995. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23:629-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoye, J. P., C. Moroni, and J. M. Coffin. 1991. Virological events leading to spontaneous AKR thymomas. J. Virol. 65:1273-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tailor, C. S., A. Nouri, C. G. Lee, C. Kozak, and D. Kabat. 1999. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc. Natl. Acad. Sci. USA 96:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss, R. A., and C. S. Tailor. 1995. Retrovirus receptors. Cell 82:531-533. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, C. A., and M. V. Eiden. 1991. Viral and cellular factors governing hamster cell infection by murine and gibbon ape leukemia viruses. J. Virol. 65:5975-5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, Y. L., L. Guo, S. Xu, C. A. Holland, T. Kitamura, K. Hunter, and J. M. Cunningham. 1999. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nat. Genet. 21:216-219. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimura, F. K., T. Wang, and S. Nanua. 2001. Mink cell focus-forming murine leukemia virus killing of mink cells involves apoptosis and superinfection. J. Virol. 75:6007-6015. [DOI] [PMC free article] [PubMed] [Google Scholar]